Significance
Following periods of famine, a reproducible spectrum of disorders often manifest long after the period of starvation. Curiously, many of these diseases arise in individuals that have no apparent genetic history of the disorder, although they do correlate with specific epigenetic modifications. We used Caenorhabditis elegans as a model to understand how acute periods of starvation might result in physiological or developmental consequences in a single generation or over multiple generations following the initial period of stress. Our data suggest that the AMP-activated protein kinase, an enzyme that mediates metabolic adjustment during starvation, is required to block germ-line gene expression during these conditions. In its absence the inappropriate activation of germ-line transcription results in sterility and transgenerational reproductive defects.
Keywords: epigenetics, AMPK, histone methyltransferase, COMPASS, C. elegans
Abstract
Life history events, such as traumatic stress, illness, or starvation, can influence us through molecular changes that are recorded in a pattern of characteristic chromatin modifications. These modifications are often associated with adaptive adjustments in gene expression that can persist throughout the lifetime of the organism, or even span multiple generations. Although these adaptations may confer some selective advantage, if they are not appropriately regulated they can also be maladaptive in a context-dependent manner. We show here that during periods of acute starvation in Caenorhabditis elegans larvae, the master metabolic regulator AMP-activated protein kinase (AMPK) plays a critical role in blocking modifications to the chromatin landscape. This ensures that gene expression remains inactive in the germ-line precursors during adverse conditions. In its absence, critical chromatin modifications occur in the primordial germ cells (PGCs) of emergent starved L1 larvae that correlate with compromised reproductive fitness of the generation that experienced the stress, but also in the subsequent generations that never experienced the initial event. Our findings suggest that AMPK regulates the activity of the chromatin modifying COMPASS complex (complex proteins associated with Set1) to ensure that chromatin marks are not established until nutrient/energy contingencies are satisfied. Our study provides molecular insight that links metabolic adaptation to transgenerational epigenetic modification in response to acute periods of starvation.
In the winter of 1944/1945, the Nazi regime blockaded regions of Holland and rationed food for all inhabitants without exception, including pregnant women and newborn children. The infamous “Hongerwinter” came to an end in the spring of 1945, with the arrival of the allied forces providing supplies and medical help for the survivors. Although birth weight and size of the Hongerwinter infants varied with the period of gestation that occurred during the famine, surprisingly few developmental anomalies were immediately observed despite the extreme mal/undernutrition of the mothers (1–3). However, years afterward, the same individuals showed an abnormally elevated frequency of obesity, cardiac disease, and even schizophrenia, despite the lack of genetic history of these diseases. Further analysis suggested that DNA methylation patterns in specific regions of the genome were altered in many of the Hongerwinter children (1, 4).
Although largely correlative, the study of the children born during and after the Hongerwinter, along with other studies of famine victims, provide compelling evidence to support a developmental origin of a broad spectrum of disorders. Some of these disorders may rely on epigenetic changes that alter gene expression downstream of a single event or prolonged environmental conditions (5, 6). At present it has proven difficult to link the observed epigenetic changes to any obvious adaptive response to the nutrient stress, or how such a response may underlie the clinical manifestations that arise in individuals several years after the initial stress. Moreover, the nature of such changes may potentially extend beyond the described changes in DNA methylation to impinge upon chromatin marks that could heritably modify gene expression in a DNA sequence-independent manner.
Although Caenorhabditis elegans does not possess the enzymes involved in cytosine methylation, it does exhibit various forms of epigenetic inheritance. These epigenetic traits are mediated largely through chromatin modifications and a heritable population of small RNAs that collectively dictate whether regions of the genome are expressed or silenced (7–9). Recently, it was shown that the composition of a large repertoire of RNAs is sensitive to starvation during the first larval (L1) diapause, and could reflect some of the adaptive changes in gene expression that are transmitted in subsequent generations (10). Furthermore, starvation during early stages of development can induce long-term phenotypic consequences for exposed animals and increase stress resistance in their descendants (11). These data therefore suggest that in C. elegans early life-history events can exert a lasting transgenerational impact associated with an adaptive response to the initial event.
We show here that the AMP-activated protein kinase (AMPK) is required not only to adjust to the stress associated with starvation during the L1 stage in C. elegans, but also to block epigenetic modifications typical of gene activation during this period of acute starvation. In its absence, the histone methyltransferase-containing COMPASS complex (complex proteins associated with Set1) becomes misregulated, generating chromatin modifications that have detrimental effects on reproductive fitness, not only in the generation of animals that experienced the starvation, but in subsequent generations that were never starved.
Results
Following embryogenesis, emergent C. elegans L1-stage larvae remain in a nondeveloping, diapause-like state until they encounter a nutrient/energy source that will trigger the onset of the postembryonic developmental program (12, 13). Whereas wild-type L1 larvae can survive up to 2 wk in this starved state, loss of activity in the metabolic regulatory kinase AMPK/aak-1/2, the tumor suppressor ortholog phosphatase and tensin homolog deleted on chromosome 10 (PTEN)/daf-18, or the histone deacetylase 1 ortholog hda-1, sensitizes animals to this stress, causing them to die prematurely en masse (Fig. S1A) (13–16). Although it is less clear how the other two genes affect survival of starved L1 larvae, AMPK becomes activated in response to starvation and phosphorylates diverse substrates to facilitate metabolic adjustment (17). These targets include key metabolic enzymes, whereas AMPK activation has also been linked to changes in gene expression through chromatin modification (18). To determine whether AMPK may establish or maintain changes within the chromatin landscape downstream of acute starvation, we examined the role of AMPK during the metabolic adaptation associated with the L1 diapause in C. elegans.
Fig. S1.
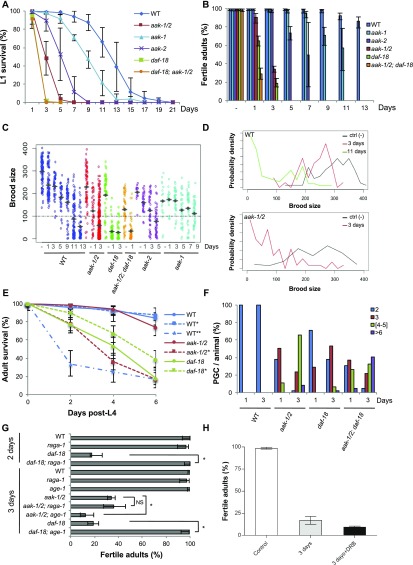
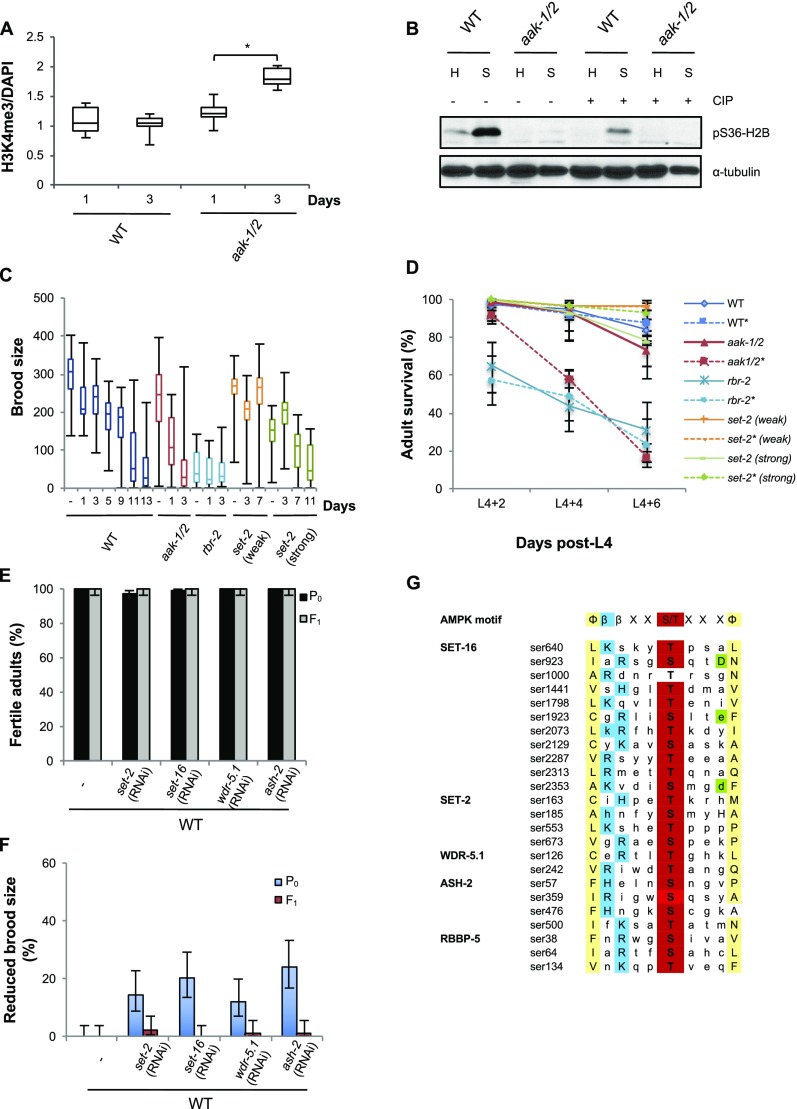
AMPK and PTEN act through independent pathways to control PGC cell cycle quiescence, germ-line integrity, and survival during the L1 diapause. (A) AMPK (aak-1/2) and PTEN/daf-18 are required to survive the stress of the L1 diapause. Animals of the indicated genotypes were hatched in the absence of food and viability was determined every 24 h following hatching. “Days” represent duration of starvation. Data represent the mean ± SD of three independent experiments. (B) AMPK (aak-1/2) and PTEN/daf-18 act independently to ensure adult reproductive fitness in post-L1 diapause animals. Animals were maintained in the L1 diapause for varying durations (Days) followed by recovery on replete plates (with OP50) until adulthood and fertility was scored in all adult animals. “Days” represent duration of starvation; “–” indicates animals that were not starved. Error bars: 95% CI. (C) Prolonged duration in the L1 diapause affects brood size of fertile adult animals. L1 animals were maintained in the L1 diapause for varying durations, recovered to replete plates, and their total F1 progeny number were counted and the distribution shown. The average brood size for each group is depicted by the horizontal bar; the dotted line represents the minimum threshold brood size used to define reduced brood size. (D) Distribution of aak-1/2 and wild-type brood sizes following varying durations in the L1 diapause. The duration of the diapause correlates negatively with the brood size of the animals tested. After a 3-d period in the diapause, ∼80% of the recovered post-L1 diapause aak-1/2 mutants produce broods of reduced size (fewer than 100 F1 progeny). For better visualization, density distribution is smoothed by grouping values in bin widths of 12 (0–11; 12–23; 24–35; …; 389–400). Ctl (–) indicates animals that were not starved; n > 40 per genotype per condition. (E) Prolonged duration in the L1 diapause results in premature adult lethality. L1 larvae of wild-type, aak-1/2, and daf-18 were maintained 3 d (*) or 11 d (**) without food in the L1 diapause, or were not starved (−), before being singled to replete plates and allowed to grow and develop. Post-L4 stage survival was scored every 2 d; n ≥100 animals per point. (F) Both AMPK (aak-1/2) and PTEN (daf-18) are required for the maintenance of PGC cell-cycle quiescence during the L1 diapause, but through independent pathways. Germ-cell numbers were scored by counting HTP-3–stained cells in starved wild-type or mutant animals after either 1 or 3 d without food in the L1 diapause; n ≥ 50 animals. (G) Differential suppression of sterility between AMPK and PTEN/daf-18 mutants. Loss of raga-1 or age-1 suppresses the sterility of post-L1 diapause daf-18 mutants, but not aak-1/2 mutants. Error bars: 95% CI; *P < 0.05 by Fisher’s exact test; NS, nonsignficant; n ≥ 50. (H) Synchronized daf-18 L1 larvae were maintained in M9 buffer without food for 3 d with or without 30 µM DRB. Animals were then transferred to regular OP50-seeded NGM plates and fertility was assessed after they reached adulthood. Nonstarved daf-18 larvae were used as a control. Assays were performed three times and the data represent the mean ± SD, where n = 50 for each condition.
