Significance
Symptoms of benign prostatic hyperplasia (BPH) affect many older men, but the etiology of the disease is largely unknown. We show that male mice develop prostatic stromal hyperplasia accompanied by lower urinary tract symptoms that appear similar to BPH with conditional homozygous deletion of the tumor suppressor gene, Stk11 (serine threonine kinase 11), in the Müllerian duct mesenchyme (MDM), which regresses in males during early fetal development. Cell lineage tracing studies confirmed that cells from the caudal MDM contribute to the stromal cell population of the dorsal periurethral area, which has been compared with the transition zone of the human prostate. These studies show that some of the stromal cells of the prostate are MD-derived, and that their mutation can lead to BPH.
Keywords: benign prostatic hyperplasia, mouse model, LUTS, Müllerian duct, liver kinase B1
Abstract
Nearly all older men will experience lower urinary tract symptoms associated with benign prostatic hyperplasia (BPH), the etiology of which is not well understood. We have generated Stk11CKO mice by conditional deletion of the liver kinase B1 (LKB1) tumor suppressor gene, Stk11 (serine threonine kinase 11), in the fetal Müllerian duct mesenchyme (MDM), the caudal remnant of which is thought to be assimilated by the urogenital sinus primordial mesenchyme in males during fetal development. We show that MDM cells contribute to the postnatal stromal cells at the dorsal aspect of the prostatic urethra by lineage tracing. The Stk11CKO mice develop prostatic hyperplasia with bladder outlet obstruction, most likely because of stromal expansion. The stromal areas from prostates of Stk11CKO mice, with or without significant expansion, were estrogen receptor positive, which is consistent with both MD mesenchyme-derived cells and the purported importance of estrogen receptors in BPH development and/or progression. In some cases, stromal hyperplasia was admixed with epithelial metaplasia, sometimes with keratin pearls, consistent with squamous cell carcinomas. Mice with conditional deletion of both Stk11 and Pten developed similar features as the Stk11CKO mice, but at a highly accelerated rate, often within the first few months after birth. Western blot analyses showed that the loss of LKB1 and phosphatase and tensin homolog deleted on chromosome 10 (PTEN) induces activation of the phospho-5′ adenosine monophosphate-activated protein kinase and phospho-AKT serine/threonine kinase 1 signaling pathways, as well as increased total and active β-catenin. These results suggest that activation of these signaling pathways can induce hyperplasia of the MD stroma, which could play a significant role in the etiology of human BPH.
Benign prostatic hypertrophy/hyperplasia (BPH) is one of the most commonly observed proliferative diseases in older men and is characterized by hyperplasia of stromal and epithelial cells of the prostate gland (1). Urinary obstruction resulting from the anatomical enlargement of the prostate in the periurethral region is one of the clinical hallmarks of BPH and the primary indication for medical intervention (2). Several studies investigating the pathophysiology of BPH indicate that the bioavailability of androgens, race, obesity, and other risk factors can contribute to progression of the disease, but age is the most prognostic factor, with estimates of up to 90% incidence in men in their ninth decade (2, 3). However, the precise mechanisms driving BPH have not been completely elucidated. The evidence does suggest that dysregulated stromal cell proliferation is a major component in symptomatic disease (1). Also, BPH appears to develop most often in the transition zone and in the periurethral region, both of which are near the base of the verumontanum (4), a remnant of the fused ends of the caudal Müllerian ducts (MDs). The tendency of BPH to develop in this anatomically distinct region of the prostate suggests the stroma has some unique qualities characteristic of its embryonic origin, which is often ascribed to the MD mesenchyme (MDM) (4–6). Speculation that the etiology of BPH might be the result of reawakening of embryonic or dedifferentiated nonprostatic stromal cell activity is consistent with this hypothesis (4, 5, 7).
Shortly after commitment of the bipotential embryonic mammalian gonadal ridge to male development, Sertoli cells differentiate in the testes and produce Müllerian inhibiting substance (also known as anti-Müllerian hormone, or AMH), a TGF-β family member that causes MD regression in males (reviewed in ref. 8). The embryonic female gonads do not produce Müllerian inhibiting substance; thus, the MDs differentiate into the oviduct, uterus, cervix, and anterior vagina. However, the caudal MD remnant, which is commonly referred to as the prostatic utricle, is not completely regressed in males and has been thought to participate in the development of the rodent and human prostate (5, 9). Prostatic stromal cells have been shown to express estrogen receptor (10, 11), particularly in BPH (12), which also suggests that estrogen-responsive cells from the MDs could contribute to prostate development and the etiology of BPH.
One of the best-studied mouse models of prostate cancer is the TRAMP mouse (13). Phosphatase and tensin homolog deleted on chromosome 10 (PTEN) is a tumor suppressor that inhibits the receptor tyrosine kinase-activated PI3K/AKT (RAC-alpha serine/threonine-protein kinase) signaling pathway, and its loss or deletion is frequently associated with a variety of cancers, including prostate cancer in humans (14, 15). Conditional deletion of Pten in prostate epithelia leads to high-grade neoplasia with complete penetrance and early onset in mice (16), supporting its essential role as a driver of prostate carcinogenesis. PTEN loss has not been reported to play a role in BPH. Another mouse model of prostate cancer has been developed by conditional deletion of Stk11 (serine threonine kinase 11), the gene for liver kinase B1 (LKB1), which is a gene linked to Peutz-Jeghers syndrome (PJS), a human hereditary cancer-prone disorder characterized by hamartomatous polyposis. Conditional deletion was accomplished in these mice by using the Cyp1A1 promoter driving Cre recombinase expression in the prostate epithelium. These mice developed intraepithelial neoplasia with nearly 100% penetrance in many of the prostate lobes examined (17).
Recent studies from our laboratory showed that conditional deletion of Stk11, along with Pten from the MDM, led to high-grade serous epithelial ovarian cancer, endometrial hyperplasia and carcinomas, and oviductal and cervical adenomas in females (18, 19). Strikingly, we observed significant expansion of the stromal compartment admixed with glandular hyperplasia in the lower reproductive tracts of all of the female mice examined in that study, a phenotype that was consistent with adenoma malignum/minimal deviation adenocarcinoma (MDA), requiring euthanasia in most cases because of tumor growth-related exstrophy. In this report, we show that male mice with conditional deletion of Stk11 in the embryonic MDM develop periurethral stromal hyperplasia, accompanied by urinary obstruction, suggesting an important etiological link between the MD remnant and BPH.
Materials and Methods
Mouse Genetics and Animal Husbandry.
All animal experimentation protocols used in this study were approved by the Institutional Animal Care and Use Committees at Massachusetts General Hospital and Michigan State University and are in compliance with the NIH Guide for the Care and Use of Laboratory Animals. Mice used in this study were kept in standard housing conditions and were maintained on a mixed genetic background (C57BL/6;129/SvEv). Amhr2tm3(cre)Bhr (Amhr2-Cre) (20), Stk11tm1Rdp (Stk11fl/fl) (21), and Ptentm1Hwu (Ptenfl/fl) (22) were mated to produce Amhr2-cre/+;Stk11Δ/Δ, Amhr2-cre/+;PtenΔ/Δ and Amhr2-Cre/+;Stk11Δ/Δ;PtenΔ/Δ, hereafter referred to as Stk11CKO, PtenCKO, and Stk11CKO;PtenCKO, respectively. Amhr2tm2(lacZ)Bhr (Amhr2-LacZ) mice for real-time Amhr2 promoter-driven LacZ expression and Amhr2-Cre/+ crosses with Rosa26;EYFP Rosa26;LacZfl/fl reporter mice have been previously described (23, 24). Tail biopsies were collected for genotyping, and PCR conditions for Amhr2-Cre, Stk11, and Pten alleles have been described (21, 22, 25, 26). Amhr2-LacZ mice were genotyped with 5′-TGC GTG ACT ACC TAC GGG TAA C and 5′-GAT CGA CAG ATT TGA TCC AGC G primers, using standard PCR amplification conditions; the presence of a 900-bp band by gel electrophoresis was considered positive for the Amhr2-LacZ allele. Gross pictures of the animals or the tumors were taken using a Nikon SMZ1500 microscope with an attached Spot camera (Diagnostic Instruments) or with a Nikon D60 digital camera and macro lens.
MDM Lineage Tracing.
