Abstract
Neuroinflammation, mediated by activated microglia and astrocytes, plays a key role in the pathogenesis of many neurological disorders. Systemically-administered dendrimers target neuroinflammation and deliver drugs with significant efficacy, without the need for ligands. Elucidating the nanoscale aspects of targeting neuroinflammation will enable superior nanodevices for eventual translation. Using a rabbit model of cerebral palsy, we studied the in vivo contributions of dendrimer physicochemical properties and disease pathophysiology on dendrimer brain uptake, diffusion, and cell specific localization. Neutral dendrimers move efficiently within the brain parenchyma and rapidly localize in glial cells in regions of injury. Dendrimer uptake is also dependent on the extent of blood-brain-barrier breakdown, glial activation, and disease severity (mild, moderate, or severe), which can lend the dendrimer to be used as an imaging biomarker for disease phenotype. This new understanding of the in vivo mechanism of dendrimer-mediated delivery in a clinically-relevant rabbit model provides greater opportunity for clinical translation of targeted brain injury therapies.
Keywords: Nanoparticle, Dendrimer, Neuroinflammation, Brain injury, Glia
1. Introduction
Neurological diseases account for 13% of the global burden of disease and, as a result, more than $760 billion a year is spent trying to treat them [1]. Drugs that are used to treat the injured or diseased brain also take about 35% longer to be developed for use in humans compared to drugs for any other type of disease [2]. This is because (i) transport of drugs and drug delivery vehicles across the blood-brain-barrier (BBB) is difficult to achieve and (ii) injury is often diffuse, making it difficult for therapeutics to reach target cells even if administered locally [3,4]. In addition, common hallmarks of neurological disease such as inflammation, excitotoxicity, and impaired fluid flow (vascular and cerebrospinal) lead to a heterogeneous, dynamic, complex environment, even within the same disease spectrum. Nanotechnology-based strategies to target specific cells involved in brain disease can potentially slow disease progression and promote repair and regeneration, enabling normal development and maturation of the brain [5]. More specifically, selective targeting of common disease hallmarks such as neuroinflammation, which has recently been elucidated as a key mediator in autism, cerebral palsy (CP), stroke, traumatic brain injury (TBI), and Alzheimer’s, to name a few, has the potential to delay the onset of disease and provide a longer therapeutic window for treatment [6].
Dendrimer-based platforms have become promising carriers for targeting inflammation [7]. Phosphorus dendrimers have been used to track macrophage polarization and fate in the presence of spinal or peripheral nerve injury [8,9], inhibit neutrophil recruitment in lung inflammation [10], and deliver therapeutics to inflammatory cells in models of arthritis [11]. Poly(amidoamine) (PAMAM) dendrimers (size ~3–12 nm) have well-defined size, branching architecture, and a high density of tailorable surface functional groups and have been shown to cross the impaired BBB in animal models [12,13]. Boridy et al. have shown celastrol incorporated PAMAM dendrimers can mediate inflammatory signaling and cytokine release from microglia in the chronically inflamed brain [14]. Systemically administered hydroxyl-modified, generation-4 (G4-OH) PAMAM dendrimers have shown significant accumulation, following systemic administration, in a rabbit model of CP [13], a mouse model of adult ischemic stroke [15], a mouse model of neonatal stroke [10], a canine model of hypothermic circulatory arrest induced brain injury [16], a rat model of retinal degeneration [17], and a primate model of ischemic optic neuropathy [18]. More importantly, when these dendrimers accumulated in specific cells that mediate inflammation, such as microglia and astrocytes, they led to profound effects when conjugated with N-acetyl cysteine (D-NAC), including dramatic improvement of phenotype [13], arrest in neurological injury [10], and neurological repair [16]. Given the strong medical need for optimizing therapeutic delivery to overcome biological barriers, reduce off-site toxicity, and achieve efficacy, it is important to explore the in vivo mechanism of how these PAMAM dendrimers, with no targeting ligands, selectively localize in cells that mediate neuroinflammation.
In this study, we use an in vivo rabbit model of CP, with features similar to CP in humans [19], to (i) characterize the impact of nanoparticle size on passage across an impaired BBB, (ii) understand how dendrimer surface functionality dictates movement in the brain parenchyma and uptake by activated microglia, and (iii) quantify dendrimer uptake and localization in the injured newborn brain as a function of disease severity. These findings are crucial for the development and improvement of disease-appropriate therapeutic nanoparticle platforms in both pediatric and adult central nervous system (CNS) disorders, where neuroinflammation is a key mediator of disease pathology [20,21].
2. Materials and methods
2.1. Materials and reagents for dendrimer synthesis and characterization
Ethylenediamine-core poly(amidoamine) (PAMAM) generation four hydroxyl-terminated dendrimer (G4-OH), amine-terminated dendrimer (G4-NH2), and generation 3.5 carboxylate dendrimer (G3.5-COOH) were purchased from Dendritech Inc. (Midland, Michigan). Cy5-mono-NHS ester was purchased from Amersham Biosciences-GE Healthcare. Benzotriazol-1-yl-oxy-tripyrrolidinophosphonium hexafluorophosphate (PyBOP), 6-(Fmoc-amino)caproic acid, N-Fmoc-1,5-diaminopentane hydrobromide, 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC.HCl), Hydroxybenzotriazole (HOBt), acetonitrile (ACN), tri-fluoroacetic acid (TFA), triethylamine (TEA) and diisopropylethylamine (DIEA) were purchased from Sigma-Aldrich (St Louis, Missouri). Dimethylformamide (DMF) and dimethyl sulfoxide (DMSO) were purchased from Fisher Scientific (St. Louis, Missouri). All other reagents and solvents were used as received. Regenerated cellulose (RC) dialysis membrane (molecular weight cut-off 1 kDa or 8 kDa) was obtained from Spectrum Laboratories Inc (Rancho Dominguez, California).
2.2. Preparation of dendrimer-Cy5 conjugates
We functionalized hydroxyl (OH) and carboxylate (COOH) dendrimers with reactive amine groups using a suitable linker and reacted with Cy5-N-hydroxysuccinamide (NHS) ester to get the corresponding Cy5-conjugates. For the amine (NH2) dendrimer, we directly reacted an NHS ester form of Cy5 with the PAMAM dendrimer. The methods for COOH dendrimer are provided below since they are previously unpublished, and the methods for NH2 and OH dendrimer have previously been published [22,23] and are provided in brief in supplemental information.
Carboxylate-functionalized PAMAM dendrimer-Cy5 conjugate
We partially functionalized G3.5 carboxylate dendrimer with reactive amine groups on the surface by reacting N-Fmoc-1,5-diaminopentane hydrobromide with a sodium salt free form of carboxylate dendrimer using a coupling reaction method to get the Fmoc-functionalized intermediate. The methanolic solution of the sodium salt of G3.5-COOH was treated with 1.25 M methanolic HCl solution till the pH of the solution reached 7.0 in cold condition. The methanol was evaporated and redissolved in water and dialyzed against water for 6 h to remove the excess of the salt. Finally the resultant water layer was freeze dried to get the white powder as free carboxylate group on the surface of the dendrimer.
The salt free form of G3.5-COOH dendrimer (100.0 mg, 0.07 mmol) was dissolved in anhydrous DMSO under nitrogen atmosphere in a 50 mL round bottom flask at 40 °C until the solid completely dissolved into the solution. EDC.HCl (44.5 mg, 0.23 mmol) and HOBt (31.5 mg, 0.23 mmol) were dissolved in DMSO and added into the reaction mixture and stirred for 1 h under nitrogen at room temperature. N-Fmoc-1,5-diaminopentane hydrobromide (47.0 mg, 0.116 mmol) was dissolved in DMF with 5% DIEA and added drop wise to the reaction mixture and stirred for 24 h. The reaction mixture was dialyzed against DMF using 1 kDa cutoff dialysis membrane for 24 h and the DMF was removed under reduced pressure to obtain yellow color oily product of Fmoc-functionalized dendrimer. The Fmoc-functionalized intermediate was dissolved in anhydrous DMSO without further purification. We deprotected the Fmoc groups using a mixture of piperidine/DMF to get the amine-terminated carboxylate dendrimer. The mixture of piperidine/DMF (2:8, 5 mL) was added drop wise and the reaction mixture was stirred for 15 min under nitrogen atmosphere. The mixture of solvents was evaporated under reduced pressure and the reaction mixture was subjected to dialysis in (2:8) DMSO/DMF for 24 h. The DMF was evaporated under reduced pressure and the traces of DMF were removed by dialyzing against DI water for 24 h, and lyophilized to obtain pale yellowish powder of G3.5-COOH-NH2. The product was characterized using 1H NMR, which suggested that ~ 3–4 molecules of linker amines were conjugated to each dendrimer. 1H NMR (DMSO-d6): δ 1.07–1.64 (m, CH2 protons of linker), 2.20–3.58 (m, CH2 protons of G3.5-COOH), 4.68 (t, CH2COO-C protons of G3.5-COOH), 7.84–7.96 (m, internal amide protons of G3.5-COOH).
2.3. Characterization of D-Cy5 conjugates
1H NMR Characterization
1H NMR spectra of the intermediates and final dendrimer-Cy5 conjugates were recorded on a Bruker (500 MHz) spectrometer using DMSO-d6 solvents. Proton chemical shifts were reported in ppm (δ).
