Abstract
We describe the surgical technique of endoscopic cyclophotocoagulation in a Boston keratoprosthesis type II patient. This patient with ocular cicatricial pemphigoid had pars plana endoscopic cyclophotocoagula through wounds created in the eyelids.
Key Words: Boston keratoprosthesis type II, endoscopic cyclophotocoagulation, glaucoma, ocular cicatricial pemphigoid
Since the Boston keratoprosthesis’ initial approval in 1992 by the Food and Drug Administration, keratoprosthesis (Kpro) surgery has experienced continued improvements in design and postoperative management, which have resulted in longer implant retention times and lower infection rates.1,2 However, visual potential can be greatly limited by glaucoma, with a high reported prevalence of 64% to 76% before Kpro surgery.3–7 In particular, glaucoma is more difficult to manage in Kpro type II patients compared with Kpro type I patients, because topical glaucoma medications have minimal ocular penetration in Kpro type II patients. Therefore, glaucoma treatment for Kpro type II patients is largely limited to oral carbonic anhydrase inhibitors and surgery.5–7 Surgical options are largely limited to tube shunt surgery and cyclodestruction.
To our knowledge, this case report is the first to describe the surgical technique of endoscopic cyclophotocoagulation (ECP) in a Kpro type II patient.
CASE REPORT
A 61-year-old woman with ocular cicatricial pemphigoid OU was referred to this hospital in April of 2011 for evaluation of possible Boston Kpro type II surgery. She had undergone multiple surgeries including amniotic membrane grafts OU and a later corneal patch graft for a perforated cornea OD. She also had glaucoma, which had been treated with brimonidine tartrate 0.2% and timolol maleate 0.5% OU. On her first visit, she was only able to see hand motions OU. She had extensive symblepharon and opacified corneas OU. Disc photography and visual fields (VFs) could not be obtained because of corneal opacification.
The patient underwent combined Kpro type II surgery, lens extraction, pars plana vitrectomy, pars plana Ahmed valve surgery, and permanent tarsorrhaphy OD. The surgery was uncomplicated but was notable for a vitreous hemorrhage which lasted for 2 months. An external eye photo OD of the patient after surgery is shown in Figure 1. At 6 months after surgery, the patient’s right eye had a best-corrected visual acuity (VA) of 20/20, normal intraocular pressures (IOPs) by digital palpation, a cup-to-disc ratio of 0.5, and a mild VF defect (Figs. 2A, B). At the time, she was on oral acetazolamide 500 mg twice a day.
FIGURE 1.
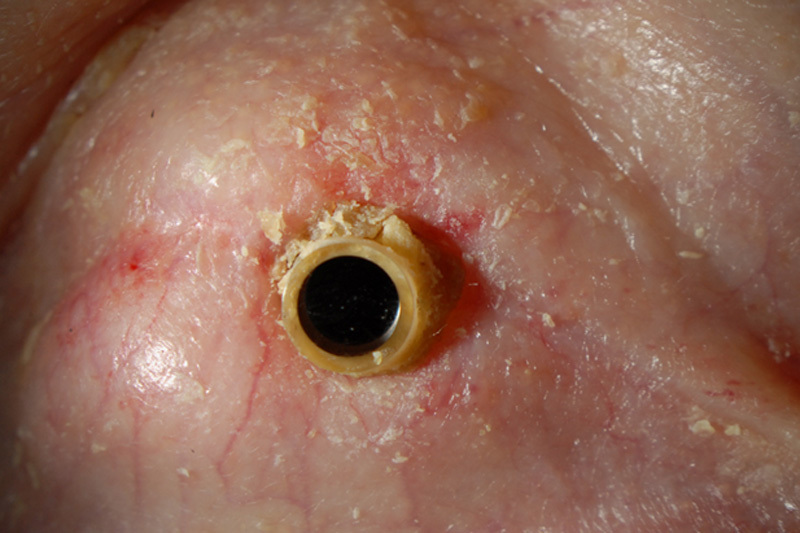
External photo of the right eye after initial Boston keratoprosthesis type II surgery.
FIGURE 2.
