Abstract
Cancer cells with defects in DNA repair are highly susceptible to DNA-damaging agents, but delivery of therapeutic agents into cell nuclei can be challenging. A subset of lupus autoantibodies is associated with nucleolytic activity, and some of these antibodies are capable of nuclear penetration. We hypothesized that such antibodies might have potential as therapeutic agents targeted towards DNA repair-deficient malignancies. We identified the lupus autoantibody 5C6 as a cell-penetrating nucleolytic antibody and found that 5C6 has a differential effect on a matched pair of BRCA2-proficient and deficient DLD1 colon cancer cells. 5C6 selectively induced γH2AX in, and suppressed the growth of, the BRCA2-deficient cells. These findings demonstrate the potential utility of 5C6 in targeted therapy for DNA repair-deficient malignancies and strengthen the rationale for studies of additional lupus autoantibodies in order to identify the best candidates for development as therapeutic agents. In addition, the toxic effect of 5C6 on BRCA2-deficient cells provides further support for the hypothesis that some lupus autoantibodies contribute to the lower risk of specific cancers associated with systemic lupus erythematosus.
Systemic lupus erythematosus (SLE) is an autoimmune disease in which inappropriate production of autoantibodies results in widespread inflammation and organ dysfunction1. A small percentage of lupus autoantibodies penetrate into the nuclei of living cells, and these antibodies have potential utility in molecular therapy2. A cell-penetrating lupus anti-DNA autoantibody, 3E10, has previously been developed as a vehicle for intracellular delivery of therapeutic cargo molecules, and this approach has proven effective in vitro and in vivo3,4,5. More recently we made the unexpected discovery that 3E10, by itself, inhibits DNA repair and is synthetically lethal to cancer cells with defects in DNA repair due to BRCA2-deficiency6. An emerging area of investigation into cell-penetrating lupus autoantibodies now focuses on their potential use as targeted therapies for cancer.
Development of tumor-selective therapies is a critical goal in cancer research. Many cancer cells harbor deficiencies in DNA repair and are more sensitive to DNA damage than normal cells7, and agents that localize to cell nuclei and inhibit DNA repair or damage DNA therefore have potential as targeted therapies for DNA repair-deficient malignancies. Effective delivery of therapeutic agents to cell nuclei is challenging, but the subset of naturally occurring lupus autoantibodies that penetrate into cell nuclei may be well suited to this role. Based on our discovery that 3E10 inhibits DNA repair and is toxic to BRCA2-deficient cells, we hypothesized that additional lupus autoantibodies may have similar potential for use as targeted cancer therapies.
Importantly, while 3E10 does not appear to directly damage DNA, cell-penetrating nucleolytic lupus autoantibodies have been previously reported8,9. The ability of these antibodies to directly localize into cell nuclei and to potentially induce DNA damage makes them intriguing candidates for development as targeted therapies for DNA repair-deficient malignancies. To the best of our knowledge the impact of a cell-penetrating nucleolytic lupus autoantibody on cells with defects in DNA repair has not been previously tested. We therefore set out to identify a cell-penetrating nucleolytic lupus autoantibody and test its effects on a matched pair of BRCA2-proficient and deficient DLD1 colon cancer cells.