Fluorescence spectroscopy
Fluorescence spectra of free Cy5, as well as all three final dendrimer-Cy5 conjugates, were recorded in methanol and in phosphate buffer [0.1 M], using a Shimadzu RF-5301 Spectrofluorometer. All measurements were done with a fixed excitation wavelength (645 nm) and emission wavelength of 662 nm.
High Performance Liquid Chromatography (HPLC)
The final dendrimer-Cy5 conjugates, as well as their intermediates, were analyzed using a Waters 1525 binary HPLC separation module, equipped with an In-Line degasser AF, a 717 plus autosampler, a 2998 photodiode array detector, and a 2475 multi λ fluorescence detector interfaced with Waters Empower software. TSK-gel ODS-80 Ts column (250 × 4.6 mm; i.d., 5 μm) and TSK-gel guard column were used for the analysis (Tosoh Bioscience LLC) with a gradient flow of 0.1% v/v TFA in H2O:ACN (90:10) to 10:90 in 30 min at flow rate of 1 mL/min.
2.4. In vivo analysis of dendrimer uptake and localization
2.4.1. Rabbit model of maternal inflammation induced cerebral palsy
All animal procedures were approved by the Institutional Animal Care and Use Committee of Johns Hopkins University, and are described previously [19,24,25]. Timed pregnant New Zealand white rabbits were obtained from Robinson Services Inc (Winston-Salem, NC). Briefly, pregnant rabbits in the endotoxin group (n = 10 dams) underwent laparotomy at gestational day 28 (G28, term pregnancy 31 days) and were injected with 1 mL of saline containing Escherichia coli endotoxin (6200 endotoxin units total) (serotype O127: B8, Sigma Aldrich) along the length of the uterus [19,25]. At this dose, the newborn kits have been shown to have uniform microglial activation in the PVR and display a phenotype of CP with predominantly hindlimb hypertonia [19,24,25]. The healthy control group (n = 6 dams) included pregnant rabbits that had no surgery or intervention. After undergoing laparotomy on G28, dams were given maintenance fluids of 60 cc normal saline subcutaneous every 12 h. On the evening of G30, dams were induced using 10 mEq/kg oxytocin to control timing of delivery, and the newborn rabbits kits were immediately removed and placed in a nesting box in an incubator maintained at 101 °F and 50–70% humidity. All kits were given 1 cc normal saline subcutaneously, weighed, and hand-fed fresh frozen rabbit milk every 6–8 h until time of euthanasia. Kits were injected with dendrimer on postnatal day 1 of life (PND1, corresponding to G31), and kept in an incubator until time of euthanasia.
2.4.2. Neurobehavioral testing
To evaluate disease severity, neurobehavioral tests were carried out on newborn healthy control kits and kits with CP on PND1. Kits were videotaped for 10 min each and scored on a scale of 0–3 (0, worst; 3, best) for posture, movements of head and limbs, and duration and intensity of movements on a flat surface, as previously described for rabbits [26]. Kits were fed rabbit milk with a syringe attached to an artificial nipple, and suck and swallow and head turn during feeding on a scale of 0–3 (worst-best) was assessed [26,27]. Olfaction was tested by the intensity of aversive response to a cotton swab soaked with 100% ethanol [27,28]. The righting reflex was assessed by placing the kits on their backs and recording the number of times they turned from the supine to the prone position in 10 tries. The hops and steps were scaled at 0–4 (0, no step or hop; 4, ≥10 steps or 4 hops) [13]. Pain sensitivity was tested by recording the time and intensity of responses to a pinch of the hind feet. Responses were scored on a 0–3 scale (from no response to a strong response, such as vigorously kicking the feet and making vocalizations) [28]. Tonicity of each limb was scored on an inverted scale of 0–4 (0, strong tone; 4, hypo or hypertonic) [26]. Scores for each behavioral test for normal healthy kits were averaged (n = 8 kits) and compared to scores for each behavioral test for all endotoxin kits (n = 18 kits) to determine statistically significant differences within these litters used in this study. Suck and swallow, head turn, posture, head, forelegs, hind limbs, forelimb tonicity, righting reflex, and hind limb tonicity were all significantly diminished (p < 0.005) in endotoxin kits compared to normal healthy control kits. These scores were combined into a composite behavioral score, providing a range of scores falling into the following classifications: severe (score 3–9), moderate (score 10–14), mild (score 15–20), normal (score >20). Tonicity, which is scored on a 0–4 scale, with 0 being normal and 4 being most severe, was inverted when incorporated into the composite score so that it aligned with the scoring for all other behavioral measures (0 = severe and 4 = normal).
2.4.3. Polystyrene nanoparticle, dextran, Evans blue, and dendrimer-Cy5 intravenous administrations
Newborn rabbits were injected with polystyrene (PS) nanoparticles (Invitrogen, 20 nm nile red), dextran-FITC (70 kDa, Invitrogen), Evans blue (Sigma), or dendrimer-Cy5 conjugate intravenously on PND1 using a 27 1/2G needle (Fisher Scientific) into one of the superficial lateral vessels visualized on the surface of the abdomen. For each dendrimer, n = 4–5 kits were injected for each time-point in both control and endotoxin groups. For PS and dextran-FITC injections, n = 3 kits were injected. For long term retention in CP kits, n = 3 kits were injected and had to be euthanized due to disease at PND9. Untreated CP kits with a moderate to severe phenotype did not live longer than PND9. The animal was lightly restrained by hand for the injection. G4-Cy5 conjugate was passed through a 0.22 μm syringe filter twice before injecting into the animal. G4-Cy5, PS, and dextran-FITC were prepared in sterile normal saline. The volume injected was 0.2 mL for each animal (approximately 5 mL/kg for an average weight of around 40 g for a PND1 kit).
2.4.4. Intraparenchymal administrations of PS NPs and dendrimers
Newborn PND1 CP kits and age-matched healthy control kits were anesthetized using a subcutaneous injection into the back of the neck of 0.1% (v/w) dextomitor. Topical lidocaine (0.4% w/w) was placed on the intended incision location, along the midline of the head. A 2 mm incision was made using a 10 blade, to expose the Bregma. A 5 μL Hamilton syringe was loaded with 2 μL of PS NP, G4-OH-Cy5, G4-OH-FITC, or G4-NH2-FITC. Injection coordinates were 1 mm lateral × 1 mm dorsal × 1 mm caudal on either side of the Bregma. PS NP and G4-OH-FITC were injected into the same animal (n = 3 kits), and G4-OH-Cy5 and G4-NH2-FITC were injected into the same animal (n = 3 kits), at a flow rate of 0.5 nL per minute. 5 ng of particle was administered. The skin was sutured, and bacitracin was placed on the incision, and animals were placed on a heating pad until they recovered consciousness, then returned to a nesting box in an incubator. Animals were euthanized using 0.2 mL pentobarbital (MWI) 4 h after injection, perfused with 10 mL cold 1x PBS (Gibco), brains removed and fixed prior to sectioning.
2.4.5. Brain uptake and cellular localization of dendrimers
Kits were euthanized at pre-determined time-points using 0.2 mL pentobarbital (MWI). The chest cavity was opened and the heart exposed. Blood was collected directly from the heart and centrifuged to separate out serum, and immediately frozen at −80 °C. The kit was then perfused with 10 mL cold 1x PBS (Gibco). The brain was immediately removed, cut down the midline to separate into two halves, and one half was placed in formalin (immunofluorescence analysis). The other half was micro-dissected to separate the PVR and cortex and frozen in 2 mL LoBind conical tubes (quantitative analysis, Eppendorf). Kidney, heart, lung, and liver were also collected and placed in 20 mL glass scintillation vials at −80 °C for further quantitative analysis. For immunofluorescence analysis, brains were placed in a formalin to 30% sucrose (Sigma) gradient over 96 h (formalin for 48 h, then 10% sucrose increase every 24 h), and sectioned on a Leica cryostat into 30 μm sections. In vivo analysis of BBB impairment in the CP brain is described in the supplemental information.
2.4.6. Immunofluorescence staining
Brain tissues were preserved in 30% sucrose at 4 °C and sectioned into 30 μm thick sections. Sections were dried overnight at room temperature. Primary antibodies for zonula occludins (ZO, 1:250 rat anti-ZO, Abcam), claudin-5 (1:250 mouse anti-claudin5, Abcam) microglia (1:250 goat anti-Iba, Abcam), astrocytes (1:250, chicken anti-GFAP, Abcam), and blood vessels (1:50, mouse anti-CD31, Abcam) were prepared in 1xPBS containing 0.01% triton-x (Sigma) and either 0.5% normal donkey serum (Sigma) or normal goal serum (Sigma). Primary antibody solutions were then added to tissue sections for 8–12 h at 4 °C. Sections were washed twice in 1x PBS (Gibco) to minimize dendrimer washing off from tissue. Secondary antibodies for microglia (1:250 donkey anti-goat Alexa488, Invitrogen), astrocytes (1:250 goat anti-chicken Alexa 488, Invitrogen), and blood vessels (1:50 donkey anti-mouse Alexa488, Invitrogen) were prepared in 1xPBS with 0.01% triton-x and added to tissue sections for 2 h. Sections were washed twice in 1x PBS, then stained with 1:1000 DAPI (Invitrogen). Slides were washed twice in 1x PBS and then allowed to dry overnight in the dark. Mountain medium (Dako) was added to each slide and a glass coverslip placed on top. Slides were stored at 4 °C until imaged and at 20 °C for long-term storage.