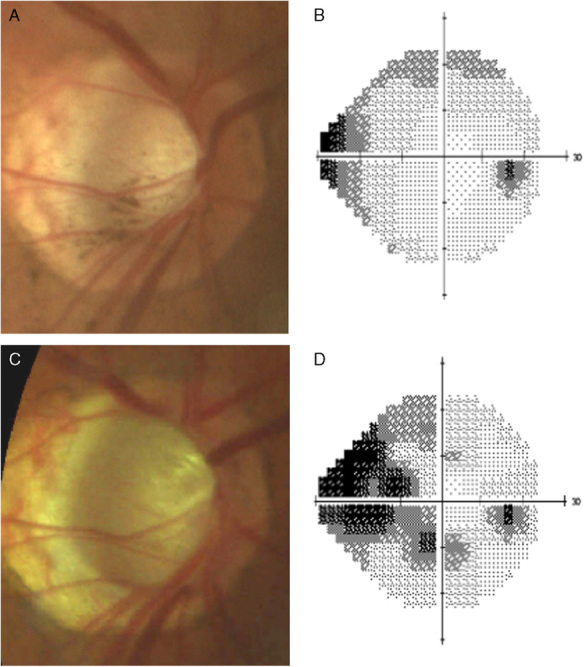
At 6 months after the patient’s initial Boston keratoprosthesis type II surgery OD, optic disc photo (A) and visual field testing (B) of the right eye were obtained. Subsequent examinations of the right eye at 3 years after initial keratoprosthesis type II surgery showed progressive cupping of the optic disc (C) and worsening of visual fields (D).
However, at 3 years after the initial Kpro type II surgery, glaucoma progression was detected in her right eye with increased cupping (cup-to-disc ratio 0.8) and worsening VFs (Figs. 2C, D), with stable 20/20 vision OD and counting finger vision OS. Because her initial Kpro type II surgery was associated with a long visual rehabilitation time of >2 months, she opted for the quicker postoperative recovery period of a pars plana ECP over repeat Ahmed valve surgery. Thus, pars plana ECP OD was performed in December of 2014.
The surgery was performed under general anesthesia. After the upper lid was injected with 1% lidocaine with epinephrine, 2 skin incisions were made through the upper lid in the superotemporal and superonasal regions 10 mm posterior from the center of the keratoprosthesis optic. The skin was dissected carefully down to bare sclera. After gentle cauterization of the scleral vessels, an infusion port was placed using a 25-G trocar and cannula in the superonasal quadrant, and another 25-G trocar and cannula in the superotemporal quadrant, both 10 mm posterior from the center of the keratoprosthesis optic. A light pipe with wide view system was inserted through the superotemporal cannula to inspect the posterior segment, which revealed a cupped nerve, no peripheral retinal breaks, and a patent Ahmed valve tube without vitreous blockage. The light pipe and cannula were then removed, and a MVR blade (20 G) was used to enlarge the superotemporal wound to a size able to accommodate an 18-G curved ECP probe (Fig. 3A). Once placed in the eye, ECP was performed with the power set to 250 mW, and the ciliary body was treated for 240 degrees. Upon completion, the superonasal infusion port and cannula was removed. The scleral wounds were closed with 7-0 vicryl sutures, and the eyelid wounds were closed with interrupted 7-0 vicryl sutures in the deep layers, and running 8-0 sutures in the superficial layers. A periocular injection of triamcinolone 30 mg was given at the end of the surgery.
FIGURE 3.
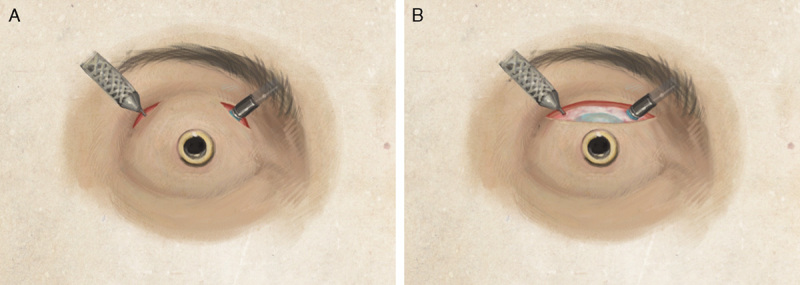
A, An illustration of the endoscopic cyclophotocoagulation (ECP) procedure performed in our patient. Two eyelid incisions were made, 1 in the superonasal and 1 in the superotemporal region. The eyelid skin tissues were dissected down to bare sclera. A 25-G infusion cannula was placed through the superonasal skin and pars plana incision, and the ECP probe similarly entered the globe superotemporally at 10 mm posterior to the center of the keratoprosthesis optic. B, An alternative approach to access the globe is via a larger single skin incision. This approach may allow for better visualization of both the superonasal and superotemporal regions to facilitate easier placement of the vitrectomy trocar and cannula and the ECP probe.
