Significance
After breaks in animal epidermal barriers, repair genes are activated in the cells adjacent to wound sites that help regenerate the barrier. The fruit fly Drosophila melanogaster is a favorable genetic system to find molecular detection systems that sense wounds and activate repair genes. In this paper, we find that the Toll signaling pathway, including the extracellular ligand Spätzle, the Toll receptor, and the Dif transcription factor, form a detection system to sense broken epidermis and then activate regeneration genes. The Toll pathway thus is involved not only in the activation of genes involved in fighting microbial invasion after epidermal breaks, but also in the activation of genes that regenerate epidermal barrier function.
Keywords: epidermal Toll, Spätzle, Drosophila, wound repair, NF-κB
Abstract
The epidermis serves as a protective barrier in animals. After epidermal injury, barrier repair requires activation of many wound response genes in epidermal cells surrounding wound sites. Two such genes in Drosophila encode the enzymes dopa decarboxylase (Ddc) and tyrosine hydroxylase (ple). In this paper we explore the involvement of the Toll/NF-κB pathway in the localized activation of wound repair genes around epidermal breaks. Robust activation of wound-induced transcription from ple and Ddc requires Toll pathway components ranging from the extracellular ligand Spätzle to the Dif transcription factor. Epistasis experiments indicate a requirement for Spätzle ligand downstream of hydrogen peroxide and protease function, both of which are known activators of wound-induced transcription. The localized activation of Toll a few cell diameters from wound edges is reminiscent of local activation of Toll in early embryonic ventral hypoderm, consistent with the hypothesis that the dorsal–ventral patterning function of Toll arose from the evolutionary cooption of a morphogen-responsive function in wound repair. Furthermore, the combinatorial activity of Toll and other signaling pathways in activating epidermal barrier repair genes can help explain why developmental activation of the Toll, ERK, or JNK pathways alone fail to activate wound repair loci.
Animal epidermal barriers can be breached by mechanical, chemical, and microbial damage. To repair wounds, many complex processes occur in tandem and sequentially. These processes include clotting to quickly seal the breach, a localized inflammatory response, and migration and proliferation of epidermal cells to regenerate a coherent epidermal layer. Finally, the epidermal layer regenerates the impermeable barrier that keeps bodily fluids in, and microbes out.
In Drosophila and other arthropods, the epidermis consists of a single cell layer that secretes a cuticular barrier at the apical surface. Mammalian epidermis typically consists of several cell layers, the outermost being an impermeable layer consisting of dead cornified epidermal cells in a sea of extracellular lipids. Despite the differences in epidermal barrier structure in different animals, there are evolutionarily conserved genetic pathways that control the development and regeneration of epidermal barriers in mammals, arthropods, and nematodes (1–5). Among many examples, the Jun N-terminal kinase (JNK) pathway regulates reepithelialization in diverse animal phyla (6–10), whereas the Grainy head (Grh) family of transcription factors, under the control of wound-activated receptor tyrosine kinases, activate effector genes that regenerate epidermal barriers in both Drosophila and mammals after wounding (1, 11–15). In Drosophila, two of the induced effector genes under the control of Grh encode the dopa decarboxylase (Ddc) and tyrosine hydroxylase (ple) enzymes, which produce reactive quinones that cross-link cuticular proteins to regenerate a barrier. Mutations in either Ddc or ple do not visibly interfere with the wound healing process, but loss of Grh, which regulates many different wound repair genes (11, 12, 15), leads to poorly healed puncture wounds (12).
The Toll/NF-κB pathway was classically defined by its role in dorsoventral patterning in early Drosophila embryos, and by its role in activating innate immune responses to infection in both mammals and Drosophila (16). In Drosophila, the Toll signaling pathway is induced by microbial or developmental signals that activate extracellular serine protease cascades that cleave pro-Spätzle to Spätzle (Spz), a ligand in the neurotrophin family. Activated Spz binds to Toll receptor, which starts a cytoplasmic signaling cascade ultimately leading to the induction of Toll target genes by the NF-κB transcription factors Dorsal and Dif (17).
Past research has suggested that the Toll/NF-κB pathway has biological roles in the Drosophila larval epidermis. Dorsal and Dif are required in larval epidermal cells to prevent melanized lesions from forming at random locations in the cuticle, suggesting that the dorsal and Dif genes are required for normal differentiation of an epidermal barrier, and/or for the normal repair of sporadic wounds that arise in the larval cuticle (18). In the Drosophila embryonic epidermis, Toll, Dif, and dorsal were also found necessary for the cytoskeletal processes that led to sealing of epidermis after laser wounding in late stage embryos (19). Surprisingly, in this context, spz function was not required (19). Other studies in late stage embryos revealed that clean puncture wounding results in the transcriptional activation of the dorsal and spz genes in epidermal cells adjacent to wounds (20), suggesting their involvement in epidermal wound healing, perhaps in an autoregulatory circuit involved in wound repair.
Our previous research showed that serine protease function is required and sufficient to activate certain epidermal barrier repair genes after puncture wounding of late stage embryos (20). The activation of epidermal barrier repair genes has been shown to require different combinations of transcription factors (11, 12); however, the entire range of signaling pathways genetically required for proper barrier regeneration is still unknown. We therefore set out to explore the possibility that the protease-activated Toll/NF-κB pathway was required for the activation of epidermal barrier repair genes after wounding.
Results
Extracellular Regulation of Epidermal Wound Transcription by the Toll Ligand.
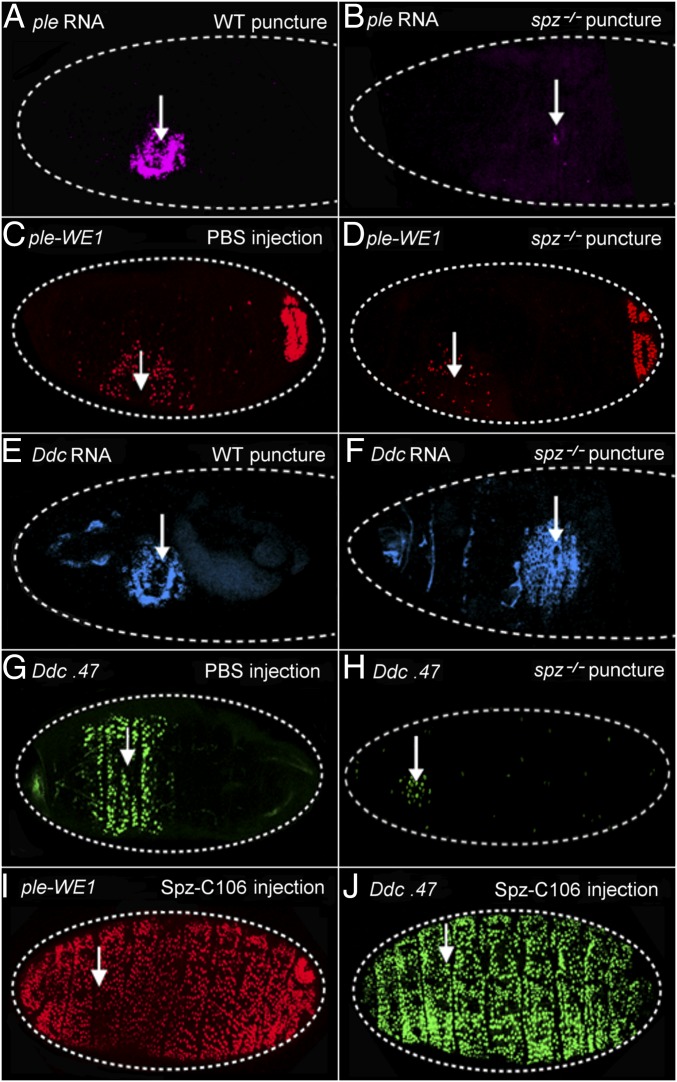
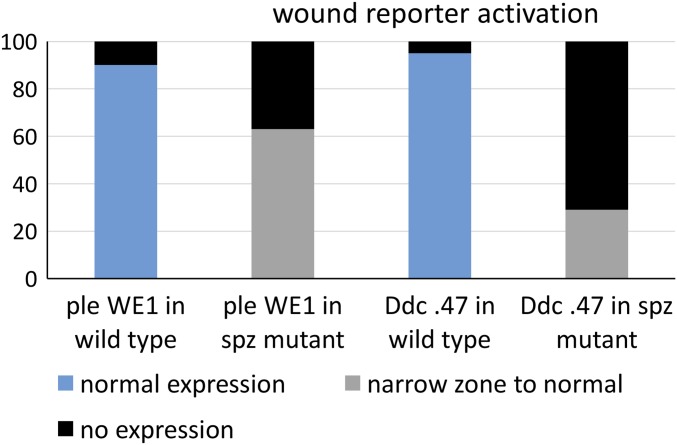
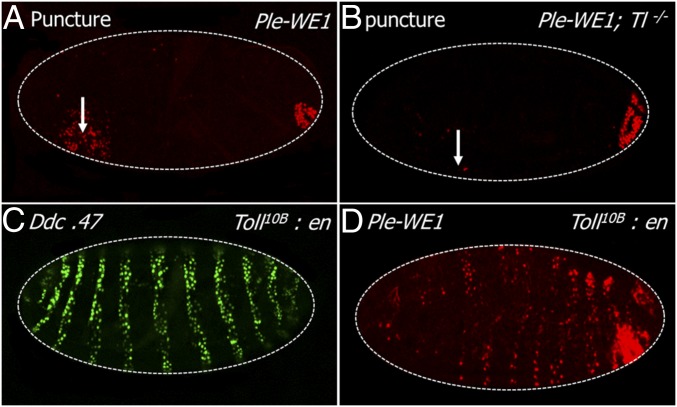