In many organisms the loss of AMPK is lethal (19, 20); however, C. elegans mutants that have no AMPK signaling because of the disruption of its two catalytic subunits (AMPK/aak-1/2) are viable but exhibit defects during periods of nutrient/energy stress. This is most notable during the L1 diapause stage and during an alternative postembryonic developmental stage called dauer, where it has been shown to affect germ-line stem cell quiescence, dauer maintenance, and survival (21–23). Mutations in the C. elegans tumor-suppressor ortholog PTEN/daf-18 cause similar defects in both dauer and L1 diapause-arrested larvae, although it most probably acts through a genetically independent pathway (15, 24, 25).
AMPK and PTEN Are Both Required for Primordial Germ Cell Quiescence During the L1 Diapause and Post-L1 Diapause Fertility, but Act Through Independent Pathways.
When starved AMPK/aak-1/2 or PTEN/daf-18 mutant L1 larvae that have been arrested in the L1 diapause are subsequently placed into replete growth conditions, the L1 larvae (which we will refer to hereafter as post-L1 diapause larvae) initiate postembryonic development, although their progression through the various larval stages is slower and more variable than wild-type L1 diapause larvae that were starved for the same duration. Once the surviving post-L1 diapause mutants reach the adult stage they show both somatic and reproductive defects, including a reduced brood size, and many mutant animals die prematurely as adults (Fig. 1A, Fig. S1 A–E, and Table S1). The survival of the L1 diapause larvae, and the severity and frequency of the somatic growth defects that manifest following recovery of the starved mutant animals are dependent on the duration of the starvation. For example, after 5 d of starvation most animals die, but after 3 d of starvation half the daf-18 and aak-1/2 animals were unable to recover, and those that did exhibited a spectrum of somatic and reproductive defects that included vulval defects, sterility, and premature adult lethality. This is not unique to the mutants because we also observe the same types of growth and reproductive abnormalities in wild-type animals that were starved for substantially longer durations (11 d) before transferring them to replete conditions (Fig. 1B, Fig. S1 D and E, and Table S1).
Fig. 1.
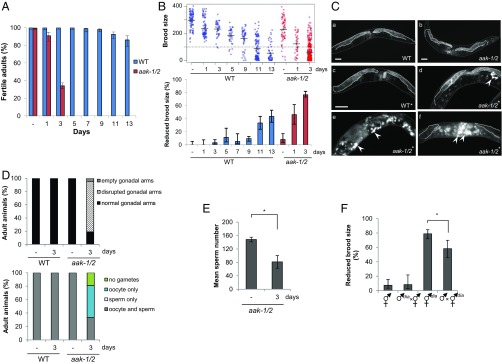
Loss of AMPK results in sensitivity to acute starvation and subsequent postrecovery reproductive effects. (A) AMPK is required to ensure adult reproductive fitness after recovering from L1 starvation. Following varying durations of starvation (“Days” refer to days of starvation here and throughout), post-L1 diapause larvae were singled to replete plates, allowed to grow to adulthood when their fertility was scored. “–” indicates nonstarved animals. Error bars represent the confidence interval at 95% (95% CI); n ≥ 80 animals were scored per experimental condition. WT, wild type. (B) Prolonged starvation in the L1 diapause results in a reduction in the F1 brood size for post-L1 diapause aak-1/2 mutant and wild-type animals. Total number of F1 progeny born from fertile post-L1 diapause parents were scored following varying durations in the L1 diapause [nonstarved (–), 1, 3, 5, 7, 9, 11, or 13 d]. (Upper) Brood size distribution for wild-type and aak-1/2 animals after varying durations of L1 diapause. The average brood size is indicated by horizontal bars for each genotype/condition. The dotted line represents the minimum number of F1 progeny used to define reduced brood size (fewer than 100 F1 progeny). (Lower) The proportion of individuals with reduced brood size is represented, n ≥ 80 animals. Error bars: 95% CI. (C) L1 starvation disrupts gonad morphology in post-L1 diapause aak-1/2 adult hermaphrodites. L1 larvae were either allowed to eat immediately (–) or maintained in the L1 diapause for varying durations before they were singled to OP50-seeded plates. Whole-animal DAPI staining was performed on wild-type or aak-1/2 adult hermaphrodites to visualize the germ cells. The gonads of (a) nonstarved wild-type controls or (b) aak-1/2 mutant adult hermaphrodites possess two symmetric, morphologically normal gonadal arms, which are also observed in (c) wild-type adults that were previously subjected to a 3-d period in the L1 diapause indicated by the asterisk (*). In contrast, young adult hermaphrodite aak-1/2 mutants that developed after a 3-d L1 diapause (*) displayed abnormal gonads that lack or have reduced germ cell numbers (d–f), where the mitosis–meiosis zone is disorganized. In addition, many germ cells undergo endomitotic cell cycles (arrowheads). Dotted lines delineate the gonad boundary, whereas solid gray lines outline the cuticle. (Scale bar, 50 μm.) (D) Morphological defects in the gonad are unique to aak-1/2 mutants that were previously subjected to 3 d in the L1 diapause. Gonadal defects observed in young-adult post-L1 diapause aak-1/2 mutant adults from C were quantified and represented graphically. Oocyte-only animals lack sperm; sperm-only animals lack oocytes. Oocytes and sperm-contain both; n ≥ 20 animals. (E) Sperm numbers are reduced in post-L1 diapause aak-1/2 mutants. Sperm were counted following DAPI staining in aak-1/2 mutant adults subjected to none (–), or a 3-d duration in the L1 diapause and subsequent recovery on replete plates until the adult stage; n ≥ 8 gonad arms. Error bars: SE, *P < 0.05 using Student’s t test. (F) Reproductive defects observed in post-L1 diapause aak-1/2 mutants are caused predominantly by compromised oocyte integrity with comparatively less contribution from the sperm. Crosses performed with post-L1 diapause hermaphrodites were partially rescued by mating with nonstarved aak-1/2 mutant males. Reciprocal crosses were performed between post-L1 diapause aak-1/2 mutant males (♂dia), or hermaphrodites (⚥dia) that were previously maintained 3 d in the L1 diapause. The resulting proportion of animals that exhibited a reduced brood size from successful mating (as judged by 50% frequency of males in the F1 progeny) was tabulated for comparison. Error bars: 95% CI; *P < 0.05 Fisher’s exact test.
Table S1.
set-2 and set-16 suppress only a subset of the vulval defects associated with the post-L1 diapause aak-1/2 mutants
| Starvation | Evl, % (95% CI) | Burst, % (95% CI) | Bag, % (95% CI) | Muv, % (95% CI) | n |
| No starvation | |||||
| Wild-type | 0 (0–3.2) | 0 (0–3.2) | 0 (0–3.2) | 0 (0–3.2) | 158 |
| aak-1/2 | 0 (0–1.8) | 1.5 (0.5–4.2) | 0 (0–1.9) | 0 (0–1.9) | 204 |
| set-2 | 0 (0–3.3 | 0 (0–3.3) | 0 (0–3.3) | 0 (0–3.3) | 116 |
| aak-1/2; set-2 | 0 (0–1.7) | 0.5 (0.1–2.6) | 0.9 (0.3–3.3) | 0 (0–1.7) | 217 |
| Three-day starvation | |||||
| Wild-type | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0 (0–1) | 200 |
| aak-1/2 | 11.8 (8.5–16.1)* | 13.2 (9.7–17.7) | 3.9 (2.2–6.9) | 2.1 (1–4.6) | 280 |
| set-2 | 0 (0–3.3) | 0 (0–3.3) | 0 (0–3.3) | 0 (0–3.3) | 111 |
| aak-1/2; set-2 | 2.6 (1.2–5.6)* | 13.6 (9.8–18.7) | 0.9 (0.2–3.1) | 0 (0–1.7) | 228 |
| Eleven-day starvation | |||||
| Wild-type | 0 (0–7.4) | 0 (0–7.4) | 25 (14.9–38.8) | 0 (0–7.4) | 48 |
| Three-day starved aak-1/2 treated with RNAi by soaking | |||||
| CTL | 62.9 (53.9–71.2)†‡ | 8.6 (4.8–15.1) | 0.9 (0.2–4.7) | 2.6 (0.9–7.3) | 116 |
| set-2 (RNAi) | 41.3 (32.4–51)† | 12.5 (7.5–20.2) | 1 (0.2–5.24) | 1.9 (9.5–6.7) | 104 |
| set-16 (RNAi) | 44 (33.5–55.3)‡ | 16 (9.2–25.9) | 0 (0–4.90 | 2.7 (0.7–9.2) | 75 |
| wdr-5 (RNAi) | 49.4 (38.9–59.9) | 15.7 (9.4–25) | 0 (0–4.4) | 1.2 (0.2–6.5) | 83 |
| ash-2 (RNAi) | 49.4 (38.9–59.9) | 8.4 (4.1–16.4) | 2.4 (0.7–8.4) | 2.4 (0.7–8.4) | 83 |
Animals of indicated genotype were starved (–, 3, or 11 d), singled to replete plates, and scored for vulva or gonadal phenotypes when they reached the adult stage. The set-2 allele [set-2(ok952)] is a weak hypomorph. For the RNAi experiments, aak-1/2 mutants were treated by soaking L1 larvae in buffer containing dsRNA that corresponded to COMPASS complex components during the L1 diapause. Animals were then recovered to replete plates, singled, and scored for vulva or gonadal phenotypes when they reached the adult stage. Control animals (CTL) are aak-1/2 L1 larvae maintained 3-d in M9 buffer containing GFP dsRNA. Results of three independent post-L1 diapause recovery experiments were pooled. Parenthesis indicate 95% CI. Letters indicate significant differences between two conditions (P < 0.0005) by Fisher’s exact test.
The frequency of Evl animals observed is significantly different between aak-1/2 and aak-1/2; set-2 mutant larvae.
The frequency of Evl animals observed is significantly different between aak-1/2 (CTL) and aak-1/2; set-2 (RNAi) larvae.
The frequency of Evl animals observed is significantly different between aak-1/2 (CTL) and aak-1/2; set-16 (RNAi) larvae.
