Abstract
The ability of human embryonic stem cells (hESCs) to differentiate into skeletal muscle cells is an important criterion in using them as a cell source to ameliorate skeletal muscle impairments. However, differentiation of hESCs into skeletal muscle cells still remains a challenge, often requiring introduction of transgenes. Here, we describe the use of WNT3A protein to promote in vitro myogenic commitment of hESC-derived cells and their subsequent in vivo function. Our findings show that the presence of WNT3A in culture medium significantly promotes myogenic commitment of hESC-derived progenitors expressing a mesodermal marker, platelet-derived growth factor receptor-α (PDGFRA), as evident from the expression of myogenic markers, including DES, MYOG, MYH1, and MF20. In vivo transplantation of these committed cells into cardiotoxin-injured skeletal muscles of NOD/SCID mice reveals survival and engraftment of the donor cells. The cells contributed to the regeneration of damaged muscle fibers and the satellite cell compartment. In lieu of the limited cell source for treating skeletal muscle defects, the hESC-derived PDGFRA+ cells exhibit significant in vitro expansion while maintaining their myogenic potential. The results described in this study provide a proof-of-principle that myogenic progenitor cells with in vivo engraftment potential can be derived from hESCs without genetic manipulation.
Over the past few decades, human embryonic stem cells (hESCs) have received much attention, owing to their potential to contribute to cell-based regenerative medicine and drug screening platforms1,2,3. Recent advancements indicate that hESC-derived myogenic progenitor cells could contribute significantly towards the regeneration of compromised skeletal muscle tissues4,5,6,7. However, there exist numerous challenges before the full potential of hESCs as a cell source for treating injured or diseased skeletal muscle tissues can be realized. Some of these challenges include low yield of myogenic progenitors and their limited in vivo engraftment efficiency upon transplantation8,9,10,11.
A number of approaches, including mRNA transfection, genetic manipulation, and small molecule treatment, have been employed to direct differentiation of hESCs into skeletal muscle cells12,13,14. A few studies have also showed that hESC-derived mesoderm progenitor cells can undergo myogenic differentiation in vitro and contribute to skeletal muscle tissue repair in vivo15,16. Recently, we have shown that platelet-derived growth factor receptor-α (PDGFRA+) positive cells derived from hESCs exhibit extensive proliferative capacity and can be differentiated into skeletal muscle cells17. Although this study demonstrated the myogenic differentiation potential of hESCs, their in vivo engraftment into injured skeletal muscle upon transplantation was minimal. Here, we sought to improve myogenic differentiation of these hESC-derived PDGFRA+ cells by incorporating Wnt signaling.
The canonical Wnt signaling pathway has been shown to play an essential role in regulating stem cell fates and in regeneration of muscle tissues18,19,20,21. Early studies by Ikeya and his colleagues have shown that Wnt-1 and Wnt-3 signaling from the developing neural tubes could promote myogenic differentiation of dorsal and medial somite cells22. Studies have also demonstrated that members of the Wnt family can play significant roles in various stages of developmental myogenesis, including the formation of dermomyotome and myotome, as well as skeletal muscle regeneration23,24,25. Moreover, the Wnt signaling pathway is shown to be critical for satellite cell activation and differentiation during skeletal muscle injury or degeneration26,27,28. Previously, Shang et al. have successfully shown the potential application of Wnt/β-catenin signaling to induce myogenic differentiation of rat mesenchymal stem cells29,30. P19 embryonal carcinoma stem cells overexpressing WNT3A have been shown to undergo spontaneous myogenic differentiation31. Another study by Ridgeway et al. have showed that P19 embryonal carcinoma stem cells cocultured with those overexpressing WNT3A undergo terminal myogenic differentiation32. In a recent study, Barberi and colleagues have used GSK3β inhibitor to generate skeletal muscle precursor cells, expressing PAX3 and PAX7, from human pluripotent stem cells33. The beneficial effect of WNT signaling on myogenic commitment of human induced pluripotent stem cells (hiPSCs) was also demonstrated by Xu et al34. Among the 2400 chemicals screened, the authors have showed that a cocktail of bFGF, forskolin, and GSK3β inhibitor induced myogenic differentiation of hiPSCs into in vivo engraftable myogenic progenitor cells.