We first asked whether the Toll ligand Spätzle (Spz) was required for the transcriptional activation of ple and Ddc wound response genes. We puncture wounded wild-type and spz null mutant embryos, fixed the embryos after an hour of recovery, and then probed for ple and Ddc RNA transcripts. At 1 h after wounding, wild-type late stage embryos showed normal activation of ple mRNA in a zone of 5- to 10-cell diameters around the sites of injury (Fig. 1A). In wounded spz mutants, the zone of ple transcript accumulation around the wounds was dramatically reduced in embryos, although there were always a few cells that showed ple transcript signal (Fig. 1B). We also tested the function of the ple-WE1 enhancer that drives the expression of the fluorescent protein dsRed in a wound-dependent fashion (11). Note that the reporter expression is not detected until 3–5 h after wounding, because the dsRed protein has to accumulate and then oxidatively mature to the fluorescent state. In wild-type embryos, we detected ple wound reporter expression in a normal zone of 5- to 10-cell diameters from the wound in 90% of embryos (Fig. 1C), whereas 10% showed no detectable expression (n = 450 embryos; Materials and Methods for an explanation of the variability in these assays from experiment to experiment). In contrast, the ple wound reporter in spz mutants was not activated in 43% of wounded embryos (5–10 cells), whereas the other 57% had ple reporter expression that ranged from weak and scattered (Fig. 1D) to normal levels (n = 120 embryos, Fig. S1).
Fig. 1.
Spätzle is required and sufficient for wound gene activation. (A) RNA FISH with a ple coding region probe on a wild-type stage 16 embryo 1 h after puncture wounding. ple transcripts are observed in a zone of 5–10 cells around the wound site. (B) The same ple probe was hybridized and visualized on a stage 16 spz mutant embryo. (C) This embryo, punctured and injected with a buffered saline solution (PBS) at stage 16, and imaged 5 h after wounding, carries the wound reporter ple-WE1, consisting of a small DNA enhancer from the ple upstream region fused to a gene for the fluorescent protein dsRed, which is fused to a nuclear localization signal (NLS) (11). (D) ple-WE1 expression in spz mutant embryos 5 h postwounding. (E) FISH using a Ddc coding region probe shows Ddc transcripts are activated in a zone of 5–10 epidermal cells is surrounding the wound site at 1 h postwounding. Developmental transcription of Ddc in differentiating cells of the head skeleton (Left, anterior end of embryo in E can also be seen). (F) Wound-induced Ddc RNA transcripts are detected in a normal zone of 5–10 epidermal cells around wounds in a spz mutant embryo 1 h after wounding at stage 16. (G) The Ddc 0.47 GFP wound reporter (12) expression pattern 5 h after wounding of a stage 16 wild type embryo. (H) Ddc 0.47 expression 5 h postwounding in stage 16 spz mutant embryos. (I and J) Wounding plus injection of stage 16 embryos with the constitutively active ligand Spz-C106 results in a global activation of the ple-WE1 and the Ddc.47 wound reporters in epidermal cells 5 h postwounding. White arrows mark wound sites. In every panel, dashed white lines outline the embryos, and anterior is to the Left. The ple-WE1 wound reporter exhibits wound-independent activation in the developing anal pad in late stage embryos, as seen in the posterior (Right) end. The individual dots of fluorescent signal in the image are nuclei of cells expressing the fluorescent protein.
Fig. S1.
The y axis of this graph shows the percentage of embryos in a wounded population that we scored with different levels of wound gene reporter activation after puncture wounding at embryonic stage 16. The x axis shows different categories based on the reporter construct (carrying wound DNA enhancers from the ple or Ddc loci fused to fluorescent proteins) carried by the embryo population being scored, and the genotype of the embryonic population (either wild type or spz mutant). See the text for a full description of how expression levels were scored and for more details on the wound reporter constructs.
When testing Ddc transcript induction in spz mutants, we did not detect a consistent difference from wild-type embryos 1 h after wounding at stage 16 (compare Fig. 1 E and F). We could not reliably test wound-induced transcription by in situ hybridization at later time points because of the increasing impermeability of the developing cuticle in late embryos. However, when we tested the Ddc 0.47 wound reporter (a 0.47-kb wound enhancer from Ddc driving GFP protein expression) in living spz mutants 5 h after wounding, we did see an effect from loss of spz function. In wild type, 95% of embryos showed normal Ddc wound reporter activation 5- to 10-cell diameters from the wound site (Fig. 1G). In contrast, 71% of spz mutants showed no Ddc-GFP expression after wounding, and 29% showed expression that ranged from a narrow zone (1–3 cells) around wound sites (Fig. 1H) to expression that was indistinguishable from wild type (Fig. S1) (n = 200 embryos).
These experiments reveal a quantitative requirement for Spz in the activation of reporters driven by ple and Ddc wound-dependent enhancer elements. The above results also suggest that either the temporal requirement for Spz on the maintenance of ple and Ddc wound-induced transcription are different or that the Ddc 0.47 wound enhancer is more sensitive to the loss of Spz function than the endogenous Ddc transcription unit in its normal chromosomal location.
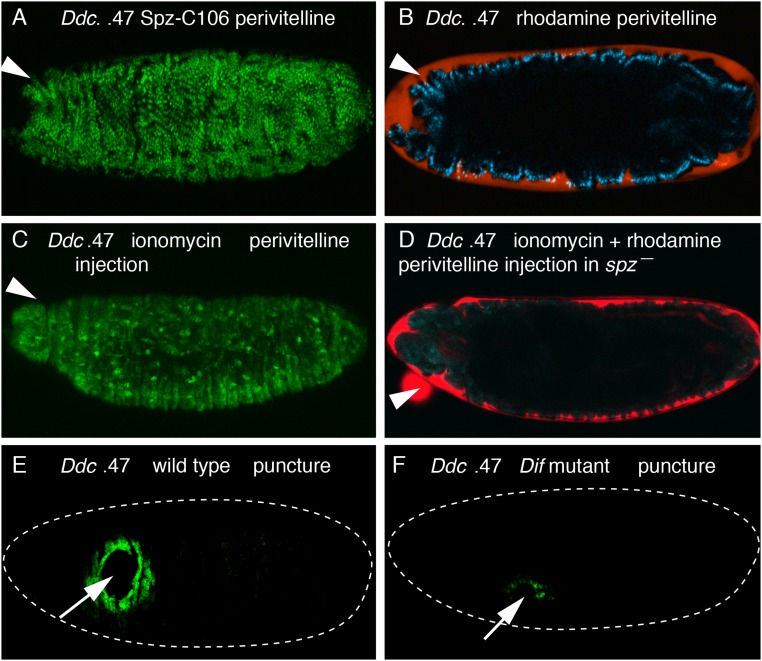
We next tested whether active, cleaved Spz protein was sufficient to induce the activation of the ple and Ddc fluorescent wound reporters. The Easter serine protease can cleave Spz in vitro, yielding a C-terminal fragment of 106 amino acids (Spz-C106) that induces Toll signaling (21). We filled microcapillary needles with Spz-C106 in PBS and injected embryos carrying the ple or Ddc wound reporters. Control embryos, which were injected with PBS alone, showed normal localized activation of the reporters around wound sites (Fig. 1 C and G). However, embryos injected with the Spz-C106 ligand showed a global activation of the Ddc and ple wound reporters in epidermal cells (Fig. 1 I and J). This activation of wound reporters did not require a breach of the epidermis, as injection of Spz-C106 into the perivitelline space on the apical side of epidermal cells also showed global activation of the Ddc and ple wound reporters (Fig. S2 B and D for dye entry controls). These results indicate that Spz, a signal apparently induced by tissue damage, is both quantitatively required and sufficient (at least when ectopically applied at high levels) to activate the ple-WE1 and Ddc 0.47 wound gene activation elements.