TRE-H2b-GFP (Tg(tetOHIST1H2BJ/GFP)47Efu) mice and Rosa26-flox-stop-tTA (Gt(ROSA)26Sortm1(tTA)Roos/J) mice were purchased from The Jackson Laboratory and mated to Amhr2-Cre mice to generate triple transgenic offspring (Amhr2-Cre;Rosa26-tTA;TRE-H2b-GFP). Cre is expressed in the MDM on embryonic day 13.5 in both male and female fetuses, leading to expression of H2B-GFP, which becomes stably incorporated into nucleosomes during DNA replication, thereby indelibly labeling MDM-derived cells. Urogenital systems from 7–9-wk-old Amhr2-Cre;Rosa-tTA;TRE-H2b-GFP and control (Rosa26-flox-stop-tTA;H2b-GFP) mice were fixed in 4% (vol/vol) PFA on ice for 30 min. After three washes in ice-cold PBS, tissues were incubated overnight at 4 °C in 15% (wt/vol) sucrose buffered in PBS. The following day, tissues were incubated at 37 °C for 1 h in gelatin [15% sucrose (wt/vol), 7.5% (wt/vol) gelatin in PBS], embedded in gelatin, then frozen at −50 °C to −65 °C in isopentane and stored at −80 °C until sectioning. Tissues were cryosectioned at 6 μm and thaw mounted. Gelatin was removed from tissue sections by incubating slides in 37 °C PBS for 10 min. After gelatin removal, slides were counterstained with DAPI (Vector Laboratories) mounting medium and viewed directly using fluorescence microscopy.
Histology and Western blot analyses are presented in SI Materials and Methods.
SI Materials and Methods
Histology, Immunohistochemistry, Immunofluorescence, and β-Galactosidase Staining.
Male urogenital tract tissues were collected postmortem and fixed, processed, sectioned, and stained with hematoxylin and eosin, as previously described (24, 25). The following primary antibodies were used: ERα (Dako), Desmin (Thermo Scientific), αSMA (Sigma Chemical Co.), NKX3.1 (Bio Care Medical). Either the Dako Envision+ system (Dako) or biotinylated donkey anti-Fab2 (Jackson ImmunoResearch Laboratories) and an ABC kit (Vector Labs) were used for immunohistochemistry detection. Immunofluorescence for NKX3.1 was used with an Alexafluor 555 secondary antibody (Thermo Scientific). LacZ reporter β-galactosidase staining was performed as described (27). Trichrome staining was performed using the Trichrome Stain and Weigert’s Iron Hematoxylin kits per manufacturer’s instructions (Sigma Chemical Co.). Photos were taken with a Nikon Eclipse Ni microscope fitted with either a Nikon DSF12 or DS-Qi1-MC camera.
Western Analyses.
Western blot analyses were performed as described previously (28). Briefly, proteins were extracted from urogenital stroma of male mice of Stk11CKO, Stk11CKO;PtenCKO and control mice (n = 3), using RIPA lysis buffer (#9806; Cell Signaling) containing a protease inhibitor mixture (#539131, MilliporeSigma) and phosphatase inhibitor mixture (#P004A, MilliporeSigma). Equal amounts of total protein (40 μg) were resolved via 4–12% (wt/vol) polyacrylamide, Bis-Tris gradient gel (Thermo Scientific) and transferred onto a nitrocellulose membrane. Membrane was blocked with 5% (wt/vol) nonfat dry milk for 1 h at room temperature and incubated with 1:1,000 diluted anti-pS6 (4857S; Cell Signaling), anti-AKT (9272; Cell Signaling), anti p-AKT (4051S; Cell Signaling), anti-pAMPK (2535P; Cell Signaling), anti-AMPK (2535S; Cell Signaling), anti-total β-catenin (610154; BD Biosciences), anti-active β-catenin (4270S; Cell Signaling), anti-GSK3-β (9315; Cell Signaling), anti-pGSK3-β (9323P; Cell Signaling), anti-LKB1 (3047S; Cell Signaling), or anti-PTEN (9188S; Cell Signaling) antibodies overnight. After washes, protein was visualized by incubation with a horseradish peroxidase-linked secondary antibody (1:10,000) for 1 h at room temperature, and signal was detected with ECL reagent (GE Healthcare Life Sciences). To control for loading, the membrane was stripped, probed with anti-β-Actin (A5228; MilliporeSigma), and developed. The band intensity was quantitated using Image J analysis software (National Institute of Health), and normalized to corresponding β-actin bands.
Results
Caudal MD-Derived Cells in the Male Reproductive Tract.
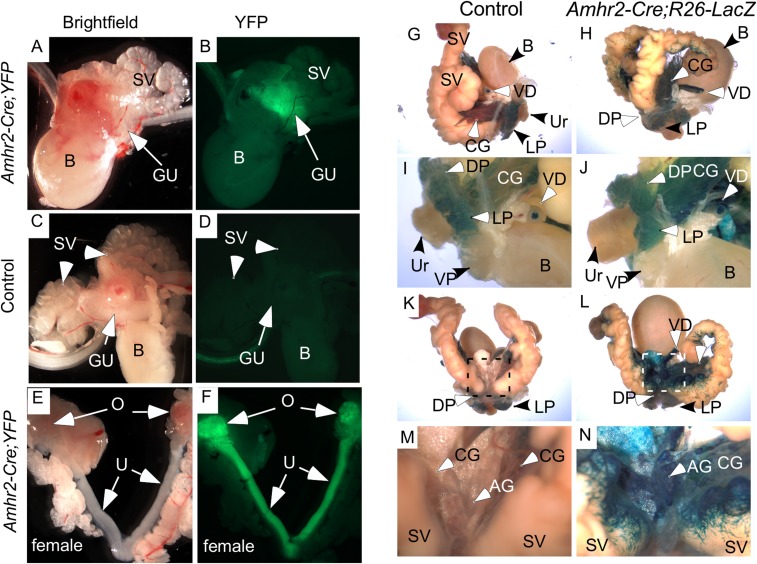
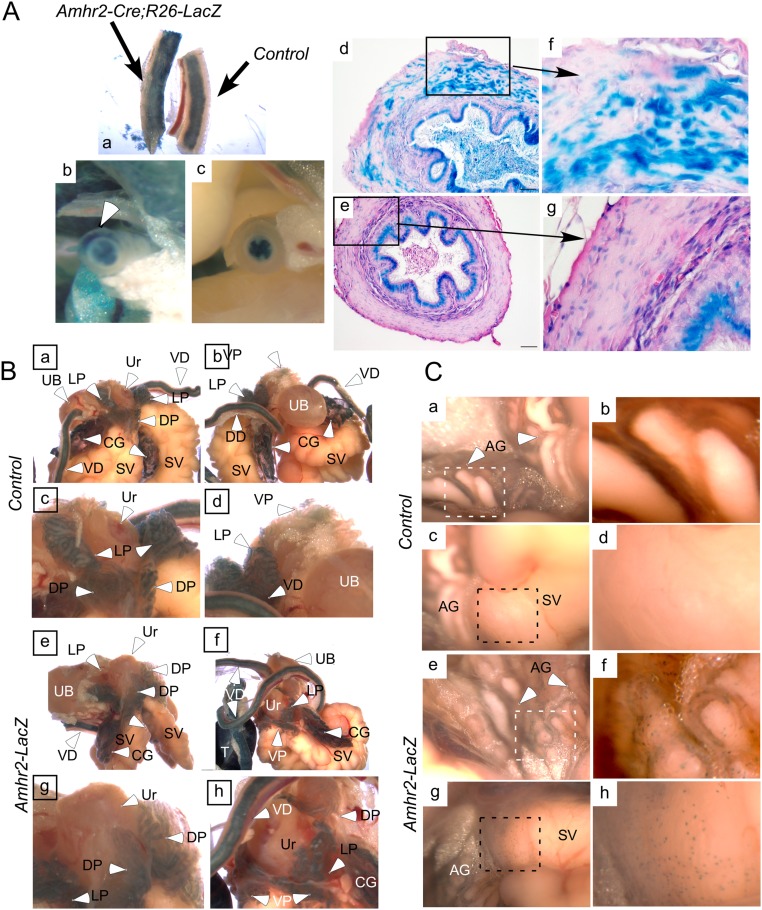
The involvement of the caudal MD remnant in male urogenital tissue development has been debated (9, 27). To study the contribution, if any, of the MDM to prostate organogenesis, we mated mice with the Amhr2-Cre allele, which is expressed in the embryonic MDM cells, with mice carrying EYFP, LacZ, or GFP floxed reporter alleles. AMH type 2 receptor (AMHR2), a bona fide receptor for AMH (28), is only expressed in the mesenchyme surrounding the fetal MDs and in the gonads of male urogenital ridges, but not in the Wolffian duct (29–32). We bred mice to contain the Amhr2-Cre;Rosa26-YFP alleles to determine whether any MD-derived tissues persisted in males and directly observed YFP activity in the dorsal prostatic region of sexually immature male mice (Fig. S1 A and B), but not in control littermates (Fig. S1 C and D). As expected, females showed YFP activity in the MD-derived tissues, the oviducts, uterine horns, cervix, and ovaries, which express Amhr2 on the surface epithelium (33) and granulosa cells (29–32) (Fig. S1 E and F). As further confirmation of the presence of MDM cells in male urogenital tissue, β-galactosidase activity was assessed in adult mice with floxed Rosa26-LacZ alleles (Fig. S1 G–N). In control littermates, variable levels of endogenous β-galactosidase activity were observed in the urogenital tracts (Figs. S1 G, I, K, and M and S2A), whereas in Amhr2-Cre;Rosa26-LacZ mice, in addition to endogenous β-galactosidase activity, strong β-galactosidase activity was observed in the ampullary glands and in stromal cells at the base of the seminal vesicles (Fig. S1 H, J, L, and N) and in the stroma and smooth muscle of the vasa deferens (Fig. S2A). Furthermore, cells expressing real-time β-galactosidase activity in which LacZ is directly under the control of the Amhr2 promoter and is only expressed in cells actively expressing AMHR2 [Amhr2-LacZ mice (23)] were observed at the base of the ampullary glands and seminal vesicles (Fig. S2 B and C), suggesting the Amhr2 locus was still active in adult mice.