The premature death that occurs in all of the post-L1 diapause animals appears to be independent of the observed morphological somatic defects, because 60% of the post-L1 diapause AMPK/aak-1/2 or PTEN/daf-18 mutant adults, and 70% of the wild-type animals that were starved 11 d, die prematurely, 6 d after reaching the L4 stage with no visible somatic defects. Unlike the post-L1 diapause AMPK/aak-1/2 mutants, daf-18 adults die earlier than wild-type in a manner that is independent of the L1 diapause (Fig. S1E). Therefore, although wild-type animals show a progressive decline in their reproductive fitness during an extended period of starvation (11 d), this same decline occurs in the AMPK/aak-1/2 and daf-18 mutants after only a short period of starvation (1–3 d) (Fig. 1 A and B and Fig. S1 C and D).
The duration of the starvation at the L1 stage has a cumulative impact on fertility in wild-type, AMPK/aak-1/2, and PTEN/daf-18 animals, whereas the sterility observed in the triple mutant combination (aak-1; daf-18; aak-2) is even more severely affected: 71 ± 6% of post-L1 diapause triple mutants became sterile after 1 d in the diapause compared with 4.6 ± 4% and 35 ± 6% for AMPK/aak-1/2 and PTEN/daf-18 mutants, respectively. No triple mutants survive after 3 d of starvation at the L1 stage, consistent with AMPK and PTEN acting through independent pathways to ensure survival, robust and timely growth, and somatic and germ-line development following starvation (Fig. S1 A–D). After being maintained 3 d in the L1 diapause stage, only 30% of the viable post-L1 diapause aak-1/2 larvae grew to become fertile adults (Fig. 1A). The majority (80%) of these fertile parents display a strikingly reduced brood size, generating fewer than 100 F1 progeny. We refer to these fertile parents as “reduced” (parents that produce broods with less than 100 F1 progeny) (Fig. 1B and Fig. S1 C and D). Surprisingly, the remaining 20% of the fertile adults produced an almost normal brood size and seem almost unaffected. We refer to these animals as “normal” (parents that produce more than 100 F1 progeny).
Because the primordial germ cells (PGCs) undergo inappropriate supernumerary divisions in both AMPK/aak-1/2– and PTEN/daf-18–starved L1 mutant larvae (Fig. S1F) (14, 15), we wondered whether this aberrant proliferation may be responsible for the compromise in fertility and brood size observed in the post-L1 diapause AMPK/aak-1/2 and PTEN/daf-18 mutant adults. Using previously described genetic suppressors that block the supernumerary PGC divisions in both PTEN/daf-18 and AMPK/aak-1/2 mutants (14, 15, 23), we determined whether they might also restore fertility in the post-L1 diapause mutant adults. Whereas, age-1 (PI 3-kinase) mutations restore both germ-line quiescence and fertility of post-L1 diapause PTEN/daf-18 mutants (Fig. S1G), age-1 did not suppress the sterility of AMPK/aak-1/2 mutants, but alternatively, enhanced sterility in the triple mutants (87± 6% of 3-d post-L1 diapause age-1; aak-1/2 adults became sterile compared with 66 ± 3% in aak-1/2 adults) (Fig. S1G). Moreover, recent data suggest that blocking the target of rapamycin complex 1 (TOR) pathway by compromising raga-1 or ragc-1 can restore PGC quiescence in both AMPK/aak-1/2– and PTEN/daf-18–starved L1 mutant larvae (14). We observed that raga-1(lf) restored the fertility of PTEN/daf-18 adults that had transited through the L1 diapause, but did not restore the fertility of post-L1 diapause AMPK/aak-1/2 mutant adults (Fig. S1G). This finding suggests that the supernumerary PGC divisions are unlikely to be the sole basis of the sterility seen in post-L1 diapause AMPK/aak-1/2 mutants, although the supernumerary PGC divisions may contribute to the sterility observed in PTEN/daf-18 mutants.
Germ-Line and Gonad Defects in Post-L1 Diapause AMPK Mutant Hermaphrodite Adults.
To better understand the basis of the AMPK/aak-1/2 reproductive defects that result from starvation at the L1 stage, we examined the germ lines of adult hermaphrodites (Fig. 1 C and D and Fig. S2 A–C). The gonads of the post-L1 diapause AMPK/aak-1/2 adults were highly disorganized, often possessing smaller or empty arms. The size of the germ-cell nuclei was variable, whereas the typical germ-cell morphology appeared abnormal in many animals. In wild-type adult hermaphrodites, germ cells exit a mitotically active zone at the distal end of the gonad and enter meiotic prophase I as they move proximally. This region of the gonad called the transition zone is cytologically distinct based on DAPI staining (26). The length of the transition zones seen in post-L1 diapause AMPK/aak-1/2 mutants was extremely variable and sometimes transition zones were completely absent. The chromosomal morphology typical of cells in the transition zone based on DAPI staining was abnormal, suggesting that the progression from mitosis to meiosis was affected. None of these features were reproducibly observed in AMPK/aak-1/2 mutant hermaphrodites that were not subjected to the L1 diapause. These germ-line abnormalities likely impinge on gamete integrity because many of the disrupted gonads possessed oocytes, while several of these appear to have undergone endomitotic cycles based on DAPI staining (Emo, 76%, n = 20). In addition, most of the post-L1 diapause aak-1/2 adults exhibit gonads with a strong reduction, or a complete absence of sperm (Fig. 1 D and E and Fig. S2C). This reduced abundance and efficiency of the sperm, compounded with the observed defects in oogenesis after starvation, likely contribute to the observed brood-size defect in post-L1 diapause AMPK/aak-1/2 mutant hermaphrodite adults.
Fig. S2.
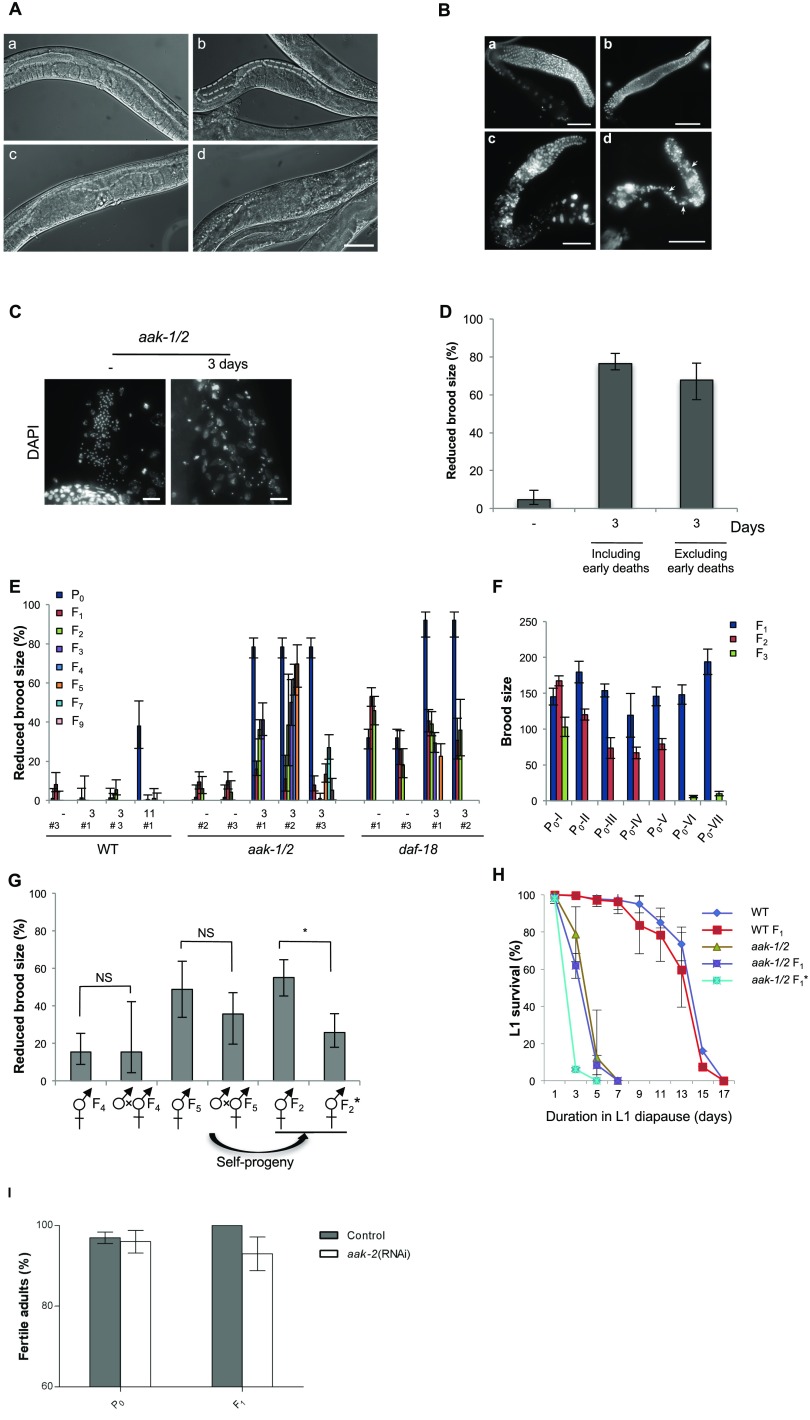
Prolonged duration in the L1 diapause results in somatic and germ-line defects in post-L1 diapause AMPK mutants. (A) DIC images of young adult hermaphrodites that include (a) a wild-type adult recovered after a 3-d period in the L1 diapause, (b–d) recovered aak-1/2 adults that were previously subjected to 3 d in the L1 diapause. Dashed lines demarcate the gonad boundary. (Scale bar, 50 μm.) (B) aak-1/2 post-L1 diapause adults display a range of germ-line defects, disruption of meiotic progression resulting in defects in gamete differentiation. Extruded gonads from adult post-L1 diapause animals that were previously subjected to 3 d without food in the L1 diapause: wild-type (a) or aak-1/2 (b–d) at L4 + 24 h were stained with DAPI. The severity of the defects in aak-1/2 gonads is associated with the observed reproductive defects gonad extruded from a post-L1 diapause adults that had a (b) normal brood size, (c) a reduced brood size, or (d) a sterile hermaphrodite. The solid line indicates the transition zone (when present) based on nuclear morphology assessed by DAPI staining, and arrows indicate inappropriate proximal germ-cell divisions. B and C are composite micrograph images that were stitched together using Adobe Photoshop (B) or Microsoft Image Composite Editor (C). (Scale bars, 50 μm.) (C) Post-L1 diapause aak-1/2 adults that were subjected to 3 d in the diapause possess gonads with reduced sperm numbers based on DAPI staining (L4 + 24 h). (Scale bars, 10 μm.) (D) AMPK is critical for the maintenance of germ-line integrity through a pathway that is independent of its effects on essential postembryonic developmental processes. Animals that demonstrated any visible developmental defect were discarded before our brood-size analysis (Table S1) and only adults that survive 4 d post-L4 stage were compared in our brood-size analysis (“excluding early deaths”). “3”: 3-d duration in the L1 diapause, or “–”: not starved. Error bars: 95% CI. (E) Loss of AMPK signaling results in a reduction in brood size that becomes progressively worse with each subsequent generation following the acute starvation. This is not observed in 11-d starved wild-type or 3-d–starved daf-18 animals. Three different methods of selection (methods #1, #2, #3) were used (Materials and Methods) and under no circumstances did we observe transgenerational defects in the subsequent generations of post-L1 diapause wild-type or PTEN/daf-18 animals. (F) aak-1/2 mutants exhibit a progressive reduction in brood size with each successive generation, similar to a Mrt phenotype. Real brood-size quantification over multiple generations from seven independent post-L1 diapause aak-1/2 mutant parents (P0-I to P0-VII) that initially exhibited a reduced F1 brood size phenotype (method #1). Lineage P0-VI and P0-VII were totally extinguished by the F3 generation. (G) Brood-size defects typical of fertile post-L1 diapause AMPK mutant adults cannot be restored by crossing F4 or F5 post-L1 diapause aak-1/2 mutant lineage (P0-VIII) with nonstarved aak-1/2 males. A partial rescue is however observed in the progeny (*) of these F1 hermaphrodites generated from this generation is allowed to self-fertilize and yield an F2 generation. Error bars: 95% CI. (H) Prolonged durations without food in the L1 diapause did not confer a survival advantage for wild-type or aak-1/2 mutants when resubjected to starvation during the L1 stage of the subsequent F1 generations. F1 generation L1 larvae obtained from fertile post-L1 diapause adults that were subjected to 3 d in the diapause. F1* represents starved L1 obtained from post-L1 diapause aak-1/2 mutant parents that showed a reduced brood size phenotype. Mean ± SD of three independent experiments. Data were obtained from ≥three independent experiments, and ≥80 animals were scored for each experiment. (I) Wild-type (N2) L1 larvae were starved in M9 buffer for 11 d after which they were transferred to plates seeded with OP50 or bacteria expressing aak-2 dsRNA. Adult aak-2 (RNAi) animals were then singled to regular NGM plates with OP50. The fertility of the treated and nontreated parents (P0) and their F1 generation were then assessed. The assays was performed twice. Data represent the mean ± SD where n = 50 for each condition.