Fig. S2.
Ddc wound reporter activation by Spz-C106 and ionomycin, and Ddc wound transcription reduction in Dif mutant embryos. (A) Wild-type embryo carrying the Ddc 0.47 wound reporter was injected with Spz-C106 and rhodamine–dextran in the perivitelline space at stage 16, and imaged 5 h later. Global activation of the fluorescent wound reporter protein is seen in epidermal cells. Each green dot is an epidermal nucleus that has accumulated the NLS-fused GFP protein. (B) The same embryo in A was imaged in the rhodamine channel and shows that the red flourescent dye has remained in the perivitelline space atop the apical side of the epidermal cells and has not penetrated into the body cavity. (C) Wild-type embryo carrying the Ddc 0.47 wound reporter was injected with ionomycin in the perivitelline space at stage 16 and imaged 5 h later. Global activation of the fluorescent wound reporter protein is seen in epidermal cells. (D) spz mutant embryo carrying the Ddc 0.47 wound reporter was injected with ionomycin and rhodamine–dextran in the perivitelline space at stage 16 and imaged 5 h later. The signal in the green GFP channel shows no activation of the Ddc wound reporter in the ionomycin-treated spz mutant epidermal cells. The overlaid red signal for rhodamine (red) shows that the red fluorescent dye has remained in the perivitelline space atop the apical side of the epidermal cells and has not penetrated into the body cavity. (E) RNA FISH with a Ddc coding region probe on a wild-type stage 16 embryo 1 h after puncture wounding. Wound induction of Ddc RNA is detected in a normal zone of 5–10 cells distant from the wound edge. (F) The same Ddc probe was hybridized and detected on a Dif mutant embryo that was 1 h postwounding at the time of fixation. Only a few cells have accumulated detectable amounts of Ddc transcripts around wound sites in the spz mutant epidermal cells. See Fig. S3 for quantitation of the effect of Dif mutation on Ddc wound-induced transcription. White arrows mark puncture wound sites, and white arrowheads mark the sites for perivitelline injections. Dashed white lines outline embryos. Anterior is always Left.
Spätzle Acts Downstream of the Wound Gene Activators Hydrogen Peroxide, Trypsin, and Calcium Influx.
Some diffusible wound signals are evolutionarily conserved between vertebrates and Drosophila. For example, in both zebrafish and Drosophila the activation of Dual oxidase (Duox) upon wounding establishes a hydrogen peroxide (H2O2) gradient that attracts blood cells to wound sites (22–24). In Drosophila the Duox gene is also required for activation of wound response genes in epidermal cells (25). Our previous studies have shown that Duox and hydrogen peroxide act upstream of serine protease(s) for the wound-dependent activation of the ple and Ddc genes (26).
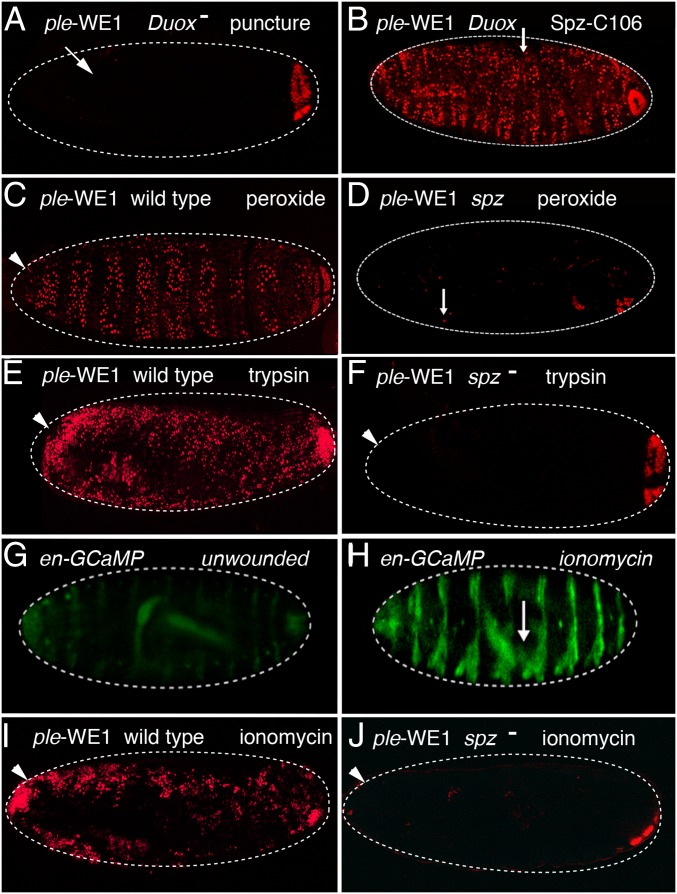
To determine the hierarchical relationships of spz, serine protease function, and hydrogen peroxide in the activation of epidermal wound response genes, we performed a series of epistasis experiments. After puncture wounding of Duox mutant embryos, ple-WE1 wound reporter expression is either barely or not detected (Fig. 2A) (25). However, all Duox mutants that were punctured and injected with the Spz-C106 ligand (either into the body cavity or the perivitelline space) showed activation of the ple wound reporter in all or nearly all epidermal cells (Fig. 2B).
Fig. 2.
Spätzle-mediated expression of ple-WE1 wound reporter is downstream of the wound gene activators: hydrogen peroxide, trypsin, and ionomycin. (A) In mutants for the Dual oxidase (Duox) gene, puncture wounding of stage 16 embryos does not induce the ple-WE1 wound reporter gene. (B) The Spz-C106 injected into the body cavity induces global activation of the ple-WE1 reporter in Duox mutants. (C) Hydrogen peroxide injection into the perivitelline space on the apical side of unwounded epidermal cells induces ple-WE1 reporter expression. (D) Injection of peroxide into the perivitelline space or the body cavity (as shown here) of spz mutant embryos does not induce ple-WE1 reporter expression. (E) Injection of trypsin into the perivitelline space of wild-type embryos induces global activation of the ple-WE1 wound reporter. (F) Injection of trypsin into the perivitelline space of spz mutants does not activate the ple-WE1 wound reporter. (G) Image of an embryo showing the background level of fluorescent signal provided by a UAS promoter-GCaMP6 calcium indicator protein driven by the engrailed-GAL4 driver in unwounded embryos. The engrailed-GAL4 driver provides striped expression of GCaMP6 under UAS promoter control. (H) Injection of the calcium ionophore ionomycin into the body cavity causes an intracellular calcium surge in epidermal cells, as shown by fluorescent activation of the GCaMP calcium indicator in its striped pattern of expression. Puncture wounding of stage 16 embryos without ionomycin causes localized intracellular calcium surges in epidermal cells in a zone of 5- to 10-cell diameters from the wound edge, as seen in ref. 24. (I) Perivitelline injection of ionomycin in wild-type embryos induces widespread activation of the ple-WE1 wound reporter in epidermal cells. Perivitelline injection of ionomycin also induces widespread activation of the Ddc 0.47 wound reporter in wild-type embryos (Fig. S2A). (J) Perivitelline injection of a stage 16 spz mutant embryo carrying the ple-WE1 wound reporter does not result in induction of the reporter gene. White arrows mark puncture wound sites, and white arrowheads mark the sites for perivitelline injections. Dashed white lines outline embryos. Anterior is always Left. The ple-WE1 wound reporter exhibits wound-independent activation in the developing anal pad in late stage embryos, as seen in the posterior (Right). The individual dots of fluorescent signal in the image are nuclei of cells expressing the fluorescent reporter protein.