Fig. S1.
Gross analysis of Amhr2-Cre-driven reporter expression. Amhr2-Cre mice were mated with YFP reporter mice, and the reproductive tracts of their young progeny were examined grossly for fluorescence. (A and B) Amhr2-Cre-driven fluorescence is detected in the prostatic or genitourinary (GU) region of male pups. The bladder (B) and seminal vesicles (SV) did not fluoresce. Fluorescence also was not observed in any of the control littermates examined (C and D). Females (E and F) showed strong fluorescence in their MD-derived organs [i.e., the uterine horns (U), cervix (C), and oviducts (Ov), as well as the ovaries (O)]. Gross analyses of older mice were done by β-galactosidase activity because fluorescence does not penetrate the older tissues. In control male reproductive tract, endogenous β-gal activity is observed in the dorsal (DP), lateral (LP), and ventral (VP) prostatic glands and in the luminal epithelial of the VD (G and I), but not in the dorsal aspect of the prostatic-periurethral area (K and M). The urethra (Ur) and bladder also showed no β-gal activity (G–N). Amhr2-Cre-driven expression of the β-gal reporter showed that MDM-derived cells persist in the dorsal aspect of the genitourinary region where the seminal vesicles (SV), coagulating glands (CG), ampullary glands (AG), and vasa deferens (VD) enter the prostatic-periurethral area (L and N). (Scale bars, 100 μm.)
Fig. S2.
β-Galactosidase activity in male mouse reproductive tracts. (A) Vasa deferens from control mice have endogenous β-gal activity in the luminal epithelium. (a) Vasa deferens from the indicated mice are shown side by side. Gross and histological analyses of the vasa deferens (b and c) show β-gal staining in the stroma of the Amhr2-Cre;R26-LacZ mice, which is absent in the stroma of control littermates (d and e). Boxes in d and e are shown at higher magnification in f and g. (B) Gross analyses and b-gal staining of reproductive tracts from Amhr2-LacZ (e–h), a knockin allele that expresses β-gal under the control of the endogenous promoter, show very little difference compared with control littermates (a–d). (C) A closer view of the dorsal aspect of the prostate/peri-urethral tissues shows some spotty and variable β-gal activity in the mutant reporter mice (e–h) that was not observed in the controls (a–d). Ag, ampullary glands; CG, coagulating glands; DD, ductus deferens; DP, dorsal prostate; LP, lateral prostate; SV, seminal vesicles; T, testis; UB, urinary bladder; Ur, periurethral; VD, vasa deferens; VP, ventral prostate.
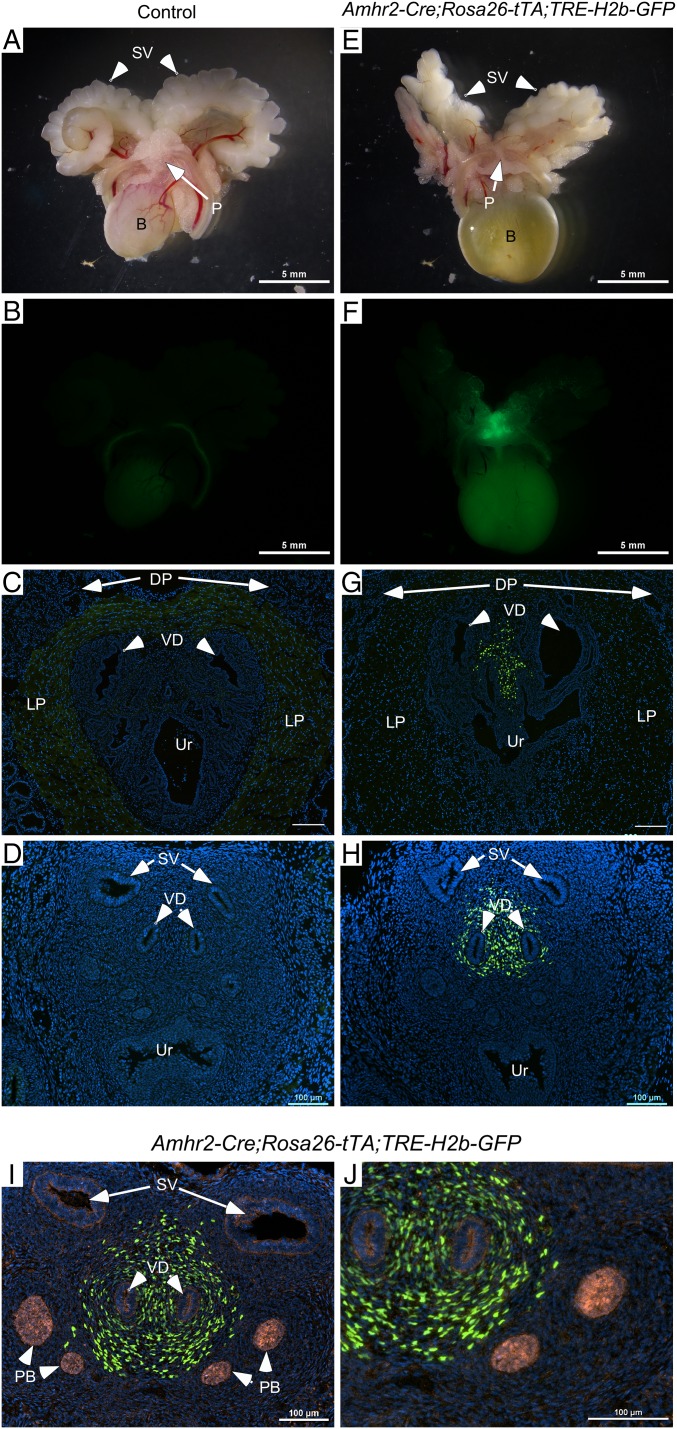
Because of the caveats associated with LacZ reporter mice, specifically the confounding endogenous β-galactosidase activity, we used a more reliable and robust reporter mouse model to further substantiate the presence of MDM cells in the male urogenital tract. Amhr2-Cre;Rosa26-tTA;TRE-H2b-GFP mice, which express nuclear GFP, showed similar gross anatomical results to that of the YFP and LacZ reporter mice with robust GFP expression in the dorsal prostatic region of adult male mice, which was absent from control littermates (Fig. 1 A, B, E, and F). To assess whether MDM cells contribute to the male prostatic stroma, we examined frozen sections of the male urogenital tissues for direct GFP activity and observed nuclear GFP activity in the dorsal periurethral stroma of Amhr2-Cre;Rosa26-tTA;TRE-H2b-GFP, but not in control littermates (Fig. 1 C and G). As discussed earlier, the Amhr2 promoter was still active in adult Amhr2-LacZ mice (Fig. S2). Because of this, we wanted to confirm that the GFP-positive cells in Amhr2-Cre;Rosa26-tTA;TRE-H2b-GFP mice were in fact derived from MDM that persisted in the male after MD regression and were not mature periurethral stromal cells that had reactivated Amhr2 epxression, and thus might not be MDM-derived. To demonstrate this, we collected postnatal day (PND) 0.5 male urogenital tracts from control and Amhr2-Cre;Rosa26-tTA;TRE-H2b-GFP mice (Fig. 1 D and H) and showed the presence of GFP-positive MDM cells in the periurethral stroma shortly after MD regression (23) and during prostate formation, which was confirmed with colocalization with NKX3.1, a prostate epithelial marker (34, 35) (Fig. 1 I and J), indicating that the MD remnant contributed to postnatal prostate development. These results reveal that AMHR2-expressing cells derived from the caudal MDM persist in the male reproductive tract, well after MD regression has occurred, and contribute to the development of these male genitourinary tissues.
Fig. 1.
