In their recent article in PNAS, Das and Krantz (1) attempt to relate the previously described (2, 3) alternate conductance substate of the anthrax toxin protective antigen (PA63) channel to the allosteric helix compression model (4). Although we do not intend to discuss the differences between this model and the extended-chain Brownian ratchet model (5), we believe that there are two experimental observations that should be specifically addressed.
First, the authors hypothesize that the observed small-conductance substate (2, 3) is related to the two states of the PA63 channel, one clamped empty state and one unclamped empty dilated state, with the dilation occurring at the ϕ-clamp (1). Although this interpretation could be relevant, we believe the arguments provided to support it are indirect. In addition to analyzing the so-called long-lived and short-lived closures induced by the lethal factor (LF) peptides, the authors could have also performed a direct statistical comparison of these closures when the channel is in one of the two conductance states.
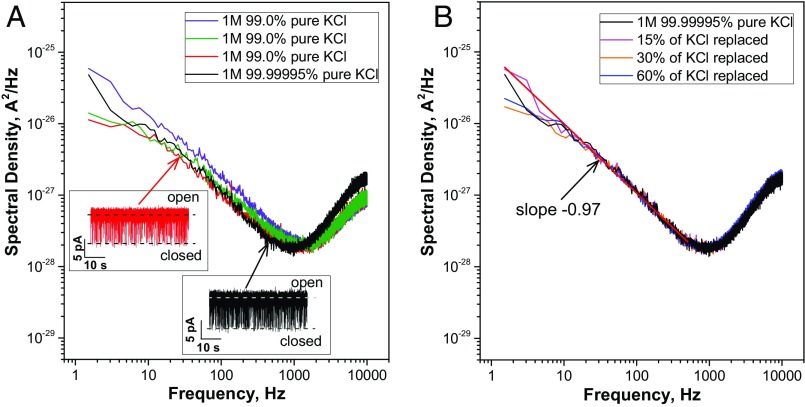
Second, the authors attribute the fast-flickering closures of PA63, that were previously characterized as a voltage-independent (2) 1/f-noise process (3), to the interaction of “a contaminant small molecule, buffer ion, or peptide” that “momentarily binds and occupies the ϕ-clamp” (1). To support this statement, the authors cite two articles (2, 6) where cationic compounds were shown to reversibly block the channel. We tested the “contaminant” hypothesis performing single PA63 recordings in two different solutions: 1 M 99.0% pure KCl prepared using Milli-Q water and 1 M 99.99995% pure KCl prepared using deionized HPLC-grade water. The power spectral density analysis of PA63 current fluctuations showed no statistically significant difference between these solutions (Fig. 1A). To rule out the possibility of contaminants resulting from protein or lipid addition, we made the measurements while gradually replacing the bathing KCl solutions with fresh KCl; however, no decrease in the density of the current power spectra was detected (Fig. 1B).
Fig. 1.
(A) Power spectral densities of single PA63 channel current fluctuations (1-ms resolution inserts) in 1 M 99.0% pure (purple, green, and red spectra) and 99.99995% pure (black spectrum) KCl solutions. The measurements in 99.0% KCl were taken in the same membrane bathing solution on single PA63 channels incorporated into three consecutively formed diphytanoyl phosphatidylcholine bilayer membranes at 50 mV of applied voltage, pH 6. At frequencies <500 Hz, a power spectrum of PA63 current fluctuations taken in the 1 M 99.99995% pure KCl/HPLC-grade water solution is within the limits of the spectral density fluctuations of individual measurements taken in the 1 M 99.0% pure KCl/type I Milli-Q water solution. (B) Power spectral density of current fluctuations through a single PA63 channel formed in 1 M 99.99995% pure KCl solution (black spectrum). No statistically significant difference in spectral density was observed when 15% (magenta spectrum), 30% (orange spectrum), and 60% (blue spectrum) of the starting solution were replaced with fresh 1 M 99.99995% pure KCl. In all solutions, PA63 displays expressed 1/f-noise behavior (fitted by a straight red line in B).
The idea that the anthrax toxin channel structural dynamics can be directly studied on a single-channel level is intriguing. However, consistency in the interpretation of the reported data (1–3) is essential. Unfortunately, the authors (1) do not mention the fact that the heptameric PA63 was also reported to insert directly in the higher conductance substate, switching to the lower conductance state only on occasion (3). Moreover, 7+β-cyclodextrins were shown to reversibly block both types of PA63 insertions with statistically indistinguishable rate constants (3). The authors report that the difference between the conductance states was threefold greater for the F427A mutant (1), but do not mention that the probability of finding PA63 in the “clamped empty state” was higher in phosphatidylserine compared with phosphatidylcholine membranes, with the substate amplitude being close to 30% of PA63 conductance (3).
In addition, 1/f current fluctuations, but not the conductance substates, were reported for channel-forming components of the binary clostridial C2 and iota toxins by Benz et al. (7, 8). These three channels share a high level of amino acid homology and numerous functional similarities related to the intracellular translocation of the enzymatic factors of these toxins.
Acknowledgments
Our research is financially supported by the National Institute of Allergy and Infectious Diseases of the NIH under the Award 1R15AI099897-01A1 and by The Catholic University startup funds (to E.M.N.).
Footnotes
The authors declare no conflict of interest.
References
- 1.Das D, Krantz BA. Peptide- and proton-driven allosteric clamps catalyze anthrax toxin translocation across membranes. Proc Natl Acad Sci USA. 2016;113(34):9611–9616. doi: 10.1073/pnas.1600624113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blaustein RO, Lea EJ, Finkelstein A. Voltage-dependent block of anthrax toxin channels in planar phospholipid bilayer membranes by symmetric tetraalkylammonium ions. Single-channel analysis. J Gen Physiol. 1990;96(5):921–942. doi: 10.1085/jgp.96.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nestorovich EM, Karginov VA, Berezhkovskii AM, Bezrukov SM. Blockage of anthrax PA63 pore by a multicharged high-affinity toxin inhibitor. Biophys J. 2010;99(1):134–143. doi: 10.1016/j.bpj.2010.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feld GK, Brown MJ, Krantz BA. Ratcheting up protein translocation with anthrax toxin. Protein Sci. 2012;21(5):606–624. doi: 10.1002/pro.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krantz BA, Finkelstein A, Collier RJ. Protein translocation through the anthrax toxin transmembrane pore is driven by a proton gradient. J Mol Biol. 2006;355(5):968–979. doi: 10.1016/j.jmb.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 6.Krantz BA, et al. A phenylalanine clamp catalyzes protein translocation through the anthrax toxin pore. Science. 2005;309(5735):777–781. doi: 10.1126/science.1113380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmid A, Benz R, Just I, Aktories K. Interaction of Clostridium botulinum C2 toxin with lipid bilayer membranes. Formation of cation-selective channels and inhibition of channel function by chloroquine. J Biol Chem. 1994;269(24):16706–16711. [PubMed] [Google Scholar]
- 8.Knapp O, Benz R, Gibert M, Marvaud JC, Popoff MR. Interaction of Clostridium perfringens iota-toxin with lipid bilayer membranes. Demonstration of channel formation by the activated binding component Ib and channel block by the enzyme component Ia. J Biol Chem. 2002;277(8):6143–6152. doi: 10.1074/jbc.M103939200. [DOI] [PubMed] [Google Scholar]