In this study, we have investigated the effect of WNT3A protein on myogenic differentiation of PDGFRA+ cells derived from hESCs. When transplanted into cardiotoxin-injured skeletal muscles of NOD/SCID mice, these committed cells were found to exhibit significantly higher cell engraftment and contribution to regenerating myofibers and satellite cell compartment compared to their untreated counterparts.
Results
WNT3A-conditioned induction medium promotes myogenic differentiation of hESC-derived PDGFRA+ cells
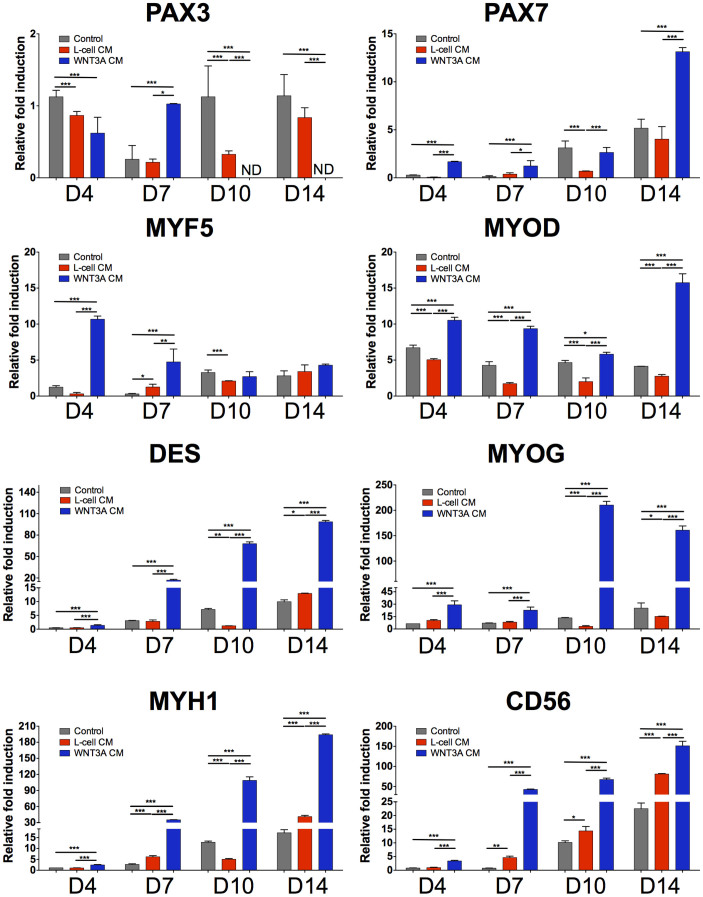
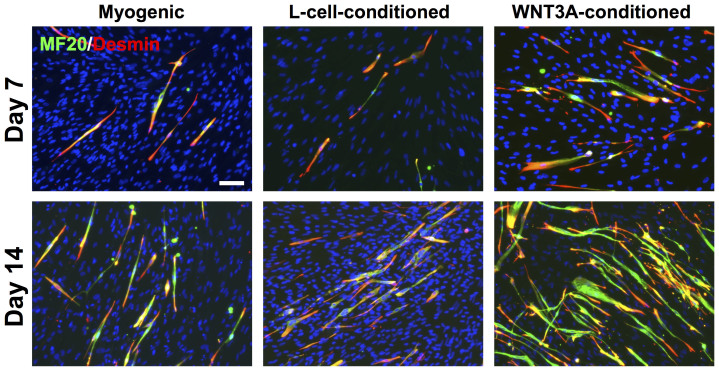
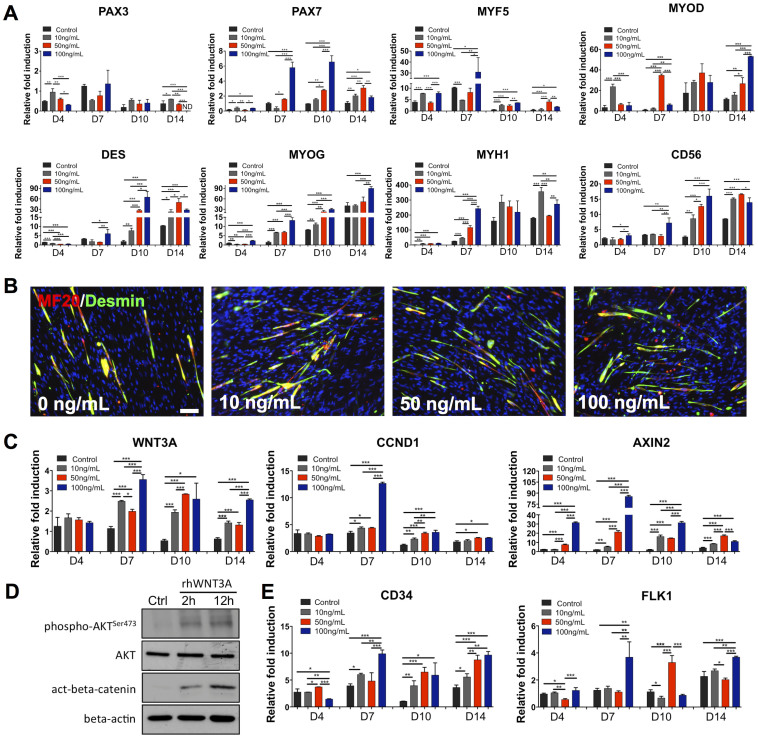
We have derived PDGFRA+ mesoderm progenitor cells, exhibiting myogenic differentiation potential, from hESCs as previously described (Fig. 1)17. Myogenic differentiation was achieved by treating hESC-derived PDGFRA+ cells in an induction medium consisting of 2 mM L-glutamine, 100 nM dexamethasone, 100 μM hydrocortisone, 1% penicillin/streptomycin, 10 μM transferrin, 860.9 nM recombinant insulin, 20 nM progesterone, 100.1 μM putrescine, and 30.1 nM selenite, albeit at a low efficiency. To determine whether exogenous WNT3A could further promote myogenic commitment of hESC-derived PDGFRA+ population, the cells were cultured in WNT3A-conditioned induction medium and compared against those cultured in induction medium or L-cell-conditioned induction medium. Supplementary Figure S1 shows the phase contrast images of PDGFRA+ cells (after passage 8) cultured in different medium conditions for 7 days. Irrespective of the medium conditions, the PDGFRA+ cells showed typical spindle shape morphology and grew to confluence with no obvious differences in cell shape. The myogenic commitment of these cells was examined for a number of early myogenic markers such as PAX3, PAX7 and MYF5 (Fig. 2). The gene expression pattern suggests that the cells cultured in WNT3A-conditioned induction medium showed an early upregulation of MYF5, followed by its downregulation as a function of culture time. The PAX3 expression of cells cultured in WNT3A-conditioned induction medium was also downregulated significantly with culture time. On the other hand, PAX7 expression in these cells showed a continuous upregulation with the highest expression at day 14. In addition, the cells cultured in WNT3A-conditioned induction medium showed upregulation of various late myogenic markers, like MYOD, DES, MYOG, and MYH1, compared to the control cultures. The cells cultured in presence of WNT3A also showed an upregulation of CD56. The immunofluorescence staining for sarcomeric myosin (MF20) and desmin (DES) further corroborated the findings from the gene expression profile (Fig. 3). The differentiation index, calculated as the fraction of total nuclei that are MF20-positive, showed that a significantly higher number of cells in WNT3A-conditioned induction medium underwent myogenic differentiation (Supplementary Figs. S2A–B). However, we have not observed any significant difference in the fusion index, which is calculated as fraction of MF20-positive cells containing 3 or more nuclei, likely because most myogenically committed cells (MF20-positive cells) in all culture conditions were multinucleated (Supplementary Fig. S2C). In addition to promoting myogenic differentiation, our time-dependent analyses revealed that myogenic differentiation of hESC-derived PDGFRA+ cells was shifted to earlier time points when cultured in WNT3A-conditioned induction medium compared to the control cultures (Fig. 2).
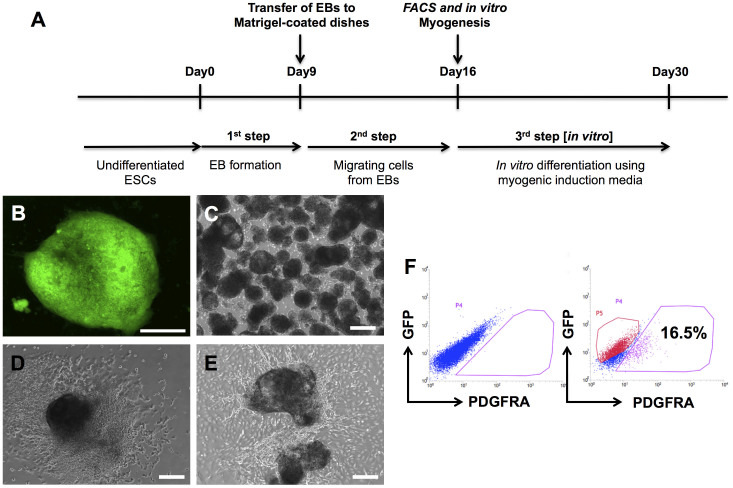
Figure 1. Derivation of PDGFRA+ cells from undifferentiated hESCs.