Caudal MDM-derived cells persist in the male reproductive tract. The reproductive tracts of adult (A–G) and PND 0.5 (D–J) progeny of Amhr2-Cre mice mated with GFP reporter mice (Amhr2-Cre;Rosa26-tTA;TRE-H2b-GFP) were examined grossly for fluorescence. Fluorescence was not observed in any of the control littermates examined (A–D). (E and F) Amhr2-Cre-driven fluorescence is detected in the prostatic or genitourinary (P) region of adult males. The bladder (B) and seminal vesicles (SV) did not fluoresce. MDM-derived cells were detected by Amhr2-Cre-driven nuclear GFP expression in the dorsal stroma of the periurethral (Ur) area between the vasa deferens (VD) in both adult (C and G) and PND 0.5 (D and H). Nkx3.1, a prostate epithelial marker, was used to show the developing prostate buds (PB) in PND 0.5 tissue (I and J). Dorsal and lateral prostate (DP, LP) are indicated in C and G for orientation. Nuclei are stained with DAPI in C, D, and G–J. [Scale bars, 5 mm (A, B, E, and F) or 100 μm (C, D, and G–J).]
Loss of LKB1 in the MDM.
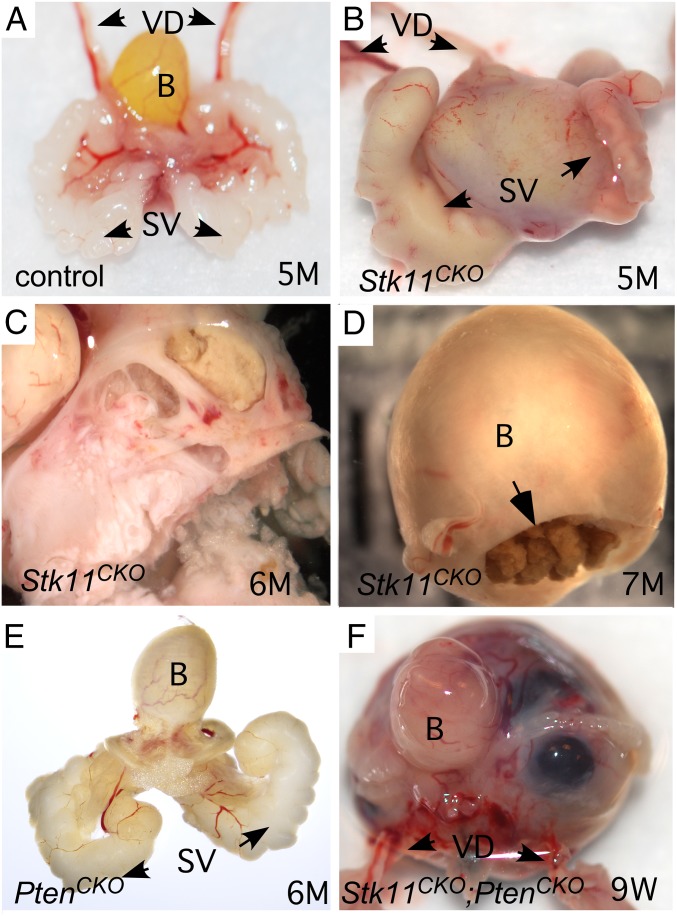
We have shown here that MDM cells contribute to the stroma of male reproductive tract tissues in mice, including the vasa deferens and the periurethral stroma. We have previously shown that deletion of tumor suppressor genes in uterine stroma can lead to hyperplasia and tumorigenesis (18). In particular, we have observed that deletion of the Stk11 gene in the MDM-derived female reproductive tract leads to significant cervical tumor burden in most mice. These tumors are stromal and resemble human adenoma malignum/MDA, and often require euthanasia of the mouse because of associated morbidities. We thus hypothesized that loss of LKB1 in the retained caudal MDM-derived stromal cells could similarly lead to the development of BPH or prostate carcinomas. To test this hypothesis, we examined the reproductive tracts of male Stk11CKO mice at various ages. From 2 to 6 wk postnatal, no gross or histological differences were observed between control and Stk11CKO mice, but by 12 wk, gross analysis of Stk11CKO mice showed that some of the mice examined had notable enlargement of the periurethral area compared with controls, which was clearly evident by 5 and 6 mo (Fig. 2 A–C). These were often accompanied by accumulation of calculi in the bladder (Fig. 2D), a diagnostic of urinary stasis and lower urinary tract obstruction in men with BPH. Additional deletion of the Pten gene, which encodes for the PTEN tumor suppressor, resulted in faster and more severe tumorigenesis (Fig. 2F). Mice with deletion of Pten alone did not show any periurethral phenotype (Fig. 2E) by 6 mo, indicating that PTEN loss accelerates tumorigenesis only in cells already prone to developing cancer.
Fig. 2.
Gross analyses of male reproductive tracts in mice with Amhr2-Cre-driven loss of LKB1 and PTEN. Control littermate male reproductive tract at 5 mo shown in A. Representative reproductive tract of similarly aged mice with loss of LKB1 in the MDM cells shown in B, with another bisected in C. Calcified debris was often observed in the bladders of Stk11CKO mice (D). Mice with loss of PTEN alone had no overt phenotype (E). Development of stromal tumors in mice with Amhr2-Cre-driven deletion of both Stk11 and Pten was accelerated (F). B, bladder; SV, seminal vesicles; VD, vasa deferens.
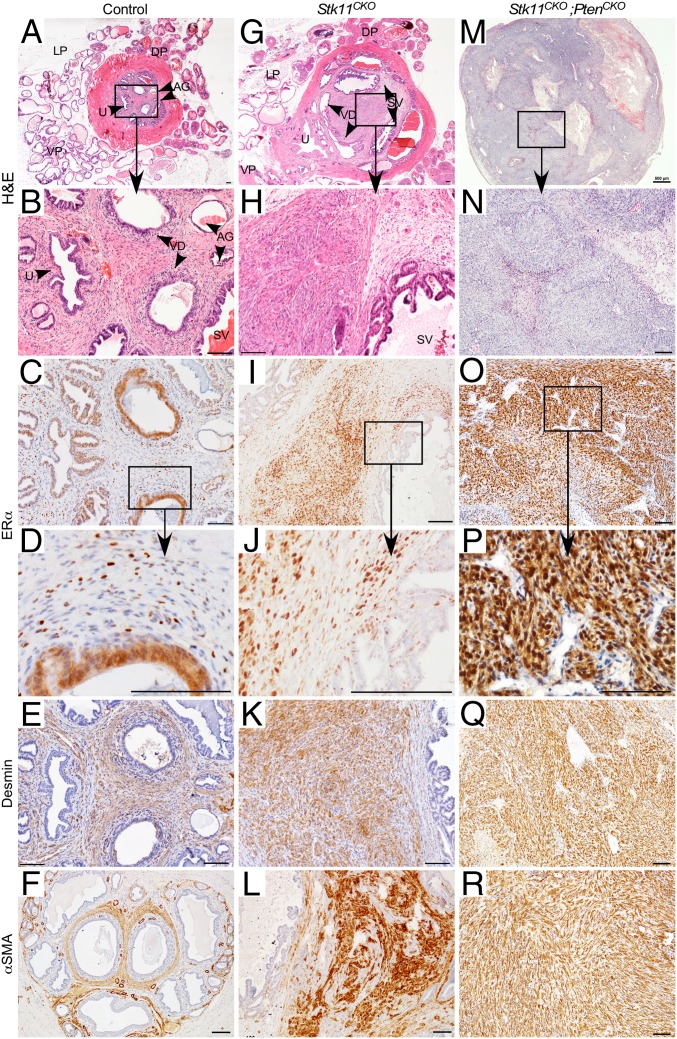
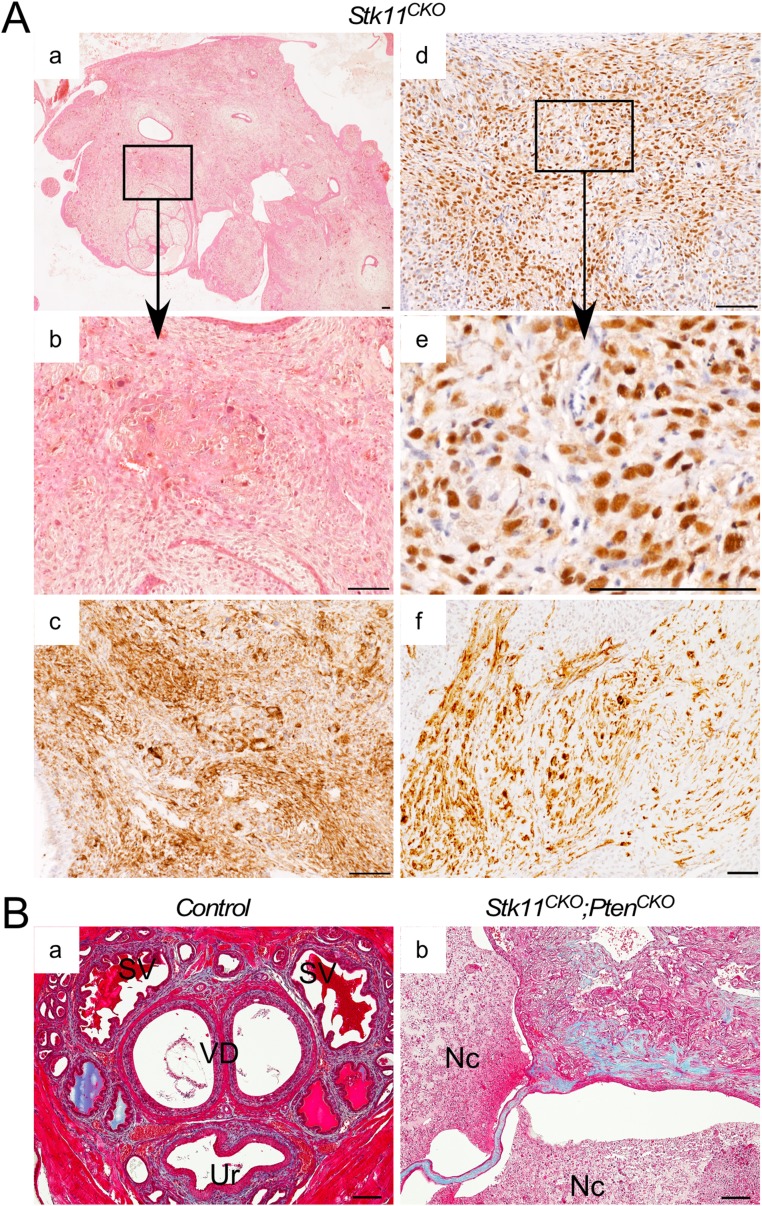
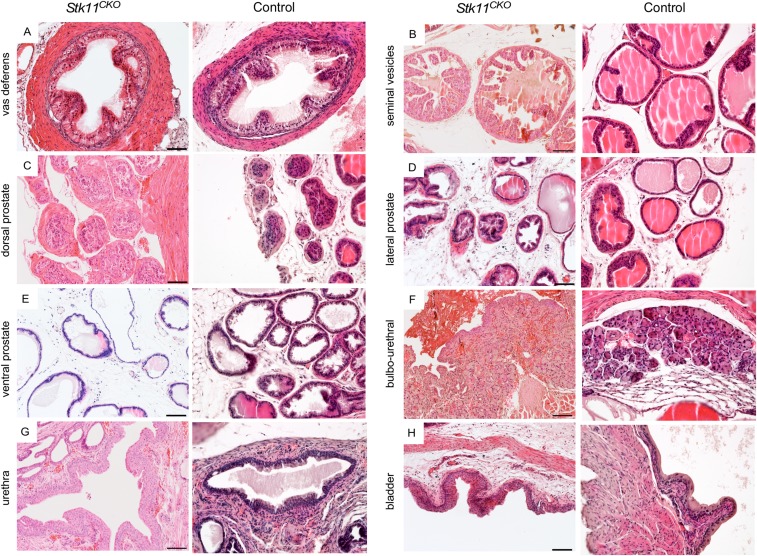
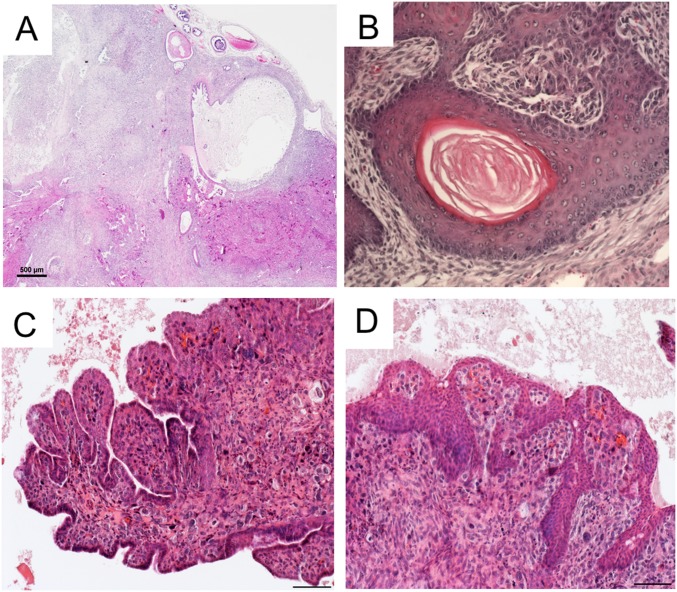
