Significance
Hydrogen cyanide (HCN) is highly volatile and among the most toxic substances known, being lethal to humans at a dosage of 1–2 mg/kg body weight. HCN blocks the respiratory chain and prevents aerobic organisms from using oxygen. In nature, HCN is produced by numerous plants that store it mainly as glycosides. Among animals, cyanogenesis is a defensive strategy that has seemed restricted to a few mandibulate arthropods (certain insects, millipedes, and centipedes), which evolved ways to store HCN in the form of stable and less volatile molecules. We found an instance of cyanogenesis in the phylogenetically distant group Chelicerata (“spider-like” arthropods), involving an aromatic ester for stable HCN storage and two degradation pathways that release HCN.
Keywords: chemical defense, cyanogenesis, Oribatida, Oribatula tibialis, toxin
Abstract
Cyanogenesis denotes a chemical defensive strategy where hydrogen cyanide (HCN, hydrocyanic or prussic acid) is produced, stored, and released toward an attacking enemy. The high toxicity and volatility of HCN requires both chemical stabilization for storage and prevention of accidental self-poisoning. The few known cyanogenic animals are exclusively mandibulate arthropods (certain myriapods and insects) that store HCN as cyanogenic glycosides, lipids, or cyanohydrins. Here, we show that cyanogenesis has also evolved in the speciose Chelicerata. The oribatid mite Oribatula tibialis uses the cyanogenic aromatic ester mandelonitrile hexanoate (MNH) for HCN storage, which degrades via two different pathways, both of which release HCN. MNH is emitted from exocrine opisthonotal oil glands, which are potent organs for chemical defense in most oribatid mites.
Chemical substances are of utmost importance in biotic interactions among plants and their herbivores/pathogens as well as among animals and their predators/parasites (1, 2). Many of these semiochemicals are emitted for defense, and one of the most deterring and toxic biogenic substances known is hydrogen cyanide (HCN, also known as hydrocyanic or prussic acid). This asphyxiant poison inhibits the cytochrome oxidase enzyme, resulting in the inability of organisms to use oxygen (3).
Biosynthesis and liberation of HCN (known as cyanogenesis) is widespread among plants, but in animals it is relatively rare. Whereas the earliest reports of HCN in arthropods are from the late 19th century (4), it was only in the early 1960s (5–8) that comprehensive chemical ecology studies began to reveal cyanogenesis as a defensive strategy of a few mandibulate arthropods, including certain species of myriapods, beetles, true bugs, and butterflies (2, 9–12). More recently, the genomic basis of cyanogenesis has been explored (13, 14).
The rarity of cyanogenesis in mandibulate arthropods (2) and its supposed absence in the other speciose arthropod subphylum, Chelicerata, may relate to the evolutionary challenge posed by using a universal toxin in defense: self-poisoning must be prevented by storing the highly volatile HCN as a safe carrier molecule or storage molecule. In case of threat or attack, the cyanogenic compounds are discharged and must be quickly degradable to release HCN. Known HCN storage compounds of mandibulate arthropods include aromatic or aliphatic glycosides, lipids, and cyanohydrins (e.g., mandelonitrile) (2, 12, 15).
Although no cyanogenic species has been known among Chelicerata, chemical defense is widespread in the group, particularly among arachnids such as whip scorpions (16, 17), harvestmen (18, 19), certain spiders (20), and mites (21, 22). Here, we demonstrate cyanogenesis in a mite of the order Oribatida, a diverse and mostly soil-dwelling group of decomposers that discharge myriad defense-related semiochemicals from a pair of large exocrine opisthonotal oil glands (22–28). The common and widespread species Oribatula tibialis stores HCN as the natural product mandelonitrile hexanoate (MNH) and releases HCN upon disturbance via two different chemical pathways.
Results and Discussion
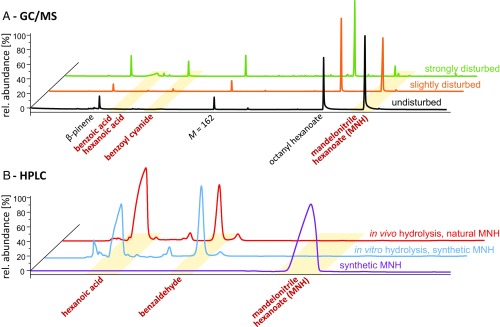
Oil gland secretions of undisturbed O. tibialis contained β-pinene, octanoyl hexanoate, MNH, and an unknown compound of molecular weight Mr = 162 g/mol (Fig. 1A). Of these compounds, only the aromatic ester MNH is involved in cyanogenesis. After gentle mechanical disturbance, specimens of O. tibialis showed a reduced amount of MNH and the presence of an additional compound, not detected in undisturbed mites: benzoyl cyanide (Fig. 1A). In intensely disturbed specimens, only traces of MNH were measured, but equimolar amounts of benzoyl cyanide and a mixture of benzoic acid and hexanoic acid were detected. Direct contact with moisture resulted in hydrolysis of MNH to hexanoic acid, benzaldehyde, and HCN (Fig. 1B).