2.4.7. Semi-quantitative analysis of confocal images using image J, Imaris and Zen software
Image analysis was performed as previously described [29], and is described in detail in the supplemental information. Briefly, for microglia cell count 40X z-stack images 10–15 μm thick, with 3–5 locations per region in 5 slices per sample were analyzed [29–31]. We analyzed three images per region in ten 30 μm thick sections and counted only cells that were Iba-1+. The PVR and a 1 mm3 region of the cortex in both healthy control and CP kit sections were analyzed. The microglia were classified as amoeboid if the surface area to volume ratio was less than 1, or ramified if the surface area to volume ratio was greater than 1 [29–31].
2.4.8. Quantification of fluorescent G4 and G6 PAMAM dendrimers
Fluorescence-based quantification of G4-and G6-Cy5 was established based on a previously published protocol, with modifications according to the dendrimer surface charge [22], and is described in detail in the supplemental information.
2.4.9. Statistical analysis
All data were presented as mean ± S.E.M. Statistical analysis was performed using two-tail Student t-test for two-sample comparison and one-way ANOVA with Fisher’s post-hoc analysis for multivariant comparisons. A value of p < 0.05 was considered statistically significant.
3. Results and discussion
3.1. Preparation of dendrimer-Cy5 conjugates
Generation-4 PAMAM dendrimers, with hydroxyl (G4-OH), amine (G4-NH2), and carboxylate (G3.5-COOH) end groups, were covalently conjugated with Cy5, a near-infrared (IR) imaging agent (details in supplemental material). Each dendrimer-Cy5 conjugate had 1–2 molecules of Cy5 on the surface of the dendrimer (5 wt%). The Cy5 conjugates were highly soluble in water, PBS buffer, and stable at physiological conditions.
3.2. Passage across an impaired BBB is dependent on the physicochemical properties of dendrimers
The neuroinflammatory process results in injury to the surrounding oligodendrocytes and neurons, and disruption of the BBB at the site of injury [32,33], which can be chronic [34,35]. Following systemic administration, dendrimers will need to cross an impaired BBB to access the brain microenvironment. PAMAM dendrimers ranging from 3 nm to 14 nm were recently used to characterize the impaired BBB pore size in ischemic stroke [15], showing that a size of less than 11 nm is desirable to cross the impaired BBB in that model. We sought to elucidate how dendrimer size and molecular weight impact ability to cross the BBB in our CP model in regions of BBB breakdown. The extent of extravasation, following systemic administration, into areas of injury in the brain of PND1 rabbit kits with CP was evaluated for 70 kDa linear polymer dextran-FITC and a hard spherical 20 nm PS nanoparticle, and compared to that of G4-OH. The physicochemical properties of these compounds, including size and surface charge, are provided in Table 1.
Table 1. Physicochemical properties of various platforms used to determine extravasation across the BBB and cellular uptake within the brain in CP kits.
Hydrodynamic diameter (size, nm) and surface charge (zeta potential, mV) were measured using dynamic light scattering in PBS, pH 7.4 at room temperature. Molecular weight (MW) was provided by the company, or determined using mass spectrometry for dendrimers. NA: not applicable or not available for platform.
| Platform | At physiological pH | MW (kDa) | Size ± SEM (nm) | Zeta potential ± SEM (mV) |
|---|---|---|---|---|
| G4-OH | Neutral | 14.1 | 4.3 ± 0.2 | +4.5 ± 0.1 |
| G4-NH2 | Cationic | 14.1 | 3.9 ± 0.3 | +19.5 ± 0.1 |
| G3.5-COOH | Anionic | 11.1 | 3.2 ± 0.4 | −12.2 ± 0.2 |
| 20 nm PS | Anionic | NA | 21 ± 1 | −23 ± 0.9 |
| Linear dextran | Neutral | 70.0 | 13.9 ± 1.3 | NA |
| G6-OH | Neutral | 58.0 | 6.7 ± 0.1 | 0.25 ± 0.4 |
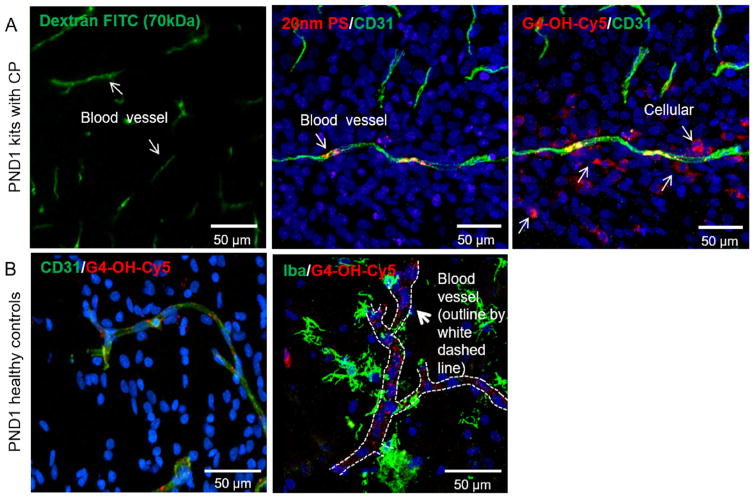
In regions of BBB impairment (Figure S1), dextran-FITC and 20 nm PS nanoparticles do not escape the blood vessel, or extravasate into the tissue 24 h following systemic administration (Fig. 1A). On the other hand, G4-OH escapes the blood vessels and localizes in cells in the periventricular region (PVR). In the brain of perfusion-fixed healthy animals, none of the materials show measurable uptake or cellular localization up to 24 h, since there is no BBB impairment (Fig. 1B, Figure S1). We analyzed the presence of tight junction (TJ) proteins ZO and claudins, which are critical to formation of tight junctions between brain microvascular endothelial cells [36]. Claudin-5 is most abundant in the brain at the BBB interface, whereas claudin-1 and claudin-3 are expressed primarily in the choroid plexus [36], and were therefore not investigated. The disruption of normal claudin-5 expression has been shown to impact the size selectivity of the BBB, specifically affecting transport of small molecules (<800 Da), but not larger molecules [37], like dendrimers (~14 kDa). We see that ZO-1, a subtype of ZO, and claudin-5 protein expression were decreased in the PVR in PND1 CP kits compared to age-matched healthy control kits (Figure S2). The ZO tight junction protein was still not present in PND9 CP kits. We also noted that in the cortex of CP kits, ZO expression was similar to that of age-matched healthy control kits, suggesting that even in an injured brain, BBB breakdown is region specific. The breakdown of TJ proteins ZO, occludin, and claudin-5 in the PVR in the presence of neuroinflammation is likely responsible for the passage of dendrimers into the brain parenchyma in that area, although increased transcytosis in the presence of inflammation is another potential mechanism of transport that should be further explored [38].
Fig. 1. Nanoparticle passage across the impaired BBB in CP kits is size dependent.
In newborn kits with CP, (A) at 24 h, 20 nm “hard” polystyrene (PS) nanoparticles and linear dextran-FITC remain trapped in blood vessels, whereas G4-OH-Cy5 is able to extravasate and localize in cells. (B) In healthy control kits at PND1, G4-OH-Cy5 remained localized in blood vessels (left panels, CD31, green) and do not penetrate into healthy normal tissue or uptake into cells (right panels, Iba-1, green). The blood vessels in all panels except the panel with dextran-FITC are stained with mouse anti-rabbit CD31 antibody, a marker for endothelial cells. There is no cell stain in the dextran-FITC panel. G4-OH (55 mg/kg) is fluorescently tagged with Cy5. Cell nuclei are stained with DAPI (blue). All scale bars are 50 μm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.3. Dendrimer selectively localizes at sites of injury in the brain
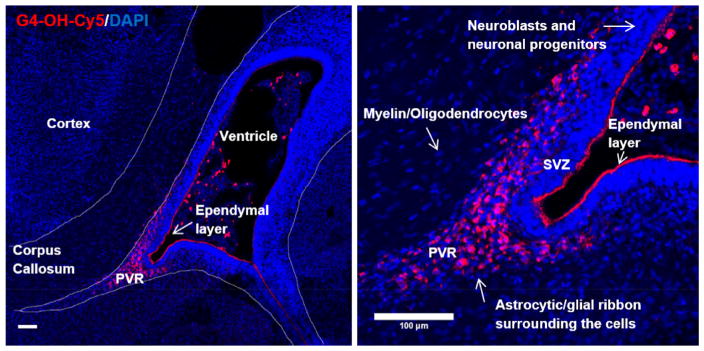
In the developing brain, new cell formation takes place, which is essential for normal development and maturation to occur. It is important to identify both the cells that do and do not uptake dendrimers. It has previously been shown that there is BBB impairment and increased pro-inflammatory microglia expression in the PVR in CP kits [24,39]. At 4 h after administration, G4-OH is present only in the activated glial ribbon of the PVR of animals with CP and in the choroid plexus, where there is significant blood vessel supply and cerebral spinal fluid (CSF)-blood exchange (Fig. 2, left). In this model, we show that G4-OH only localizes in this region of injury, and not in the subventricular zone (SVZ), where neuronal progenitor cells are present, or in the corpus callosum and cortex (Fig. 2, right). This pattern of localization is observed even at later time points.