More thorough analyses of the periurethral tissues were performed by histology (Fig. 3). The periurethral area of control littermates (n = 13) between 3 and 8 mo postnatal showed normal histology (36) with well-differentiated ducts, glands, and stroma (Fig. 3 A and B). In contrast, most mice with loss of LKB1 in MDM showed either expansion of the stroma between 3 and 7 mo postnatal (11/17; Fig. 3 G and H), often accompanied by deformation of the ducts and glands, or frank stromal tumorigenesis (4/17; Fig. S3 A and B). In the periurethral expansions or smaller tumors, the histology of prostatic and accessory glands and ducts appeared normal in mutant mice (Fig. S4), suggesting the mutant stroma was not affecting adjacent epithelial cell homeostasis, at least during the early stages. The bladder urothelium of some of the mice appeared abnormally thickened and/or edematous (Fig. S4H), probably as a result of the urethral obstruction caused by adjacent stroma. Histological analyses of the periurethral tissues of Stk11CKO;PtenCKO mice was more challenging because of the rapid growth of these tumors and their total overgrowth of adjacent accessory ducts and glands. The stroma appeared as greatly expanded (Fig. 3 M and N and Fig. S5A) versions of larger tumors from older Stk11CKO mice, as shown above (Fig. S3 A and B). In a minority of both large Stk11CKO tumors and Stk11CKO;PtenCKO tumors, various forms of epithelial metaplasia were observed (Fig. S5B), including stratified squamous epithelia with and without keratin pearls and transitional epithelium that resembles the urothelium. Invasive and phyllodial epithelial metaplasia were also observed in some tumors (Fig. S5 C and D). All this suggests the expansion of the mutant stroma can induce tumorigenesis in the epithelial compartment.
Fig. 3.
Histological and immunohistochemistry analyses of prostatic/periurethral region in mice with Amhr2-Cre-driven loss of LKB1 and PTEN. H&E of prostatic area of controls (A) and mice with Stk11 deletion (G) or Stk11 and Pten (M) deletion in the MDM cells shows typical stromal expansion (G) and tumor formation (M) observed over time compared with control littermates (A). Boxed areas in A, G, and M are shown at higher magnification in B, H, and N. Nuclear ERα is observed in fewer than 50% of the stromal cells in the prostatic/periurethral area of control mice by immunohistochemistry (C). Nearly all the stromal cells in the Stk11CKO (I) and Stk11CKO;PtenCKO (O) prostatic/periurethral area are positive for nuclear ERα. Boxed areas in C, I, and O are shown at higher magnification in D, J, and P. Desmin and αSMA are expressed by immunohistochemistry in the control (E and F) and mutant (K, Q, L, and R) stromal areas. AG, ampullary glands; DP, dorsal prostate; LP, lateral prostate; SV, seminal vesicles; U, urethra; VD, vasa deferens; VP, ventral prostate. Nuclei counterstained with hematoxylin. [Scale bars, 100 μm; 500 μm (M).]
Fig. S3.
Histological and immunohistochemistry analyses of prostatic/periurethral region in mice with Amhr2-Cre-driven loss of LKB1 or LKB1 and PTEN with advanced stromal expansion/tumor formation. (A) H&E of prostatic area of mice with Stk11 deletion in the MDM cells shows advanced stromal expansion/tumor formation (a) similar to mice with additional Pten deletion. Nuclear ERα is observed in almost all the stromal cells in the prostatic/periurethral area of mutant mice by immunohistochemistry (d). Boxed areas in a and d are shown at higher magnification in b and e. Desmin (c) and αSMA (f) are expressed by immunohistochemistry in the mutant stromal areas. (B) Trichrome staining of control tissues shows the presence of collagen (blue staining) in the periurethral (Ur) stromal cells, which is more prominent in Stk11CKO;PtenCKO tumor regions (b). Nc, necrotic tissue; SV, seminal vesicles; VD, vasa deferens. Nuclei were counterstained with hematoxylin. (Scale bars, 100 μm.)
Fig. S4.