Fig. 1.
GC/MS (A) chromatograms of oil gland secretions of Oribatula tibialis and HPLC (B) chromatograms of mandelonitrile hexanoate (MNH) hydrolysis. (A) Black: profile of secretions from undisturbed mites; orange: profile of slightly disturbed mites (brush stimulus); green: profile of strongly disturbed mites (vortex mixer). (B) Lilac: synthetic MNH; blue: in vitro hydrolysis of synthetic MNH; red: in vivo hydrolysis of natural MNH. Yellow bands highlight compounds involved in cyanogenesis.
Release of Hydrogen Cyanide by MNH Degradation.
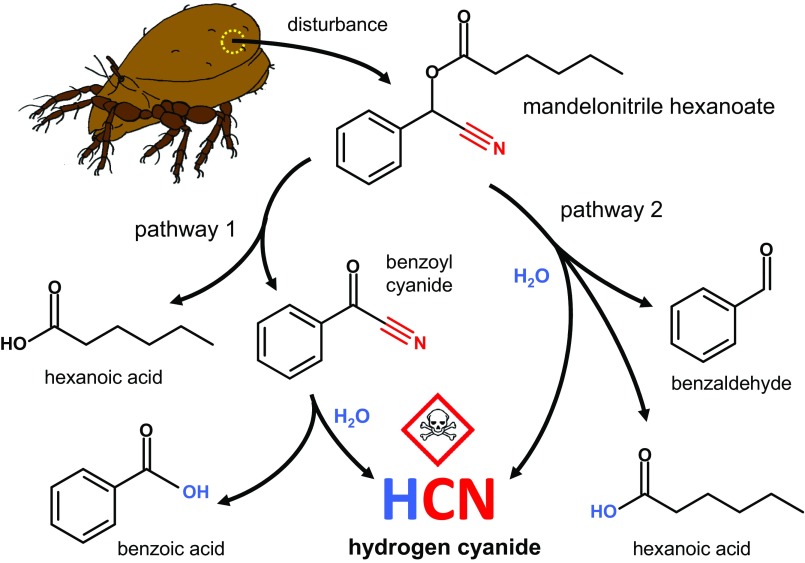
The detected compounds indicate that HCN is released, and we propose the following two degradation pathways of mandelonitrile hexanoate (Fig. 2).
Fig. 2.
Expulsion and degradation of MNH from opisthonotal oil glands in the oribatid mite, Oribatula tibialis. Pathway 1: MNH is cleaved by a catalytic oxidation to benzoyl cyanide and hexanoic acid on the mite´s body surface. Subsequently, benzoyl cyanide hydrolyzes to benzoic acid and HCN. Pathway 2: Direct hydrolysis of the ester bond in MNH in the presence of moisture, resulting in the release of HCN, benzaldehyde, and hexanoic acid.
Pathway 1.
Mechanical disturbance of O. tibialis results in the active expulsion of oil gland contents, including MNH. MNH is cleaved and catalytically oxidized to benzoyl cyanide and hexanoic acid on the mite's body surface. These two products are measurable quickly after disturbance in mite whole-body extracts, indicating rapid and spontaneous chemical reactions. The mechanism of this breakdown is unknown, but it has been shown that mites possess surface-associated enzymes that contribute to previously unsuspected chemical reactions (29–31). Finally, when exposed to water in the humid environment, benzoyl cyanide hydrolyzes to benzoic acid and HCN (32, 33).
Pathway 2.
A second breakdown reaction directly releases HCN from MNH and is based on the hydrolysis of the ester bond in MNH in the presence of moisture without oxidation. MNH is spontaneously cleaved by an addition–elimination reaction resulting in the release of HCN and the residual products benzaldehyde and hexanoic acid. Compared with pathway 1 the reaction is slower, but MNH is continuously hydrolyzed to HCN and the byproducts (Fig. 1).
These results demonstrate that O. tibialis solved the problem of storing and releasing HCN in a previously unknown manner, using the natural cyanogenic compound MNH, which shows both low volatility and high stability in the water-free chemical environment of the oil gland reservoirs. The major constituents of this ester are mandelonitrile and hexanoic acid. Mandelonitrile (or its oxygenated form benzoyl cyanide) is probably derived from the aromatic amino acid phenylalanine (34, 35), whereas hexanoic acid is most probably synthesized de novo using acetyl–CoA in a Claisen condensation (36, 37).
HCN in Predator Defense.
The large oil glands, which represent the major exocrine system in oribatid mites and which secrete MNH in O. tibialis, are potent organs for chemical defense, thus playing an important role in the structuring of feeding interactions in soil food webs (24–26). As with arthropod defensive compounds in general, most known oil gland secretions of oribatid mites—mainly hydrocarbons, terpenes, aromatics, esters, and alkaloids—are probably “class II compounds” (38); i.e., they irritate or repel potential predators without substantial harm (22–28). By contrast, benzoyl cyanide and HCN are “class I compounds” (38), true and harmful toxins (3, 6, 39, 40). The amount of MNH produced by O. tibialis is about 1.0–1.5 ng per individual mite (estimated by GC/MS), resulting in 0.1–0.15 ng HCN, if MNH is completely degraded (LD50, rat, oral: 5 ng/mg (41)).