Fig. 2. G4-OH localizes only in regions of BBB impairment and glia cell activation.
Following systemic administration in PND1 CP kits, G4-OH-Cy5 (red) uptake is only found in regions of BBB impairment (PVR) and localized in glial cells. In this model of white matter injury, G4-OH-Cy5 is not found in the cortex, corpus callosum, or in the subventricular zone (SVZ), where new cell formation is present. Cell nuclei are stained with DAPI (blue). Scale bars: 100 μm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.4. Movement within the brain parenchyma is governed by nanoparticle size and surface functionality
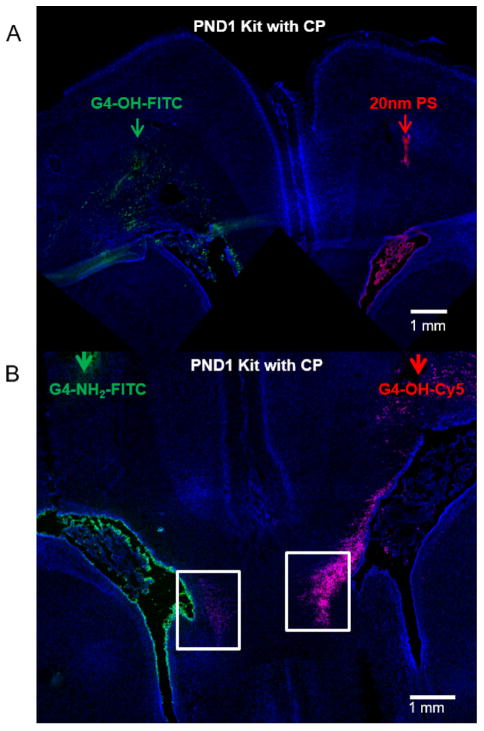
After crossing an intact or impaired BBB, the brain extracellular space (ECS) is a conduit through which drug delivery platforms must diffuse. Activated microglia/astrocytes are often distributed diffusely throughout the brain in the ECS, and can be several microns from the nearest blood vessel [40–42]. Even in regions of BBB impairment, both size and surface charge are critical to the ability of a drug delivery platform to cross the BBB [43,44], penetrate within the brain parenchyma [45], and reach diffuse cells often associated with CNS disorders to have maximum therapeutic effect. We found that, unlike G4-OH, 20 nm PS nanoparticles injected intraparenchymally in PND1 CP kits were not able to penetrate within the brain parenchyma away from the site of injection (Fig. 3A). This result is similar to what has been previously demonstrated with unmodified (negatively charged) PS nanoparticles of sizes ranging from 40 nm to 200 nm [45]. We injected G4-OH and G4-NH2 intraparenchymally in newborn kits with CP, and found G4-OH is able to rapidly diffuse several millimeters away from the point of injection within 4 h, and localize in cells only in regions of injury, whereas G4-NH2 remains trapped at the site of injection (Fig. 3B). Based on screening the brain using confocal imaging, PS nanoparticles and G4-NH2 were only able to follow routes of CSF flow, back along the injection track, into the sub-arachnoid space or into the choroid plexus, where they remained despite the presence of BBB impairment in the PVR.
Fig. 3. Dendrimer movement within the brain parenchyma is governed by nanoparticle size and surface functionality.
(A) ‘Large’, hard 20 nm PS nanoparticles (red, injection indicated by red arrow) do not move within the brain microenvironment following local administration, whereas 4 nm G4-OH-Cy5 (green, injection site indicated by green arrow) are capable of moving away from the site of injection within 4 h (B) G4-OH-Cy5 (red) can move and penetrate within brain parenchyma, reaching more diffuse sites, compared to charged dendrimers (cationic, green) of similar size. Arrows indicate the injection sites of the particles in the same brain, on opposing hemispheres, and the white boxes highlight the PVR, which is the target area of microglia activation. Scale bars: 1 mm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.5. Dendrimer uptake and cellular localization in the injured brain is a function of time and dendrimer surface functionality
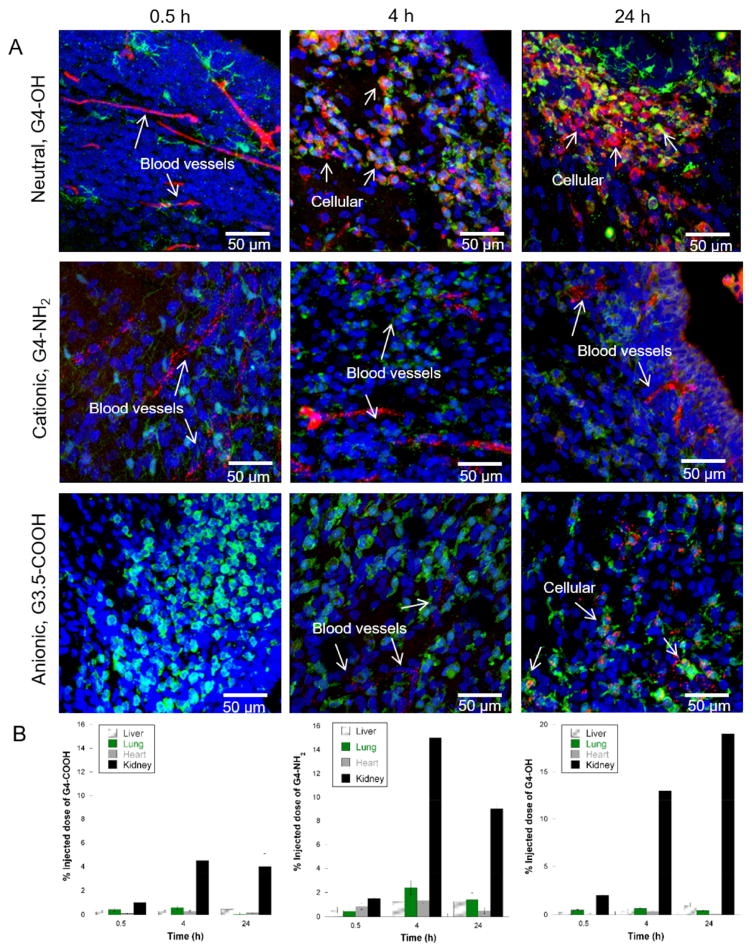
It is important to understand the effect of dendrimer surface functionality on the dendrimer’s ability to extravasate and localize in activated glial cells. We systematically investigated the time dependence of G4-NH2, G3.5-COOH, and G4-OH uptake in the brain following systemic administration on PND1. These three dendrimers have approximately the same size and molecular weight, but different surface functionalities and zeta potentials at physiological pH (Table 1). All animals were perfused with 1xPBS at time of sacrifice. We show that G4-OH was able to extravasate and rapidly localize in activated microglia within 4 h in regions of BBB impairment (Fig. 4A). At all the time points investigated in this study, G4-NH2 remains trapped within blood vessels, likely due to charge interactions with negatively charged endothelial cell membranes [46]. G3.5-COOH was not present in cells or blood vessels in the brain at 0.5 h after injection, and was present in blood vessels at 4 h and 24 h, and in microglia cells at 24 h. The delay in G3.5-COOH uptake in microglia cells compared to G4-OH uptake suggests that the neutral surface functionality on a dendrimer may be desirable for rapid escape from blood vessels. In the confocal images, the varying pattern of intracellular distribution between G4-OH and G3.5-COOH is supported by previous intracellular trafficking studies, which showed G4-OH traffics to late lysosomes and G3.5-COOH sequesters in endosomes [47]. G3.5-COOH could be useful for application in neuroinflammation since it also co-localizes in microglia, albeit in a delayed manner, and the different method of internalization compared to G4-OH could lead to targeting of specific intracellular pathways. In the brain of healthy PND1 kits, dendrimers did not cross the intact BBB, and remained localized within blood vessel structures, independent of dendrimer surface functionality. In CP kits, biodistribution in the heart, liver, and lungs, as well as clearance from the body via the kidneys, was similar for all G4 dendrimers studied (Fig. 4B). As expected based on previous biodistribution analysis of G4-OH [22], accumulation in the kidneys occurred up to 24 h, as G4-OH was cleared from circulation. There was no significant difference in biodistribution in the heart, liver, lungs and kidneys in control verse CP kits at this age.
Fig. 4. Dendrimer localization and retention in activated glia in CP kits.