Urogenital and reproductive tract tissues appear normal in male Stk11CKO mice. H&E analyses of sections from the indicated urogenital and reproductive tract tissues suggest the phenotype is limited to the periurethral stroma during early stages of the disease before the stroma absorbs the surrounding tissues. A–H show sections from mutant tissues; sections of the same tissues from control mice are shown to the Right.
Fig. S5.
Advanced stromal tumors can alter adjacent epithelia. H&E of pathological sections from advanced Stk11CKO tumors show that the stroma can absorb the adjacent epithelia (A), can induce squamous metaplasia, with (B) or without keratin pearls. Some of the stroma/epithelia took on leafy or frond structures (C), or else the stroma could be invaded by the stratified epithelia (D). (Scale bar, 100 μm.)
The most caudal MD-derived tissue in females is the vagina, which expresses estrogen receptor-α (ERα) in both the epithelial and stromal compartments (37, 38) and has been observed in both compartments of the prostate as well (10, 11). We examined whether the periurethral stromal cells of control male mice similarly expressed ERα, and whether expression was altered in the stromal cells of Stk11CKO and Stk11CKO;PtenCKO mice by immunohistochemistry. In control mice (n = 7/9), strong ERα expression was observed in the ductal, glandular, and urethral epithelial cells (Fig. 3C) and in scattered stromal cells (Fig. 3D). In contrast, strong ERα expression was observed in nearly all the stromal cells in half the Stk11CKO mice (n = 4/8) (Fig. 3 I and J and Fig. S3 D and E), and in all the Stk11CKO;PtenCKO mice (Fig. 3 O and P) that were examined. Weaker or scattered expression was observed in the adjacent epithelial cells in Stk11CKO mice (n = 8/8) (Fig. 3 I and J) compared with controls (Fig. 3 C and D), suggesting the mutant stromal cells could be controlling the fate of the nearby, unmutated epithelium. Expression of desmin and α-smooth muscle actin, smooth muscle cell markers, was observed throughout the stroma of control, Stk11CKO, and Stk11CKO;PtenCKO mice (Fig. 3 E, F, K, L, Q, and R and Fig. S3 A–C and F), indicating that the affected cells were more likely a myofibroblast phenotype. In addition, the stromal cells of control and Stk11CKO;PtenCKO mice showed the presence of collagen by trichrome staining (Fig. S3B), which was more abundant in the Stk11CKO;PtenCKO mice as a result of tumorigenesis, further substantiating the likelihood of these cells being myofibroblast-like. Taken together, these data are consistent with what is observed in human BPH (39).
Disrupted Signaling in the Stk11CKO;PtenCKO Stroma.
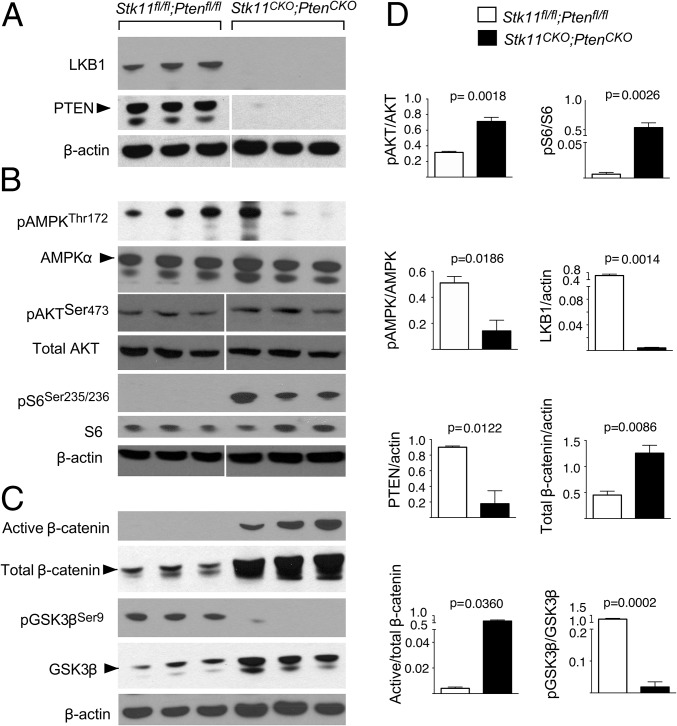
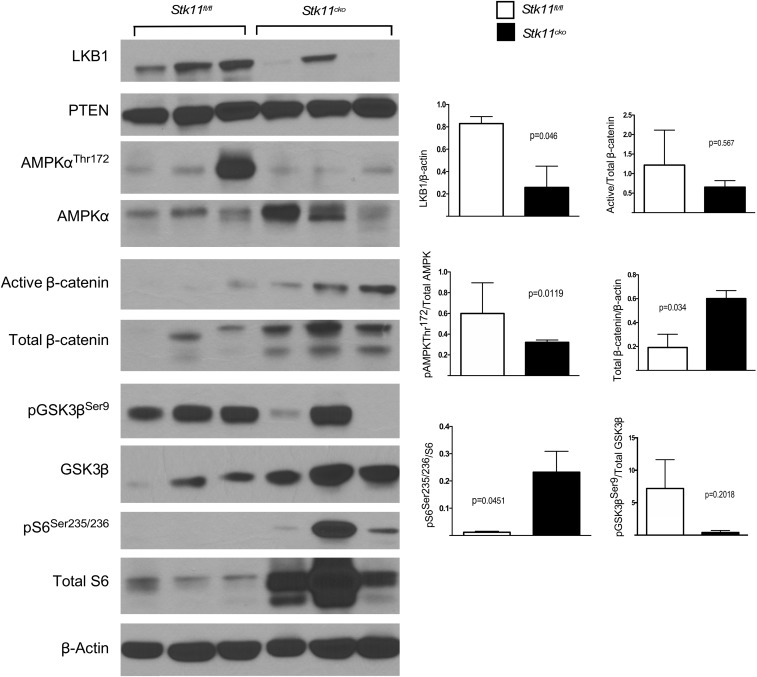
To investigate the mechanisms that could be driving tumorigenesis in the periurethral stroma, we assessed activation of known signaling cascades downstream of LKB1 and PTEN tumor suppressors. Western blot analysis confirmed gene deletion and protein reduction of both LKB1 and PTEN in Stk11CKO;PtenCKO prostatic/periurethral tissues (Fig. 4A). LKB1 mediates its tumor suppressor activity by phosphorylating AMPK at Thr172 (40). In contrast to Stk11+/+;Pten+/+ control tissues, phosphorylated AMPK was reduced in Lkb1CKO;PtenCKO mice (Fig. 4B), indicating a loss in control of the mTOR pathway. PTEN antagonizes PI3K activity by dephosphorylating PIP3 and sequestering AKT from the membrane, thus inhibiting phosphorylation and activation of the kinase (41). Levels of Ser473-phosphorylated AKT were elevated in tumors, consistent with activation of AKT (Fig. 4B). On the basis of these data, we assayed phosphorylation of S6 at Ser235/236 and saw increased levels, consistent with hyperactive mTOR signaling (Fig. 4B). Deletion of Stk11 alone affected both AMPK and S6 phosphorylation compared with control tissues, confirming the role of LKB1 in these pathways (Fig. S6).
Fig. 4.
Conditional loss of LKB1 and PTEN leads to activation of mTOR and Wnt signaling. (A) Western blots of protein lysate from the male urogenital tissues of Stk11+/+;Pten+/+ controls and Stk11CKO;PtenCKO mutants were performed in triplicate. Strong reductions in LKB1 and PTEN were observed in the double mutant. (B) Western blots show increased levels of downstream signaling partners of pAKTSer473, pS6Ser235/236, and pAMPKThr172 for PTEN and LKB1, respectively, in the double mutants. No change was observed in total AKT and total AMPK. (C) Alternative LKB1 signaling was also observed with increased total and active β-catenin, as well as reduced pGSK3βSer9 in the double mutants. Control and tumor extracts were immunoblotted with indicated antibodies. β-Actin was used as loading control. Arrows indicate the correct band. Phosphorylation is indicated by p, and corresponding residues are identified. (D) Semiquantification of the bands in A, B, and C was performed, averaged, and graphed. P values were calculated by the unpaired t test. Error bars represent SEM.
Fig. S6.
Conditional loss of LKB1 leads to activation of mTOR signaling. Western blots of protein lysate from the male urogenital tissues of Stk11fl/fl controls and Stk11CKO single mutants were isolated from n = 3 mice. LKB1 protein levels were reduced in Stk11CKO mice, whereas PTEN levels remains unchanged. Western blots show increased levels of LKB1 downstream signaling with pS6 and decreased pAMPKThr172 for Stk11CKO tissues. Loss in LKB1 signaling lead to increased total β-catenin expression levels in the single mutants. Control and mutant extracts were immunoblotted with antibodies, as detailed in the Materials and Methods, and β-actin was used as loading control. Semiquantification of the bands in was performed using Image J analysis and graphed using Prism. P values were calculated by the unpaired t test. Error bars represent SEM.