(A) Schematic describing the derivation of PDGFRA+ cells. (B) Undifferentiated hESC colony with OCT4-GFP expression. (C) EB formation. (D) EB attached to the Matrigel-coated plates. (E) Migrating cells from EBs. (F) Representative flow cytometric plots showing PDGFRA+ and OCT4-GFP− populations. Scale bar = 200 μm.
Figure 2. In vitro myogenic differentiation of PDGFRA+ cells in WNT3A-conditioned induction medium.
Gene expression profiles of PDGFRA+ cells cultured in induction medium, L-cell-conditioned, and WNT3A-conditioned induction media. Statistical analysis was performed among cells cultured in different media within the same time point. *p < 0.05, **p < 0.01, and ***p < 0.001.
Figure 3. Terminal myogenic differentiation of cells cultured in WNT3A-conditioned induction medium.
Immunofluorescence staining for MF20 (green) and DES (red) of PDGFRA+ cells cultured in induction medium, L-cell-conditioned, and WNT3A-conditioned induction media as a function of time. Scale bar = 100 μm.
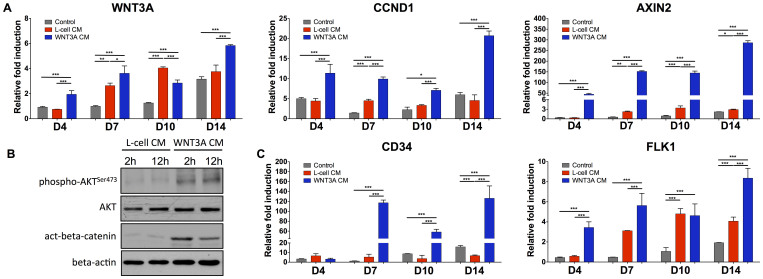
We further examined the effect of WNT3A-conditioned induction medium on endogenous gene expressions of WNT3A and its downstream targets such as cyclin D1 (CCND1) and AXIN2. As shown in Fig. 4A, the cells cultured in WNT3A-conditioned induction medium consistently showed upregulation of WNT3A, CCND1, and AXIN2 compared to the control cultures at all experimental time points. This is preceded by the markedly elevated levels of phosphorylated AKT and accumulation of beta-catenin, as evident by the western blot analyses (Fig. 4B). Additionally, cells cultured in WNT3A-conditioned induction medium also exhibited upregulation of CD34 and FLK1 (Fig. 4C), which are established markers for hematopoietic stem cells and have been shown to promote the in vivo cell viability and engraftment35,36,37.
Figure 4. Gene expression profile of cells cultured in WNT3A-conditioned induction medium and protein analysis of WNT3A signaling.
(A), (C) Gene expression profiles of PDGFRA+ cells cultured in induction medium, L-cell-conditioned, and WNT3A-conditioned induction media. Statistical analysis was performed among cells cultured in different media within the same time point. (B) Expression of AKT phosphorylation at Ser473 and active beta-catenin in PDGFRA+ cells cultured in L-cell-conditioned or WNT3A-conditioned induction medium at 2 and 12 hours. Equal amount of protein loading was verified by beta-actin. Images were cropped to show the indicated bands and uncropped images of Western blots are presented in Supplementary Fig. S5. *p < 0.05, **p < 0.01, and ***p < 0.001.
Recombinant human WNT3A protein promotes myogenesis of hESC-derived PDGFRA+ cells
To further verify the role of WNT3A protein on myogenic commitment of PDGFRA+ cells, we have cultured the cells in induction medium containing varying amounts (0, 10, 50, and 100 ng/mL) of human recombinant WNT3A protein (rhWNT3A) (Supplementary Fig. S3). As shown in Fig. 5A, early myogenic markers, including PAX3, PAX7, and MYF5, were found to be downregulated in cells cultured in the presence of rhWNT3A protein. In contrast, these cells showed significant upregulation of other myogenic markers, such as MYOD, DES, MYOG, MYH1, and CD56. The cells cultured in medium containing rhWNT3A protein also stained positive for MF20 and DES, suggesting terminal differentiation of the cells (Fig. 5B, Supplementary Fig. S2D–E). Similar to the WNT3A-conditioned induction medium, cells cultured with rhWNT3A protein resulted in the upregulation of endogenous WNT3A and its target genes CCND1 and AXIN2 (Fig. 5C). Similar to WNT3A-conditioned medium, Western blot analyses showed higher levels of phosphorylation of AKT at Ser473 and active beta-catenin in cells cultured in media containing 50 ng/mL of rhWNT3A protein (Fig. 5D). The cells cultured in medium supplemented with rhWNT3A also exhibited an upregulation of CD34 and FLK1 (Fig. 5E).
Figure 5. In vitro myogenic differentiation of PDGFRA+ cells in induction medium containing recombinant human WNT3A protein.
(A), (C), (E) Gene expression profiles of PDGFRA+ cells cultured in induction medium, and medium supplemented with varying amount of recombinant human WNT3A protein. Statistical analysis was performed among cells cultured in different media within the same time point. *p < 0.05, **p < 0.01, and ***p < 0.001. (B) Immunofluorescence staining for MF20 (green) and DES (red) of PDGFRA+ cells cultured in induction medium, and medium supplemented with different amounts of recombinant human WNT3A protein for 14 days in vitro. (D) Phosphorylation of AKT at Ser473 and active beta-catenin expression in PDGFRA+ cells cultured in induction medium containing rhWNT3A (50 ng/mL) at 2 and 12 hours. Equal amount of protein loading was verified by beta-actin. Images were cropped to show the indicated bands and uncropped images of Western blots are presented in Supplementary Fig. S5. Scale bar = 100 μm.