The observed reproductive defects could result from either somatic or germ-cell deficiencies that arise during or following the L1 diapause. To determine if the defects were a result of the integrity of the oocytes, the sperm, or both, we performed reciprocal crosses and assessed the brood size of the resulting cross progeny (Fig. 1F). When we mated AMPK/aak-1/2 mutant hermaphrodites that had never been starved with post-L1 diapause AMPK/aak-1/2 males, the resulting brood sizes were normal and we did not observe any significant increase in sterility, suggesting that the abundance or the quality of the sperm in post-L1 diapause AMPK/aak-1/2 adult hermaphrodites is not a major factor in the observed sterility. However, when the crosses were performed with post-L1 diapause AMPK/aak-1/2 mutant hermaphrodites and nonstarved male AMPK/aak-1/2 mutant animals, we noted that the brood-size defects could only be partially restored. A similar partial rescue was observed when nonstarved wild-type males were mated with post-L1 diapause AMPK/aak-1/2 mutant hermaphrodites. This finding suggests that only some of the reproductive defects (sterility or reduced brood size) of the post-L1 diapause AMPK/aak-1/2 mutants are derived from the sperm defects described above (Fig. 1E). These results collectively suggest that the germ line of the hermaphrodite is particularly sensitive to starvation during this period and that when the PGCs are affected, a defect occurs in the oocytes such that fertility cannot be fully restored through crossing with unaffected males. The memory of the starvation remains molecularly recorded and ultimately affects the integrity of the oocyte.
Transgenerational Reproductive Defects Arise in Post-L1 Diapause AMPK Mutants.
This phenomenon is consistent with epigenetic modifications that alter the chromatin, ultimately affecting cellular outcomes over the course of one or more cell divisions or even generations. To test whether a single bout of starvation during the L1 stage could result in transgenerational epigenetic defects in subsequent generations of progeny that never experienced the initial starvation, we carried out three distinct multigenerational analyses based on different hypotheses (Fig. 2 A and B and Fig. S2E). Using the first method (method #1 in Fig. 2A), we postulated that post-L1 diapause parents that have a reduced brood size (fewer than 100 F1 progeny) might have an increased chance of transmitting these epigenetic changes transgenerationally compared with post-L1 diapause parents that exhibit a normal brood size (more than 100 F1 progeny). To test this hypothesis, we followed animals through multiple generations by continuously selecting L4 larvae born from parents that produced a reduced brood size at each generation. The larvae were then allowed to develop, produce progeny, and their individual brood sizes were evaluated and recorded for each generation (Fx).
Fig. 2.
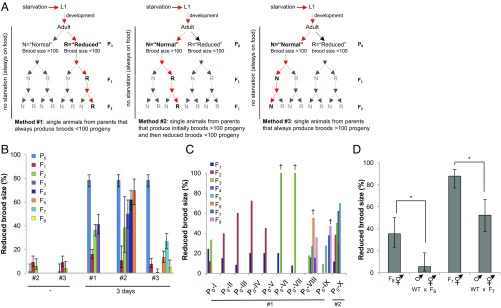
Post-L1 diapause AMPK mutants display variable transgenerational reproductive defects. (A) Schematic representation of the three selection methods used for our transgenerational analyses. Method #1: transgenerational analysis performed using fertile parents (P0) that have a reduced brood size of less than 100 F1 progeny. Methods #2 and #3: Multigenerational analyses were carried out first using parents that produce a normal brood size of more than 100 F1 progeny (Materials and Methods for additional details). Red arrows indicate the selection at each successive generation. (B) Loss of AMPK causes transgenerational reproductive defects following a short duration in the L1 diapause. The percentage of post-L1 diapause animals that generated descendants that bore smaller broods (≤100 progeny) after either no starvation (−), or 3 d in the diapause. For multigenerational analyses, the F1 progeny generated from reduced parents using method #1, or parents with a normal brood size using methods #2 and #3 were analyzed over multiple generations (Fig. S2E for additional information). Error bars: 95% CI. (C) aak-1/2 mutants exhibit a progressive transgenerational reduction in brood size. Phenotypic variability in brood size is inherent in subsequent generations analyzed from fertile post-L1 diapause aak-1/2 parents. Brood sizes were analyzed over multiple generations in the descendants of 10 independent post-L1 diapause aak-1/2 mutant parents. Brood-size defects were monitored in lineages P0-I to P0-IX using section method #1, whereas for comparison, the analysis in P0-X was performed using method #2. Descendants that showed spontaneous mutant phenotypes that did not subsequently breed true (Him, Lon, Dpy or Sma) are shown by a dagger (†). (D) The multigenerational reproductive defects that occur in post-L1 diapause aak-1/2 mutant animals arise because of adverse epigenetic changes that occur as a result of the absence of AMPK. aak-1/2 transgenerational brood-size defects are only partially rescued by the introduction of a wild-type copy of AMPK. Well-fed wild-type males (nonstarved) were crossed with aak-1/2 hermaphrodites that were selected from two independently maintained transgenerationally compromised lineages (P0-VIII and P0-IX) that produced smaller broods (≤100 progeny) after seven or eight generations following the initial diapause (F7 and F8, respectively). The brood sizes of cross progeny were determined and represented as the percentage of the total population of animals born from the same successful cross. Error bars: 95% CI; *P < 0.05 Fisher's exact test.
Alternatively, it is possible that all of the post-L1 diapause AMPK/aak-1/2 mutant adults are affected by the stress of starvation during the L1 diapause, regardless of whether the parents exhibit reproductive defects initially. We noted that some (20%) of the fertile post-L1 diapause AMPK/aak-1/2 mutant adults had normal broods, but their progeny fell into two categories: those that generated F2 broods that were reduced in size and those that produced normal F2 broods. By systematically selecting L4 larvae from each generation (Materials and Methods), we then tracked individuals from the reduced and normal categories and constructed multigenerational lineages that were based on whether they were initially selected from parents that had a reduced brood size (method #2), or whether they were generated from parents that had a normal brood size (method #3) (Fig. 2A; additional information in Materials and Methods).
All of the animals that were tracked through successive generations using method #3 generated progeny with comparatively less impact on brood size. The minor effects that did manifest in the early generations were resolved rapidly, but in later generations (F5–F9) reproductive defects did appear, despite our continuous selection of normal parents. This finding suggests that even though most of these post-L1 diapause parents were either phenotypically unaffected by the diapause, or that any initially occurring defects may have been resolved in the first generation, some heritable record of the initial event persisted and was manifested, albeit at low penetrance, up to nine generations after the initial event.
Alternatively, when we continuously selected post-L1 diapause AMPK/aak-1/2 descendants from parents that had reduced brood sizes using methods #1 or #2, we consistently observed transgenerational brood-size defects (Fig. 2B). Curiously, this transmissible brood-size defect occurred despite the fact that these successive generations of progeny had never been starved or, in some cases, whether their parents appeared reproductively compromised or not (method #2). Therefore, regardless of whether the initially affected aak-1/2 post-L1 diapause animals had a normal or reduced brood size, both types of parents are very likely to transmit this reduction in brood size to their descendants (Fig. 2B). This progressive transgenerational reduction in reproductive fitness is never observed in nonstarved AMPK/aak-1/2 mutant animals, in 3-d post-L1 diapause PTEN/daf-18 animals, or in 3-d or 11-d starved wild-type animals (Fig. S2E).
There is a substantial degree of variability in the brood-size defects between these parental lineages that may represent a graded threshold effect in response to the epigenetic stimulus (starvation). However, this variability seems typical of an epigenetic response where not all of the germ cells are affected by starvation in an identical way, potentially because of individual differences in availability to nutrient/energy levels within the PGCs. Nevertheless, most AMPK/aak-1/2 mutant animals that survive the acute stress associated with starvation in the L1 diapause will produce progeny that will eventually be adversely affected by the initial challenge, even though the affected animals in subsequent generations may never have experienced the stress directly.
To determine the extent to which each individual animal was affected we quantified the brood size of 10 individual fertile post-L1 diapause AMPK/aak-1/2 mutant parents and that of their progeny over several generations (Fig. 2C and Fig. S2F). Nine of these lineages (P0-I to P0-IX) were analyzed using method #1 and one lineage (P0-X) was analyzed using method #2. In each of the parental lineages analyzed by method #1 (P0-I to P0-IX) we observed a similar progressive reduction in brood size that varied in expressivity and in the delay that preceded either the correction of the reproductive defects or the eventual terminal arrest of the lineage. This progressive brood size reduction is similar to the progressive mortal germ-line phenotypes (Mrt) that remain fertile indefinitely at permissive temperature but become progressively sterile after growth for multiple generations at higher temperatures (27). These phenotypes are typically caused by mutations in genes involved in genome integrity or epigenetic regulation (27–30). Consistent with this, in four independent lineages (see † in Fig. 2C), we noted that animals became very sickly in late generations, segregating a high percentage of male progeny (Him) and other spontaneous mutant phenotypes [Dumpy (Dpy), Roller (Rol), Long (Lon), Small (Sma)]: in the F6 plates of P0-VIII and the F8 plates of lineage P0-IX, 9% and 14% of the animals segregate a strong Him phenotype in next generation; 6% and 14% segregate a Lon phenotype; whereas 3% and 5% give rise to a Dpy phenotype, respectively, before laying a large number of dead eggs. In the animals present on the F3 plates of the P0-VI and P0-VII lineages, up to 33% of the progeny segregate a Sma phenotype, 4–8% give rise to a Him phenotype, and 80–92% of the remaining viable progeny eventually arrested between the L1 and L3 stages.