G4 dendrimer with various surface functionalities (red in all panels) were injected systemically in PND1 CP kits. Animals were euthanized and perfused with normal saline at 0.5 h, 4 h, or 24 h after administration into the lateral vein. (A) G4-OH-Cy5 is able to escape the blood vessels by 4 h and localize in activated glia cells (Iba+, green in all panels). By 24 h, any G4-OH-Cy5 dendrimer in the tissue and not in cells has been cleared. G4-NH2-Cy5 remains in blood vessels at all time points studied, and does not localized in activated glia in the tissue parenchyma. G3.5-COOH-Cy5 shows delayed cellular uptake in the brain, with localization in the brain blood vessels at 4 h, but not at 0.5 h after injection. By 24 h, the G3.5-COOH-Cy5 is localized in activated microglia, but is sequestered in endosomes, whereas G4-OH-Cy5 is sequestered in late lysosomes. Cell nuclei are stained with DAPI (blue). All scale bars are 50 μm. (B) G4-OH, G3.5-COOH, and G4-NH2 distribution in off-site organs in newborn kits with CP. Dendrimer accumulation in the heart, liver, lungs, and kidney as a function of time was measured following 55 mg/kg injection of G4-OH-Cy5, G3.5-COOH-Cy5, or G4-NH2-Cy5 in newborn kits with CP. Data is represented as the percent injected dose (%ID) of dendrimer per gram of tissue. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The uptake and specific cellular localization of the dendrimer platforms can play a significant role in targeted delivery, especially if toxicity is of concern. Cationic PAMAM dendrimers have been shown to uptake in the brain when administered intraparenchymally or intraventricularly [48], but are also toxic at higher generations and higher concentrations through these aforementioned, systemic, and intranasal administration routes. This can lead to a negative effect on gene expression [49] and the induction of autophagy due to increased intracellular reactive oxygen species generation [50]. The inability of cationic dendrimers to diffuse within the brain parenchyma is also limiting, even if no toxicity for G4 or lower cationic dendrimers at low concentrations has been reported in vivo [51]. It is important to emphasize that minimal or no G4-OH dendrimer uptake is seen in regions of healthy tissue, or in regions with new cell formation critical to normal brain development and function, which will reduce off-site toxicity and minimize long term negative impact. The ability of the neutral G4-OH to deliver drugs to activated glia, without associated toxicity, may offer new avenues for targeted delivery.
3.6. Semi-quantitative analysis of dendrimer uptake and cellular localization
We next sought to quantify the amount of dendrimer in the PVR of the brain, after perfusion. We calculated the percent injected dose (% ID) of each dendrimer as the total amount of dendrimer in the brain (μg) over the total amount of brain tissue analyzed (g tissue). We show peak uptake for all G4 dendrimers at 4 h after administration in PND1 CP kits, and a decrease in total amount in the brain by 24 h (Fig. 5A). G4-NH2 was the most abundant in the brain at all time-points, yet was never present in cells within the parenchyma, as seen in Fig. 4. G3.5-COOH and G4-OH had similar amounts in the brain at all time-points; however, as seen by the confocal images, the cellular localization of G3.5-COOH and G4-OH at each time point varied. The maximum %ID of G4-OH in the brain of kits with CP was 0.05%, compared to 0.003% ID of G4-OH in the brain of healthy control kits (>10-fold overall uptake in the brain of CP kits). Importantly, the amount of G4-OH in the brain is 100-fold higher than that of a free drug (NAC), and the G4-OH is predominantly localized in target cells. We have previously published data that shows that systemically administered D-NAC (containing ~55 mg/kg of dendrimer and 10 mg/kg of drug NAC) lead to significant and profound efficacy in newborn rabbits with CP [13]. The dose of G4-OH dendrimer in this study, with a Cy5 molecule instead of NAC, is comparable to that of the dose of D-NAC that produced motor function improvement in CP [13], suggesting that targeting the injured region of the brain, and specific cells, can lead to a profound effect.
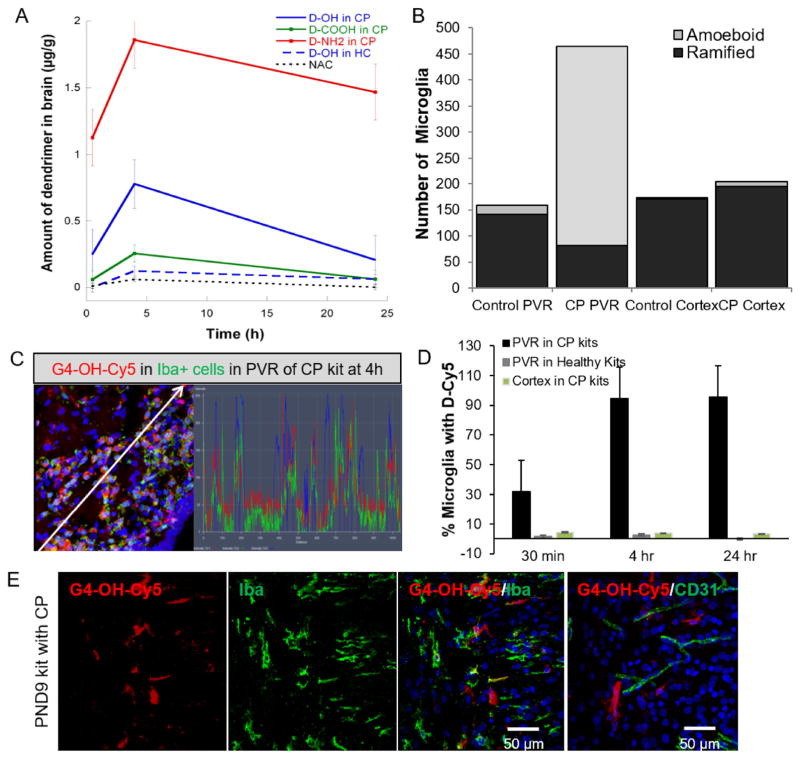
Fig. 5. G4-OH quantities are 100-fold higher compared to free drug in the brain and localize in amoeboid microglia up to 9 days after administration.
G4 dendrimers with various surface functionalities were systemically injected (55 mg/kg) into PND1 CP kits and age-matched healthy control (HC) kits. Animals were euthanized at specified time points, perfused, and the brains collected for dendrimer (A) quantification using spectrophotometry or (B–D) for semi-quantitative analysis using confocal imaging. (A) Amount of dendrimer is calculated based on a standard curve for each dendrimer compound, G4-NH2-Cy5, G3.5-COOH-Cy5, and G4-OH-Cy5, in both CP brains and healthy control brains at 0.5 h, 4 h, and 24 h after systemic administration, with n = 4–5 kits per time point per dendrimer per group. (B) The total number of ramified and amoeboid Iba1+ microglia were counted in a 1 mm3 section of the PVR and cortex of healthy control kits and CP kits. (C) Co-localization of G4-OH-Cy5 (red) in microglia (green) at 4 h after administration. White arrows represent path through which co-localization histograms were analyzed. (D) Dendrimer localization in Iba-1+ cells was quantified from confocal images taken in the PVR and in the cortex in both newborn kits with CP and age-matched healthy controls. Localization is expressed as a percent of cells positive for the respective cell stain and G4-OH-Cy5. There were no significant number of microglia co-localized with G4-OH-Cy5 in the cortex of healthy kits with CP. (E) G4-OH-Cy5 (red) was injected systemically in PND1 kits with CP. Animals were euthanized at PND9, eight days after dendrimer administration. In regions of white matter injury, G4-OH-Cy5 is localized (yellow) with microglia (Iba1+, green, left panels) and not in blood vessels (CD31, green right panel). Cell nuclei are stained with DAPI (blue). Scale bar: 50 μm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
We further analyzed cellular localization of dendrimer using semi-quantitative analysis of the confocal images. In recent years, a number of in vitro and in vivo studies have implicated microglial cells in the development of CP [13,52]. In the healthy brain, microglia are involved in surveillance functions, and monitoring of neuronal well-being [53]. Upon activation after an injury, microglia undergo a pronounced change in morphology from ramified to an amoeboid structure, a decrease in migration ability, and proliferate, increasing in number [54–56]. The number of total microglia showed a 3.5-fold increase in the PVR of CP kits compared with healthy controls. However, the number of microglia in the cortex of CP kits remained comparable to that of healthy controls (Fig. 5B). In the PVR of PND1 CP kits, the amoeboid population of microglia was 83% of the total microglia, compared to only 11% of total microglia in the PVR of healthy controls. In the rabbit model of CP, the number of microglia increases in the presence of inflammation, and there is an associated decrease in ramified “resting” microglia and an increase in amoeboid “activated” microglia. The microglia morphology in the cortex of both healthy and CP kits was predominantly ramified, with less than 4% of microglia classified as amoeboid. Given the rapid uptake and previous use of G4-OH-drug conjugate in efficacy studies in CP [13], we analyzed the cell specific change in localization of G4-OH over time in the PVR and cortex of both healthy newborn kits and CP kits. The difference in co-localization of G4-OH over time corresponds to G4-OH movement from blood vessels at 0.5 h to intracellular localization within microglia by 4 h. Analysis of a representative region in the PVR showed co-localization of the G4-OH only with Iba-1 stained microglia, with no co-localization seen in the parenchyma (Fig. 5C). By analyzing a subset of 30 μm thick sections within the PVR, we counted the number of microglia that were positive for both G4-OH and Iba-1 at each time point. The number of Iba-1+ microglia with G4-Cy5 increases in the PVR of kits with CP from 0.5 h to 4 h (Fig. 5D), and reaches a maximum of 90% of cells containing G4-OH. There is no uptake in microglia in the cortex of CP kits, or in the PVR or cortex of healthy control kits, due to the lack of BBB impairment, as shown in Fig. 1 and Figure S1. Based on previous cytokine data analysis in brains of kits with CP, it can be extrapolated that the dendrimer is localizing in “activated” microglia.