Because mites are highly derived chelicerates, and because O. tibialis is a member of the highly derived oribatid mite family Oribatulidae (42, 43), we believe cyanogenesis is a relatively recent defense in Chelicerata. Considering its scattered phylogenetic distribution, HCN-based chemical defense obviously evolved multiple times in arthropods. Our findings highlight how convergence on a simple, yet particularly effective, chemical defense has taken different evolutionary pathways, leaving as footprints a diversity of storage compounds for this simple, well-known poison.
Materials and Methods
Adult specimens of Oribatula tibialis (Nicolet) were collected from moss and litter taken from a mixed forest stand near Groß-Gerau, Hesse, Germany, using a thermal-gradient extractor over 24 h (for further methods and habitat characterization, 44).
Oil gland exudates were extracted by submersing groups of 15–45 living mites in 20–50 µL solvent (hexane or water), a well-established method to obtain oil gland components (21, 28, 29, 45). After 3 min the solvent was separated from the mites. The two extraction solvents related to tests of two potential defense reactions—in water-free or humid conditions—and their corresponding MNH breakdown pathways. (i) To simulate predator attacks, without aqueous saliva contact, mites were mechanically stressed using a fine brush (equals slight disturbance) or briefly shaken in an otherwise empty GC vial using a vortex mixer (equals strong disturbance) before hexane extraction. (ii) To simulate a predator attack with aqueous saliva contact, mites were submerged in water. As a control, mites were directly immersed in hexane without mechanical stimulation. In addition, synthetic MNH (see below) was directly hydrolyzed in water as an in vitro control (Fig. 1). Crude hexane sample aliquots (2–5 µL) were analyzed with a GC/MS-QP2010 Ultra gas-chromatography mass-spectrometry system (Shimadzu) equipped with a ZB-5MS column (Phenomenex) according to a protocol given elsewhere (45). Electron ionization mass spectra were recorded at 70 eV from m/z = 40–400. Crude aqueous extracts of the mites (in vivo) and 1 µL synthetic MNH (in 50 µL water; in vitro) were further hydrolyzed from 30 min up to 1 h. The breakdown of MNH was monitored using an Agilent 1100 HPLC system (Agilent Technologies; Fig. 1). The separation of 20-µL sample aliquots was performed using an isocratic elution with 40% phosphate-buffer (pH = 3.2) and 60% methanol:water mixture (85:15; vol/vol) for 10 min with a flow rate of 1 mL/min, on a Discovery HS C18 (15 cm × 4 mm, 5 µm) column (Sigma-Aldrich), at 25 °C. Chromatograms were recorded using a VWD-UV/VIS detector at λ = 245 nm (0–5 min) and at λ = 490 nm (5–10 min). Retention indices (46) and literature mass spectra (47, 48) for GC, as well as authentic standards for GC and HPLC, were used for compound identification.
Mandelonitrile hexanoate (MNH; IUPAC name is Cyano-(phenyl)-methyl hexanoate) was synthesized from mandelonitrile (2-hydroxy–2-phenylacetonitrile) and hexanoyl chloride by an Einhorn acylation (49): 1 g (0.9 mL, 7.5 mmol, 1 eq) mandelontrile was dissolved in 6 mL pyridine. Two grams (4.2 mL, 15 mmol, 2 eq) hexanoyl chloride were added drop-wise while stirring at 0 °C. After heating the mixture for 10 min to 50 °C under exclusion of moisture (using a drying tube filled with anhydrous CaCl2), the reaction was terminated with ice water. HCl was added, and the mixture was extracted with hexane. The organic phase was washed three times with saturated sodium bicarbonate solution and dried with sodium sulfate. Finally, hexane was removed under a nitrogen atmosphere, resulting in 1.2 g crude, unpurified product (yield of ∼50%, containing hexane and carboxylic acid derivatives as byproducts, calculated based on NMR data; Figs. S3 and S4). This residual product was partly redissolved in hexane and stored at ‒28 °C until GC/MS or HPLC analyses and further experiments. For NMR spectroscopy the product was redissolved in CDCl3 and measured directly afterward. All chemicals used for synthesis were analytical GC/MS grade (≥99.9%) purchased from Merck KGaA.