In vivo engraftment of hESC-derived myogenic progenitors in a cardiotoxin-injury model
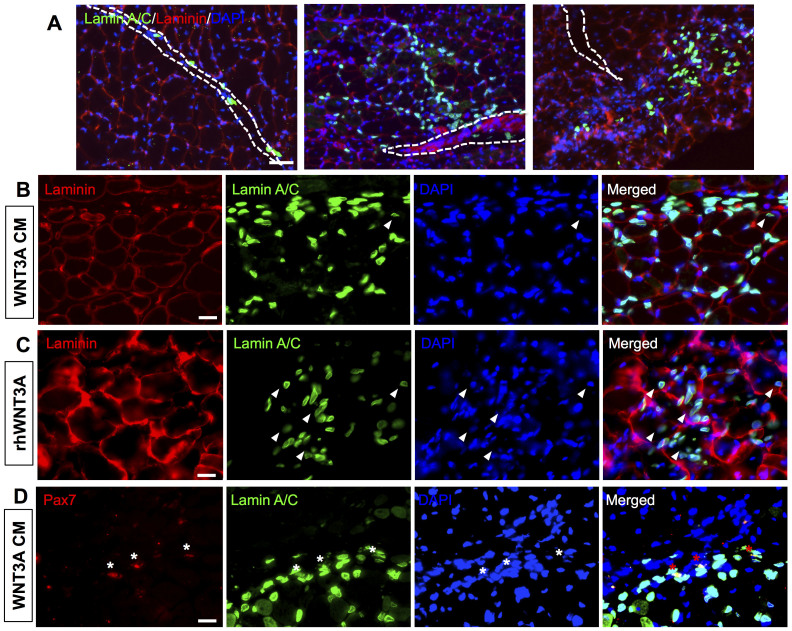
We next evaluated the in vivo engraftment potential of hESC-derived myogenic progenitor cells. We used three cell populations with varying levels of preconditioning prior to transplantation— hESC-derived PDGFRA+ cells cultured for 14 days in (i) induction medium, (ii) WNT3A-conditioned induction medium, or (iii) induction medium supplemented with 50 ng/mL of rhWNT3A. The preconditioned cells were subsequently transplanted into cardiotoxin-injured TA muscles of 2-month-old immunodeficient NOD/SCID mice. Fourteen days after transplantation, the TA muscles were characterized to assess the viability and in vivo contribution of the donor cells. The harvested TA muscles were immunostained for human-specific lamin A/C and mouse laminin to visualize the donor cells within the host tissues. Regardless of the differences in preconditioning, histological analyses of the host tissue identified presence of donor cells 14 days post-transplantation (Fig. 6A). However, significant differences were observed in their engraftment efficiency and ability to migrate and contribute to tissue repair. A significantly higher number of donor cells were found when the transplanted cells were preconditioned with either rhWNT3A protein or WNT3A-conditioned induction medium (Fig. 6A and Supplementary Fig. S4). In addition to contributing to the survival of the transplanted cells, preconditioning also had a significant effect on the in vivo contribution of the transplanted cells. Most of the cells cultured in induction medium prior to transplantation were found to be in the interstitial space near the muscle fibers (Fig. 6A, left panel). On the contrary, cells cultured in medium containing Wnt components (rhWNT3A protein or WNT3A-conditioned induction medium) prior to their transplantation were found to disseminate away from the injection site (Fig. 6A, center and right panels). The presence of donor cell-positive nuclei located in the center of the muscle fibers indicates the contribution of donor cells to the regeneration of host muscle fibers (Fig. 6B–C). This contribution of donor cells to the regeneration of damaged muscle fibers was observed only with cell populations that were cultured in medium containing WNT3A protein prior to transplantation. We also determined the contribution of transplanted cells to the satellite cell compartment by staining serial muscle sections for PAX7, a satellite cell marker, human-specific lamin A/C, and mouse laminin. As seen from Fig. 6D, we have detected both PAX7 and human-specific lamin A/C positive cells that were located at the basal membrane of the muscle fibers in the case of donor cells preconditioned in WNT3A-conditioned induction medium. This indicates contribution of donor cells into the satellite cell compartment. No such contribution to the satellite cell compartment was observed in our experiment with cells preconditioned with induction medium or induction medium containing rhWNT3A.
Figure 6. Engraftment of myogenic progenitors in cardiotoxin-injured NOD/SCID mice.
(A) Immunofluorescence staining of TA muscle sections of NOD/SCID mice injected with cells cultured in induction medium (left), WNT3A-conditioned induction medium (middle), and induction medium supplemented with 50 ng/mL of recombinant human WNT3A protein (right) for 14 days in vitro prior to the transplantation. The dotted white line within the images indicates the needle injection site. Muscle sections were stained for mouse laminin (red), human lamin A/C (green), and nuclei (blue). Corresponding high magnification images for muscles treated with cells cultured for 14 days in WNT3A-conditioned induction medium (B), and induction medium supplemented with 50 ng/mL (C). The white arrowheads within the images indicate the centerally-located nuclei of donor cells. (D) Immunofluorescence staining of TA muscle sections from NOD/SCID mice injected with cells cultured in WNT3A-conditioned induction medium for 14 days in vitro for PAX7 (red) and human lamin A/C (green), and nuclei (blue). The white stars indicate the presence of both Lamin A/C+ and PAX7+ nuclei at the basal membrane. Scale bar = 200, 20, 20, and 20 μm, respectively.
Discussion
Previously, we have devised a derivation protocol to generate myogenic progenitor cells from hESCs17. In this study, we have harnessed Wnt signaling to promote myogenic differentiation of hESC-derived PDGFRA+ cells by using WNT3A-conditioned induction medium or induction medium containing rhWNT3A protein. Our findings show that both WNT3A-conditioned induction medium and induction medium containing rhWNT3A protein promoted the myogenic differentiation of hESC-derived PDGFRA+ cells. These results are in accordance with previous reports33,38. While presence of WNT3A moieties in culture medium promoted myogenic commitment of the hESC-derived cells, there were culture condition-dependent (WNT3A-conditioned induction medium vs. induction medium containing rhWNT3A) differences in the gene expression pattern of the cells and the percentage of cells expressing MF20. These differences could be attributed to various reasons such as the concentration of exogenous proteins, presence of additional cell-secreted factors in the WNT3A-conditioned induction medium, etc. A previous report has suggested that WNT3A-conditioned medium from WNT3A-overexpressing L cells contains ~100–200 ng of WNT3A per mL39; this indicates a higher amount of WNT3A in conditioned medium compared to our cultures containing rhWNT3A. However, our finding from the dose-dependent effect of rhWNT3A on myogenic differentiation shows no significant effect beyond ~50 ng/mL. Taken together, the findings suggest that the difference between WNT3A-conditioned medium and rhWNT3A could be attributed to the presence of other cell-secreted factors having a beneficial effect to myogenic lineage specification in the conditioned medium.