3.7. Dendrimer is retained in the injured newborn brain
The uptake, long term retention, and release kinetics of dendrimer-drug conjugates will dictate both the timing of administration, as well as initial design of dendrimer-therapies. To determine if dendrimer is still present in microglia many days after administration, we further investigated the retention of G4-OH in activated microglia in CP kits. The mortality of untreated CP kits is very high after PND9- PND10 of life. At PND9 (8 days after systemic administration), G4-OH remained localized in microglia in the PVR (Fig. 5E). Unlike in PND1 kits, G4-OH was not present in blood vessels in PND9 CP kits, suggesting G4-OH that had not internalized in cells is not present in the brain. The qualitative amount of G4-OH in the brain of PND9 kits was also reduced compared to 4 h after systemic administration.
3.8. Dendrimer uptake correlates to disease severity in rabbits with CP
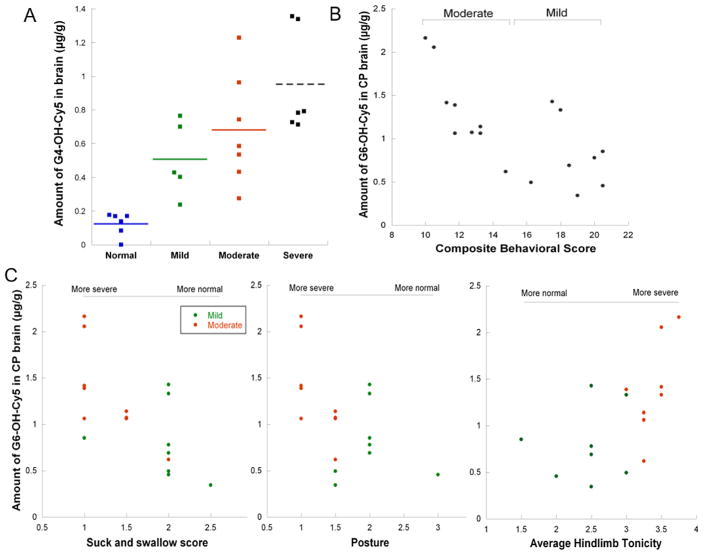
The toxicity of G4-OH, even at high doses, is minimal compared to cationic dendrimers, particularly cationic dendrimers of generations G6 and above [51,57–59]. G4-OH dendrimer is cleared intact on the order of hours from blood circulation, and over 24–48 h from the kidney [22]. G4-OH dendrimers did not show any toxicity at 10-fold higher doses than those used in this study [13]. As we have shown, G4-OH only accumulates in regions of injury where there is BBB impairment and cell activation, and not in normal healthy tissue or non-activated cells (Figs. 1, 2 and 4). Therefore, the extent of dendrimer uptake can be correlated to the extent of disease in the brain. Animals were evaluated in a blinded manner for neurobehavioral measures, prior to dendrimer injection on PND1. We generated a composite behavioral score based on behavioral tests that were significantly different at PND1 between control kits and CP kits used in this study (Table S1). Newborn kits with CP (n = 18 total) were classified into the following categories: severe (n = 6 kits, composite score 3–9), moderate (n = 7 kits, composite score 10–14), and mild (n = 5 kits, composite score 15–20). Normal healthy kits (n = 8) had a composite behavioral score greater than 23. No kits with CP had a composite behavioral score greater than 20.
We first use G4-OH to look at dendrimer uptake as a function of disease severity. In normal healthy control kits, we see minimal dendrimer accumulation (0.004% ID) in the brain. In CP kits, we see up to 13-fold higher accumulation in kits with a severe phenotype, as assessed by composite behavioral score (Fig. 6A). We found that the amount of G4-OH uptake in the newborn CP brain was statistically greater in the severe group compared to normal (p < 0.001) and mild kits (p < 0.05). The G4-OH uptake in moderate and mild CP kits was significantly higher than healthy kits (p < 0.005). However, there was no significant difference in the amount of G4-OH uptake in the severe kits compared to moderate kits, or in the moderate kits compared to mild kits.
Fig. 6. Dendrimer uptake correlates to disease severity.
G4-OH-Cy5 and G6-OH-Cy5 was injected systemically in PND1 CP kits, and age-matched healthy control kits. Kits were assessed for behavioral performance in a blinded manner prior to injection. For the purpose of the composite score used for classification in (A) and (B), average limb tonicity was inverted so that the scoring aligned with that of all other behavioral measurements, where 0 = severe and 3 or 4 = normal. The amount of G4-OH-Cy5 and G6-OH-Cy5 in the brain per gram of tissue at 4 h and 24 h, respectively, was quantified for each kit, also in a blinded manner. (A) The amount of G4-OH-Cy5 uptake strongly correlates to disease severity, defined by severe (composite score 3–9), moderate (composite score 10–14) or mild CP phenotype (composite score 15–20), or normal for healthy controls (composite score >20). (B) When assessing animals within the mild-moderate phenotype, the amount of G6-OH-Cy5 uptake in individual kits is correlated (R2 = 0.51) to composite behavioral score. (C) In addition, the amount of G6-OH-Cy5 uptake in the brain of newborn kits with CP can delineate individual behavior scores that are significantly different (p < 0.001) between mild and moderate classified animals, including the suck and swallow score (scale 0–3), indicative of how well the kits can latch on to the syringe nipple and suckle milk, the posture (scale 0–3) of the head and upper body when on all four limbs, and the average tonicity of the hindlimbs (scale 0–4), where a higher score indicates more hypertonic limbs.
Therefore we sought to further determine if we could better delineate phenotype in the mild-moderate range based on dendrimer uptake in the CP brain. We used a Cy5-labeled, generation-6 dendrimer (G6-OH-Cy5) to evaluate uptake as a function of disease severity in CP kits (n = 17 kits total) that fell into the mild (n = 8) and moderate phenotype (n = 9), with the same composite behavioral score ranges as described above. G6-OH has a longer circulation time compared to G4-OH (Figure S3A) [7] and thus has greater uptake in the CP brain (Figure S3B). However, G6-OH is still small enough in size and possesses neutral surface functionality (Table 1) to pass the impaired BBB and localize within microglial cells in the PVR of CP kits (Figure S3C). We see a correlation (R2 = 0.51) between amount of G6-OH dendrimer in the brain (μg/ g) and an increase in disease severity from mild to moderate (Fig. 6B). More importantly, the average amount of G6-OH uptake in moderate kits (1.33 μg/g) is significantly greater (p < 0.05) than the average amount of G6-OH uptake in mild kits (0.79 μg/g). This trend is less when assessing individual behavioral scores (R2 < 0.50) in moderate CP kits that are statistically worse than mild CP kits (Fig. 6C). This suggests that a comprehensive behavioral analysis, as performed clinically, is a more accurate assessment of disease severity than a single behavioral test in pre-clinical studies.
There is a significant lack of effective translationally-relevant biomarkers of disease states. These assays of disease are not only essential for improving our understanding of neurological mechanisms that underlie disease, but also for providing patient screening tools to increase the probability of success for drug delivery platforms as they enter clinical trials. Previously, PET and MRI agents have been utilized to non-invasively measure inflammation in the epileptic brain [60], schizophrenia [61], CP [24], Alzheimer’s [62], and following stroke [63]. PAMAM dendrimers show specific localization with atherosclerotic plaques using a PET-conjugated dendrimer [64] and have been used to track pharmacokinetics using dendrimer-conjugated MRI agents [65]. We have demonstrated that Cy5-labeled dendrimer uptake is selective and specific to regions of injury and to the cells involved in injury. As seen in healthy control newborn rabbits, minimal dendrimer accumulation is present in the brain if there is no BBB impairment and no glial cell activation. There is also a strong correlation between the extent of the disease, determined based on neurobehavioral scoring, and the amount of dendrimer uptake in the brain, with greater dendrimer uptake in the brain in more sick animals. G6-OH dendrimer was more capable of delineating disease phenotype as a function of dendrimer uptake in the brain. This is likely due to the longer circulation of the G6-OH dendrimer, leading to significantly greater access to the injured brain [66]. Hydroxyl modified dendrimers have been shown to be non-toxic if generation 6.5 or lower, and if given at a low dose (less than 200 mg/kg) [67]. When we assess area under the curve (AUC) in CP kits for G4-OH uptake from 0.5 to 24 h and for G6-OH uptake from 4 to 48 h, we obtain a ratio of G6/G4 AUC of 2.8. This indicates a much higher amount of G6 uptake in the brain compared to G4. Nonetheless, these dendrimers are cleared intact via the kidneys, and there is minimal uptake in all other organs in the body [22]. Therefore, this correlation between amount of dendrimer uptake in the brain and the disease severity can represent a potential imaging biomarker of disease. This may allow for more accurate diagnosis via patient screening for better timing of therapy, and to follow response to therapy.