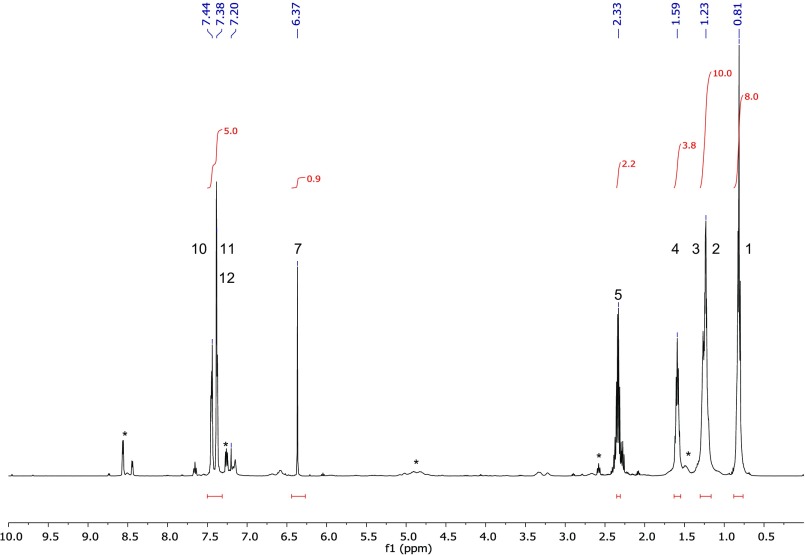
Fig. S3.
Synthetic MNH: complete 500 MHz 1H NMR in CDCl3 (ref. 7.20 ppm) of MNH. *, Byproduct contaminations (hexane, carboxylic acid derivatives).
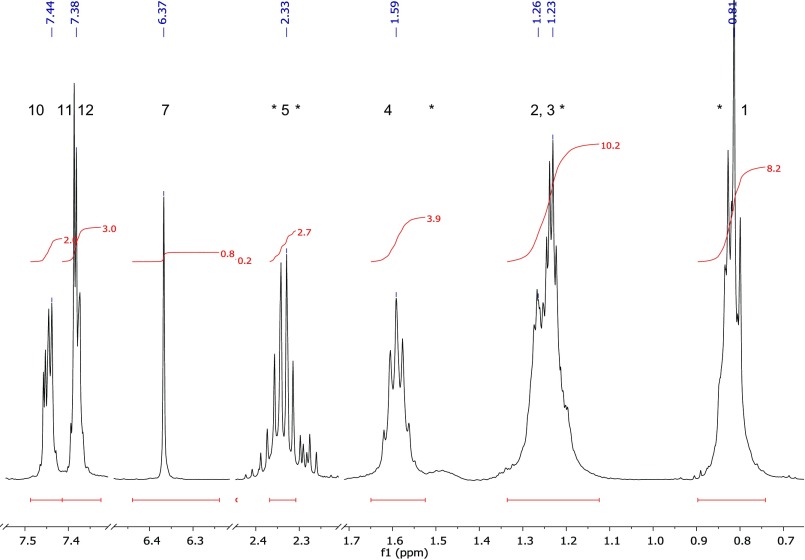
Fig. S4.
Synthetic MNH: 1H NMR only showing expanded ranges of essential signals. *, Byproduct contaminations (hexane, carboxylic acid derivatives).
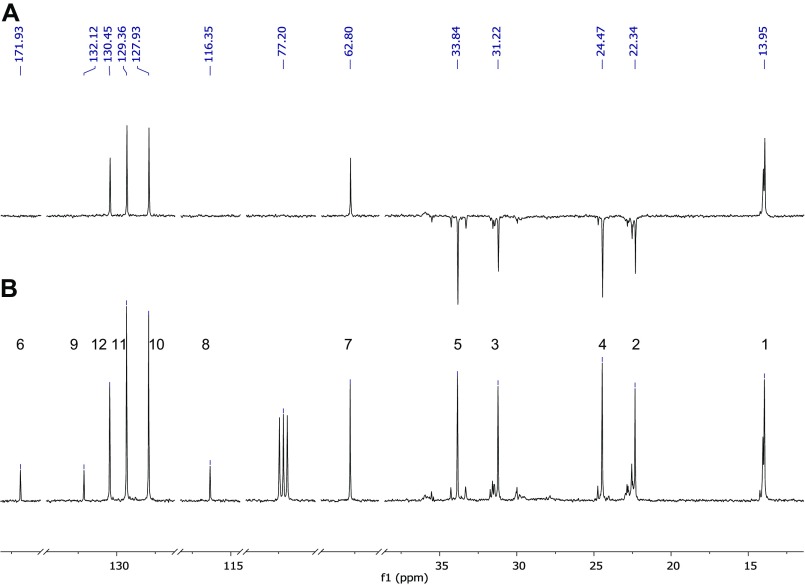
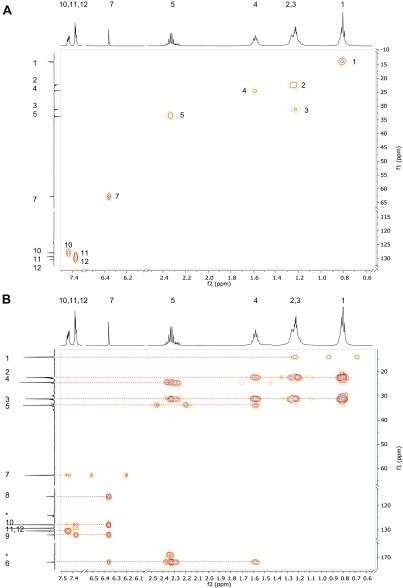
MNH was characterized by combining electron ionization mass spectrometry (EI-MS; for conditions, see above) and NMR spectroscopy. NMR spectroscopy was performed on a Bruker Avance DRX 500 spectrometer (Bruker Biospin). All 1H and 13C -NMR experiments were performed with standard conditions, using CDCl3 as solvent. The chloroform signals were used as internal standard for 1H- (7.20 ppm) and 13C-NMR (77.20 ppm) spectra. The reliable assignment of all 1H and 13C-signals was ascertained from 2D NMR measurements (13C-DEPT, 13C-HMBC und 13C-HSQC spectra). (Figs. S5 and S6) Raw data were processed with the MestReNova vers. 8.0 software (Mestrelab Research).