Wild-type embryos injected with a hydrogen peroxide solution into the body cavity or perivitelline space showed activation of the ple wound reporter in all or nearly all epidermal cells (Fig. 2C) (25). In contrast, all spz mutant embryos that were punctured and injected with a hydrogen peroxide solution into the perivitelline space showed extremely weak ple reporter expression either locally or globally in epidermal cells (Fig. 2D). These results suggest that spz functions downstream of hydrogen peroxide within the epidermal wound response pathway.
Previous developmental and innate immune studies have shown that pro-Spz has to be cleaved by extracellular proteases before it can bind and activate Toll receptor. We have not identified the endogenous protease that activates pro-Spz in the context of wound gene activation, so we tested the epistatic relationship of protease activation and spz by treating spz mutant embryos with a concentration of trypsin in the perivitelline space that is sufficient to activate wound repair genes without breaching the epidermal barrier (20).
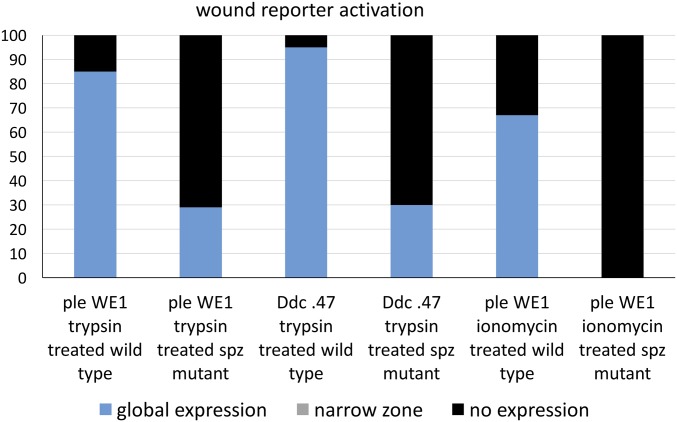
In wild-type embryos, trypsin treatment globally activated the ple wound reporter 85% of the time (Fig. 2E), with 15% showing low or no reporter expression (n = 113). In contrast, only 29% of the spz homozygous mutants treated with trypsin globally activated the ple wound reporter, whereas 71% of the mutants (n = 48) showed no ple wound reporter expression (Fig. 2F and Fig. S3). For the Ddc wound reporter activation treated with trypsin, 95% of wild-type embryos showed global activation (n = 200), whereas 70% of spz mutants showed no activation of the Ddc reporter, (n = 60) (Fig. S3). These results suggest that trypsin activates barrier repair genes either by cleaving pro-Spz directly or by cleaving another proprotease upstream of Spz activation. Previous studies have shown that calcium ion influx occurs rapidly around laser wound sites and triggers a Duox-dependent local inflammatory response (24). As seen in Razzell et al. (24), we also find a rapid calcium ion intracellular “flash” around puncture wound sites, and in addition observe that injection of ionomycin (a calcium ionophore) into either the body cavity or the pervitelline space triggers widespread calcium influx into epidermal cells, activating GCaMP6 driven by an engrailed promoter that produces striped expression in epidermal cells (Fig. 2 G and H). Ionomycin injection into the perivitelline space onto the apical side of epidermal cells induces ple wound reporter activation broadly in 67% of wild-type embryos (n = 40) (Fig. 2I and Fig. S3). Neither local nor extended ple wound reporter activation was observed in any of the spz mutant embryos treated with perivitelline injections of ionomycin (n = 40) (Fig. 2J). The Ddc wound reporter was also activated by perivitelline injection of ionomycin in wild-type embryos, and ionomycin did not activate the Ddc reporter in spz mutants (Figs. S2 C and D and S3). These results are consistent with calcium ion influx acting upstream of the Toll pathway in the activation of wound transcription in the epidermis.
Fig. S3.
The y axis of this graph shows the percentage of embryos in a wounded population that we scored with different levels of wound gene reporter activation after treatment at embryonic stage 16 with the wound-gene–inducing enzyme trypsin or the calcium ionophore ionomycin. The x axis shows different categories based on the reporter construct (carrying wound DNA enhancers from the ple or Ddc loci fused to fluorescent proteins) carried by the embryo population being scored and the genotype of the embryonic population (either wild type or spz mutant). See the text for a full description of how expression levels were scored and for more details about the wound reporter constructs.
The Toll Receptor Regulates Epidermal Wound Response Gene Activation.
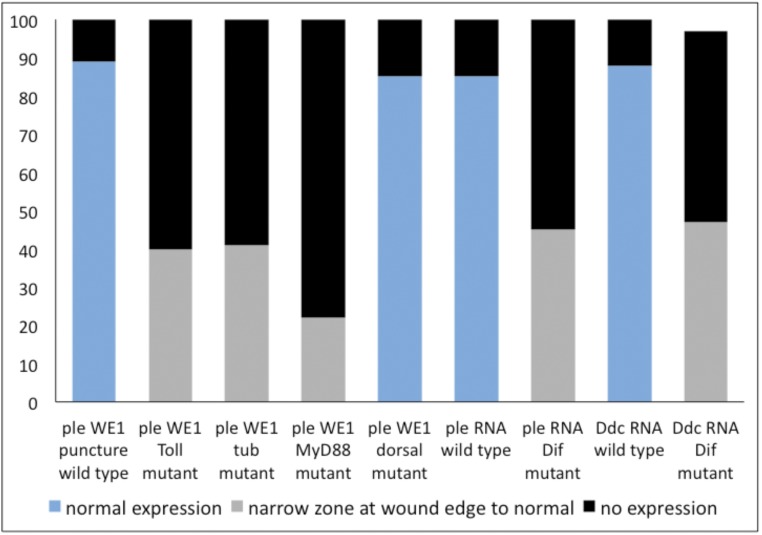
Having found that spz function is required to activate wound-induced gene expression, we next tested whether Toll receptor function was required to transduce the spz signal in epidermal wound gene activation. In wild-type embryos, 89% activated the ple-WE1 reporter around wound sites (n = 260) (Fig. 3A). There was no detectable reporter expression in 60% of the Toll mutant embryos, and in the remaining 40%, ple reporter expression ranged from a few cells around a wound site (Fig. 3B) to a normal amount of wound reporter expression (n = 52, Fig. S4). This result suggests that the Toll receptor is required for robust transcriptional activation of the ple gene during the barrier repair process. However, the fact that elimination of Toll function does not block all Ddc and ple reporter expression is consistent with a combinatorial and quantitative contribution in the activation of these barrier regeneration genes.
Fig. 3.
Toll receptor is required and sufficient for substantial epidermal wound response. (A) Localized ple-WE1 reporter activation 5 h after puncture wounding a stage 16 wild-type embryo. (B) A Toll mutant embryo showing strongly reduced expression of ple-WE1 reporter 5 h after wounding this stage 16 embryo. Toll loss of function does not affect the developmental expression of the ple reporter gene in the developing anal pads (Right end). (C and D) Ectopic overexpression of the constitutively active Toll10B receptor under the striped pattern provided by an engrailed-GAL4 driver (Toll10B:en) is sufficient to activate the Ddc 0.47 and ple-WE1 wound reporters in unwounded stage 16 embryos. White arrows mark wound sites. White dashed lines outline embryos. Anterior is to the Left in all panels. The ple-WE1 wound reporter exhibits wound-independent activation in the developing anal pad in late stage embryos, as seen in the posterior (Right). The individual dots of fluorescent signal in the image are nuclei of cells expressing the fluorescent reporter protein.
Fig. S4.
The y axis of this graph shows the percentage of embryos in a wounded population that we scored with different levels of wound gene reporter activation (or wound gene transcriptional activation) after puncture wounding embryonic stage 16. The x axis shows different categories based on the reporter construct (carrying wound DNA enhancers from the ple or Ddc loci fused to fluorescent proteins) or the gene being assayed for wound-induced transcription and by the genotype of the embryonic population (either wild type or mutant for the Toll, tube, MyD88, dorsal, or Dif genes). See the text for a full description of how expression levels were scored and for more details about the wound reporter constructs and detection of wound gene transcription.