4. Conclusions
Dendrimer-mediated delivery of therapeutics can improve efficacy by greater accumulation of the therapeutic at sites of injury, and only at sites of injury, also reducing off-target side effects. Building on significant, translationally relevant efficacy studies, we sought to understand the nanoscale attributes of PAMAM dendrimers relevant for targeting neuroinflammation. We have demonstrated that, when administered systemically, nanoparticle size and dendrimer surface functionality, as well as the presence of neuroinflammatory disease hallmarks such as BBB impairment and glial activation, govern the ability of a dendrimer to cross an impaired BBB, and selectively localize in activated glia cells involved in the neuroinflammatory process. We show that neutral dendrimer uptake is rapid, with 100-fold greater brain accumulation compared to free drug, and localization is primarily to activated microglia within regions of injury. In addition, when systemically administered, the amount of dendrimer uptake is directly and strongly correlated with disease severity in newborn kits with CP. Understanding this mechanism of dendrimer-mediated delivery provides greater opportunity and ease for clinical translation of therapies for pediatric brain injury. Given the underlying role of neuroinflammation in many brain diseases, including CP, stroke, and autism, we expect these findings to translate to multiple models of pediatric and adult brain injury.
Supplementary Material
Acknowledgments
This study was partly funded by the NICHD R01HD069562 (S. Kannan) and NIBIB R01EB018306 (Rangaramanujam M. Kannan) and NICHD R01HD076901 (Rangaramanujam M. Kannan). Dr. Nance would like to thank the Hartwell Foundation and the Burroughs Wellcome Fund (BWF) Career Award at Scientific Interfaces for funding for her postdoctoral fellowship. The authors thank the Wilmer Core Module for Microscopy and Imaging for allowing us to use LSM710 confocal. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, the Hartwell Foundation, or BWF.
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.biomaterials.2016.05.044.
References
- 1.Organization WH. Neurological Disorders: Public Health Challenges. 2006. [Google Scholar]
- 2.Alavijeh MS, Chishty M, Qaiser MZ, Palmer AM. Drug metabolism and pharmacokinetics, the blood-brain barrier, and central nervous system drug discovery. NeuroRx J Am Soc Exp Neurother. 2005;2(4):554–571. doi: 10.1602/neurorx.2.4.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel T, Zhou J, Piepmeier JM, Saltzman WM. Polymeric nanoparticles for drug delivery to the central nervous system. Adv drug Deliv Rev. 2012;64(7):701–705. doi: 10.1016/j.addr.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nance E, et al. Non-invasive delivery of stealth, brain-penetrating nanoparticles across the blood-brain barrier using MRI-guided focused ultrasound. J Control Release. 2014;189:123–132. doi: 10.1016/j.jconrel.2014.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang F, Lin YA, Kannan S, Kannan RM. Targeting specific cells in the brain with nanomedicines for CNS therapies. J Control release Off J Control Release Soc. 2015 doi: 10.1016/j.jconrel.2015.12.013. http://dx.doi.org/10.1016/j.jconrel.2015.12.013 (epub is ahead of print) [DOI] [PMC free article] [PubMed]
- 6.Dammann O. Persistent neuroinflammation in cerebral palsy: a therapeutic window of opportunity? Acta Paediatr. 2007;96(1):6–7. doi: 10.1111/j.1651-2227.2007.00097.x. [DOI] [PubMed] [Google Scholar]
- 7.Kannan RM, Nance E, Kannan S, Tomalia DA. Emerging concepts in dendrimer-based nanomedicine: from design principles to clinical applications. J Intern Med. 2014;276(6):579–617. doi: 10.1111/joim.12280. [DOI] [PubMed] [Google Scholar]
- 8.Shakhbazau A, et al. Fluorescent phosphorus dendrimer as a spectral nanosensor for macrophage polarization and fate tracking in spinal cord injury. Macromol Biosci. 2015;15(11):1523–1534. doi: 10.1002/mabi.201500150. [DOI] [PubMed] [Google Scholar]
- 9.Shakhbazau A, et al. Doxycycline-regulated GDNF expression promotes axonal regeneration and functional recovery in transected peripheral nerve. J Control Release Off J Control Release Soc. 2013;172(3):841–851. doi: 10.1016/j.jconrel.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Nance E, et al. Systemic dendrimer-drug treatment of ischemia-induced neonatal white matter injury. J Control Release Off J Control Release Soc. 2015;214:112–120. doi: 10.1016/j.jconrel.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayder M, et al. A phosphorus-based dendrimer targets inflammation and osteoclastogenesis in experimental arthritis. Sci Transl Med. 2011;3(81):81ra35. doi: 10.1126/scitranslmed.3002212. [DOI] [PubMed] [Google Scholar]
- 12.Sarin H, et al. Effective transvascular delivery of nanoparticles across the blood-brain tumor barrier into malignant glioma cells. J Transl Med. 2008;6:80. doi: 10.1186/1479-5876-6-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kannan S, et al. Dendrimer-based postnatal therapy for neuroinflammation and cerebral palsy in a rabbit model. Sci Transl Med. 2012;4(130) doi: 10.1126/scitranslmed.3003162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boridy S, Soliman GM, Maysinger D. Modulation of inflammatory signaling and cytokine release from microglia by celastrol incorporated into dendrimer nanocarriers. Nanomed (Lond) 2012;7(8):1149–1165. doi: 10.2217/nnm.12.16. [DOI] [PubMed] [Google Scholar]
- 15.Zheng S, et al. Multimodal nanoprobes evaluating physiological pore size of brain vasculatures in ischemic stroke models. Adv Healthc Mater. 2014;3(11):1909–1918. doi: 10.1002/adhm.201400159. [DOI] [PubMed] [Google Scholar]
- 16.Mishra MK, et al. Dendrimer brain uptake and targeted therapy for brain injury in a large animal model of hypothermic circulatory arrest. ACS nano. 2014;8(3):2134–2147. doi: 10.1021/nn404872e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kambhampati SP, et al. Systemic and intravitreal delivery of dendrimers to activated microglia/macrophage in ischemia/reperfusion mouse retina. Investig Ophthalmol Vis Sci. 2015;56(8):4413–4424. doi: 10.1167/iovs.14-16250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo Y, et al. Dendrimers target the ischemic lesion in rodent and primate models of nonarteritic anterior ischemic optic neuropathy. PloS one. 2016;11(4):e0154437. doi: 10.1371/journal.pone.0154437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saadani-Makki F, et al. Intrauterine administration of endotoxin leads to motor deficits in a rabbit model: a link between prenatal infection and cerebral palsy. Am J obstet Gynecol. 2008;199(6):651, e651–657. doi: 10.1016/j.ajog.2008.06.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maragakis NJ, Rothstein JD. Mechanisms of Disease: astrocytes in neurodegenerative disease. Nat Clin Pract Neurol. 2006;2(12):679–689. doi: 10.1038/ncpneuro0355. [DOI] [PubMed] [Google Scholar]
- 21.Fuller S, Munch G, Steele M. Activated astrocytes: a therapeutic target in Alzheimer’s disease? Expert Rev Neurother. 2009;9(11):1585–1594. doi: 10.1586/ern.09.111. [DOI] [PubMed] [Google Scholar]
- 22.Lesniak WG, et al. Biodistribution of fluorescently labeled PAMAM dendrimers in neonatal rabbits: effect of neuroinflammation. Mol Pharm. 2013;10(12):4560–4571. doi: 10.1021/mp400371r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benchaala I, et al. Folate-functionalized dendrimers for targeting Chlamydia-infected tissues in a mouse model of reactive arthritis. Int J Pharm. 2014;466(1–2):258–265. doi: 10.1016/j.ijpharm.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 24.Kannan S, et al. Magnitude of [(11)C]PK11195 binding is related to severity of motor deficits in a rabbit model of cerebral palsy induced by intrauterine endotoxin exposure. Dev Neurosci. 2011;33(3–4):231–240. doi: 10.1159/000328125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kannan S, et al. Microglial activation in perinatal rabbit brain induced by intrauterine inflammation: detection with 11C-(R)-PK11195 and small-animal PET. J Nucl Med Off Publ Soc Nucl Med. 2007;48(6):946–954. doi: 10.2967/jnumed.106.038539. [DOI] [PubMed] [Google Scholar]
- 26.Derrick M, et al. Preterm fetal hypoxia-ischemia causes hypertonia and motor deficits in the neonatal rabbit: a model for human cerebral palsy? J Neurosci Off J Soc Neurosci. 2004;24(1):24–34. doi: 10.1523/JNEUROSCI.2816-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eixarch E, et al. Neonatal neurobehavior and diffusion MRI changes in brain reorganization due to intrauterine growth restriction in a rabbit model. PloS one. 2012;7(2):e31497. doi: 10.1371/journal.pone.0031497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Georgiadis P, et al. Characterization of acute brain injuries and neurobehavioral profiles in a rabbit model of germinal matrix hemorrhage. Stroke; a J Cereb Circ. 2008;39(12):3378–3388. doi: 10.1161/STROKEAHA.107.510883. [DOI] [PubMed] [Google Scholar]