In addition to suppression of the mTOR pathway, LKB1 is also known to regulate wingless-related integration site (WNT)/β-catenin signaling, and conditional deletion of Stk11 in mouse prostrate has been reported to cause an increase in WNT signaling (17, 42, 43). Increased expression of both active and total β-catenin, the canonical downstream effector protein in the WNT signaling cascade, was observed in the Stk11CKO;PtenCKO stromal tumor (Fig. 4C). Interestingly, we observed loss of phosphorylation at Ser9 of pGSK3β (Fig. 4C) and increased total GSK3β levels (Fig. 4C) in the periurethral stroma, indicating that LKB1 and/or PTEN are important for maintaining GSK3β phosphorylation levels in these tissues. Analyses of Stk11CKO prostate tissues compared with controls suggests that LKB1 affected β-catenin and GSK3β phosphorylation and expression (Fig. S6).
Discussion
LKB1 has multiple functions in cells. It is best known as a tumor suppressor whose kinase is active during cellular metabolic stress conditions such as low ATP. Mutations of the gene (STK11) for LKB1 are responsible for PJS, an autosomal dominant disease that leaves patients at an increased risk of developing benign and malignant tumors with age (44). Patients typically present with hamartomous polyps. LKB1 would normally phosphorylate and activate AMPK family kinases in response to stress, which subsequently activates TSC2 and inhibits mTOR, the master regulator of proliferation. Thus, loss of PTEN, another tumor suppressor upstream of mTOR, would be expected to synergize with loss of LKB1 and lead to uncontrolled cellular proliferation, which is what we observe in our Stk11CKO;PtenCKO mice, as shown by accelerated tumorigenesis. LKB1 appears to also have an evolutionary conserved role in regulating cellular polarity and structure (45), which can also be important for constraining any effects of mitogens on proliferation, and possibly tumorigenesis. We have shown that mTOR activity is induced in the tumors of Stk11CKO mice (Fig. 4), which suggests that unrestrained proliferation is an immediate trigger to tumorigenesis in the MDM, but we cannot rule out an effect on cellular organization contributing to the phenotype.
The loss of LKB1 in the MDM results in a male phenotype that is characterized by the growth of periurethral stroma in mice, which is akin to BPH in humans, a disease whose etiology is essentially unknown (1). These results in mice suggest two relatively understudied areas that could be important for understanding how BPH develops in humans. First, it is clear that MD regression is not complete in males and that some of the MD mesenchyme persists postnatally and differentiates into the periurethral stroma. The implications on BPH biology of this observation are notable. MD mesenchyme in females develops into the stroma of the female reproductive tract, which is a highly estrogen-responsive tissue. In BPH, the prostatic stroma is thought to become more estrogen-responsive, which would suggest that the stroma derived from the MDM could be de-differentiating or “re-awakening” in a more primitive state (4, 5, 7). Although hypothesized for some time, we believe our results shown here provide strong evidence that this indeed might be the case.
We have also observed that β-catenin levels are elevated in the stromal tumor tissues (Fig. 4B), which is consistent with previous reports that loss of LKB1 can induce β-catenin (17, 43, 46, 47). LKB1 physically associates with GSK3β and regulates WNT signaling pathway by modulating phosphorylation of pGSK3βSer9 (43). In our mice with conditional deletion of Stk11 and Pten, we observed that loss of LKB1 led to down-regulation of pGSK3βSer9 and increased expression of both total and active forms of β-catenin. This indicates that LKB1 antagonizes the stability of β-catenin by activating GSK3β, decreasing protein levels of β-catenin, and turning off WNT target genes. Deregulation of β-catenin is associated with a number of cancers and other diseases (48). This inverse correlation highlights the multifunctional role of LKB1 and confirms its role in regulation of β-catenin and its contribution to tumorigenesis in the MDM.
Another important aspect of our study is that although we have deleted many different genes, including other tumor suppressor genes using Amhr2-Cre, we have observed this BPH-like phenotype only with conditional deletion of Stk11, the gene that is linked with PJS. In patients with PJS, the incidence of adenoma malignum of the uterine cervix (MDA), a usually rare form of cervical cancer, is estimated at between 15% and 30%, and patients with PJS account for 10% of the total cases (49). In another study of 11 MDA tumors from patients without PJS, six had mutations in STK11 with loss of heterozygosity. MDA was observed in our female mice with conditional deletion of Stk11 and Pten with 100% penetrance (18), indicating that, as with humans, Stk11 mutation renders the caudal MD highly susceptible to MDA tumorigenesis in mice. In this context, development of the periurethral stromal tumors from the caudal remnant of the MDM with Stk11 deletion in male mice makes sense and strengthens the hypothesis that dysregulation of STK11 expression, or more likely downstream signaling events, could be involved in the human caudal MDM developing into BPH. Determining whether the LKB1/AMPK signal transduction cascade is dysregulated in BPH, although challenging, will be needed to confirm this hypothesis.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1612284114/-/DCSupplemental.
References
- 1.McNeal J. Pathology of benign prostatic hyperplasia. Insight into etiology. Urol Clin North Am. 1990;17(3):477–486. [PubMed] [Google Scholar]
- 2.Sarma AV, Wei JT. Clinical practice. Benign prostatic hyperplasia and lower urinary tract symptoms. N Engl J Med. 2012;367(3):248–257. doi: 10.1056/NEJMcp1106637. [DOI] [PubMed] [Google Scholar]
- 3.Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. J Urol. 1984;132(3):474–479. doi: 10.1016/s0022-5347(17)49698-4. [DOI] [PubMed] [Google Scholar]
- 4.McNeal JE. Origin and evolution of benign prostatic enlargement. Invest Urol. 1978;15(4):340–345. [PubMed] [Google Scholar]
- 5.Cai Y. Benign prostatic hyperplasia is a reawakened process of persistent Müllerian duct mesenchyme. BJU Int. 2001;87(3):177–182. doi: 10.1111/j.1464-410x.2001.00024.x. [DOI] [PubMed] [Google Scholar]
- 6.Cunha GR, et al. The endocrinology and developmental biology of the prostate. Endocr Rev. 1987;8(3):338–362. doi: 10.1210/edrv-8-3-338. [DOI] [PubMed] [Google Scholar]
- 7.Cunha GR, Ricke WA. A historical perspective on the role of stroma in the pathogenesis of benign prostatic hyperplasia. Differentiation. 2011;82(4-5):168–172. doi: 10.1016/j.diff.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teixeira J, Maheswaran S, Donahoe PK. Müllerian inhibiting substance: An instructive developmental hormone with diagnostic and possible therapeutic applications. Endocr Rev. 2001;22(5):657–674. doi: 10.1210/edrv.22.5.0445. [DOI] [PubMed] [Google Scholar]
- 9.Cai Y. Participation of caudal müllerian mesenchyma in prostate development. J Urol. 2008;180(5):1898–1903. doi: 10.1016/j.juro.2008.07.025. [DOI] [PubMed] [Google Scholar]
- 10.Stumpf WE, Sar M. Autoradiographic localization of estrogen, androgen, progestin and glucocorticosteroid in “target tissues” and non-target tissues. In: Pasqualini JR, editor. Receptors and Mechanism of Action of Steroid Hormones. Marcel Dekker; New York: 1976. pp. 41–84. [Google Scholar]
- 11.Cooke PS, Young P, Hess RA, Cunha GR. Estrogen receptor expression in developing epididymis, efferent ductules, and other male reproductive organs. Endocrinology. 1991;128(6):2874–2879. doi: 10.1210/endo-128-6-2874. [DOI] [PubMed] [Google Scholar]
- 12.Ehara H, et al. Expression of estrogen receptor in diseased human prostate assessed by non-radioactive in situ hybridization and immunohistochemistry. Prostate. 1995;27(6):304–313. doi: 10.1002/pros.2990270603. [DOI] [PubMed] [Google Scholar]
- 13.Gingrich JR, et al. Metastatic prostate cancer in a transgenic mouse. Cancer Res. 1996;56(18):4096–4102. [PubMed] [Google Scholar]