Results
5C6 is a nucleolytic lupus autoantibody
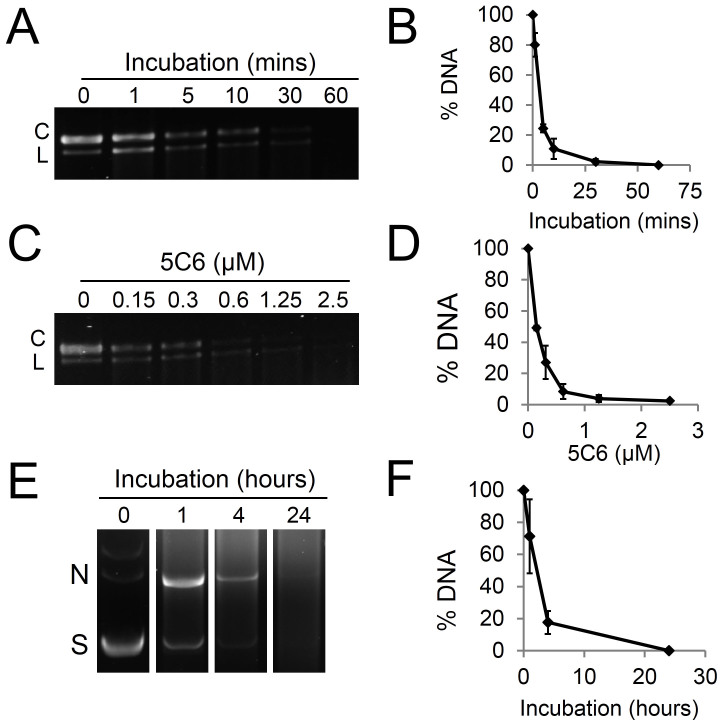
We screened a panel of lupus anti-DNA antibodies for nucleolytic activity in order to identify a candidate for testing on the BRCA2-deficient cells. Anti-DNA autoantibodies produced by hybridomas generated from the MRL-mpj/lpr mouse model of SLE10 were incubated with DNA in vitro, and most did not significantly impact the integrity of the DNA (not shown). The IgG2a-κ antibody 5C6, however, was found to be associated with significant degradation of both single and double-stranded DNA. When incubated with M13mp18 single-stranded DNA, 5C6 catalyzed degradation of the single-stranded DNA in a time and dose-dependent manner, with near complete degradation of the single-stranded DNA by 10 minutes at doses of 1.25 μM and higher (Fig. 1A–D). When incubated with double-stranded plasmid DNA (pBluescript) 5C6 similarly catalyzed a time-dependent degradation of the plasmid DNA (Fig. 1E–F). These data indicated that 5C6 is associated with nucleolytic activity, and we therefore proceeded to test the effects of 5C6 on cells.
Figure 1. 5C6 is a nucleolytic lupus autoantibody.
(A): 5C6 degrades single-stranded DNA in a time-dependent manner. Single-stranded M13mp18 circular DNA was incubated with buffer containing 2.5 μM 5C6 for 0–60 minutes, followed by visualization of DNA on an agarose gel. (B): The percentage of M13mp18 DNA remaining after incubation with 5C6 was quantified relative to untreated M13mp18 DNA. (C): 5C6 degrades single-stranded DNA in a dose-dependent manner. M13mp18 DNA was incubated with buffer containing 0–2.5 μM 5C6 for 10 minutes, followed by visualization on an agarose gel. (D): The percentage of M13mp18 DNA remaining after incubation with 5C6 as described in C was quantified relative to untreated M13mp18 DNA. (E): 5C6 degrades double-stranded DNA. pBluescript double-stranded plasmid DNA was incubated with buffer containing 6.6 µM 5C6 for 0–24 hours followed by visualization on an agarose gel. (F): The percentage of pBluescript plasmid DNA remaining after incubation with 5C6 as described in E was quantified relative to untreated pBluescript. C = circular conformation. L = linear conformation. N = nicked conformation. S = supercoiled conformation. Error bars: SEM.
5C6 penetrates into cell nuclei
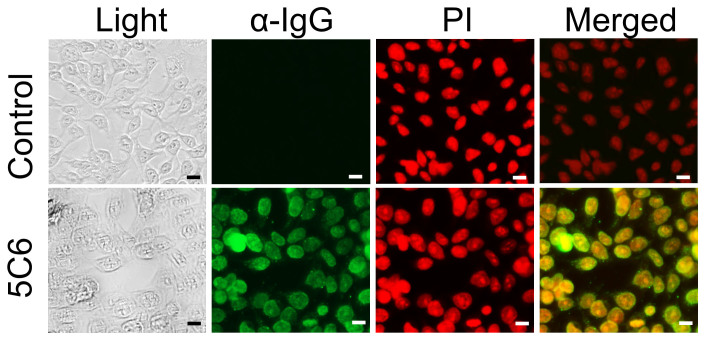
We first sought to confirm that 5C6 penetrates into cell nuclei. DLD1 colon cancer cells were treated with control media or media containing 5C6. Cells were then washed, fixed, and immunostained for murine IgG. Propidium iodide (PI) counterstaining allowed direct visualization of cell nuclei, and overlay of anti-IgG and PI fluorescent images confirmed nuclear localization by 5C6 in the DLD1 cells (Fig. 2).