We were able to correct the brood-size defects of post-L1 diapause AMPK/aak-1/2 mutant descendants from later generations in P0-VIII and P0-IX by crossing them with nonstarved wild-type (N2) males (Fig. 2D). However, although AMPK/aak-1/2 mutant males could partially suppress the brood-size defect in the first generation of affected animals born from post-L1 diapause AMPK/aak-1/2 mutant parents (Fig. 1F), crossing nonstarved AMPK mutant males with these later generation (F4 or F5) AMPK/aak-1/2 mutants did not suppress the brood-size defects in the resulting cross progeny (Fig. S2G; compare with Fig. 1F). These observations are consistent with an epigenetic phenomenon that affects germ-line integrity in the post-L1 diapause AMPK/aak-1/2 mutants that becomes progressively worse with each successive generation. Furthermore, although this transgenerational defect can be corrected by wild-type gene activity, the inability of nonstarved AMPK/aak-1/2 mutant males to rescue the transgenerational brood size defects of the post-L1 diapause AMPK/aak-1/2 mutant hermaphrodites suggests that AMPK activity may also be required to resolve these epigenetic modifications once they are established.
The possibility that the stress of an initial starvation event might confer increased resistance to a subsequent bout of starvation as an evolutionary trade-off was excluded because F1 animals obtained from fertile post-L1 diapause AMPK/aak-1/2 mutant adults exhibited similar L1 survival rates to animals that had never been previously starved. The F1 animals that arose from post-L1 diapause AMPK/aak-1/2 mutant parents with reduced broods did, however, die earlier than nonstarved mutant controls (Fig. S2H; see asterisks, F1*).
Our data therefore indicate that both PTEN/daf-18 and AMPK are required for survival and adult fertility in response to acute starvation during the early L1 stage. Whereas PTEN/daf-18 is required acutely in a pathway that is independent of AMPK, its effects are resolved in the first generation following the starvation. In AMPK/aak-1/2 mutants however, the reproductive consequences that manifest in the post-L1 diapause animals persist throughout multiple generations after the initial event resulting in the progressive extinction of the affected lineages.
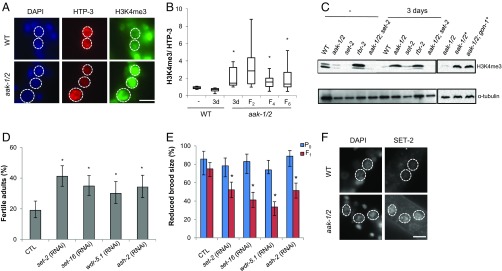
H3K4me3 Levels Are Abnormally High in the PGCs of Post-L1 Diapause AMPK Mutants.
Changes in chromatin modification have been linked to epigenetic transmission in several contexts (31). To determine whether chromatin marks are affected in AMPK/aak-1/2 mutants following starvation in the L1 stage and whether these marks persist in the subsequent affected generations, we examined the levels of various histone modifications in the PGCs of post-L1 diapause wild-type and mutant L1 larvae. Consistent with recent reports, the levels of phosphorylated H2B were reduced in starved AMPK/aak-1/2 mutants (18), whereas the levels of H3K4me3 were increased in the PGCs of starved AMPK/aak-1/2 mutants (Fig. 3 A and B and Fig. S3 A and B). Emergent L1 larvae in the subsequent F2, F4, and F6 generation derived from post-L1 diapause AMPK/aak-1/2 mutants also possess increased levels of H3K4me3 (Fig. 3B).
Fig. 3.
H3K4me3 levels accumulate and persist over multiple generations in the primordial germ cells of post-L1 diapause AMPK mutants. (A) H3K4me3 is increased in the PGCs of post-L1 diapause aak-1/2 mutants. Emergent wild-type and aak-1/2 L1 larvae were starved 3 d before fixation and immunostaining with H3K4me3 (green) and the PGC-specific marker HTP-3 (red) antibodies. Dashed lines mark the PGCs; note that the PGCs undergo supernumerary divisions in the aak-1/2 mutants, as previously described. (Scale bar, 5 μm.) (B) H3K4me3 is elevated through multiple generations in the progeny of post-L1 diapause aak-1/2 L1 mutants. Quantification of H3K4me3 levels normalized to HTP-3 signal following immunostaining in the PGCs of nonstarved (−), 3 d starved (3d), and in subsequent generations (F2, F4, and F6) of post-L1 diapause animals. n ≥ 8 different animals from which all PGCs were used for the quantification. *P < 0.05 (one-tailed t test). (C) The elevated H3K4me3 levels that arise in PGCs of starved aak-1/2 mutants occur in both the germ line and in the soma. The modification persists into later stages of development and is dependent on the SET-2 histone methyltransferase. Wild-type, aak-1/2, set-2, and rbr-2 post-L1 diapause larvae that spent 3 d in the diapause, or not (−), were recovered to replete plates and collected at the mid-L4 stage for immunoblot analysis using an anti-H3K4me3 antibody. Forty percent more post-L1 diapause (*) aak-1/2; gon-1(RNAi) animals were required to match the levels of the α-tubulin loading control. (D and E) Increased COMPASS complex activity contributes to sterility and brood-size defects in post-L1 diapause aak-1/2 mutants. Compromise of COMPASS complex components by dsRNA soaking during the period of starvation improves fertility (D), and the frequency of animals that exhibit brood size defects (E) in the F1 descendants of post-L1 diapause aak-1/2 mutants. Control animals (CTL) are aak-1/2 L1 larvae maintained 3 d in M9 buffer containing GFP dsRNA. Error bars: 95% CI; *P < 0.05 Fisher’s exact test, (D) n ≥ 150, from three independent experiments. (E) For P0 analysis n ≥ 40, and for the F1 analysis n ≥ 120 from three independent experiments. (F) PGCs of starved aak-1/2 mutants have abnormally high levels of SET-2. Immunostaining of SET-2 after 3 d of starvation in wild-type and aak-1/2 mutant L1 larvae. Animals were counterstained with DAPI. Dotted white lines delineate the boundaries of the PGC nuclei. (Scale bar, 5 μm.)
Fig. S3.
Post L1-diapause AMPK mutant animals accumulate H3K4me3 that is dependent on SET-2 histone methyltransferase. (A) Post-L1 diapause aak-1/2 L1 larvae have abnormally high levels of H3K4me3 in their PGCs. Wild-type and aak-1/2 L1 larvae were maintained either 1 or 3 d without food in the L1 diapause then immunostained with antibodies that recognize H3K4me3 and counterstained with DAPI. H3K4me3 levels were quantified and normalized to DNA content (DAPI) in the PGCs; n ≥ 15 different animals. *P < 0.05 (one-tailed t test). (B) H2B phosphorylation is induced by starvation and is AMPK-dependent. Western blot performed using antibodies against the H2 phosphorylated S36 epitope (ECM Biosciences) using whole-animal extracts from animals subjected to 3 d in the L1 diapause (“S” for starved) or nonstarved L1 extracts (“H” for healthy) treated with CIP phosphatase (+), or not (−), to show specificity for the phosphorylated isoform. (C and D) A misregulation of histone methyltransferase results in brood-size defects and premature lethality in post-L1 diapause AMPK mutants. L1 larvae of the following genotypes: set-2 (n4589) (a strong allele) or set-2 (ok952) (a weak allele), or the demethylase rbr-2 (tm1941), were subjected to varying periods in the L1 diapause then recovered on plates with food and grown to reproductive maturity. (C) The F1 brood size of resulting fertile adults was scored and represented in box plot format; n ≥ 40 per genotype per condition. (D) rbr-2 mutants phenocopy the premature adult death of post-L1 diapause aak-1/2 animals. Wild-type, aak-1/2, set-2, and rbr-2 L1 larvae were maintained in the L1 diapause for 3 d (*) or not starved (–), then recovered to replete plates and their post-L4 stage survival was scored every 2 d; n ≥100 animals per point. (E and F) Reducing the COMPASS activity by soaking animals in dsRNA that corresponds to each of the COMPASS components during the 3-d period in the diapause does not affect (E) the fertility of wild-type animals either in post-L1 diapause animals or, (F) the brood size of their subsequent F1 generation. Error bars: 95% CI; n ≥ 100 per condition from three independent experiments. (G) Two histone methyltransferases (SET-2 and SET-16) and associated components of the COMPASS complex are enriched for consensus AMPK phosphorylation sites. Peptide sequences were compared with the described AMPK consensus site described by Gwinn et al. (40) (AMPK motif) and aligned for comparison. There is a significant enrichment (Fisher exact test, P = 2.8 × 10−7) in proteins with at least one AMPK consensus phosphorylation recognition motif within the COMPASS complex. Red, phosphoacceptor site (Ser/Thr); yellow, hydrophobic residues; blue, basic residues; green, acidic residues. To perform Fisher’s exact test: 11,353 proteins were identified that have a single AMPK consensus site among a total of 20,405 Caenorhabditis elegans proteins (excluding isoforms) according to Wormbase release WS246. This enrichment is still valid even when protein size is taken into consideration: 24 of 11,353 AMPK consensus sites are found in COMPASS complex components (2.1%). This is significantly different from the number expected according to random distribution. All of the COMPASS components together contain 11,130 aa for a total of 8,378,701 aa (1.3%); exact binomial test P = 0.00211.
In many organisms H3K4me3 is catalyzed by a COMPASS-like complex, the catalytic core of which includes a histone methyltransferase of the Su(var)3-9/enhancer of zeste/trithorax domain protein (SET1)/mixed lineage leukaemia (MLL) family (32–34). The SET1/MLL orthologs in C. elegans are SET-2 and SET-16 (35, 36) and they exist in a large multisubunit assembly (34, 37). Mutations in the COMPASS-like complex exhibit somatic phenotypes that include disruptions in vulva development by attenuating LET-60/RAS signaling, and a reduced brood size (35, 36). We noted that increases in H3K4me3 levels, such as those seen in mutants that harbor mutations in rbr-2: a member of a family of the histone demethylases involved in removing methyl groups from H3K4, lead to somatic defects, reduced brood size, and premature adult death (38). These phenotypes mirror those we observed in our post-L1 diapause AMPK/aak-1/2 mutants, suggesting that the misregulation of H3K4me3 levels may be linked to the observed defects (Fig. S3 C and D).
Consistent with the increases in H3K4me3 observed in the PGCs of the starved AMPK/aak-1/2 mutants, we also observed a global increase in H3K4me3 in later-stage (L4) post-L1 diapause animals, suggesting that early modifications that occur in the PGCs are not reduced to a baseline threshold more typical of wild-type upon the onset of postembryonic development, but rather remain abnormally elevated (Fig. 3C). The increased H3K4me3 levels were not unique to the germ cells, but also occurred in the soma. Larvae that were subjected to gon-1(RNAi), which compromises germ-cell proliferation without affecting nongonadal cells (39), retained significantly high levels of H3K4me3 despite a reduction in the number of germ cells at this stage (Fig. 3C).