- 14.Pourmand G, et al. Role of PTEN gene in progression of prostate cancer. Urol J. 2007;4(2):95–100. [PubMed] [Google Scholar]
- 15.Taylor BS, et al. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010;18(1):11–22. doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Backman SA, et al. Early onset of neoplasia in the prostate and skin of mice with tissue-specific deletion of Pten. Proc Natl Acad Sci USA. 2004;101(6):1725–1730. doi: 10.1073/pnas.0308217100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pearson HB, McCarthy A, Collins CM, Ashworth A, Clarke AR. Lkb1 deficiency causes prostate neoplasia in the mouse. Cancer Res. 2008;68(7):2223–2232. doi: 10.1158/0008-5472.CAN-07-5169. [DOI] [PubMed] [Google Scholar]
- 18.Tanwar PS, et al. Stromal liver kinase B1 [STK11] signaling loss induces oviductal adenomas and endometrial cancer by activating mammalian Target of Rapamycin Complex 1. PLoS Genet. 2012;8(8):e1002906. doi: 10.1371/journal.pgen.1002906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanwar PS, et al. Loss of LKB1 and PTEN tumor suppressor genes in the ovarian surface epithelium induces papillary serous ovarian cancer. Carcinogenesis. 2014;35(3):546–553. doi: 10.1093/carcin/bgt357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jamin SP, Arango NA, Mishina Y, Hanks MC, Behringer RR. Requirement of Bmpr1a for Müllerian duct regression during male sexual development. Nat Genet. 2002;32(3):408–410. doi: 10.1038/ng1003. [DOI] [PubMed] [Google Scholar]
- 21.Bardeesy N, et al. Loss of the Lkb1 tumour suppressor provokes intestinal polyposis but resistance to transformation. Nature. 2002;419(6903):162–167. doi: 10.1038/nature01045. [DOI] [PubMed] [Google Scholar]
- 22.Lesche R, et al. Cre/loxP-mediated inactivation of the murine Pten tumor suppressor gene. Genesis. 2002;32(2):148–149. doi: 10.1002/gene.10036. [DOI] [PubMed] [Google Scholar]
- 23.Arango NA, et al. A mesenchymal perspective of Müllerian duct differentiation and regression in Amhr2-lacZ mice. Mol Reprod Dev. 2008;75(7):1154–1162. doi: 10.1002/mrd.20858. [DOI] [PubMed] [Google Scholar]
- 24.Tanwar PS, Zhang L, Roberts DJ, Teixeira JM. Stromal deletion of the APC tumor suppressor in mice triggers development of endometrial cancer. Cancer Res. 2011;71(5):1584–1596. doi: 10.1158/0008-5472.CAN-10-3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanwar PS, et al. Mammalian target of rapamycin is a therapeutic target for murine ovarian endometrioid adenocarcinomas with dysregulated Wnt/β-catenin and PTEN. PLoS One. 2011;6(6):e20715. doi: 10.1371/journal.pone.0020715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanaka Y, et al. Deletion of tuberous sclerosis 1 in somatic cells of the murine reproductive tract causes female infertility. Endocrinology. 2012;153(1):404–416. doi: 10.1210/en.2011-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shapiro E, et al. The prostatic utricle is not a Mullerian duct remnant: Immunohistochemical evidence for a distinct urogenital sinus origin. J Urol. 2004;172:1753–1756. doi: 10.1097/01.ju.0000140267.46772.7d. [DOI] [PubMed] [Google Scholar]
- 28.Mishina Y, et al. Genetic analysis of the Müllerian-inhibiting substance signal transduction pathway in mammalian sexual differentiation. Genes Dev. 1996;10(20):2577–2587. doi: 10.1101/gad.10.20.2577. [DOI] [PubMed] [Google Scholar]
- 29.Baarends WM, et al. A novel member of the transmembrane serine/threonine kinase receptor family is specifically expressed in the gonads and in mesenchymal cells adjacent to the müllerian duct. Development. 1994;120(1):189–197. doi: 10.1242/dev.120.1.189. [DOI] [PubMed] [Google Scholar]
- 30.di Clemente N, et al. Cloning, expression, and alternative splicing of the receptor for anti-Müllerian hormone. Mol Endocrinol. 1994;8(8):1006–1020. doi: 10.1210/mend.8.8.7997230. [DOI] [PubMed] [Google Scholar]
- 31.Arango NA, et al. Conditional deletion of beta-catenin in the mesenchyme of the developing mouse uterus results in a switch to adipogenesis in the myometrium. Dev Biol. 2005;288(1):276–283. doi: 10.1016/j.ydbio.2005.09.045. [DOI] [PubMed] [Google Scholar]
- 32.Teixeira J, et al. Developmental expression of a candidate müllerian inhibiting substance type II receptor. Endocrinology. 1996;137(1):160–165. doi: 10.1210/endo.137.1.8536608. [DOI] [PubMed] [Google Scholar]
- 33.Tanwar PS, et al. Constitutive activation of Beta-catenin in uterine stroma and smooth muscle leads to the development of mesenchymal tumors in mice. Biol Reprod. 2009;81(3):545–552. doi: 10.1095/biolreprod.108.075648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brechka H, McAuley EM, Lamperis SM, Paner GP, Vander Griend DJ. Contribution of caudal Müllerian duct mesenchyme to prostate development. Stem Cells Dev. 2016;25(22):1733–1741. doi: 10.1089/scd.2016.0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanaka M, et al. Nkx3.1, a murine homolog of Drosophila bagpipe, regulates epithelial ductal branching and proliferation of the prostate and palatine glands. Dev Dyn. 2000;219(2):248–260. doi: 10.1002/1097-0177(2000)9999:9999<::aid-dvdy1054>3.3.co;2-5. [DOI] [PubMed] [Google Scholar]
- 36.Shappell SB, et al. Prostate pathology of genetically engineered mice: Definitions and classification. The consensus report from the Bar Harbor meeting of the Mouse Models of Human Cancer Consortium Prostate Pathology Committee. Cancer Res. 2004;64(6):2270–2305. doi: 10.1158/0008-5472.can-03-0946. [DOI] [PubMed] [Google Scholar]
- 37.Yamashita S, Newbold RR, McLachlan JA, Korach KS. Developmental pattern of estrogen receptor expression in female mouse genital tracts. Endocrinology. 1989;125(6):2888–2896. doi: 10.1210/endo-125-6-2888. [DOI] [PubMed] [Google Scholar]
- 38.Gould SF, Shannon JM, Cunha GR. The autoradiographic demonstration of estrogen binding in normal human cervix and vagina during the menstrual cycle, pregnancy, and the menopause. Am J Anat. 1983;168(2):229–238. doi: 10.1002/aja.1001680209. [DOI] [PubMed] [Google Scholar]
- 39.Nicholson TM, Ricke WA. Androgens and estrogens in benign prostatic hyperplasia: Past, present and future. Differentiation. 2011;82(4-5):184–199. doi: 10.1016/j.diff.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shaw RJ, et al. The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell. 2004;6(1):91–99. doi: 10.1016/j.ccr.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 41.Carracedo A, Pandolfi PP. The PTEN-PI3K pathway: Of feedbacks and cross-talks. Oncogene. 2008;27(41):5527–5541. doi: 10.1038/onc.2008.247. [DOI] [PubMed] [Google Scholar]
- 42.Ollila S, Mäkelä TP. The tumor suppressor kinase LKB1: Lessons from mouse models. J Mol Cell Biol. 2011;3(6):330–340. doi: 10.1093/jmcb/mjr016. [DOI] [PubMed] [Google Scholar]
- 43.Ossipova O, Bardeesy N, DePinho RA, Green JB. LKB1 (XEEK1) regulates Wnt signalling in vertebrate development. Nat Cell Biol. 2003;5(10):889–894. doi: 10.1038/ncb1048. [DOI] [PubMed] [Google Scholar]
- 44.Hearle N, et al. Frequency and spectrum of cancers in the Peutz-Jeghers syndrome. Clin Cancer Res. 2006;12(10):3209–3215. doi: 10.1158/1078-0432.CCR-06-0083. [DOI] [PubMed] [Google Scholar]
- 45.Hezel AF, Bardeesy N. LKB1; linking cell structure and tumor suppression. Oncogene. 2008;27(55):6908–6919. doi: 10.1038/onc.2008.342. [DOI] [PubMed] [Google Scholar]
- 46.Liu K, et al. The tumor suppressor LKB1 antagonizes WNT signaling pathway through modulating GSK3β activity in cell growth of esophageal carcinoma. Tumour Biol. 2014;35(2):995–1002. doi: 10.1007/s13277-013-1133-0. [DOI] [PubMed] [Google Scholar]
- 47.Lin-Marq N, Borel C, Antonarakis SE. Peutz-Jeghers LKB1 mutants fail to activate GSK-3beta, preventing it from inhibiting Wnt signaling. Mol Genet Genomics. 2005;273(2):184–196. doi: 10.1007/s00438-005-1124-y. [DOI] [PubMed] [Google Scholar]
- 48.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17(1):9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Banno K, et al. Hereditary gynecological tumors associated with Peutz-Jeghers syndrome (Review) Oncol Lett. 2013;6(5):1184–1188. doi: 10.3892/ol.2013.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]