Fig. S5.
Synthetic MNH: Distortionless enhancement by polarization transfer (DEPT) 135 (A) and broadband decoupled (B) 13C NMR spectra (ref. CDCl3 77.2 ppm). The DEPT spectrum shows a negative phase for the CH2 signals.
Fig. S6.
Synthetic MNH: 2D NMR, 1H-13C-heteronuclear single quantum correlation (HSQC) (A) and 1H-13C-heteronuclear multiple-bond correlation (HMBC) (B) spectra show the hydrogen–carbon connectivity over one and two or three covalent bonds, respectively.
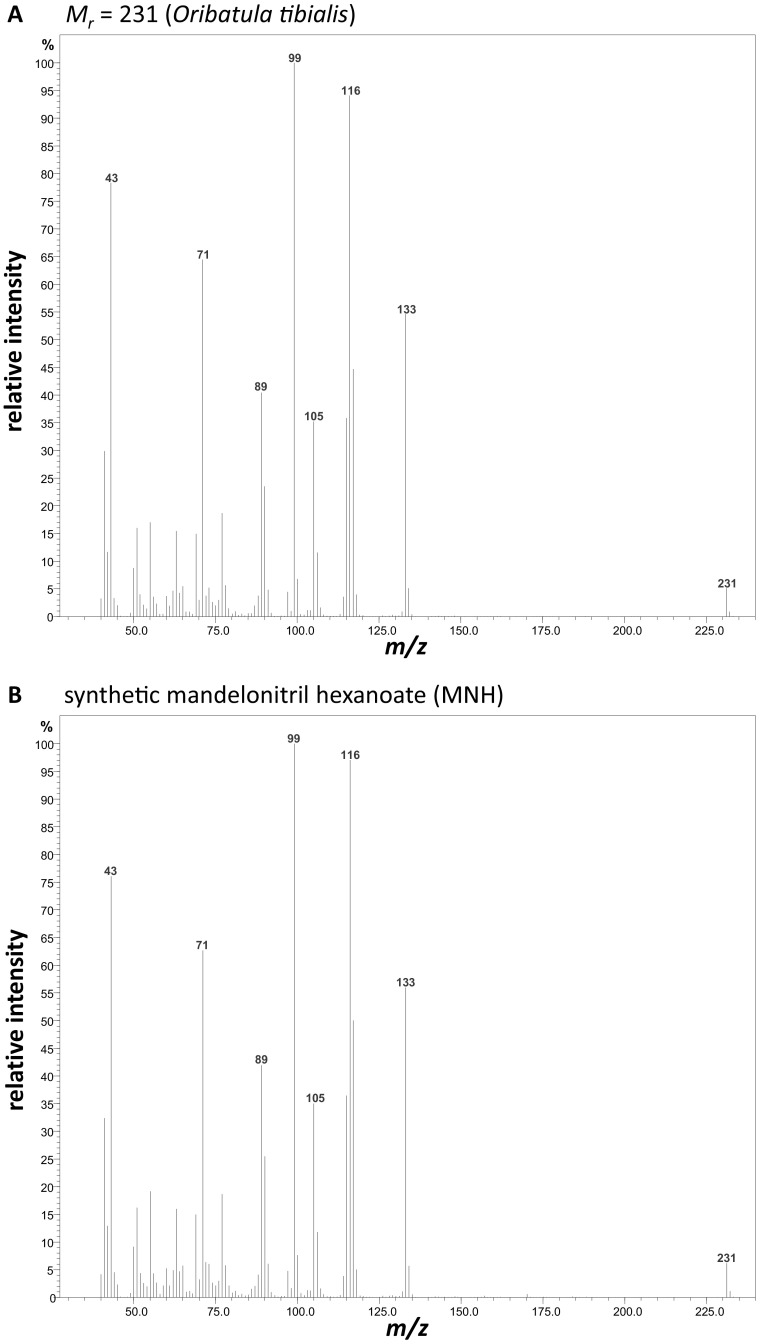
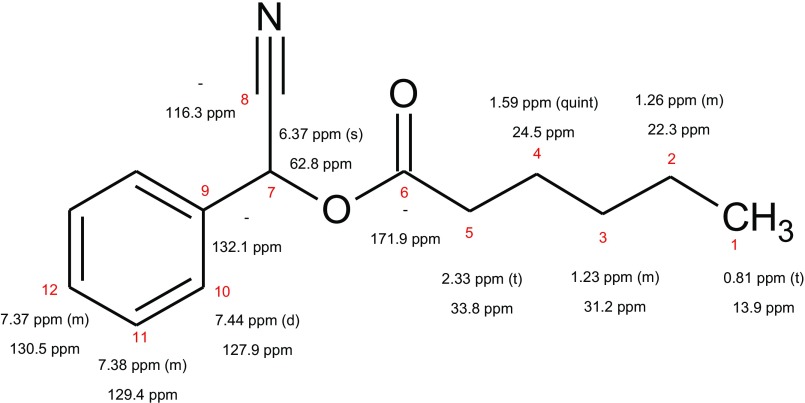
Mass spectrometric investigation resulted in the following mass signals (and their relative abundances): 231 (M+, 9), 133 (53), 117 (46), 116 (93), 115 (36), 105 (36), 99 (100), 89 (39), 71 (55), 43 (68). The linear retention index (46) was 1730. The 1H and 13C NMR gave the following chemical shifts: 1H 500 MHz, CDCl3: 7.44 (2H, m), 7.38 (2H, m), 7.37 (1H, m), 6.36 (1H, s), 2.32 (2H, t), 1.58 (2H, quin), 1.22 (4H, m), 0.81 (3H, t); 13C 125 MHz, CDCl3: 171.9 (C), 132.1 (C), 130.4 (CH), 129.4 (2CH), 127.9 (2CH), 116.3 (C), 62.8 (CH), 33.8 (CH2), 31.2 (CH2), 24.5 (CH2), 22.3 (CH2), 14.0 (CH3). Detailed mass spectrometric data of natural and synthetic MNH, as well as NMR data of synthetic MNH, are published as supporting information (Figs. S1–S6). Synthetic MNH (equals in vitro) dissolved in water for 30–60 min readily hydrolyzed to hexanoic acid, benzaldehyde, and HCN (Fig. 1).
Fig. S1.
Mass spectra of mandelonitrile hexanoate (MNH) extracted from the oil glands of Oribatula tibialis (A) and from synthetic MNH (B).
Fig. S2.
Structural formula of MNH with observed NMR shift values for 1H (upper values) and 13C (lower values). Peak multiplicity: d, doublet; m, multiplet; quint, quintet; s, singlet; t, triplet. Differences between predicted and experimental chemical shifts (in ppm) based on the evaluation report from the CSEARCH database (nmrpredict.orc.univie.ac.at/c13robot/robot.php): 1 (0.1 ppm), 2 (0.1 ppm), 3 (3.2 ppm), 4 (3.0 ppm), 5 (0.7 ppm), 6 (0.7 ppm), 7 (1.6 ppm), 8 (1.3 ppm), 9 (1.2 ppm), 10 (0.1 ppm), 11 (0.3 ppm), 12 (1.0 ppm).
Acknowledgments
Adrian Brückner was supported by a PhD scholarship from the German National Academic Foundation. This study was partly funded by the German Research Foundation (DFG; HE 4593/5-1).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1618327114/-/DCSupplemental.