If the abnormal accumulation of H3K4me3 in the PGCs during starvation is responsible for the fertility defects and reduced brood size in the post-L1 diapause AMPK/aak-1/2 mutants, then reducing their levels should suppress the reproductive defects seen in both post-L1 diapause AMPK/aak-1/2 mutants and their descendants. Consistent with a misregulation of the COMPASS complex in the observed starvation-dependent sterility, soaking emergent L1 larvae in buffer containing double-stranded RNA (dsRNA) corresponding to any one of the COMPASS components set-2, set-16, ash-2, or wdr-5.1 for a 3-d period during the L1 diapause was sufficient to partially suppress both the sterility of the post-L1 diapause AMPK/aak-1/2 mutant larvae and the reduced brood size observed in the F1 generation. Conversely, the same treatment did not affect the fertility or the brood size of wild-type animals (Fig. 3 D and E and Fig. S3 E and F). Furthermore, the compromise of set-2 was sufficient to dramatically reduce the global H3K4me3 levels in the post-L1 diapause AMPK/aak-1/2 mutant larvae (Fig. 3C).
Curiously, most of the vulval defects of starved AMPK/aak-1/2 mutants were not suppressed when L1 larvae were treated with dsRNA corresponding to set-2 or set-16, suggesting that other H3K4me3-independent pathways may be disrupted in the post-L1 diapause AMPK/aak-1/2 larvae (Table S1). Although the suppression that we achieve by removing components of the COMPASS complex is partial, this may reflect our inability to remove all of the target protein through RNAi. On the other hand we cannot rule out that other factors that may contribute to the observed defect that are not downstream of inappropriate histone methyltransferase activity.
AMPK has been demonstrated to drive adaptive metabolic adjustment to energy stress by phosphorylating key cellular proteins, ultimately blocking anabolic pathways while simultaneously enhancing pathways involved in energy production or conservation. Bioinformatic analysis revealed that all of the members of the COMPASS-like complex components, except DPY-30, possess amino acid signatures that match the optimal AMPK substrate motif (Fig. S3G) (40). This finding represents a significant enrichment of these sites within this complex, suggesting that AMPK may regulate the activity of the complex during environmental challenges. Despite our efforts we could never acquire the quantity of PGCs to confirm this possibility biochemically. Moreover, the transient nature of the phosphorylation makes it unlikely that it would be present in the germ cells of later-stage post-L1 diapause larvae/adults.
To determine if AMPK might affect COMPASS function within the starved PGCs by altering its abundance, we examined the levels of SET-2 in the PGCs in starved L1 mutant larvae to assess if its stability or its localization might be affected in an AMPK-dependent manner. We noted that SET-2 levels were consistently higher in the PGCs of starved L1 larvae that lack AMPK compared with starved wild-type PGCs, indicating that AMPK compromise may affect its accumulation in the PGC nuclei and consequently increase the levels of H3K4me3 in the germ cells of the starved AMPK/aak-1/2 mutants (Fig. 3F).
par-5 Acts with AMPK to Ensure Germ-Line Integrity in a Manner That Is Independent of set-2.
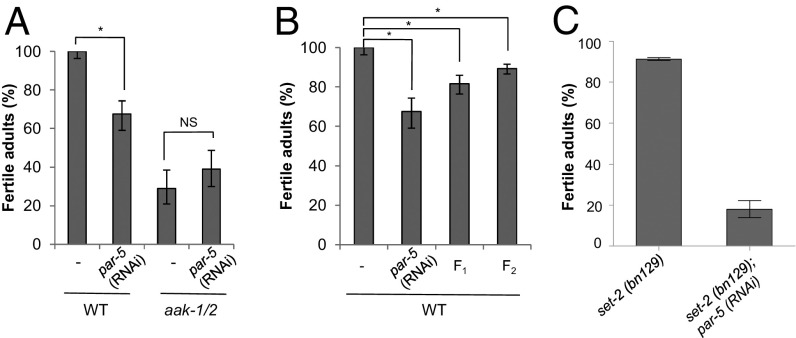
AMPK commonly regulates its targets by generating phosphorylated motifs that are recognized and bound by 14-3-3 proteins (40, 41). The 14-3-3 proteins are involved in many diverse biological processes and affect their targets by multiple mechanisms. If AMPK-dependent phosphorylation of SET-2 or other components of the COMPASS complex results in recognition of the phosphotargets by 14-3-3 proteins to mediate its effects downstream of nutrient/energy stress, then loss of the C. elegans 14-3-3 proteins should recapitulate the phenotypes typical of AMPK compromise in post-L1 diapause animals. In C. elegans there are two predicted 14-3-3 proteins: ftt-2 and par-5, but only par-5 is expressed in the germ line (42, 43). Because par-5 is an essential gene we performed RNA soaking experiments with dsRNA corresponding to par-5 during the L1 diapause. Post-L1 diapause wild-type larvae subjected to par-5(RNAi) exhibited pronounced sterility compared with control animals, and this effect could not be compounded by removing AMPK function, indicating that the two gene products likely work in a linear genetic pathway to affect adult fertility following starvation (Fig. 4A). In addition, the fertility of both the F1 and F2 generation descendants that were derived from post-L1 diapause wild-type animals subjected to par-5(RNAi) were also affected (Fig. 4B). Therefore, the effect of the single par-5(RNAi) treatment during the L1 diapause spanned at least two generations. The similarity of the par-5(RNAi) phenotype to that of the observed AMPK post-L1 diapause mutants, and the observation that the compound mutations are not additive, is consistent with a role for PAR-5 in mediating the ability of AMPK to protect germ-line integrity during periods of starvation.
Fig. 4.
The 14-3-3 protein PAR-5 acts with AMPK, but in parallel to the COMPASS complex, to protect germ-line integrity. (A) Wild-type and aak-1/2 mutant L1 larvae were hatched then maintained in M9 containing par-5 dsRNA for 3 d before recovery on food. Adult fertility was assessed in control nonstarved and par-5(RNAi) animals; n = 100. Error bars: 95% CI; *P < 0.05 Fisher’s exact test; NS, nonsignificant. (B) The 14-3-3 compromise results in transgenerational reproductive defects. Starved wild-type L1 larvae subjected to par-5(RNAi) soaking or not (−) for 3 d were recovered and allowed to produce F1 and F2 progeny. The number of fertile adult animals in each generation was evaluated and represented; n ≥ 250. Errors bars: 95% CI; *P < 0.05 Fisher’s exact test. (C) set-2 and par-5 function in parallel pathways to establish or maintain post-L1 diapause germ-line integrity. Genetic analysis was performed by soaking set-2(bn129) mutants in dsRNA corresponding to par-5, as described above.
If AMPK triggers a PAR-5–mediated response to modulate COMPASS activity through its regulation of SET-2 accumulation in the PGC nuclei, then the complete removal of set-2 from par-5–compromised starved animals should suppress any inappropriate H3K4me3 caused by accumulation of SET-2, and ultimately improve the fertility of post-L1 diapause mutant animals. This is, however, not what we observe in the set-2; par-5(RNAi) animals, which exhibit a substantially greater frequency of sterility than either single mutant alone following a 3-d period in the L1 diapause (Fig. 4C). These data strongly suggest that par-5 and set-2 do not function in a linear genetic pathway, but rather the two gene products work independently of one another, whereby both are dependent on AMPK function. Therefore, in response to acute starvation AMPK regulates at least two different pathways that ultimately affect germ-line integrity, and its absence during the L1 diapause has reproductive consequences that can persist through multiple generations. One branch appears to be mediated by the 14-3-3 protein PAR-5, which likely targets effectors that have yet to be elucidated, whereas a second branch impinges on the chromatin-writing COMPASS complex.
We propose that during starvation AMPK blocks the catalysis of H3K4me3 in the two PGCs through its ability to modify one or more of the COMPASS components (SET-16, SET-2, ASH-2, WDR-5.1). In post-L1 diapause AMPK mutant larvae, the consequent increase of H3K4me3 in the PGCs is associated with a progressive transgenerational deterioration in reproductive fitness.
Misregulated Transcriptional Elongation Contributes to the Sterility of Post-L1 Diapause AMPK Mutants.
The early stages of animal development are critical for reading and writing new epigenetic information directed by maternal and/or paternal instruction (44, 45). How this information is transduced to affect gene expression in response to a given stimulus is currently speculative at best. During starvation in C. elegans L1 larvae, a specific gene-expression program is initiated (46) and during this period RNA polymerase II is observed in different configurations: in the “docked” state RNA polymerase II is on proximal promoters waiting for cues to initiate, whereas in the “paused” configuration the complex remains in a postinitiated state, presumably on hold until a physiological or developmental contingency is satisfied (47, 48).
This developmental pausing is not exceptional and in many organisms RNA polymerase II is maintained in a postinitiated state where subsequent elongation is regulated by factors that associate with the phosphorylated carboxyl-terminal domain of the RNA polymerase II large subunit (49). In the PGCs the role of carboxyl-terminal domain phosphorylation appears to be less critical than in the somatic cells, whereas orthologs of some of the critical known elongation regulators are absent from the C. elegans genome, suggesting that regulation of elongation is independent of many of the mechanisms described in somatic cells (50). The COMPASS complex may nevertheless contribute to this transcriptional switch in the PGCs in a manner that is independent of the soma-specific controls and therefore must be neutralized in the PGCs during starvation.
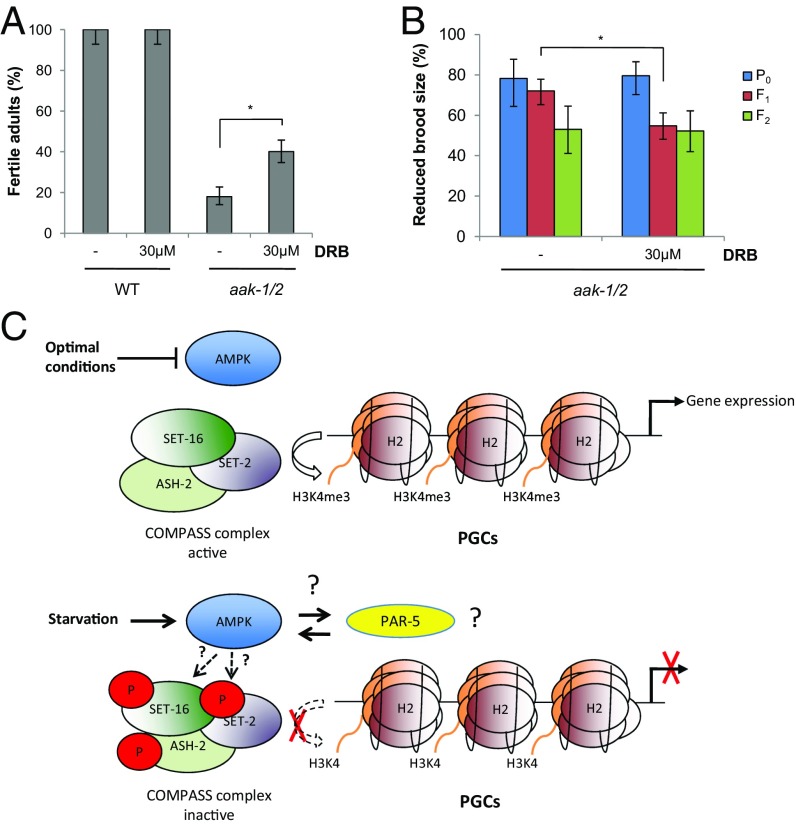
To determine if the defects observed in AMPK-compromised starved animals are related to the link between increased H3K4me3 and aberrant resumption of transcriptional elongation, we blocked any elongating complexes during the period of starvation using 5,6-dichloro-1-β-d-ribofuranosylbenzimidazole (DRB), a potent, highly specific, elongation inhibitor (51, 52) and quantified the effects on fertility in the treated animals and on the brood-size defects in subsequent generations. Treatment of starved AMPK/aak-1/2 mutant L1 stage larvae with DRB partially suppressed the sterility observed in post-L1 diapause AMPK/aak-1/2 mutant adult hermaphrodites by 49% (n = 100) (Fig. 5A), without affecting the fertility of treated post-L1 diapause wild-type. Moreover, consistent with AMPK and PTEN affecting two independent pathways that control PGC integrity, DRB treatment had no effect on the sterility observed in post-L1 diapause PTEN/daf-18 mutants (Fig. S1H).