On postoperative day 1, the eye pressure by palpation was around 15 mm Hg, and there was a moderate amount of vitreous haze, which was thought to be sterile vitritis, that obscured the view of the fundus. However, the vitritis resolved in one month after an oral regimen of prednisolone 60 mg/d tapering to 10 mg/d over 8 days. At 18 months after ECP, the patient’s best-corrected VA was 20/30, with IOPs ranging between 12 and 15 mm Hg, and with stable VFs.
DISCUSSION
Two types of Boston keratoprosthesis surgeries are currently available: type I and type II. Although Kpro type I surgery is more common, Kpro type II surgery is beneficial in patients with very severe ocular surface disease involving both the eyelids and ocular surface, such as those with extensive symblepharon, ankyblepharon, and ocular surface keratinization.5,7 The surgical method in type II keratoprosthesis implantation involves additional steps to the type I keratoprosthesis method,8 and consists of stripping of the bulbar and palpebral conjunctiva, shaping of the eyelids around the anterior extension of Kpro type II optic, and tarsorrhaphy to permanently close the eyelids around the keratoprosthesis optic.5
The high glaucoma prevalence in Kpro patients is most likely caused by the significant inflammation associated with not only their preexisting ocular surface disease but also the keratoprosthesis device.6 Because Kpro surgery can cause both new onset glaucoma and preexisting glaucoma progression, both type I and type II procedures are often performed concomitantly with prophylactic Ahmed valve surgery,5–7,9 with or without pars plana vitrectomy, depending if the tube is inserted into the anterior chamber or pars plana. However, despite these efforts, glaucoma progression still commonly occurs in 22% to 42% of patients after Kpro surgery.4,6,10
Management of glaucoma can be considerably more challenging in Kpro type II patients compared with Kpro type I patients, because the penetration of topical glaucoma medications through the permanently closed eyelids in Kpro type II patients is very low. Therefore, treatment options for Kpro type II patients are largely limited to oral acetazolamide or surgery.5–7 Glaucoma surgical options after Kpro type II surgery are also limited. In these patients, tube shunt surgery or cyclodestructive procedures are the main options available after the conjunctiva has been removed during the initial Kpro type II surgery.
In our patient, ECP was performed, because it is believed to have a shorter recovery period and is less invasive compared with a second tube shunt surgery, which would require a larger eyelid wound opening. ECP and Ahmed valve surgery have been shown to have similar success rates in controlling IOP in refractory glaucomas, however, Ahmed valve surgery may be associated with a higher rate of complications and worsened VA, as reported by Lima et al.11 Furthermore, extensive exposure of the entire ocular surface may be necessary for both Ahmed valve implantation and trans-scleral cyclophotocoagulation. Separating the eyelids from the globe can be a challenge as the normal tissue plane may have been lost after the union of eyelid submucosal tissue to the ocular surface.5 Creating a large wound by reopening the tarsorrhaphy to expose a large area of the globe surface for repeat tube shunt surgery may also contribute to a greater amount of postoperative inflammation. Because patients who undergo Kpro type II surgery often have ocular surface disease resulting from an autoimmune or severe inflammatory condition, it would be desirable to induce as little surgical trauma as possible to avoid an exaggerated postoperative inflammatory response.