References
- 1.Dossey AT. Chemical defense of insects: A rich resource for chemical biology in the tropics. In: Vivanco JM, Weir T, editors. Chemical Biology of the Tropics. Springer-Verlag; Berlin: 2011. [Google Scholar]
- 2.Zagrobelny M, Bak S, Møller BL. Cyanogenesis in plants and arthropods. Phytochemistry. 2008;69(7):1457–1468. doi: 10.1016/j.phytochem.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 3.Chauhan S, et al. Chemical warfare agents. Environ Toxicol Pharmacol. 2008;26(2):113–122. doi: 10.1016/j.etap.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Guldensteeden-Egeling C. Über Bildung von Cyanwasserstoffsäure bei einem Myriapoden. Pflügers Arch. 1882;28(1):576–579. [Google Scholar]
- 5.Blum MS, Woodring JP. Secretion of benzaldehyde and hydrogen cyanide by the millipede Pachydesmus crassicutus. Science. 1962;138(3539):512–513. doi: 10.1126/science.138.3539.512. [DOI] [PubMed] [Google Scholar]
- 6.Eisner HE, Alsop DW, Eisner T. Defense mechanisms of arthropods. XX. Quantitative assessment of hydrogen cyanide production in two species of millipedes. Psyche (Stuttg) 1967;74(2):107–117. [Google Scholar]
- 7.Eisner T, Eisner HE, Hurst JJ, Kafatos FC, Meinwald J. Cyanogenic glandular apparatus of a millipede. Science. 1963;139(3560):1218–1220. doi: 10.1126/science.139.3560.1218. [DOI] [PubMed] [Google Scholar]
- 8.Jones DA, Parsons J, Rothschild M. Release of hydrocyanic acid from crushed tissues of all stages in the life-cycle of species of the Zygaeninae (Lapidoptera) Nature. 1962;193:52–53. doi: 10.1038/193052a0. [DOI] [PubMed] [Google Scholar]
- 9.Blum MS. Chemical Defenses of Arthropods. Acad Press; New York: 1981. [Google Scholar]
- 10.Eisner T. For Love of Insects. Harvard Univ Press; Cambridge, MA: 2003. [Google Scholar]
- 11.Eisner T, Eisner M, Siegler M. Secret Weapons. Harvard Univ Press; Cambridge, MA: 2005. [Google Scholar]
- 12.Nahrstedt A. Cyanogenesis and the role of cyanogenic compounds in insects. Ciba Found Symp. 1988;140:131–150. doi: 10.1002/9780470513712.ch9. [DOI] [PubMed] [Google Scholar]
- 13.Consortium THG. Heliconius Genome Consortium Butterfly genome reveals promiscuous exchange of mimicry adaptations among species. Nature. 2012;487(7405):94–98. doi: 10.1038/nature11041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jensen NB, et al. Convergent evolution in biosynthesis of cyanogenic defence compounds in plants and insects. Nat Commun. 2011;2:273. doi: 10.1038/ncomms1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuwahara Y, Shimizu N, Tanabe T. Release of hydrogen cyanide via a post-secretion Schotten-Baumann reaction in defensive fluids of polydesmoid millipedes. J Chem Ecol. 2011;37(3):232–238. doi: 10.1007/s10886-011-9920-9. [DOI] [PubMed] [Google Scholar]
- 16.Eisner T, Meinwald J, Monro A, Ghent R. Defence mechanisms of arthropods I - The composition and function of the spray of the whipscorpion, Mastigoproctus giganteus (Lucas) (Arachnida, Pedipalpida) J Insect Physiol. 1961;6(4):272–298. [Google Scholar]
- 17.Haupt J, Höhne G, Weiske T. Acetic acid esters, n-hexanol, n-octanol, and capronic acid as ingredients in the defense secretion product of whip scorpions. Boll Acc Gioenia Sci Nat. 1993;26:175–180. [Google Scholar]