Fig. 5.
Inappropriate transcriptional elongation during periods of starvation contributes to the reproductive defects seen in AMPK mutants. (A) The elongation inhibitor DRB can partially suppress the sterility observed in post-L1 diapause AMPK mutant hermaphrodites. Emergent wild-type and aak-1/2 larvae were maintained in M9 buffer containing 30 μM DRB or not (−) for 3 d before recovery on replete plates. The proportion of fertile adult animals is represented, n ≥ 150. Error bars: 95% CI; *P < 0.05 Fisher’s exact test. (B) Transgenerational brood-size defects are resolved more efficiently in the progeny of post-L1 diapause AMPK mutant larvae that were treated with DRB during the period of starvation. The frequency of animals with reduced brood size in aak-1/2 mutants and in the subsequent generation (F1 and F2) is represented, n ≥ 50. Errors bars: 95% CI; *P < 0.05 by Fisher’s exact test. (C) AMPK affects quiescence and integrity of the PGCs during acute starvation. During replete conditions, AMPK is inactive permitting the COMPASS complex to actively methylate H3K4, thereby enhancing transcriptional elongation. During periods of acute starvation, AMPK becomes activated and targets components of the COMPASS complex, effectively blocking the histone methyltransferase and ultimately inhibiting transcriptional elongation, and hence germ-line gene expression. In addition, AMPK functions with 14-3-3/PAR-5 to affect germ-line integrity through a SET-2–independent pathway.
The brood-size defects typical of the F1 generation of post-L1 diapause AMPK mutants were also reduced, but the effect in the F2 generation is also accompanied by a significant number of affected F1 progeny animals that resolve their brood-size defects, making it difficult to conclude whether the transgenerational defects are indeed dependent on aberrant elongation in the starved PGCs of AMPK mutants (Fig. 5B). Nevertheless, by specifically targeting the aberrant elongating complexes we were able to correct many of the reproductive defects typical of the post-L1 diapause AMPK/aak-1/2 mutants.
Discussion
By regulating its various protein targets, AMPK ensures that the PGCs respond efficiently to starvation during the L1 diapause by eliciting both cell cycle and developmental/reproductive quiescence. In its absence, the nutrient/energy contingency is relaxed, allowing a critical chromatin writer, such as the COMPASS complex, to aberrantly activate a postembryonic transcriptional program in the PGCs during a period when all cells should remain quiescent (Fig. 5C).
It is unclear why the chromatin modifications that arise in the post-L1 diapause AMPK mutants cannot be resolved in subsequent generations, but instead accumulate to progressively extinguish the germ line. The heritable effects of set-2 mutations on lifespan are largely resolved in a limited number of generations (53). In contrast, the transgenerational defects of post-L1 diapause AMPK mutant descendants often worsen and cannot be corrected by crossing with mutants that were never starved. In addition, the sterility observed in wild-type animals that were subjected to an 11-d L1 diapause is completely corrected in the next generation. However, if AMPK is reduced in these animals as they grow to adulthood, the fertility of the next generation of animals is visibly affected, albeit not significantly (Fig. S2I). This finding suggests that similar epigenetic modifications can occur even in wild-type animals after severe starvation, and AMPK may be important for the resolution of these modifications in the subsequent generation. In the absence of AMPK function, these chromatin modifications become more abundant and can be transmitted to subsequent generations. The germ-line defects we describe herein resemble those seen in spr-5 mutants that have compromised demethylase function, where the germ line becomes mortalized over several generations (28, 54). Understanding the mechanism through which AMPK functions with PAR-5 to ensure PGC integrity may provide some insight regarding the potential targets that exacerbate the progressive germ-line extinction that occurs in the descendants of post-L1 diapause AMPK mutants.
We have shown that during periods of acute starvation in the L1 stage, the absence of AMPK results in the inappropriate writing of epigenetic marks that compromise germ-line integrity because of this change in gene expression. The L1 diapause, and potentially other diapause states, such as the dauer, may be critical developmental buffer zones, allowing the animal to couple appropriate stasis of the chromatin landscape to environmental conditions. Our data suggest that AMPK is a pivotal molecular link to couple the chromatin environment and consequent adaptive changes in gene expression, with the metabolic status of the animal to ensure proper regulation of gene expression during this extended developmental hiatus. Recent data have demonstrated that starvation can induce the expression of specific RNAs that may provide some selective advantage in subsequent generations (10). It will be particularly exciting to determine if the expression of this suite of RNAs may be coupled to AMPK function to trigger a heritable adaptive change in the germ line during periods of severe energy stress.
Our data delineate how an AMPK-mediated adaptation during acute starvation includes the buffering of epigenetic writers and is critical to preserve germ-line integrity. This is presumably achieved by adjusting energy resources accordingly in part by limiting enzyme activities that could otherwise compromise the fitness of the animal. How general this AMPK function may be is currently unknown, nor can we speculate on the limits of its buffering capacity. At present, there are no polymorphisms that have been characterized that affect AMPK function and that are associated with the affected Hongerwinter victims or their children. However, our findings suggest that failure at any level of this complex cascade downstream of AMPK activation could have far-reaching implications. Variations in an AMPK-mediated response could potentially account for the frequency and spectrum of observed genetic and epigenetic anomalies that manifest in these individuals and in other survivors of famine.
Materials and Methods
C. elegans Strains and Culture.
C. elegans were cultured at 20 °C on OP50, as previously described (55) unless otherwise indicated. Strains used in this study include: Bristol N2 wild type, aak-1(tm1944)I, aak-2(ok524)X, daf-18 (ok480)IV, raga-1(ok386)II, age-1(hx546)II, set-2(ok952)III, set-2(n4589)III, set-2(bn129)III, rbr-2(tm1231)IV.
Postrecovery Defects Following the L1 Diapause.
The L1 starvation assay was adapted from previously described protocols (56, 57). Briefly, animals were fed with OP50 for at least five generations before any analysis. Gravid hermaphrodites were treated with alkaline hypochlorite and the resulting eggs were hatched and cultured in sterile M9 medium in 15-mL tubes with rotation at 20 °C for the duration of the diapause. After 24 h, the density of newly hatched L1 larvae was adjusted to 6–10 L1 larvae per microliter. To determine viability, 10-μL aliquots were distributed on 6-cm nematode growth medium (NGM) plates seeded with OP50 every 48 h and the number of L1 larvae was counted (initial). The following day, the number of moving animals was recorded as viable, and the survival rate was calculated as viable animals per initial number seeded. This experiment was repeated twice per time point. A minimum of three independent experiments was performed per analysis; the average survival at each time point was determined and used to generate the survival curve where error bars indicate SD.
Analysis of Fertility, Brood Size, and Viability.
Newly hatched larvae were maintained in the absence of food and every 48 h groups of L1 larvae were transferred to OP50-seeded NGM plates and maintained at 20 °C until the L2 stage, when they were singled onto seeded NGM plates and allowed to grow to adulthood. Larval growth and other postembryonic developmental phenotypes (Bag, Burst, Evl, Muv) were monitored. Fertility and other phenotypic abnormalities (presence of dead embryos, males, long adults, premature death) were assessed up until 1-wk post-L4 stage. All experiments were performed at least three times; 200–800 recovered animals were analyzed per genotype per time point were monitored.
Brood-size analysis was performed by counting progeny born from fertile parents of varying genotype. Fertile post-L1 diapause AMPK mutant adults were either parents that generated a reduced brood size (fewer than 100 F1 progeny), or those parents that produced a normal brood size (more than 100 F1 progeny). To quantify brood size, post-L1 diapause hermaphrodites were allowed to develop to the L4, after which they were singled to plates and were allowed to reproduce and were transferred daily throughout their entire reproductive period (3 d). The number of F1 offspring that were present on each plate that reached the L4/young adult stage was thereafter counted, allowing us to infer whether the parent had a normal or reduced brood size. Fifty to 150 animals were analyzed per genotype per time point from three independent experiments.
The viability of post-L1 diapause hermaphrodites was scored every 48 h following the L4 stage after varying durations in the diapause (0, 1, 3, 11 d); 100–300 animals were evaluated from three independent experiments.
Progressive Brood-Size Reduction in Individual Parental Lineages.
Ten independent post-L1 diapause aak-1/2 mutant lineages were analyzed independently. For each lineage, the entire population of F1 progeny from post-L1 diapause aak-1/2 fertile parents (P0) was singled onto fresh plates with food. Brood-size defects were quantified from individual fertile parents by counting progeny. Plates with animals that exhibited a reduced brood size were kept to continue the analysis and 100–200 individuals per parental lineage were singled every generation onto replete plates. The transgenerational progeny obtained from individual starting lineages were maintained separately for the duration of the analysis. In the final generations of several lines, the animals became very sick and the proportions of plates that possessed animals that showed a Him, Lon, Dpy, or Sma were assessed.
Transgenerational Rescue.
To determine the role of sperm in the post-L1 diapause reproductive defects 3-d post-L1 diapause aak-1/2 L4 hermaphrodites were crossed with healthy nonstarved aak-1/2 males, and the reciprocal cross was also performed. The resulting F1 brood size was evaluated and the percentage of parents that exhibit a reduced brood size was calculated from plates with successful crosses. A minimum of 40 successful crosses was analyzed. To determine the ability of wild-type gene copies to rescue observed reproductive defects in our transgenerational rescue experiments, L4 hermaphrodites from late-generation (F7 or F8) post-L1 diapause aak-1/2 mutants that exhibited severe brood-size defects were crossed with healthy nonstarved wild-type or aak-1/2 males. Reproductive defects were determined in the resulting cross progeny, as described above.
RNAi Soaking During the L1 Diapause.
dsRNAs that corresponded to set-2, set-16, ash-2, wdr-5, par-5, and GFP (as a control), were prepared as described previously (58). Wild-type and aak-1/2 L1 larvae were obtained by alkaline hypochlorite treatment and were maintained in M9 buffer containing dsRNA of the query gene (1–1.5 μg/μL) during the 3-d diapause. L1 larvae were then transferred to seeded NGM plates to recover to the L4 stage. Subsequently, 50 larvae were collected for each dsRNA tested, maintained at 20 °C for 3 or 4 d, after which their fertility and brood size were scored. Adults were sterile or fertile, whereas fertile animals could either be “reduced brood size” (<100 F1), or “normal brood size” (>100 F1). For transgenerational analysis (from F1 to F3), 50 F1 L4-stage larvae obtained from parents with reduced brood size were singled to seeded NGM plates and their fertility and brood size were scored as above. Each experiment was performed three independent times.