The upregulation of Wnt target genes such as CCND1 and AXIN2 indicates that both WNT3A-conditioned induction medium and induction medium containing rhWNT3A protein activate the Wnt signaling pathway. The active Wnt signaling pathway promotes myogenic commitment of hESC-derived PDGFRA+ cells through accumulation of beta-catenin, which is consistent with previous studies that showed activation of the canonical Wnt signaling pathway during postnatal myogenesis and muscle regeneration26,27. Studies by Brack et al. demonstrated Wnt signaling-mediated myogenic lineage progression of progenitor cells in presence of either WNT3A protein or GSK3β inhibitor27. Our study further reveals that the WNT3A-mediated myogenic differentiation of hESC-derived PDGFRA+ cells is accompanied by increased AKT activity. This observation is consistent with a previous study that showed treatment with either recombinant WNT3A protein or overexpression of WNT3A protein in myoblasts enhanced insulin-stimulated AKT activation at Ser47340. In addition, von Maltzahn et al. have showed that WNT7A signaling directly activates AKT/mTOR growth pathway in C2C12 cells41. These studies also suggest the discrete effect of cell-context dependency and culture conditions on Wnt-mediated activation of AKT.
Our in vivo results indicate a strong correlation between preconditioning of the cells with their in vivo survival and contribution to muscle tissue repair. In addition to differences in the viability of transplanted cells in vivo, the culture conditions used to prime the cells prior to their transplantation were found to have a pivotal role in their contribution to skeletal muscle cells. Though 14 days of preconditioning using induction medium supported viability of a few donor cells, they were localized within the interstitial space near the muscle fibers and did not show any apparent contribution to the host myofiber repair or to the satellite cell compartment. On the contrary, a significantly higher number of donor cells were observed in the host tissue when the transplanted cells were preconditioned in medium containing WNT3A components. This could be attributed to the differential extent of differentiation commitment/phenotype of the transplanted cells.
While cells preconditioned with WNT3A were found to repopulate the host tissue and contribute to regeneration of myofibers, only cells preconditioned with WNT3A-conditioned induction medium were found to differentiate into satellite cells, as evident by PAX7 staining. The differences in in vivo function of transplanted cells might be attributed to the differences in cell populations resulting from activation or repression of genes by different culture conditions. For example, cells treated with WNT3A-conditioned induction medium for 14 days have shown the highest level of PAX7 expression compared to their counterparts, which could contribute to the satellite cell fractions, in accordance with recent studies42,43,44. The cells cultured in WNT3A-conditioned induction medium also showed a significant upregulation of CD56, a neural cell adhesion molecule (NCAM). Emerging studies suggest CD56 as a crucial marker of myogenic progenitor cells and the presence of CD56-positive cells has increasingly been identified to be associated with muscle tissue repair36,37,45,46.
In summary, we demonstrate that culture conditions containing WNT3A protein promote myogenic differentiation of hESC-derived PDGFRA+ cells into multinucleated myocytes in vitro. When transplanted into cardiotoxin-injured skeletal muscle tissue, the donor cells showed a culture condition-dependent contribution to host tissue repair. No teratoma formation was observed, indicating the potential application of hESC-derived myogenic progenitor cells for clinical purposes. Such ESC-derived myogenic progenitors with the ability to contribute to myofibers and satellite cells could have significant impact in the treatment of various muscle injuries and degenerative diseases.
Methods
Expansion of hESCs
The OCT4-GFP reporter cell line was created as described previously47. Cells were expanded on mitomycin C-treated MEF (mouse embryonic fibroblast) feeder cells with Knockout DMEM containing 10% KSR (knockout serum replacement), 10% human plasmanate (Talecris Biotherapeutics), 1% NEAA (non-essential amino acids), 1% penicillin/streptomycin, 1% Gluta-MAX, and 55 μM 2-mercaptoethanol47. 30 ng/mL of bFGF (basic fibroblast growth factor, Life Technologies) was added daily into the growth medium and cells were passaged using Accutase (Millipore) at ~80% confluency.
Derivation of mesoderm progenitor cells expressing PDGFRA
The mesoderm progenitor cells expressing PDGFRA was derived as described earlier17. Briefly, undifferentiated HUES9 cells were treated with Accutase for 5 mins to create a suspension of single cells. Approximately 1.0 × 106 cells were suspended in high glucose DMEM containing 5% FBS, 2 mM L-glutamine, 100 nM dexamethasone, 100 μM hydrocortisone, 1% penicillin/streptomycin, 10 μM transferrin, 860.9 nM recombinant insulin, 20 nM progesterone, 100.1 μM putrescine, and 30.1 nM selenite (Life Technologies). The cells were cultured on ultra low attachment plates for 9 days to allow them to form embryoid bodies (EBs). The medium was changed every other day. The EBs were split 1 to 6, transferred to a 10 cm dish coated with growth factor-reduced Matrigel (1:25 diluted in KnockOut DMEM; BD Biosciences), and cultured further with the afore-mentioned medium. Within 24 hours, the cells adhered onto the surface. After 7 days of culture, the migrating cells were trypsinized and filtered using a 40 μm cell strainer. The cells were sorted for a PDGFRA+/OCT4-GFP− (termed as PDGFRA+ cell) population by FACS. The sorted cells were cultured in high glucose DMEM supplemented with 10% FBS, 2 mM L-glutamine, and 1% penicillin/streptomycin before characterization.
FACS analysis
The hESC-derived single cells were dissociated in DPBS (BD Biosciences) with 2% FBS and 0.09% sodium azide, and then stained with Alexa Fluor 647-conjugated PDGFRA or Alexa Fluor 647-conjugated mouse IgM,K isotype control antibodies (Biolegend). The cells were stained for 30 mins on ice, after which they were washed and resuspended in the above buffer prior to loading on the FACSCanto (BD Biosystems). Data were analyzed with the CellQuest Pro software.
Preparation of WNT3A-conditioned induction medium
WNT3A-conditioned induction medium was generated using L-Wnt3A cells (ATCC® CRL-2647™) according to the manufacturer's protocol39. Conditioned medium from the corresponding L-cells (CRL-2648™) was collected and used as a control. The cell lines were cultured following manufacturer's protocol39. Briefly, the cells were grown in 10 mL high glucose DMEM supplemented with 2 mM L-glutamine, 100 nM dexamethasone, 100 μM hydrocortisone, 1% penicillin/streptomycin, 10 μM transferrin, 860.9 nM recombinant insulin, 20 nM progesterone, 100.1 μM putrescine, and 30.1 nM selenite with 10% FBS for 4 days prior to collecting conditioned medium. To these cells, another 10 mL of fresh medium was added and cultured for three days to collect second batch of conditioned medium. The two batches of conditioned media were mixed at a 1:1 ratio and filtered using 0.22 μm filter and stored at 4°C until usage.