Figure 2. 5C6 penetrates into DLD1 cell nuclei.
DLD1 cells were treated with control media or media containing 3.3 μM 5C6 for 1.5 hours. Cells were then washed, fixed, and immunostained for presence of IgG, followed by counterstaining with PI to allow visualization of the nucleus. Light and fluorescent images under GFP and RFP filters are presented. Merged images confirm nuclear localization by 5C6. Scale bar: 20 µm.
5C6 has a differential impact on BRCA2-proficient and BRCA2-deficient DLD1 cells
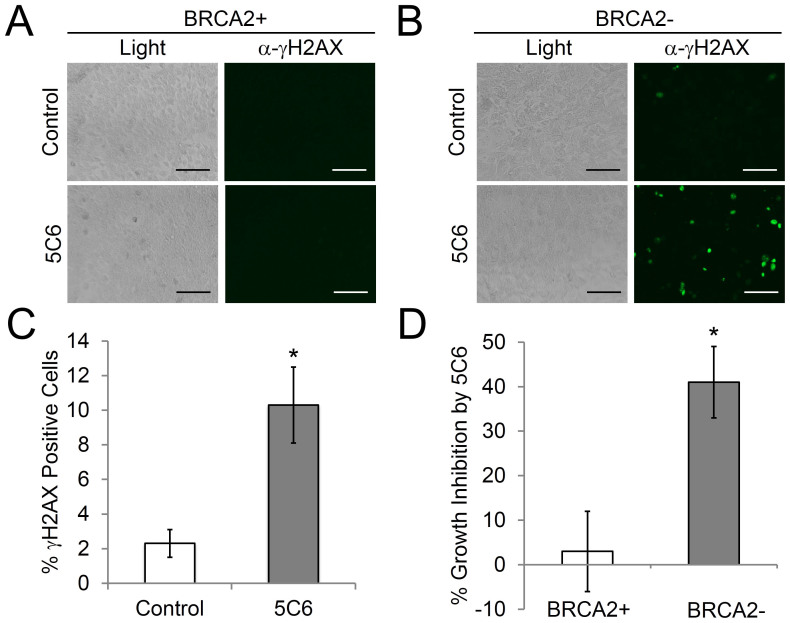
The effect of 5C6 on the matched pair of BRCA2-proficient (BRCA2+) and BRCA2-deficient (BRCA2-) DLD1 cells was assessed. Cells were treated with control media or media containing 10 μM 5C6 for one hour, followed by evaluation of the percentage of cells positive for γH2AX (a marker of DNA double-strand breaks) by immunofluorescence. 5C6 did not increase the percentage of γH2AX-positive BRCA2+ cells but did increase the percentage of γH2AX-positive BRCA2- cells approximately 5-fold compared to control cells (2.3% versus 10.3%; p = 0.03) (Fig. 3A, B, and C). The observed increase in percentage of γH2AX-positive BRCA2- cells after treatment with 5C6 may reflect direct DNA damage induced by 5C6, and the differential impact of 5C6 on γH2AX expression in the BRCA2+ and BRCA2- cells suggests that defective DNA repair in the BRCA2- cells makes them more susceptible to the effects of the 5C6 nucleolytic antibody.
Figure 3. 5C6 has a differential impact on BRCA2-proficient and deficient DLD1 cells.
(A) and (B): BRCA2+ and BRCA2- DLD1 cells were treated with control media or media containing 10 µM 5C6 for 1 hour. Cells were then washed, fixed, and probed for the presence of γH2AX with an Alexa-488 conjugated antibody. Light and immunofluorescence images are presented. Scale bar = 100 μm. (C): The percentage of γH2AX-positive BRCA2- cells after treatment with control or 5C6 was quantified. 5C6 increased the percentage of γH2AX-positive cells ~5-fold relative to control media. *p = 0.03 (n = 4). (D): 5C6 is toxic to BRCA2- DLD1 cells. BRCA2+ and BRCA2- DLD1 cells in subconfluent monolayers were treated with control media or media containing 10 µM 5C6 for 4 days. Cells were then harvested and counted using trypan blue. Percent growth inhibition relative to cells treated with control was determined. Percent growth inhibition is presented. 5C6 did not notably impact the relative percentage of viable BRCA2+ cells but significantly suppressed the growth of the BRCA2- cells. *p = 0.01 (n = 6). Error bars: SEM.