We next tested whether Toll activity was sufficient for ple and Ddc wound reporter activation. Toll receptor is normally expressed in the late embryonic epidermis at modest levels (27), and in unwounded zygotic mutants carrying the Toll10b mutation under the control of its endogenous promoter, we did not detect global activation of wound reporter expression in unwounded embryos. The Toll10b allele encodes a mutant version of Toll protein that activates the pathway without input from the Spz ligand (28). High-level expression of Toll10b protein driven by the engrailed-GAL4 driver resulted in the activation of both the ple and Ddc wound reporters in epidermal stripes correlating with the localized activity of the engrailed driver (Fig. 3 C and D). This result indicates constitutively activated Toll is sufficient to activate both of these wound response genes when expressed at sufficiently high levels. Taking into account all of the above, we conclude Toll signaling has a quantitative effect on the activation of wound response genes and must require other signaling pathways to robustly activate wound response gene expression.
Intracellular Components of the Toll Signaling Pathway Regulate ple Wound Gene Transcription.
To further investigate innate immune regulation of epidermal wound gene activation, we next focused on the analysis of Toll/NF-κB pathway proteins acting downstream of the Toll receptor. To determine their effect on ple-WE1 wound reporter, we wounded embryos homozygous mutant for null mutations in either tube or Myd88, both of which encode intracellular adaptor proteins (29). We also wounded embryos in mutants for dorsal (dl) and Dif, which encode NF-κB family transcription factors.
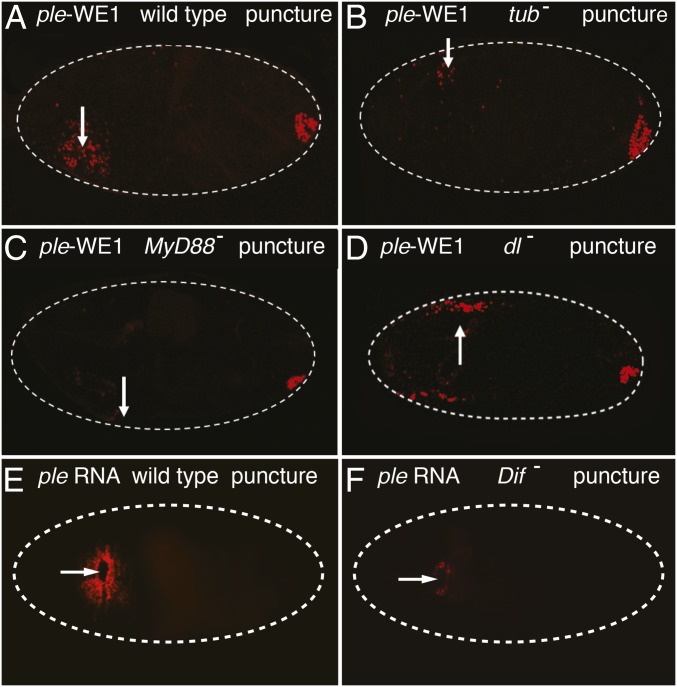
In the wounded controls, 89% of wild-type embryos (n = 260) had normal ple reporter expression around the wound, and 11% had no detectable reporter activity (Fig. 4A). In wounded mutants for tube (n = 700), 61% had no activation of the ple wound reporter, and 39% (n = 700) had ple reporter expression that ranged from a few cells near the wound edge, as seen in Fig. 4B, to normal expression (Fig. S4). In wounded MyD88 mutants, 78% had no ple wound reporter activation (Fig. 4C), whereas 22% activated ple reporter in a zone from a few cells to a normal range (n = 270, Fig. S4). Wounded embryos that were mutant for dorsal had ple reporter activity around wound sites that was indistinguishable from wounded controls (85% positive, n = 200) (Fig. 4D and Fig. S4). In Dif mutants, we tested the wound induction of Ddc and ple transcripts. In the wild-type controls, 85% (n = 118) had normal wound-induced transcription of ple 5- to 10-cell diameters from the wound edge (Fig. 4E). In Dif mutant embryos, 55% had no detectable ple transcripts around wound sites, whereas 45% showed expression ranging from one to two rows of ple gene activation around wound edges (Fig. 4F) to a normal zone of 5–10 cells (Fig. S4).
Fig. 4.
Intracellular components of the Toll signaling pathway are required for activation of the ple wound reporter. (A) Wild-type embryo carrying the ple-WE1 wound reporter was puncture wounded at stage 16 and shows the normal ple wound reporter expression (5- to 10-cell diameters from the wound edge) when imaged 5 h after wounding. (B) This tube2 homozygous mutant embryo shows strongly reduced ple reporter expression around puncture wound sites. (C) This mutant in the MyD88 (aka Kra1) gene has barely detectable expression of ple wound reporter around a puncture wound site. (D) This wounded dorsal mutant embryo shows no detectable difference in ple wound reporter activation compared with wild-type embryos. (E) This image of a stage 16 wild-type embryo shows the typical extent of ple RNA staining (Materials and Methods) around puncture wound sites 1 h after wounding. (F) Stage 16, wounded Dif mutant embryo, stained for ple RNA 1 h after wounding. Only a few cells bordering the wound site show ple transcript accumulation. White arrows mark the wound sites. White dashed lines outline embryos. Anterior is to the Left in all panels. The ple-WE1 wound reporter exhibits wound-independent activation in the developing anal pad in late stage embryos, as seen in the posterior (Right). Fig. S4 for quantitative data on wound-induced gene expression in tube, MyD88, dorsal, and Dif mutant embryos.
In wild-type embryos, 88% of wild-type embryos (n = 135) showed normal wound-induced Ddc transcription (Figs. S2E and S4). In contrast, 50% of punctured Dif mutants showed no activation of Ddc around wound sites, whereas the remaining 47% showed expression ranging from one to two rows from the wound edge (Fig. S2F) to normal Ddc induction at wound sites (Fig. S4). Cumulatively, these results indicate that intracellular Toll signaling, acting through Dif, is required for robust activation of the wound-induced ple reporter in epidermal cells.
Discussion
Both septic and clean wounding treatments result in the transcriptional activation of barrier repair and innate immunity genes in Drosophila epithelia (20, 30, 31). In this paper, we have shown that numerous signaling components in the Toll pathway, from the extracellular Spz ligand to the Dif transcription factor in the nucleus, are required to robustly induce expression of the Ddc and ple epidermal barrier repair genes after wounding late stage embryos. In addition, overexpression of constitutively activated forms of Toll receptor and injection of the activated Spz-C106 ligand are sufficient to induce wound repair reporter genes in undamaged epidermis. These results establish Spz as an epidermal wound response ligand. We found that in the hierarchy of wound-induced signals, the Spz ligand appears to act downstream of Duox and hydrogen peroxide, two previously identified activators of the epidermal wound response pathway (25).
Based on our results, we propose a model where wounds result in the production of hydrogen peroxide, which activates as-yet-unknown proteases that cleave pro-Spz to locally activate repair gene transcription (Fig. 5). A concentration increase of calcium ions into cells near wound sites is likely to be the initiating event to trigger the activation of the Duox enzyme and extracellular hydrogen peroxide (24). The calcium flash in Drosophila epidermal cells nearby laser or puncture wound sites persists for only 10–50 min (24) (also Materials and Methods), but might trigger a more stable expression pattern of wound repair gene expression through a Dif-dependent autoregulatory circuit, because both dorsal and Dif genes are transcriptionally activated in epidermal cells around wound sites (20). A serpin (serine protease inhibitor) is a possible candidate for the role of a redox sensor in this pathway, because some serpins have cysteines that are redox sensitive (32, 33).