In vitro myogenic differentiation
For in vitro myogenic differentiation, passage 8, PDGFRA+ cells were plated at 1 × 104 cells/cm2 and cultured in different media conditions. (i) An induction medium (high glucose DMEM supplemented with 2 mM L-glutamine, 100 nM dexamethasone, 100 μM hydrocortisone, 1% penicillin/streptomycin, 10 μM transferrin, 860.9 nM recombinant insulin, 20 nM progesterone, 100.1 μM putrescine, and 30.1 nM selenite with 10% FBS), (ii) an induction media containing varying amounts of human recombinant WNT3A protein (rhWNT3A; 10, 50, and 100 ng/mL) (R&D Systems, Inc., Cat#: 5036-WN-010), and (iii) a WNT3A-conditioned induction medium and its control conditioned induction medium collected from L-cells. The extent of myogenic differentiation of the cells in response to different media conditions was analyzed as a function of culture time (4–14 days).
Immunofluorescence staining
Primary antibodies used in staining consisted of the following: PAX7, MF20 (1:200; Developmental Studies Hybridoma Bank), DES (1:250; Abcam), human-specific lamin A/C (1:250; Abcam), and mouse laminin (1:200; Millipore). Secondary antibodies used for staining consisted of the following: goat anti-rat Alexa Fluor 546, goat anti-mouse Alexa Fluor 546, goat anti-mouse Alexa Fluor 488, goat anti-rabbit Alexa Fluor 488, goat anti-rabbit Alexa 546 (1:250; Life Technologies). For staining of cells cultured on TCPS, they were first fixed in 4% PFA for 10 mins at room temperature. Just prior to staining, the cells were permeabilized with 0.1% (v/v) Triton X-100 and blocked with 3% (w/v) BSA for 30 mins, stained with primary antibodies in 1% BSA for overnight at 4°C. After washing 3 times with PBS, cells were stained with secondary antibodies for 1 hr at room temperature. Nuclei were visualized by staining with Hoechst 33342 (2 μg/ml; Life Technologies) for 5 mins at room temperature. For immunohistochemistry, the tibialis anterior (TA) muscles were dissected and placed in optimal temperature cutting compound (OCT). Muscles were serially cryosectioned into 10 μm-thick sections, fixed with 4% PFA for 10 mins at room temperature, permeabilized with 0.3% Triton X-100, and blocked with 20% normal goat serum for 1 hr at room temperature. Finally, the muscle sections were stained with human lamin A/C and laminin primary antibodies. The muscle section from NOD/SCID mice injected with the same volume of PBS devoid of any cells was used as a control to determine the specificity of human-specific lamin A/C antibody (Supplementary Fig. S6). For antigen retrieval for PAX7 staining, the sections were first stained with human lamin A/C, then post-fixed with 4% PFA for 10 mins at room temperature. These sections were then immersed in preheated (90°C) 10 mM citric acid (pH 6) for 15 mins, washed with PBS thrice, and incubated with PAX7 antibody, followed by incubation with secondary antibody for 1 hr at room temperature. The images acquired using a fluorescence microscope (Carl Zeiss; Axio Observer A1).
Image analysis
The differentiation index was determined as the ratio of MF20-positive cells to the total number of cells. The fusion index was determined as the ratio of multinucleated myotubes having 3 or more nuclei to the total number of MF20-positive nuclei48. The number of MF20-positive cells was counted manually from four random fields of view from 4–5 different images. Nuclei were counted by filtering images, adjusting thresholds, and calculating the total number of DAPI-positive nuclei.
RNA extraction and qPCR
TRIzol (Invitrogen) and iScript cDNA synthesis kit (BioRad) were used to isolate RNA and prepare cDNA, respectively. Real-time polymerase chain reaction (qPCR) was performed using SYBR Select Master Mix (Life Technologies) and the ABI Prism 7300 Sequence Detection System (Applied Biosystems). Gene expression was normalized to GAPDH expression as reference and delta Ct values were calculated as Cttarget − Ctreference. All experiments were performed with three biological replicates and the relative fold inductions were calculated as 2−ΔΔCt 49. The list of primers used in this study is presented in Supplementary Table S1.
Western blot
Cells were lysed in lysis buffer (Sigma-Aldrich, cat# R0278) containing protease and phosphatase inhibitors (Sigma-Aldrich). Total cell protein was harvested by centrifuging cell lysate at 15000 rcf at 4°C and protein concentration was measured by Bradford assay. Gel electrophoresis was carried out on 8% polyacrylamide gels at 100 V and transferred to polyvinylidene fluoride (PVDF) membranes at 350 mA and 4°C. Membranes were blocked with 3% bovine serum albumin and incubated with active beta-catenin (1 μg/ml; Millipore), phospho-AKTSer473 (1:1000; Cell Signaling), AKT (1:1000; Cell Signaling), and β-actin (1:1000; Sigma-Aldrich) primary antibodies overnight on a shaker at 4°C. Membranes were incubated with horseradish peroxidase-conjugated secondary antibodies in the next day for 1 hr in room temperature, and then covered in chemiluminescent reagent for subsequent exposure on X-ray film or chemiluminescence imager (Protein Simple).
Cell transplantation
Animal experiments were carried out according to the protocols approved by Institutional Animal Care and Use Committee (IACUC) of the University of California, San Diego and National Institute of Health (NIH). Twenty four hrs prior to cell transplantation, 2-month-old immunodeficient NOD.CB17-Prkdcscid/J mice were injected intraperitoneally with ketamine (100 mg/kg) and xylazine (10 mg/kg), and their TA muscles were injured with 30 μL of cardiotoxin from Naja mossambica mossambica (0.5 mg/mL, Sigma). Approximately 5.0 × 105 cells of preconditioned hESC-derived PDGFRA+ cell populations – cells cultured for 14 days in (i) induction medium, (ii) WNT3A-conditioned induction medium, and (iii) induction medium supplemented with 50 ng/mL rhWNT3A – were resuspended in 10 μL of physiological saline solution (PBS), and intramuscularly injected into the TA muscles. Two weeks after transplantation, TA muscles were harvested and embedded in OCT for cryosectioning. Engraftment of the transplanted cells was analyzed histologically.