- 18.Raspotnig G. Scent gland chemistry and chemosystematics in harvestmen. Biologia Serbica. 2012;34:5–18. [Google Scholar]
- 19.Raspotnig G, et al. Chemosystematics in the Opiliones (Arachnida): A comment on the evolutionary history of alkylphenols and benzoquinones in the scent gland secretions of Laniatores. Cladistics. 2015;31(2):202–209. doi: 10.1111/cla.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang S, et al. A novel property of spider silk: Chemical defence against ants. Proc Biol Sci. 2012;279(1734):1824–1830. doi: 10.1098/rspb.2011.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuwahara Y. Chemical ecology of astigmatid mites. In: Cardé RT, Millar JG, editors. Advances in Insect Chemical Ecology. Cambridge Univ Press; Cambridge, UK: 2004. pp. 76–109. [Google Scholar]
- 22.Raspotnig G, Norton RA, Heethoff M. Oribatid mites and skin alkaloids in poison frogs. Biol Lett. 2011;7(4):555–556. doi: 10.1098/rsbl.2010.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brückner A, Stabentheiner E, Leis HJ, Raspotnig G. Chemical basis of unwettability in Liacaridae (Acari, Oribatida): Specific variations of a cuticular acid/ester-based system. Exp Appl Acarol. 2015;66(3):313–335. doi: 10.1007/s10493-015-9914-3. [DOI] [PubMed] [Google Scholar]
- 24.Heethoff M, Koerner L, Norton RA, Raspotnig G. Tasty but protected--first evidence of chemical defense in oribatid mites. J Chem Ecol. 2011;37(9):1037–1043. doi: 10.1007/s10886-011-0009-2. [DOI] [PubMed] [Google Scholar]
- 25.Heethoff M, Rall BC. Reducible defence: Chemical protection alters the dynamics of predator–prey interactions. Chemoecology. 2015;25(2):53–61. [Google Scholar]
- 26.Raspotnig G. Chemical alarm and defence in the oribatid mite Collohmannia gigantea (Acari: Oribatida) Exp Appl Acarol. 2006;39(3-4):177–194. doi: 10.1007/s10493-006-9015-4. [DOI] [PubMed] [Google Scholar]
- 27.Saporito RA, Spande TF, Garraffo HM, Donnelly MA. Arthropod alkaloids in poison frogs: A review of the dietary hypothesis. Heterocycles. 2009;79:277–297. [Google Scholar]
- 28.Shimano S, Sakata T, Mizutani Y, Kuwahara Y, Aoki J. Geranial: The alarm pheromone in the nymphal stage of the oribatid mite, Nothrus palustris. J Chem Ecol. 2002;28(9):1831–1837. doi: 10.1023/a:1020517319363. [DOI] [PubMed] [Google Scholar]
- 29.Heethoff M, Raspotnig G. Is 7-hydroxyphthalide a natural compound of oil gland secretions? - Evidence from Archegozetes longisetosus (Acari, Oribatida) Acarologia. 2011;51:229–236. [Google Scholar]
- 30.Kizawa Y, Kuwahara Y, Matsuyama S, Suzuki T. Chemical ecology of astigmatid mites XXXVI: Mite body catalyzes isomerization and reduction of neral (alarm pheromone component): Common phenomenon? J Acarol Soc Jpn. 1993;2(2):67–74. [Google Scholar]
- 31.Kuwahara Y, Suzuki H, Matsumoto K, Wada Y. Pheromone study on acarid mites XI. Function of mite body as geometric isomerization and reduction of citral (the alarm pheromone) Appl Entomol Zool (Jpn) 1983;18(1):30–39. [Google Scholar]
- 32. German Social Accident Insurance (2016) GESTIS Substance database. gestis.itrust.de.