With pars plana ECP, only 2 small wounds through the eyelid and sclera are needed to accommodate the ECP probe and the infusion port (Fig. 3A). A larger skin incision can also be used, as this allows for easier insertion of the vitrectomy cannulas and ECP probe (Fig. 3B), but this skin incision is still smaller than that required for tube shunt surgery. Although the limbus cannot be easily identified after Kpro type II surgery for ECP, the location of the pars plana can be easily estimated at 10 mm from the center of the Kpro optic (ie, the limbus is 6 mm from the center of the 3 mm diameter Kpro optic, with an additional 4 mm from the limbus to the pars plana). The use of ECP has been described in Kpro type I patients,4,10,12 however, because the lids of Kpro type II patients are sutured shut and because the limbus cannot easily be directly visualized, ECP surgery in Kpro type II patients require a different surgical approach.
In conclusion, we describe the general surgical approach of performing ECP in Kpro type II patients, while also describing 2 possible incision types (Fig. 3). Compared with Ahmed valve implantation which requires reopening the initial complete tarsorraphy, ECP has the advantages of a smaller incision, shorter surgery time, and quicker recovery. Furthermore, the minimally invasive approach through the eyelid minimizes potential excessive surgical trauma to the eye.
ACKNOWLEDGMENTS
The authors thank Hsiang-Ling Hsu for her assistance in creating Figure 3.
Footnotes
Disclosure: T.C.C.—Fidelity Charitable Fund (Harvard University), Massachusetts Lions Eye Fund, American Glaucoma Society Mid-Career Award, National Institutes of Health UL 1RR 025758. C.H.D. is a full-time academic employee of the Massachusetts Eye and Ear Infirmary, manufacturer of the Boston keratoprosthesis. The remaining authors do not have any financial disclosures or conflicts of interest related to this manuscript.
REFERENCES
- 1.Khan BF, Harissi-Dagher M, Khan DM, et al. Advances in Boston keratoprosthesis: enhancing retention and prevention of infection and inflammation. Int Ophthalmol Clin. 2007;47:61–71. [DOI] [PubMed] [Google Scholar]
- 2.Traish AS, Chodosh J. Expanding application of the Boston type I keratoprosthesis due to advances in design and improved post-operative therapeutic strategies. Semin Ophthalmol. 2010;25:239–243. [DOI] [PubMed] [Google Scholar]
- 3.Chew HF, Ayres BD, Hammersmith KM, et al. Boston keratoprosthesis outcomes and complications. Cornea. 2009;28:989–996. [DOI] [PubMed] [Google Scholar]
- 4.Talajic JC, Agoumi Y, Gagne S, et al. Prevalence, progression, and impact of glaucoma on vision after Boston type 1 keratoprosthesis surgery. Am J Ophthalmol. 2012;153:267–274. [DOI] [PubMed] [Google Scholar]
- 5.Pujari S, Siddique SS, Dohlman CH, et al. The Boston keratoprosthesis type II: the Massachusetts Eye and Ear Infirmary experience. Cornea. 2011;30:1298–1303. [DOI] [PubMed] [Google Scholar]
- 6.Netland PA, Terada H, Dohlman CH. Glaucoma associated with keratoprosthesis. Ophthalmology. 1998;105:751–757. [DOI] [PubMed] [Google Scholar]
- 7.Lee R, Khoueir Z, Tsikata E, et al. Long-term visual outcomes and complications of Boston keratoprosthesis type II implantation. Ophthalmology. 2017;124:27–35. [DOI] [PubMed] [Google Scholar]
- 8.Aquavella JV, Qian Y, McCormick GJ, et al. Keratoprosthesis: the Dohlman-Doane device. Am J Ophthalmol. 2005;140:1032–1038. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen P, Chopra V. Glaucoma management in Boston keratoprosthesis type I recipients. Curr Opin Ophthalmol. 2014;25:134–140. [DOI] [PubMed] [Google Scholar]
- 10.Kamyar R, Weizer JS, de Paula FH, et al. Glaucoma associated with Boston type I keratoprosthesis. Cornea. 2012;31:134–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lima FE, Magacho L, Carvalho DM, et al. A prospective, comparative study between endoscopic cyclophotocoagulation and the Ahmed drainage implant in refractory glaucoma. J Glaucoma. 2004;13:233–237. [DOI] [PubMed] [Google Scholar]
- 12.Li JY, Greiner MA, Brandt JD, et al. Long-term complications associated with glaucoma drainage devices and Boston keratoprosthesis. Am J Ophthalmol. 2011;152:209–218. [DOI] [PubMed] [Google Scholar]