Statistical analysis
All values are shown as mean ± standard deviation and statistical significance was assessed by two-tailed unpaired Student's t-test or single-factor analysis of variance (ANOVA) with Tukey's Multiple Comparison Test (*p < 0.05, **p < 0.01, and ***p < 0.001).
Supplementary Material
Supplementary Information
Acknowledgments
This work was funded by the California Institute of Regenerative Medicine (RN2-00945). The authors acknowledge Dr. Alexey Terskikh for kindly providing the lentiviral construct that was used to generate the OCT4-GFP reporter line and Karl E Marquez for experimental assistance with FACS. The authors acknowledge Vikram Rao and Hyunwoo Park for valuable discussions. We would also like to acknowledge the Developmental Studies Hybridoma Bank for providing the PAX7 antibody generated by Kawakami Lab, and the MF20 antibody generated by Fischman Lab. The antibodies are developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA 52242.
Footnotes
The authors declare no competing financial interests.
Author Contributions Y.H. and S.V.: conception and design of the study, performed experiments, data collection and analysis, manuscript writing; S.S., Y.R.S., T.S., B.D., Y.X., Z.L.: performed experiments, data collection and analysis, discussion.
References
- Thomson J. A. et al. Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147 (1998). [DOI] [PubMed] [Google Scholar]
- Trounson A. The production and directed differentiation of human embryonic stem cells. Endocr Rev 27, 208–219, 10.1210/er.2005-0016 (2006). [DOI] [PubMed] [Google Scholar]
- Crook J. M. et al. The Generation of Six Clinical-Grade Human Embryonic Stem Cell Lines. Cell Stem Cell 1, 490–494, 10.1016/j.stem.2007.10.004 (2007). [DOI] [PubMed] [Google Scholar]
- Darabi R. et al. Human ES- and iPS-Derived Myogenic Progenitors Restore DYSTROPHIN and Improve Contractility upon Transplantation in Dystrophic Mice. Cell Stem Cell 10, 610–619, 10.1016/j.stem.2012.02.015 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberi T. et al. Derivation of engraftable skeletal myoblasts from human embryonic stem cells. Nat Med 13, 642–648, 10.1038/nm1533 (2007). [DOI] [PubMed] [Google Scholar]
- Darabi R. et al. Functional skeletal muscle regeneration from differentiating embryonic stem cells. Nat Med 14, 134–143, 10.1038/nm1705 (2008). [DOI] [PubMed] [Google Scholar]
- Ryan T. et al. Retinoic acid enhances skeletal myogenesis in human embryonic stem cells by expanding the premyogenic progenitor population. Stem Cell Rev 8, 482–493, 10.1007/s12015-011-9284-0 (2012). [DOI] [PubMed] [Google Scholar]
- Salani S. et al. Generation of skeletal muscle cells from embryonic and induced pluripotent stem cells as an in vitro model and for therapy of muscular dystrophies. J Cell Mol Med 16, 1353–1364, 10.1111/j.1582-4934.2011.01498.x (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darabi R. et al. Engraftment of embryonic stem cell-derived myogenic progenitors in a dominant model of muscular dystrophy. Exp Neurol 220, 212–216, 10.1016/j.expneurol.2009.08.002 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filareto A., Darabi R. & Perlingeiro R. C. Engraftment of ES-Derived Myogenic Progenitors in a Severe Mouse Model of Muscular Dystrophy. J Stem Cell Res Ther 10, 10.4172/2157-7633.S10-001 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagavati S. & Xu W. Generation of skeletal muscle from transplanted embryonic stem cells in dystrophic mice. Biochem Biophys Res Commun 333, 644–649, 10.1016/j.bbrc.2005.05.135 (2005). [DOI] [PubMed] [Google Scholar]
- Goudenege S. et al. Myoblasts derived from normal hESCs and dystrophic hiPSCs efficiently fuse with existing muscle fibers following transplantation. Mol Ther 20, 2153–2167, 10.1038/mt.2012.188 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao L. et al. Highly efficient derivation of skeletal myotubes from human embryonic stem cells. Stem Cell Rev 8, 1109–1119, 10.1007/s12015-012-9413-4 (2012). [DOI] [PubMed] [Google Scholar]
- Warren L. et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell 7, 618–630, 10.1016/j.stem.2010.08.012 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberi T., Willis L. M., Socci N. D. & Studer L. Derivation of multipotent mesenchymal precursors from human embryonic stem cells. PLoS Med 2, e161, 10.1371/journal.pmed.0020161 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai H., Okawa Y., Inami Y., Nishio N. & Isobe K. Paraxial mesodermal progenitors derived from mouse embryonic stem cells contribute to muscle regeneration via differentiation into muscle satellite cells. Stem Cells 26, 1865–1873, 10.1634/stemcells.2008-0173 (2008). [DOI] [PubMed] [Google Scholar]
- Hwang Y. et al. Directed in vitro myogenesis of human embryonic stem cells and their in vivo engraftment. PLoS One 8, e72023, 10.1371/journal.pone.0072023 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P., Polesskaya A. & Rudnicki M. A. Adult stem cell specification by Wnt signaling in muscle regeneration. Cell Cycle 2, 418–419 (2003). [PubMed] [Google Scholar]
- von Maltzahn J., Chang N. C., Bentzinger C. F. & Rudnicki M. A. Wnt signaling in myogenesis. Trends Cell Biol 22, 602–609, 10.1016/j.tcb.2012.07.008 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munsterberg A. E., Kitajewski J., Bumcrot D. A., McMahon A. P. & Lassar A. B. Combinatorial signaling by Sonic hedgehog and Wnt family members induces myogenic bHLH gene expression in the somite. Genes Dev 9, 2911–2922 (1995). [DOI] [PubMed] [Google Scholar]
- Cisternas P., Henriquez J. P., Brandan E. & Inestrosa N. C. Wnt signaling in skeletal muscle dynamics: myogenesis, neuromuscular synapse and fibrosis. Mol Neurobiol 49, 574–589, 10.1007/s12035-013-8540-5 (2014). [DOI] [PubMed] [Google Scholar]
- Ikeya M. & Takada S. Wnt signaling from the dorsal neural tube is required for the formation of the medial dermomyotome. Development 125, 4969–4976 (1998). [DOI] [PubMed] [Google Scholar]