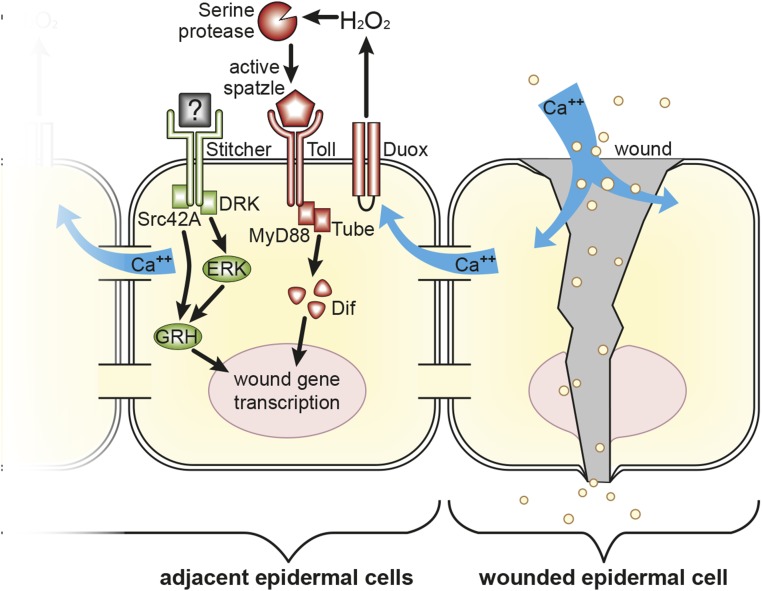
Fig. 5.
Model of wound-induced gene expression signaling in Drosophila epidermal cells. Based on our results and past studies, we propose that epidermal injury leads to influx of extracellular calcium ions into cells neighboring a wound, activating the dual oxidase enzyme (Duox), which produces a burst of extracellular hydrogen peroxide (24). In some manner this leads to the activation of a protease or proteases that cleave and activate the Spätzle ligand (20, 25). The Spätzle/Toll signaling complex transmits a signal through the adaptors MyD88 and Tube that results in activation of wound gene transcription that is dependent on the Dif transcription factor. The loss of Toll pathway components does not result in a complete loss of wound gene transcription, because there is at least one other signaling pathway acting in parallel that also has a combinatorial role in helping to activate wound gene transcription. One such pathway involves the Stitcher receptor tyrosine kinase, the downstream effectors Src42A, DRK, and ERK, and the transcription factor Grainy head (GRH) (11, 12, 32, 33, 54).
Carvahlo et al. (19) discovered that Toll receptor and either the Dif or Dorsal transcription factors (or both) were required for normal epidermal closure of laser-induced wounds in late embryos, but surprisingly found that spz gene function was not required for the laser wound closure process. Thus, it seems that two different processes during wound healing, reepithelialization and barrier repair gene activation, are distinguished by the use of different signals that activate Toll receptor signaling in epidermal cells near the wound edge. Dual oxidase function, which we find is required for the activation of some barrier repair genes in a Spz-dependent fashion, is also required for the attraction of hemocytes to laser-induced wounds in the late embryonic epidermis (24). How the signals activating this inflammatory response are integrated with the signals for epidermal wound closure and activation of barrier repair genes is as yet unknown, but our results that many of the signals to activate these wound-induced processes are shared, and integrated control of these processes will be a fascinating subject for future studies.
Other pathways besides Toll are crucially important for epidermal wound gene activation (Fig. 5). One pathway operates via the Stitcher Receptor Tyrosine Kinase through the downstream effectors DRK, Src42A, and ERK to the Grainy head transcription factor (12, 34, 35). DNA binding sites for Grainy head are commonly found in, and are required for the function of some wound-activated epidermal repair genes, in Drosophila and mammals (11, 12, 14). AP-1 sequence motifs are also required for wound-induced gene expression of Ddc and ple, although it is still unclear in Drosophila embryos whether the JNK pathway acts upstream of the AP-1 (Jun/Fos DNA binding) sites in this context (11, 12). There are many other epidermal wound-induced genes for which the JNK pathway is unquestionably crucial (6, 7, 9, 10, 36). It seems logical that many overlapping signaling pathways generate the specificity to regulate wound-induced genes, because it would be catastrophic to activate the entire suite of wound repair genes whenever the individual pathways involving ERK, JNK, or Toll are activated (Fig. 5). Relevant to the model in Fig. 5, a recent study showed that Src42A can be directly activated by a wound-induced peroxide burst, leading to the recruitment of hemocytes to a wound site (37). Our results show that peroxide injected into spz mutant embryos fails to activate wound reporter genes. This finding suggests to us that peroxide is not sufficient to independently activate the Src42A protein in the context of wound gene activation, although it may have a quantitative role.
Early innate immune systems evolved to recognize potential pathogens by conserved molecular signatures such as peptidoglycans of Gram-positive bacteria and lipopolysaccharides of Gram-negative bacteria. Collectively these are called pathogen-associated molecular patterns (PAMPs) (38). Concomitant with the cellular invasion or damage triggered by pathogens bearing PAMPs there are conserved “self” molecules that are released by damaged host cells. These signals have been called damage-associated molecular patterns (DAMPs) (39). In the danger model, proposed by Matzinger (40), optimal activation of the immune system requires both damage signals and nonself signals from pathogens (40–42). This model thus nicely aligns with the data we present here to provide an explanation as to why the epidermal wound response and innate immune signaling pathways are both activated in response to barrier damage, even in the absence of microbes.
In mammals, there is evidence that alarm or DAMP signals include heat shock proteins, extracellular matrix components (hyaluronic acid), and a chromatin-associated protein HMGB1 (43). Although the signaling pathways elicited by DAMPs are not fully defined, there is evidence that their damage signaling occurs via receptors such as the multiligand receptor for advanced glycation end products (RAGE receptors), and Toll-like receptors (43). The common feature of these pattern recognition receptors is their multivalency, which may be achieved by having multiple receptor isoforms, or different receptor paralogs. Although not traditionally put in the DAMP category, a local increase in hydrogen peroxide can be a direct or indirect signature of pathogen-induced or wound-induced damage. Immunocompetent cells are known to use reactive oxygen species to incapacitate invaders (44), and reactive oxygen species like hydrogen peroxide can signal surrounding tissues of a nearby wound (22, 23).
In mammals, Toll-like receptors in epidermal cells can be activated by the direct binding of individual PAMPs or DAMPs (45, 46). However, in Drosophila, the Toll receptor indirectly senses PAMPs by their ability to activate protease cascades that produce the Spz-106 ligand (47). It seems that signaling through the Toll receptor of Drosophila and probably other insects is a stripped down model of PAMP and DAMP recognition, with Toll receptor functioning as a secondary receptor hub. Acting in this way, the PAMPS recognized indirectly by Toll receptor can integrate inputs coming from different sources. In addition, and/or in parallel, Spz can serve as a proteolytic activity sensor that can be activated by proteases secreted by microbes (48, 49), or as shown here, by endogenous protease activities that are activated by tissue damage.
The protease(s) that activates pro-Spz in the activation of epidermal wound repair genes is as yet unknown. There are two serine proteases encoded by the genes, persephone (psh) and ModSP genes, that function immediately upstream of Spz activation in the context of the innate immune response, and another Spz activating protease, encoded by the easter (ea) gene, that, is required for ventral patterning in early embryos (50). We tested individual mutants in these three genes and observed no consistent changes in the wound-induced activation of the ple and Ddc genes. However, some combination of these three proteases might be involved in cleaving pro-Spz during epidermal wound gene activation.
Materials and Methods
Drosophila Stocks.
The wild-type strain used was w1118. Ddc 0.47 and ple-WE1 were previously described (11, 12) and we refer to Ddc 0.47 and ple-WE1 as the Ddc and ple wound reporter lines in this paper. The following mutant lines: ea1, ModSPG4511, spz4, dl1, DuoxKG07745, Dif1, Dif2, en-GAL4, and green fluorescent balancers CKG, TKG, and TM3 Ser GFP, were obtained from the Bloomington Stock Center. The following lines were kindly provided as follows: psh4 from J. M. Reichhart, Institut for Molecular and Cellular Biology, Strasbourg, France; tub2, tub R5.6, Tlr4, Toll10b, UAS-Toll10b, Kra1 (MyD88) from Steven Wasserman, University of California, San Diego, La Jolla, CA; GCaMP6 from Chih-Ying Su, University of California, San Diego, La Jolla, CA; and the Df(2L)J4 (Dif dl deficiency line) from Tony Ip, University of Massachusetts Medical School, Worcester, MA. Mutant flies were crossed to Ddc 0.47 or ple-WE1 wound reporter lines and balanced with chromosomes expressing GFP.
Wounding and Microinjections.