5C6 selectively suppresses the growth of the BRCA2- DLD1 cells
To confirm that 5C6 is more toxic to BRCA2- than BRCA2+ cells, we tested the effect of 5C6 on the proliferation of BRCA2+ and BRCA2- DLD1 cells growing as subconfluent monolayers. BRCA2+ and BRCA2- DLD1 cells were treated with control media or media containing 10 μM 5C6. Four days later total viable cell counts were determined. 5C6 did not significantly inhibit the growth of the BRCA2+ cells (percent growth inhibition of 2.8% ± 9). However, 5C6 significantly impaired the growth of the BRCA2- cells (percent growth inhibition of 41% ± 8) (Fig. 3D). These results are consistent with our finding that 5C6 selectively induced an increase in γH2AX in BRCA2- cells and demonstrate that 5C6 is more toxic to BRCA2- than BRCA2+ cells.
5C6 induces senescence in the BRCA2-deficient DLD1 cells
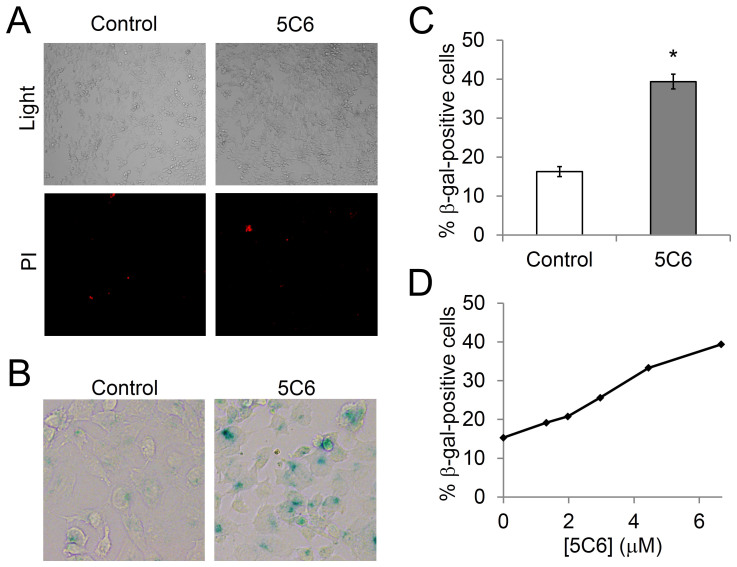
To investigate the mechanism by which 5C6 suppresses the growth of BRCA2- DLD1 cells we examined the effect of 5C6 on membrane integrity as a marker for apoptosis or necrosis. BRCA2- DLD1 cells were treated with control or 10 μM 5C6 and then treated with propidium iodide (PI). No significant increase in the percentage of PI-positive cells in the presence of 5C6 relative to control media was observed (Fig. 4A), which suggests that neither apoptosis nor necrosis are the primary mechanisms responsible for the effect of 5C6 on BRCA2- cells. We therefore proceeded to test the effect of 5C6 on induction of cell senescence by examining the relative expression of β-galactosidase (β-gal) in cells treated with 5C6. As shown in Fig. 4B–D, 5C6 yielded a significant and dose dependent increase in β-gal expression in the BRCA2- DLD1 cells, which suggests that 5C6 suppresses the growth of the cells by inducing senescence. At dose of 6.6 μM 5C6 increased the percentage of β-gal-positive cells to 39.3% ± 1.8 compared to 16.3% ± 1.3 in cells treated with control media.
Figure 4. 5C6 induces senescence in BRCA2-deficient DLD1 cells.
(A): 5C6 does not appear to induce apoptosis or necrosis of BRCA2- DLD1 cells. Cells were treated with control media or media containing 10 μM 5C6, and cell membrane integrity was then examined by visualization of PI uptake. No difference in PI uptake was observed between the cells treated with control or 5C6, suggesting that apoptosis or necrosis are not the primary mechanisms by which 5C6 is toxic to the BRCA2- cells. (B), (C), and (D): 5C6 induces senescence in the BRCA2- DLD1 cells. Cells were treated with control media or media containing 6.6 μM 5C6 and were then stained for β-gal as a marker of senescence. Representative images are shown in B, and the percentage of β-gal-positive cells was quantified in C. *p = 0.009 (n = 2). The impact of 5C6 on the percentage of β-gal-positive cells was dose dependent (D).