- 29.Zhang F, et al. Uniform brain tumor distribution and tumor associated macrophage targeting of systemically administered dendrimers. Biomaterials. 2015;52:507–516. doi: 10.1016/j.biomaterials.2015.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stence N, Waite M, Dailey ME. Dynamics of microglial activation: a confocal time-lapse analysis in hippocampal slices. Glia. 2001;33(3):256–266. [PubMed] [Google Scholar]
- 31.Orr AG, Orr AL, Li XJ, Gross RE, Traynelis SF. Adenosine A(2A) receptor mediates microglial process retraction. Nat Neurosci. 2009;12(7):872–878. doi: 10.1038/nn.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li J, Baud O, Vartanian T, Volpe JJ, Rosenberg PA. Peroxynitrite generated by inducible nitric oxide synthase and NADPH oxidase mediates microglial toxicity to oligodendrocytes. Proc Natl Acad Sci U S A. 2005;102(28):9936–9941. doi: 10.1073/pnas.0502552102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stolp HB, et al. Effects of neonatal systemic inflammation on blood-brain barrier permeability and behaviour in juvenile and adult rats. Cardiovasc Psychiatry Neurol. 2011;2011:469046. doi: 10.1155/2011/469046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Vries HE, Kuiper J, de Boer AG, Van Berkel TJ, Breimer DD. The blood-brain barrier in neuroinflammatory diseases. Pharmacol Rev. 1997;49(2):143–155. [PubMed] [Google Scholar]
- 35.Petty MA, Lo EH. Junctional complexes of the blood-brain barrier: permeability changes in neuroinflammation. Prog Neurobiol. 2002;68(5):311–323. doi: 10.1016/s0301-0082(02)00128-4. [DOI] [PubMed] [Google Scholar]
- 36.Goncalves A, Ambrosio AF, Fernandes R. Regulation of claudins in blood-tissue barriers under physiological and pathological states. Tissue Barriers. 2013;1(3):e24782. doi: 10.4161/tisb.24782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nitta T, et al. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J cell Biol. 2003;161(3):653–660. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knowland D, et al. Stepwise recruitment of transcellular and paracellular pathways underlies blood-brain barrier breakdown in stroke. Neuron. 2014;82(3):603–617. doi: 10.1016/j.neuron.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saadani-Makki F, et al. Intrauterine endotoxin administration leads to white matter diffusivity changes in newborn rabbits. J Child Neurol. 2009;24(9):1179–1189. doi: 10.1177/0883073809338213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bickel U, Yoshikawa T, Pardridge WM. Delivery of peptides and proteins through the blood-brain barrier. Adv Drug Deliv Rev. 2001;46(1–3):247–279. doi: 10.1016/s0169-409x(00)00139-3. [DOI] [PubMed] [Google Scholar]
- 41.Pawlik G, Rackl A, Bing RJ. Quantitative capillary topography and blood flow in the cerebral cortex of cats: an in vivo microscopic study. Brain Res. 1981;208(1):35–58. doi: 10.1016/0006-8993(81)90619-3. [DOI] [PubMed] [Google Scholar]
- 42.Schlageter KE, Molnar P, Lapin GD, Groothuis DR. Microvessel organization and structure in experimental brain tumors: microvessel populations with distinctive structural and functional properties. Microvasc Res. 1999;58(3):312–328. doi: 10.1006/mvre.1999.2188. [DOI] [PubMed] [Google Scholar]
- 43.Mayhan WG, Heistad DD. Permeability of blood-brain barrier to various sized molecules. Am J Physiol. 1985;248(5 Pt 2):H712–H718. doi: 10.1152/ajpheart.1985.248.5.H712. [DOI] [PubMed] [Google Scholar]
- 44.Pardridge WM. Drug transport across the blood-brain barrier. J Cereb blood flow Metab Off. 2012;32(11):1959–1972. doi: 10.1038/jcbfm.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nance EA, et al. A dense poly(ethylene glycol) coating improves penetration of large polymeric nanoparticles within brain tissue. Sci Transl Med. 2012;4(149):149ra119. doi: 10.1126/scitranslmed.3003594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jallouli Y, Paillard A, Chang J, Sevin E, Betbeder D. Influence of surface charge and inner composition of porous nanoparticles to cross blood-brain barrier in vitro. Int J Pharm. 2007;344(1–2):103–109. doi: 10.1016/j.ijpharm.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 47.Perumal OP, Inapagolla R, Kannan S, Kannan RM. The effect of surface functionality on cellular trafficking of dendrimers. Biomaterials. 2008;29(24–25):3469–3476. doi: 10.1016/j.biomaterials.2008.04.038. [DOI] [PubMed] [Google Scholar]
- 48.Albertazzi L, et al. In vivo distribution and toxicity of PAMAM dendrimers in the central nervous system depend on their surface chemistry. Mol Pharm. 2013;10(1):249–260. doi: 10.1021/mp300391v. [DOI] [PubMed] [Google Scholar]
- 49.WinShwe TT, et al. Effects of PAMAM dendrimers in the mouse brain after a single intranasal instillation. Toxicol Lett. 2014;228(3):207–215. doi: 10.1016/j.toxlet.2014.04.020. [DOI] [PubMed] [Google Scholar]
- 50.Wang S, et al. The role of autophagy in the neurotoxicity of cationic PAMAM dendrimers. Biomaterials. 2014;35(26):7588–7597. doi: 10.1016/j.biomaterials.2014.05.029. [DOI] [PubMed] [Google Scholar]
- 51.Shcharbin D, et al. How to study dendrimers and dendriplexes III. Bio-distribution, pharmacokinetics and toxicity in vivo. J Control Release Off J Control Release Soc. 2014;181:40–52. doi: 10.1016/j.jconrel.2014.02.021. [DOI] [PubMed] [Google Scholar]
- 52.Mallard C, et al. Astrocytes and microglia in acute cerebral injury underlying cerebral palsy associated with preterm birth. Pediatr Res. 2014;75(1–2):234–240. doi: 10.1038/pr.2013.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Billiards SS, et al. Development of microglia in the cerebral white matter of the human fetus and infant. J Comp Neurol. 2006;497(2):199–208. doi: 10.1002/cne.20991. [DOI] [PubMed] [Google Scholar]
- 54.Perry VH, Nicoll JA, Holmes C. Microglia in neurodegenerative disease. Nat Rev Neurol. 2010;6(4):193–201. doi: 10.1038/nrneurol.2010.17. [DOI] [PubMed] [Google Scholar]
- 55.Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8(1):57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- 56.Zhang F, Nance E, Alnasser Y, Kannan R, Kannan S. Microglial migration and interactions with dendrimer nanoparticles are altered in the presence of neuroinflammation. J Neuroinflammation. 2016;13(1):65. doi: 10.1186/s12974-016-0529-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jones CF, et al. Cationic PAMAM dendrimers aggressively initiate blood clot formation. ACS nano. 2012;6(11):9900–9910. doi: 10.1021/nn303472r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jones CF, et al. Cationic PAMAM dendrimers disrupt key platelet functions. Mol Pharm. 2012;9(6):1599–1611. doi: 10.1021/mp2006054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Greish K, et al. Size and surface charge significantly influence the toxicity of silica and dendritic nanoparticles. Nanotoxicology. 2012;6(7):713–723. doi: 10.3109/17435390.2011.604442. [DOI] [PubMed] [Google Scholar]
- 60.Amhaoul H, Staelens S, Dedeurwaerdere S. Imaging brain inflammation in epilepsy. Neuroscience. 2014;279C:238–252. doi: 10.1016/j.neuroscience.2014.08.044. [DOI] [PubMed] [Google Scholar]
- 61.Pasternak O, Westin CF, Dahlben B, Bouix S, Kubicki M. The Extent of Diffusion MRI Markers of Neuroinflammation and White Matter Deterioration in Chronic Schizophrenia. Schizophrenia research. 2014 doi: 10.1016/j.schres.2014.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mach RH. New targets for the development of PET tracers for imaging neurodegeneration in alzheimer disease. J Nucl Med Off Publ Soc Nucl Med. 2014;55(8):1221–1224. doi: 10.2967/jnumed.114.127811. [DOI] [PubMed] [Google Scholar]
- 63.Scheller A, et al. Imaging neuroinflammation after brain injuries by ultra-sensitive MRI and two-photon laser-scanning microscopy. Romanian J Morphol Embryol Revue roumaine de Morphol Embryol. 2014;55(3):735–743. [PubMed] [Google Scholar]
- 64.Seo JW, et al. (64)Cu-labeled LyP-1-dendrimer for PET-CT imaging of atherosclerotic plaque. Bioconjugate Chem. 2014;25(2):231–239. doi: 10.1021/bc400347s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tomalia DA, Reyna LA, Svenson S. Dendrimers as multi-purpose nano-devices for oncology drug delivery and diagnostic imaging. Biochem Soc Trans. 2007;35(Pt 1):61–67. doi: 10.1042/BST0350061. [DOI] [PubMed] [Google Scholar]
- 66.Grimm JC, et al. Nanotechnology approaches to targeting inflammation and excitotoxicity in a canine model of hypothermic circulatory arrest-induced brain injury. Ann Thorac Surg. 2016 doi: 10.1016/j.athoracsur.2016.02.077. http://dx.doi.org/10.1016/j.athor-acsur.2016.02.077 (epub is ahead of print) [DOI] [PMC free article] [PubMed]
- 67.Thiagarajan G, Greish K, Ghandehari H. Charge affects the oral toxicity of poly(amidoamine) dendrimers. Eur J Pharm Biopharm. 2013;84(2):330–334. doi: 10.1016/j.ejpb.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.