Drosophila embryos were puncture wounded as described in Juarez et al. (51). The embryonic stage of wounding was from stages 15–16, and live imaging of wound reporters was done 5 h later. Fixed and stained images of whole mount in situ were done 1 h after wounding. All fluorescent images were collected using either a Leica SP2 or SP5 laser-scanning confocal microscope, with instrument settings at nonsaturated gain levels for both experimental and control samples, although to obtain visible photomicrographs, the images for experimental results were taken at 1.3–2.0, the gain-of-control images. Optical sections were scanned at 1-mm thicknesses, and maximum projection images are shown.
Embryonic puncture wounding, injection into the body cavity, and (especially) injection into the perivitelline space are manually demanding operations whose success varies based on the level of experience and the level of meticulous attention to detail shown by different experimenters. This variation explains most of the variation in frequency of gene or reporter activation reported in different experiments, and so the quantitation of control and experimental reporter or gene activation that we report are those obtained by the same experimenter on the same days. Although many mutants have a large proportion of embryos that show no expression, we think it best to show images of those with visible, albeit weak, wound-induced RNA or reporter expression. Even so, we usually have to boost the camera gain for mutants about 1.5- to 2-fold compared with wild type to see the weak expression we show in this paper on photomicrographs. Regarding quantitation, in the mutant backgrounds where there was a significant diminution of wound gene expression after puncture wounding, only two classes could be honestly called: class A, no expression and class B, variable expression that ranged “smoothly” between a few cells near the wound edge to normal appearing expression. This is what we observed, so that is what we report in the text and graphs. In wounded wild-type embryos, we observe either normal expression of wound genes 5- to 10-cell diameters from the wound edge (the radius varies somewhat based on the size of the wound) or no expression (which we believe is due to embryonic death immediately after wounding). Almost all embryos survive puncture wounding under oil and go on to develop into first instar larvae. Trypsin and hydrogen peroxide microinjection details are reported in Juarez et al. (25) and Patterson et al. (20). Spz-C106 protein and the calcium ion ionophore, ionomycin, were injected in a similar fashion. For injections, trypsin was used at 2 mg/mL concentration, hydrogen peroxide at 2% (vol/vol), ionomycin at 1 mg/mL (from DMSO stock), and Spz-C106 protein at 50 μg/mL in 1× PBS. Embryos carrying the GCaMP6 fluorescent indicator (52), which allowed the detection of cytoplasmic influxes of intracellular calcium ion after wounding or ionophore treatment, were imaged 5 min after injection/wounding. A calcium ion-induced fluorescent signal was visible at 2 min after wounding, the earliest time we could image, and it faded to background levels by 50 min. Spz-C106 protein was kindly provided by Rob DeLotto, Rutgers University, Piscataway, NJ, and is described in DeLotto and DeLotto (21).
Fluorescent in Situ Hybridization.
Ddc and ple RNA probes were generated from partial or full-length cDNA clones obtained from the Drosophila Genomics Resource Center. Probe labeling, hybridization, and detection protocols were as described in Kosman et al. (53). RNA probes were labeled with digoxigenin (DIG)-conjugated ribonucleotides, and anti-DIG coupled to tyramide were used to detect transcripts in situ. Stained embryos were mounted in DTG [2.5% (wt/vol) DABCO-Sigma #D-2522, 50 mM Tris (pH 8.0), 90% (vol/vol glycerol] and imaged with a Leica SP5 confocal microscope as described above.
Acknowledgments
We thank Bonnie Weems for the design and preparation of Fig. 5 and for help preparing the other figures; Steven A. Wasserman and Chih-Ying Su, who generously provided several fly mutant and transgenic lines as well as anti-Dorsal antiserum; Robert DeLotto for the Spz-C106 protein; Steven Wasserman and Scott Lindsay for their comments on the manuscript; and the Bloomington Drosophila Stock Center (NIH P40OD018537) for many fly lines used in this study. This research was supported by NIH Grant R01HD28315 (to W.M.), and NIH Grants R03AI117671 and G12MD007603 (to M.J.T.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1613917114/-/DCSupplemental.
References
- 1.Harden N. Cell biology. Of grainy heads and broken skins. Science. 2005;308(5720):364–365. doi: 10.1126/science.1112050. [DOI] [PubMed] [Google Scholar]
- 2.Razzell W, Wood W, Martin P. Swatting flies: Modelling wound healing and inflammation in Drosophila. Dis Model Mech. 2011;4(5):569–574. doi: 10.1242/dmm.006825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belacortu Y, Paricio N. Drosophila as a model of wound healing and tissue regeneration in vertebrates. Dev Dyn. 2011;240(11):2379–2404. doi: 10.1002/dvdy.22753. [DOI] [PubMed] [Google Scholar]
- 4.Lee W-J, Miura M. Mechanisms of systemic wound response in Drosophila. Curr Top Dev Biol. 2014;108:153–183. doi: 10.1016/B978-0-12-391498-9.00001-2. [DOI] [PubMed] [Google Scholar]
- 5.Chisholm AD. Epidermal wound healing in the nematode Caenorhabditis elegans. Adv Wound Care (New Rochelle) 2015;4(4):264–271. doi: 10.1089/wound.2014.0552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galko MJ, Krasnow MA. Cellular and genetic analysis of wound healing in Drosophila larvae. PLoS Biol. 2004;2(8):E239. doi: 10.1371/journal.pbio.0020239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rämet M, Lanot R, Zachary D, Manfruelli P. JNK signaling pathway is required for efficient wound healing in Drosophila. Dev Biol. 2002;241(1):145–156. doi: 10.1006/dbio.2001.0502. [DOI] [PubMed] [Google Scholar]
- 8.Li G, et al. c-Jun is essential for organization of the epidermal leading edge. Dev Cell. 2003;4(6):865–877. doi: 10.1016/s1534-5807(03)00159-x. [DOI] [PubMed] [Google Scholar]
- 9.Lesch C, Jo J, Wu Y, Fish GS, Galko MJ. A targeted UAS-RNAi screen in Drosophila larvae identifies wound closure genes regulating distinct cellular processes. Genetics. 2010;186(3):943–957. doi: 10.1534/genetics.110.121822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stevens LJ, Page-McCaw A. A secreted MMP is required for reepithelialization during wound healing. Mol Biol Cell. 2012;23(6):1068–1079. doi: 10.1091/mbc.E11-09-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pearson JC, Juarez MT, Kim M, Drivenes Ø, McGinnis W. Multiple transcription factor codes activate epidermal wound-response genes in Drosophila. Proc Natl Acad Sci USA. 2009;106(7):2224–2229. doi: 10.1073/pnas.0810219106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mace KA, Pearson JC, McGinnis W. An epidermal barrier wound repair pathway in Drosophila is mediated by grainy head. Science. 2005;308(5720):381–385. doi: 10.1126/science.1107573. [DOI] [PubMed] [Google Scholar]
- 13.Stramer B, Martin P. Cell biology: Master regulators of sealing and healing. Curr Biol. 2005;15(11):R425–R427. doi: 10.1016/j.cub.2005.05.034. [DOI] [PubMed] [Google Scholar]
- 14.Ting SB, et al. A homolog of Drosophila grainy head is essential for epidermal integrity in mice. Science. 2005;308(5720):411–413. doi: 10.1126/science.1107511. [DOI] [PubMed] [Google Scholar]
- 15.Wang S, Samakovlis C. Grainy head and its target genes in epithelial morphogenesis and wound healing. Curr Top Dev Biol. 2012;98:35–63. doi: 10.1016/B978-0-12-386499-4.00002-1. [DOI] [PubMed] [Google Scholar]
- 16.Silverman N, Maniatis T. NF-kappaB signaling pathways in mammalian and insect innate immunity. Genes Dev. 2001;15(18):2321–2342. doi: 10.1101/gad.909001. [DOI] [PubMed] [Google Scholar]
- 17.Weber AN, et al. Binding of the Drosophila cytokine Spätzle to Toll is direct and establishes signaling. Nat Immunol. 2003;4(8):794–800. doi: 10.1038/ni955. [DOI] [PubMed] [Google Scholar]