Discussion
We have shown that a cell-penetrating nucleolytic lupus autoantibody, 5C6, has a differential effect on BRCA2+ and BRCA2- DLD1 cells. Specifically, 5C6 induces γH2AX in BRCA2- but not BRCA2+ cells and selectively suppresses the growth of the BRCA2- cells. Mechanistically, 5C6 appears to induce senescence in the BRCA2- cells. Senescence is a well-known response to DNA damage, and DNA damaging agents, including many chemotherapeutics, induce senescence after prolonged exposure11,12,13. Taken together, the observations listed above provide strong support for the hypothesis that 5C6 penetrates cell nuclei and damages DNA, and that cells with pre-existing defects in DNA repair due to BRCA2-deficiency are more sensitive to this damage than cells with intact DNA repair.
We previously found that the cell-penetrating lupus anti-DNA antibody 3E10 inhibits DNA repair and is selectively toxic to BRCA2- cancer cells6, which revealed the possibility of using select lupus antibodies as targeted cancer therapies. However, a key question remained regarding whether the effect of 3E10 on BRCA2- cancer cells was a unique phenomenon or if there are other lupus autoantibodies that have similar or even greater potential for use in cancer therapy. We have now answered this question by showing that the nucleolytic lupus autoantibody 5C6 also has a selective effect on BRCA2-deficient cancer cells.
The finding that 5C6 has a selective impact on BRCA2- cells may also provide further insight into the unusual cancer risk profile associated with SLE. SLE is associated with an overall increased risk of malignancy, but lower than expected rates of tumors associated with defects in BRCA2 such as breast, ovarian, and prostate cancers14,15,16. The pathophysiology underlying this risk profile is unknown and is likely multifactorial17,18. We previously suggested that cell-penetrating lupus autoantibodies such as 3E10 that inhibit DNA repair might contribute to this phenomenon by suppressing growth of BRCA2- cells6, and our present findings now raise the possibility that nucleolytic lupus autoantibodies may also contribute to inhibiting the growth of BRCA2-associated tumors in patients with SLE. Additional epidemiologic and laboratory studies are needed before any definitive conclusions may be drawn in this regard.
The primary significance of this work is in providing proof of principle to support additional studies into the application of nucleolytic lupus autoantibodies as targeted therapies for DNA repair-deficient malignancies. A wide range of human malignancies harbor deficiencies in DNA repair7, and this therapeutic strategy therefore has potential for applications in the treatment of numerous tumors. The findings presented herein also strengthen the rationale for continuing the search for lupus autoantibodies that may have even greater and more selective effects on cancer cells. Given the diversity of autoantibodies associated with SLE19 we are confident that additional lupus autoantibodies with therapeutic potential await discovery.
Methods
Hybridomas and cell lines
A panel of hybridomas, including the 5C6 hybridoma, was previously generated from the MRL-mpj/lpr lupus mouse model and DNA binding activity was evaluated10,20. Hybridomas were maintained in serum free hybridoma media (BD Cell MAb medium; BD Biosciences, San Jose, CA) supplemented with 2 mM L-glutamine (Life Technologies, Carlsbad, CA). Antibodies were harvested in hybridoma supernatants and exchange-dialyzed into appropriate media. BRCA2+ and BRCA2- DLD1 colon cancer cells (Horizon Discovery Ltd, Cambridge, UK) were grown in RPMI 1640 (Life Technologies) supplemented with 10% fetal bovine serum (FBS; Sigma-Aldrich, Saint Louis, MO).
Cell-penetration assays
DLD1 cells grown on glass coverslips were treated with control buffer or 3.3 µM 5C6 for 1.5 hours. Cells were then washed with PBS, fixed with chilled 100% ethanol for 5 minutes, washed again with PBS, and then probed with Alexa488-conjugated goat anti-mouse IgG antibody overnight at 4°C (Cell Signaling, Danvers, MA). To counterstain the nucleus, PI at 1 µg/ml was added to the cells for 30 minutes. Nuclear penetration by the antibodies was then imaged using an EVOS fl digital fluorescence microscope (Advanced Microscopy Group, Bothell, WA) using green fluorescent protein (GFP) filter (40× magnification; Life Technologies).