- Shi X. & Garry D. J. Muscle stem cells in development, regeneration, and disease. Genes Dev 20, 1692–1708, 10.1101/gad.1419406 (2006). [DOI] [PubMed] [Google Scholar]
- Wagner J., Schmidt C., Nikowits W. Jr & Christ B. Compartmentalization of the somite and myogenesis in chick embryos are influenced by wnt expression. Dev Biol 228, 86–94, 10.1006/dbio.2000.9921 (2000). [DOI] [PubMed] [Google Scholar]
- Bentzinger C. F., Wang Y. X. & Rudnicki M. A. Building muscle: molecular regulation of myogenesis. Cold Spring Harb Perspect Biol 4, 10.1101/cshperspect.a008342 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto A. et al. Canonical Wnt signalling induces satellite-cell proliferation during adult skeletal muscle regeneration. J Cell Sci 121, 2939–2950, 10.1242/jcs.026534 (2008). [DOI] [PubMed] [Google Scholar]
- Brack A. S., Conboy I. M., Conboy M. J., Shen J. & Rando T. A. A temporal switch from notch to Wnt signaling in muscle stem cells is necessary for normal adult myogenesis. Cell Stem Cell 2, 50–59, 10.1016/j.stem.2007.10.006 (2008). [DOI] [PubMed] [Google Scholar]
- Fujimaki S., Hidaka R., Asashima M., Takemasa T. & Kuwabara T. Wnt Protein-mediated Satellite Cell Conversion in Adult and Aged Mice Following Voluntary Wheel Running. J Biol Chem 289, 7399–7412, 10.1074/jbc.M113.539247 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Y. C. et al. Wnt3a signaling promotes proliferation, myogenic differentiation, and migration of rat bone marrow mesenchymal stem cells. Acta Pharmacol Sin 28, 1761–1774, 10.1111/j.1745-7254.2007.00671.x (2007). [DOI] [PubMed] [Google Scholar]
- Shang Y. et al. Activated beta-catenin induces myogenesis and inhibits adipogenesis in BM-derived mesenchymal stromal cells. Cytotherapy 9, 667–681, 10.1080/14653240701508437 (2007). [DOI] [PubMed] [Google Scholar]
- Petropoulos H. & Skerjanc I. S. Beta-catenin is essential and sufficient for skeletal myogenesis in P19 cells. J Biol Chem 277, 15393–15399, 10.1074/jbc.M112141200 (2002). [DOI] [PubMed] [Google Scholar]
- Ridgeway A. G., Petropoulos H., Wilton S. & Skerjanc I. S. Wnt signaling regulates the function of MyoD and myogenin. J Biol Chem 275, 32398–32405, 10.1074/jbc.M004349200 (2000). [DOI] [PubMed] [Google Scholar]
- Borchin B., Chen J. & Barberi T. Derivation and FACS-Mediated Purification of PAX3+/PAX7+ Skeletal Muscle Precursors from Human Pluripotent Stem Cells. Stem Cell Reports 1, 620–631, 10.1016/j.stemcr.2013.10.007 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C. et al. A zebrafish embryo culture system defines factors that promote vertebrate myogenesis across species. Cell 155, 909–921, 10.1016/j.cell.2013.10.023 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. et al. Flk-1+ adipose-derived mesenchymal stem cells differentiate into skeletal muscle satellite cells and ameliorate muscular dystrophy in mdx mice. Stem Cells Dev 16, 695–706, 10.1089/scd.2006.0118 (2007). [DOI] [PubMed] [Google Scholar]
- Zheng B. et al. Prospective identification of myogenic endothelial cells in human skeletal muscle. Nat Biotechnol 25, 1025–1034, 10.1038/nbt1334 (2007). [DOI] [PubMed] [Google Scholar]
- Mahmood A., Harkness L., Schroder H. D., Abdallah B. M. & Kassem M. Enhanced differentiation of human embryonic stem cells to mesenchymal progenitors by inhibition of TGF-beta/activin/nodal signaling using SB-431542. J Bone Miner Res 25, 1216–1233, 10.1002/jbmr.34 (2010). [DOI] [PubMed] [Google Scholar]
- Leung M. et al. Nanofiber-based in vitro system for high myogenic differentiation of human embryonic stem cells. Biomacromolecules 14, 4207–4216, 10.1021/bm4009843 (2013). [DOI] [PubMed] [Google Scholar]
- Willert K. et al. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature 423, 448–452, 10.1038/nature01611 (2003). [DOI] [PubMed] [Google Scholar]
- Yoon J. C. et al. Wnt signaling regulates mitochondrial physiology and insulin sensitivity. Genes Dev 24, 1507–1518, 10.1101/gad.1924910 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Maltzahn J., Bentzinger C. F. & Rudnicki M. A. Wnt7a-Fzd7 signalling directly activates the Akt/mTOR anabolic growth pathway in skeletal muscle. Nat Cell Biol 14, 186–191, 10.1038/ncb2404 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darabi R. et al. Human ES- and iPS-derived myogenic progenitors restore DYSTROPHIN and improve contractility upon transplantation in dystrophic mice. Cell Stem Cell 10, 610–619, 10.1016/j.stem.2012.02.015 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P., Ishibashi J., Scime A. & Rudnicki M. A. Pax7 is necessary and sufficient for the myogenic specification of CD45+:Sca1+ stem cells from injured muscle. PLoS Biol 2, E130, 10.1371/journal.pbio.0020130 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P. et al. Pax7 is required for the specification of myogenic satellite cells. Cell 102, 777–786 (2000). [DOI] [PubMed] [Google Scholar]
- Castiglioni A. et al. Isolation of Progenitors that Exhibit Myogenic/Osteogenic Bipotency In Vitro by Fluorescence-Activated Cell Sorting from Human Fetal Muscle. Stem Cell Reports 2, 92–106, 10.1016/j.stemcr.2013.12.006 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng J., Adkin C. F., Xu S. W., Muntoni F. & Morgan J. E. Contribution of human muscle-derived cells to skeletal muscle regeneration in dystrophic host mice. PLoS One 6, e17454, 10.1371/journal.pone.0017454 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brafman D. A. et al. Long-term human pluripotent stem cell self-renewal on synthetic polymer surfaces. Biomaterials 31, 9135–9144, 10.1016/j.biomaterials.2010.08.007 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangaj N. et al. Heparin mimicking polymer promotes myogenic differentiation of muscle progenitor cells. Biomacromolecules 11, 3294–3300, 10.1021/bm101041f (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K. J. & Schmittgen T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408, 10.1006/meth.2001.1262 (2001). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information