- 18.Matova N, Anderson KV. Drosophila Rel proteins are central regulators of a robust, multi-organ immune network. J Cell Sci. 2010;123(Pt 4):627–633. doi: 10.1242/jcs.060731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carvalho L, Jacinto A, Matova N. The Toll/NF-κB signaling pathway is required for epidermal wound repair in Drosophila. Proc Natl Acad Sci USA. 2014;111(50):E5373–E5382. doi: 10.1073/pnas.1408224111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patterson RA, et al. Serine proteolytic pathway activation reveals an expanded ensemble of wound response genes in Drosophila. PLoS One. 2013;8(4):e61773. doi: 10.1371/journal.pone.0061773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeLotto Y, DeLotto R. Proteolytic processing of the Drosophila Spätzle protein by easter generates a dimeric NGF-like molecule with ventralising activity. Mech Dev. 1998;72(1–2):141–148. doi: 10.1016/s0925-4773(98)00024-0. [DOI] [PubMed] [Google Scholar]
- 22.Niethammer P, Grabher C, Look AT, Mitchison TJ. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature. 2009;459(7249):996–999. doi: 10.1038/nature08119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moreira S, Stramer B, Evans I, Wood W, Martin P. Prioritization of competing damage and developmental signals by migrating macrophages in the Drosophila embryo. Curr Biol. 2010;20(5):464–470. doi: 10.1016/j.cub.2010.01.047. [DOI] [PubMed] [Google Scholar]
- 24.Razzell W, Evans IR, Martin P, Wood W. Calcium flashes orchestrate the wound inflammatory response through DUOX activation and hydrogen peroxide release. Curr Biol. 2013;23(5):424–429. doi: 10.1016/j.cub.2013.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Juarez MT, Patterson RA, Sandoval-Guillen E, McGinnis W. Duox, Flotillin-2, and Src42A are required to activate or delimit the spread of the transcriptional response to epidermal wounds in Drosophila. PLoS Genet. 2011;7(12):e1002424. doi: 10.1371/journal.pgen.1002424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Juarez MT. Drosophila embryos as a model for wound-induced transcriptional dynamics: Genetic strategies to achieve a localized wound response. Adv Wound Care (New Rochelle) 2016;5(6):262–270. doi: 10.1089/wound.2014.0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lund VK, DeLotto Y, DeLotto R. Endocytosis is required for Toll signaling and shaping of the Dorsal/NF-kappaB morphogen gradient during Drosophila embryogenesis. Proc Natl Acad Sci USA. 2010;107(42):18028–18033. doi: 10.1073/pnas.1009157107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schneider DS, Hudson KL, Lin T-Y, Anderson KV. Dominant and recessive mutations define functional domains of Toll, a transmembrane protein required for dorsal-ventral polarity in the Drosophila embryo. Genes Dev. 1991;5(5):797–807. doi: 10.1101/gad.5.5.797. [DOI] [PubMed] [Google Scholar]
- 29.Lindsay SA, Wasserman SA. Conventional and non-conventional Drosophila Toll signaling. Dev Comp Immunol. 2014;42(1):16–24. doi: 10.1016/j.dci.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Gregorio E, Spellman PT, Rubin GM, Lemaitre B. Genome-wide analysis of the Drosophila immune response by using oligonucleotide microarrays. Proc Natl Acad Sci USA. 2001;98(22):12590–12595. doi: 10.1073/pnas.221458698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Onfelt Tingvall T, Roos E, Engström Y. The imd gene is required for local Cecropin expression in Drosophila barrier epithelia. EMBO Rep. 2001;2(3):239–243. doi: 10.1093/embo-reports/kve048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Przygodzka P, Ramstedt B, Tengel T, Larsson G, Wilczynska M. Bomapin is a redox-sensitive nuclear serpin that affects responsiveness of myeloid progenitor cells to growth environment. BMC Cell Biol. 2010;11:30. doi: 10.1186/1471-2121-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morris EC, et al. Murine serpin 2A is a redox-sensitive intracellular protein. Biochem J. 2003;371(Pt 1):165–173. doi: 10.1042/BJ20021567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang S, et al. The tyrosine kinase Stitcher activates Grainy head and epidermal wound healing in Drosophila. Nat Cell Biol. 2009;11(7):890–895. doi: 10.1038/ncb1898. [DOI] [PubMed] [Google Scholar]
- 35.Tsarouhas V, Yao L, Samakovlis C. Src kinases and ERK activate distinct responses to Stitcher receptor tyrosine kinase signaling during wound healing in Drosophila. J Cell Sci. 2014;127(Pt 8):1829–1839. doi: 10.1242/jcs.143016. [DOI] [PubMed] [Google Scholar]
- 36.Brock AR, et al. Transcriptional regulation of Profilin during wound closure in Drosophila larvae. J Cell Sci. 2012;125(Pt 23):5667–5676. doi: 10.1242/jcs.107490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Evans IR, Rodrigues FSLM, Armitage EL, Wood W. Draper/CED-1 mediates an ancient damage response to control inflammatory blood cell migration in vivo. Curr Biol. 2015;25(12):1606–1612. doi: 10.1016/j.cub.2015.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schnare M, Holt AC, Takeda K, Akira S, Medzhitov R. Recognition of CpG DNA is mediated by signaling pathways dependent on the adaptor protein MyD88. Curr Biol. 2000;10(18):1139–1142. doi: 10.1016/s0960-9822(00)00700-4. [DOI] [PubMed] [Google Scholar]
- 39.Oppenheim JJ, Yang D. Alarmins: Chemotactic activators of immune responses. Curr Opin Immunol. 2005;17(4):359–365. doi: 10.1016/j.coi.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 40.Matzinger P. The danger model: A renewed sense of self. Science. 2002;296(5566):301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 41.Seong SY, Matzinger P. Hydrophobicity: An ancient damage-associated molecular pattern that initiates innate immune responses. Nat Rev Immunol. 2004;4(6):469–478. doi: 10.1038/nri1372. [DOI] [PubMed] [Google Scholar]
- 42.Stuart LM, Paquette N, Boyer L. Effector-triggered versus pattern-triggered immunity: How animals sense pathogens. Nat Rev Immunol. 2013;13(3):199–206. doi: 10.1038/nri3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ. HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol. 2010;28:367–388. doi: 10.1146/annurev.immunol.021908.132603. [DOI] [PubMed] [Google Scholar]
- 44.Rada B, Leto TL. Oxidative innate immune defenses by Nox/Duox family NADPH oxidases. Contrib Microbiol. 2008;15:164–187. doi: 10.1159/000136357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lai Y, Gallo RL. Toll-like receptors in skin infections and inflammatory diseases. Infect Disord Drug Targets. 2008;8(3):144–155. doi: 10.2174/1871526510808030144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Borkowski AW, Park K, Uchida Y, Gallo RL. Activation of TLR3 in keratinocytes increases expression of genes involved in formation of the epidermis, lipid accumulation, and epidermal organelles. J Invest Dermatol. 2013;133(8):2031–2040. doi: 10.1038/jid.2013.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kurata S. Peptidoglycan recognition proteins in Drosophila immunity. Dev Comp Immunol. 2014;42(1):36–41. doi: 10.1016/j.dci.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.El Chamy L, Leclerc V, Caldelari I, Reichhart JM. Sensing of ‘danger signals’ and pathogen-associated molecular patterns defines binary signaling pathways ‘upstream’ of Toll. Nat Immunol. 2008;9(10):1165–1170. doi: 10.1038/ni.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gottar M, et al. Dual detection of fungal infections in Drosophila via recognition of glucans and sensing of virulence factors. Cell. 2006;127(7):1425–1437. doi: 10.1016/j.cell.2006.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ferrandon D, Imler J-L, Hetru C, Hoffmann JA. The Drosophila systemic immune response: Sensing and signalling during bacterial and fungal infections. Nat Rev Immunol. 2007;7(11):862–874. doi: 10.1038/nri2194. [DOI] [PubMed] [Google Scholar]
- 51.Juarez MT, Patterson RA, Li W, McGinnis W. Microinjection wound assay and in vivo localization of epidermal wound response reporters in Drosophila embryos. J Vis Exp. 2013;81(81):e50750. doi: 10.3791/50750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen TW, et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499(7458):295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kosman D, et al. Multiplex detection of RNA expression in Drosophila embryos. Science. 2004;305(5685):846. doi: 10.1126/science.1099247. [DOI] [PubMed] [Google Scholar]
- 54.Kim M, McGinnis W. Phosphorylation of Grainy head by ERK is essential for wound-dependent regeneration but not for development of an epidermal barrier. Proc Natl Acad Sci USA. 2011;108(2):650–655. doi: 10.1073/pnas.1016386108. [DOI] [PMC free article] [PubMed] [Google Scholar]