γH2AX assays
BRCA2+ and BRCA2- DLD1 cells were treated with control media or media containing 10 μM 5C6 for one hour. Cells were then washed with PBS, fixed with chilled 100% ethanol for 5 minutes, washed again with PBS, blocked with 1% BSA/PBS for 45 minutes and then probed with Alexa488-conjugated anti-γH2AX antibody overnight at 4°C (Cell Signaling). Fluorescent signal corresponding to γH2AX was then imaged using an EVOS fl digital fluorescence microscope using a GFP filter.
Catalytic assays
For single-stranded plasmid DNA assays, single-stranded M13mp18 DNA (New England Biolabs, Ipswich, MA) was incubated with 0–2.5 µM 5C6 for 0–60 minutes in binding buffer (50 mM Tris-HCl, 100 mM NaCl, 10 mM MgCl2). The reaction was terminated by the addition of 1% SDS. Samples were boiled and then run on 1% agarose gels and stained with SYBR Gold (Life Technologies) for 30 minutes. The proportion of DNA remaining after treatment relative to untreated M13mp18 DNA was then calculated by band densitometry using ImageJ (National Institutes of Health, Bethesda, MA). pBluescript (Agilent Technologies, Englewood, CO) was used in double-stranded plasmid DNA assays by incubating with 6.6 µM 5C6 for 0–24 hours in binding buffer at 37°C. Samples were then boiled, and DNA conformations were visualized on 1% agarose gels with ethidium bromide. The proportion of DNA remaining after treatment relative to untreated pBluescript DNA was then calculated by band densitometry using ImageJ.
Cell growth assays
BRCA2+ and BRCA2- DLD1 cells were plated in 48 well plates at 1 × 104 and allowed to adhere overnight. The cells were then treated with control media or media containing 10 μM 5C6. Cell counts were determined four days later by trypan blue extrusion. Percent growth inhibition was then calculated by comparing the total number of viable cells treated with 5C6 relative to cells treated with control media.
Propidium iodide uptake
BRCA2- DLD1 cells were plated in 96 well plates at 3 × 104 and allowed to adhere overnight. The cells were then treated with control media or media containing 10 μM 5C6 overnight at 37°C. Antibody was then removed and cells were stained with 1 μg/ml PI for 15 minutes at room temperature. Cells were then imaged for PI uptake using an EVOS fl digital fluorescence microscope using an RFP filter.
Cellular senescence assay
BRCA2- DLD1 cells were plated in 12 well plates at 2 × 104 and allowed adhere overnight. The cells were then treated with media containing 0–6.6 μM 5C6. Cells were incubated at 37°C for 4 days. The Senescence-βGal Staining Kit (Cell Signaling Technology) was used for β-gal staining. After treatment cells were washed, fixed and stained for the presence of β-gal at pH 6.0. Cells were then imaged (Olympus IX70, Tokyo, Japan) and percentage of β-gal positive cells calculated.
Statistics
P values were determined by two-tailed Student's t-test.
Acknowledgments
This work was supported in part by the Yale School of Medicine Department of Therapeutic Radiology and ACS-IRG #58-012-55 from the American Cancer Society to J.E.H.
Footnotes
The authors declare no competing financial interests.
Author Contributions P.W.N. designed and performed experiments, analyzed data, and wrote the manuscript. M.R.Y. and S.B. reviewed and contributed to writing the manuscript. R.H.W. generated hybridomas, identified the anti-DNA antibodies, and reviewed and contributed to writing the manuscript. J.E.H. conceived the study, designed and supervised the experiments, analyzed data, and wrote the manuscript.