- 33.Jones TH, Conner WE, Meinwald J, Eisner HE, Eisner T. Benzoyl cyanide and mandelonitrile in the cyanogenetic secretion of a centipede. J Chem Ecol. 1976;2(4):421–429. [Google Scholar]
- 34.Duffey SS, Towers GHN. On the biochemical basis of HCN production in the millipede Harpaphe haydeniana (Xystodesmidae: Polydesmida) Can J Zool. 1978;56(1):7–16. [Google Scholar]
- 35.Towers GHN, Duffey SS, Siegel SM. Defensive secretion: Biosynthesis of hydrogen cyanide and benzaldehyde from phenylalanine by a millipede. Can J Zool. 1972;50(7):1047–1050. [Google Scholar]
- 36.Morgan ED. Biosynthesis in Insects. RSC Publ; Cambridge, UK: 2010. [Google Scholar]
- 37.Shimizu N, Naito M, Mori N, Kuwahara Y. De novo biosynthesis of linoleic acid and its conversion to the hydrocarbon (Z,Z)-6,9-heptadecadiene in the astigmatid mite, Carpoglyphus lactis: Incorporation experiments with 13C-labeled glucose. Insect Biochem Mol Biol. 2014;45:51–57. doi: 10.1016/j.ibmb.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 38.Gullan PJ, Cranston PS. The Insects: An Outline of Entomology. Wiley-Blackwell; Oxford, UK: 2005. [Google Scholar]
- 39.Nishio N, Eguchi N, Fujiyama S. On the amounts of cyanides contained in an adult of Parafontaria laminata armigera (Verhoeff), and Epanerchodus orientalis (Attems) Edaphologia. 1995;53:25–29. [Google Scholar]
- 40.Stebbins RC. Lizards killed by a millipede. Amer Midl Bat. 1944;32:777–778. [Google Scholar]
- 41.Subcommittee on Acute Exposure Guideline Levels 2002. Hydrogen cyanide: Acute exposure guideline levels. in Acute Exposure Guideline Levels for Selected Airborne Chemicals, ed Natl Res Counc USA (Natl Acad Press, Washington, DC)
- 42.Dunlop JA. Geological history and phylogeny of Chelicerata. Arthropod Struct Dev. 2010;39(2-3):124–142. doi: 10.1016/j.asd.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 43.Norton RA, Behan-Pelletier V. Oribatida. In: Krantz GW, Walter DE, editors. A Manual of Acarology. Texas Tech Univ Press; Lubbock, TX: 2009. [Google Scholar]
- 44.Wehner K, Norton RA, Blüthgen N, Heethoff M. Specialization of oribatid mites to forest microhabitats - the enigmatic role of litter. Ecosphere. 2016;7(3):e01336. [Google Scholar]
- 45.Brückner A, Heethoff M. Scent of a mite: Origin and chemical characterization of the lemon-like flavor of mite-ripened cheeses. Exp Appl Acarol. 2016;69(3):249–261. doi: 10.1007/s10493-016-0040-7. [DOI] [PubMed] [Google Scholar]
- 46.van den Dool H, Kratz PD. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J Chromatogr A. 1963;11:463–471. doi: 10.1016/s0021-9673(01)80947-x. [DOI] [PubMed] [Google Scholar]
- 47.Adams RP. 1995. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. (Allured Publ Corp, Carol Stream, IL)
- 48.Stein SE. 2015 Mass spectra by NIST Mass Spec Data Center. eds Linstrom PJ, Mallard WG. NIST Chemistry Webbook, NIST standard reference database number 69, Natl Inst of Standards and Technol, Gaithersburg, MD, 20899. webbook.Nist.Gov.
- 49.Scheme BGR. Einhorn acylation. Comprehensive Organic Name Reactions and Reagents. 2010;209:967–970. [Google Scholar]