References
- Rahman A. & Isenberg D. A. Systemic lupus erythematosus. N. Engl. J. Med. 358, 929–939, 10.1056/NEJMra071297 (2008). [DOI] [PubMed] [Google Scholar]
- Alarcon-Segovia D. Antinuclear antibodies: to penetrate or not to penetrate, that was the question. Lupus 10, 315–318 (2001). [DOI] [PubMed] [Google Scholar]
- Hansen J. E. et al. Antibody-mediated p53 protein therapy prevents liver metastasis in vivo. Cancer Res. 67, 1769–1774, 10.1158/0008-5472.can-06-3783 (2007). [DOI] [PubMed] [Google Scholar]
- Lawlor M. W. et al. Enzyme replacement therapy rescues weakness and improves muscle pathology in mice with X-linked myotubular myopathy. Hum. Mol. Genet. 22, 1525–1538, 10.1093/hmg/ddt003 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan X. et al. Recombinant Fv-Hsp70 protein mediates neuroprotection after focal cerebral ischemia in rats. Stroke 41, 538–543, 10.1161/strokeaha.109.572537 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J. E. et al. Targeting cancer with a lupus autoantibody. Science translational medicine 4, 157ra142, 10.1126/scitranslmed.3004385 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeijmakers J. H. DNA damage, aging, and cancer. N. Engl. J. Med. 361, 1475–1485, 10.1056/NEJMra0804615 (2009). [DOI] [PubMed] [Google Scholar]
- Shuster A. M. et al. DNA hydrolyzing autoantibodies. Science 256, 665–667 (1992). [DOI] [PubMed] [Google Scholar]
- Lee E. J. et al. Cell-penetrating autoantibody induces caspase-mediated apoptosis through catalytic hydrolysis of DNA. Bioorg. Med. Chem. 15, 2016–2023, 10.1016/j.bmc.2006.12.037 (2007). [DOI] [PubMed] [Google Scholar]
- Zack D. J. et al. DNA mimics a self-protein that may be a target for some anti-DNA antibodies in systemic lupus erythematosus. J. Immunol. 154, 1987–1994 (1995). [PubMed] [Google Scholar]
- Sliwinska M. A. et al. Induction of senescence with doxorubicin leads to increased genomic instability of HCT116 cells. Mech. Ageing Dev. 130, 24–32, 10.1016/j.mad.2008.04.011 (2009). [DOI] [PubMed] [Google Scholar]
- te Poele R. H., Okorokov A. L., Jardine L., Cummings J. & Joel S. P. DNA damage is able to induce senescence in tumor cells in vitro and in vivo. Cancer Res. 62, 1876–1883 (2002). [PubMed] [Google Scholar]
- Achuthan S., Santhoshkumar T. R., Prabhakar J., Nair S. A. & Pillai M. R. Drug-induced senescence generates chemoresistant stemlike cells with low reactive oxygen species. J. Biol. Chem. 286, 37813–37829, 10.1074/jbc.M110.200675 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernatsky S., Ramsey-Goldman R., Foulkes W. D., Gordon C. & Clarke A. E. Breast, ovarian, and endometrial malignancies in systemic lupus erythematosus: a meta-analysis. Br. J. Cancer 104, 1478–1481, 10.1038/bjc.2011.115 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernatsky S., Ramsey-Goldman R., Gordon C. & Clarke A. E. Prostate cancer in systemic lupus erythematosus. Int. J. Cancer 129, 2966–2969, 10.1002/ijc.25956 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernatsky S. et al. Cancer risk in systemic lupus: An updated international multi-centre cohort study. J. Autoimmun. 42, 130–135, 10.1016/j.jaut.2012.12.009 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernatsky S. et al. Decreased breast cancer risk in systemic lupus erythematosus: the search for a genetic basis continues. Lupus 21, 896–899, 10.1177/0961203312443992 (2012). [DOI] [PubMed] [Google Scholar]
- Bernatsky S. et al. The relationship between cancer and medication exposures in systemic lupus erythaematosus: a case-cohort study. Ann. Rheum. Dis. 67, 74–79, 10.1136/ard.2006.069039 (2008). [DOI] [PubMed] [Google Scholar]
- Kostrikina I. A., Kolesova M. E., Orlovskaya I. A., Buneva V. N. & Nevinsky G. A. Diversity of DNA-hydrolyzing antibodies from the sera of autoimmune-prone MRL/MpJ-lpr mice. J. Mol. Recognit. 24, 557–569, 10.1002/jmr.1067 (2011). [DOI] [PubMed] [Google Scholar]
- Gu L. et al. Genetic determinants of autoimmune disease and coronary vasculitis in the MRL-lpr/lpr mouse model of systemic lupus erythematosus. J. Immunol. 161, 6999–7006 (1998). [PubMed] [Google Scholar]