DRB Treatment.
We determined our working concentration of 30 μM DRB through a typical LE50 drug dosage. Wild-type and aak-1/2 L1 larvae obtained by alkaline hypochlorite treatment, were maintained in M9 buffer containing 30 μM DRB, or not, during the 3-d duration in the diapause. Following the starvation, postdiapause L1 larvae were transferred to seeded NGM plates and when they reached the L4 stage 50 larvae per condition were isolated and incubated at 20 °C for 3 or 4 d to assess their fertility and brood size. The reproductive defects fall into three different categories: sterile, fertile with reduced brood size (≤100 F1), or fertile with normal brood size (>100 F1). For transgenerational analysis (from F1 to F3), 50 F1 animals at the L4 stage, obtained from parents with reduced brood size, were singled onto NGM plates seeded with OP50, and their fertility and brood size were scored as described above. Each experiment was performed minimally three independent times.
SI Materials and Methods
Immunofluorescence Microscopy and Quantification.
DAPI and immunostaining were performed as described previously (59) with some modifications: L1 larvae were first fixed in paraformaldehyde, freeze-cracked, and then immersed in cold MeOH. Primary antibodies were used at the following dilutions: purified guinea-pig and rabbit anti-HTP-3 (1:500, 1:300; a gift from M. Zetka, McGill University, Montreal) (59), rabbit anti-SET-2 (1:200; a gift from S. Strome, University of California, Santa Cruz, CA) (35), rabbit anti-H3K4me3 (1:500; Abcam, cat#ab8580), and rabbit anti-H2B phospho S36 (1:500; Cell Signaling, BL7546). The following secondary antibodies were used at a dilution of 1:500: Alexa Fluor-488 anti-rabbit, Alexa Fluor-555 anti-guinea pig, Alexa Fluor-555 anti-mouse (Molecular Probes).
For the quantification of PGC fluorescence, images were taken at 63× magnification as stacks covering the entire thickness of the PGCs using a Zeiss Axioplan (Imager Z1 microscope coupled to a HAMAMATSU Digital C4742-95 camera). Histone fluorescence intensity was measured on each PGC using the ImageJ software package as described previously (30). Measurements were taken from 8 to 15 gonads for each genotype and averaged.
Western Blot Analysis.
Animals were grown synchronously to appropriate stages and washed with M9 buffer. L1 protein extracts were sonicated 11 times for 10 s at ∼11W in PBST, then centrifuged at 16,500 × g at 4 °C for 10 min to collect the supernatant. L4 protein extracts were sonicated in HB buffer (15 mM Hepes pH7.6, 10 mM KCl, 1.5 mM MgCl2, 0.1 mM EDTA, 0.5 mM EGTA, 44 mM sucrose, 1 mM DTT, and protease inhibitors) 10 times for 10 s at 15W. Protein concentrations were measured by absorbance to calibrate gel loading. Nitrocellulose membranes were incubated with primary antibodies: rabbit anti-H3K4me3 (1:1,000; Diagenode CS-003-100); mouse anti–α-tubulin (1:5,000; Sigma); Proteins were visualized using horseradish peroxidase conjugated anti-rabbit or anti-mouse secondary antibody (Bio-Rad), and ECL Plus (Amersham Biosciences).
Transgenerational Analysis.
Our transgenerational analyses were performed according to three different selection methods to rigorously test whether a single exposure to starvation during the L1 stage results in reproductive defects in the descendants of these starved animals throughout multiple generations. The three independent methods were based on the severity of the reproductive defects in the post-L1 diapause hermaphrodite parents (see details of Fig. 2A).
In the first selection (method #1), we postulated that post-L1 diapause parents that have a reduced brood size might have a higher probability of transmitting transgenerational defects compared with post-L1 diapause parents with a normal brood size. Therefore, in method #1 we assessed brood size in descendants from parents that had a reduced number of progeny (reduced). In each subsequent generation we selected descendants that, like their reduced parents, also had a reduced brood size.
After 3 d in the L1 diapause, L1 larvae were subsequently placed on OP50 and after 6 d their fertility and brood size were evaluated as described above. This allowed us to define the parents as reduced or normal. We then selected four representative plates from among the plates of F1 progeny obtained from parents that had reduced broods. We then singled 50 F1 L4 larvae to individual plates and allowed them to reproduce (200 individual animals from four reduced lineages). The number of F2 progeny generated by each F1 descendant was evaluated. Again, four representative plates were selected from among the plates of F2 progeny that exhibited a reduced F2 brood size. From these plates 50 F2 L4 larvae were singled to individual plates and were allowed to reproduce (200 individual animals from 4 reduced lineages) an F3 brood. These steps were repeated successively such that the brood size of 200 individual animals (4 plates of 50 individuals) was quantified at each generation.
In the other selection methods (methods #2 and #3) we tested whether visibly healthy normal animals might nevertheless be affected epigenetically by the acute starvation during the L1 diapause. To test this possibility, we selected visibly normal parents (those that generated more than 100 F1 progeny) and then, using a similar multigenerational analysis as described above, we then selected F2 progeny that were generated from an F1 parent that showed a reduced brood size (method #2). We then exclusively selected progeny from parents that have a reduced brood size (4 plates of 50 individuals) at every generation, just as we described in method #1.
In method #3 we began our analysis using parents with a normal brood size that appeared unaffected to determine if these normal animals would ever resegregate animals with brood-size defects. For selection method #3 (transgenerational analysis from apparently visibly normal parents), 50 L4 F1 larvae were singled from four plates that harbored normal parents based on the above criteria. These animals were allowed to reproduce and their brood size was evaluated by counting F2 generation animals. Four plates that harbored normal parents were selected for subsequent transgenerational analysis. The adult F2 progeny were allowed to produce eggs, and 50 F3 L1 larvae from of each of these plates were singled and their fertility was scored. This analysis was repeated until the F9 generation.
Acknowledgments
We thank Florence Couteau, Monique Zetka, Brandon Faubert, Rusty Jones, Bill Kelly, Francesca Palladino, Susan Strome, Vincent Calcagno, and Anne Brunet for strains, reagents, and discussion; and Victor Ambros, Monique Zetka, and Stephanie Crane-Weber for critically reading the manuscript. Many of the strains used here were generated or provided by the Gene Knockout Consortium or the Caenorhabditis Genetics Center, respectively. This work was supported by funding from the Canadian Institutes of Health Research.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1616171114/-/DCSupplemental.
References
- 1.Ahmed F. Epigenetics: Tales of adversity. Nature. 2010;468(7327):S20. doi: 10.1038/468S20a. [DOI] [PubMed] [Google Scholar]
- 2.Stein AD, Zybert PA, van de Bor M, Lumey LH. Intrauterine famine exposure and body proportions at birth: The Dutch Hunger Winter. Int J Epidemiol. 2004;33(4):831–836. doi: 10.1093/ije/dyh083. [DOI] [PubMed] [Google Scholar]
- 3.Roseboom T, de Rooij S, Painter R. The Dutch famine and its long-term consequences for adult health. Early Hum Dev. 2006;82(8):485–491. doi: 10.1016/j.earlhumdev.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Heijmans BT, et al. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci USA. 2008;105(44):17046–17049. doi: 10.1073/pnas.0806560105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tobi EW, et al. Prenatal famine and genetic variation are independently and additively associated with DNA methylation at regulatory loci within IGF2/H19. PLoS One. 2012;7(5):e37933. doi: 10.1371/journal.pone.0037933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lumey LH, Stein AD, Susser E. Prenatal famine and adult health. Annu Rev Public Health. 2011;32:237–262. doi: 10.1146/annurev-publhealth-031210-101230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelly WG. Transgenerational epigenetics in the germline cycle of Caenorhabditis elegans. Epigenetics Chromatin. 2014;7(1):6. doi: 10.1186/1756-8935-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seth M, et al. The C. elegans CSR-1 argonaute pathway counteracts epigenetic silencing to promote germline gene expression. Dev Cell. 2013;27(6):656–663. doi: 10.1016/j.devcel.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wedeles CJ, Wu MZ, Claycomb JM. Protection of germline gene expression by the C. elegans Argonaute CSR-1. Dev Cell. 2013;27(6):664–671. doi: 10.1016/j.devcel.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 10.Rechavi O, et al. Starvation-induced transgenerational inheritance of small RNAs in C. elegans. Cell. 2014;158(2):277–287. doi: 10.1016/j.cell.2014.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jobson MA, et al. Transgenerational effects of early life starvation on growth, reproduction, and stress resistance in Caenorhabditis elegans. Genetics. 2015;201(1):201–212. doi: 10.1534/genetics.115.178699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hong Y, Roy R, Ambros V. Developmental regulation of a cyclin-dependent kinase inhibitor controls postembryonic cell cycle progression in Caenorhabditis elegans. Development. 1998;125(18):3585–3597. doi: 10.1242/dev.125.18.3585. [DOI] [PubMed] [Google Scholar]
- 13.Baugh LR, Sternberg PW. DAF-16/FOXO regulates transcription of cki-1/Cip/Kip and repression of lin-4 during C. elegans L1 arrest. Curr Biol. 2006;16(8):780–785. doi: 10.1016/j.cub.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 14.Fukuyama M, et al. C. elegans AMPKs promote survival and arrest germline development during nutrient stress. Biol Open. 2012;1(10):929–936. doi: 10.1242/bio.2012836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fukuyama M, Rougvie AE, Rothman JH. C. elegans DAF-18/PTEN mediates nutrient-dependent arrest of cell cycle and growth in the germline. Curr Biol. 2006;16(8):773–779. doi: 10.1016/j.cub.2006.02.073. [DOI] [PubMed] [Google Scholar]
- 16.Lee I, Hendrix A, Kim J, Yoshimoto J, You YJ. Metabolic rate regulates L1 longevity in C. elegans. PLoS One. 2012;7(9):e44720. doi: 10.1371/journal.pone.0044720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hardie DG, Ross FA, Hawley SA. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13(4):251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bungard D, et al. Signaling kinase AMPK activates stress-promoted transcription via histone H2B phosphorylation. Science. 2010;329(5996):1201–1205. doi: 10.1126/science.1191241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Celenza JL, Carlson M. A yeast gene that is essential for release from glucose repression encodes a protein kinase. Science. 1986;233(4769):1175–1180. doi: 10.1126/science.3526554. [DOI] [PubMed] [Google Scholar]
- 20.Lee JH, et al. Energy-dependent regulation of cell structure by AMP-activated protein kinase. Nature. 2007;447(7147):1017–1020. doi: 10.1038/nature05828. [DOI] [PubMed] [Google Scholar]
- 21.Apfeld J, O’Connor G, McDonagh T, DiStefano PS, Curtis R. The AMP-activated protein kinase AAK-2 links energy levels and insulin-like signals to lifespan in C. elegans. Genes Dev. 2004;18(24):3004–3009. doi: 10.1101/gad.1255404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Narbonne P, Roy R. Inhibition of germline proliferation during C. elegans dauer development requires PTEN, LKB1 and AMPK signalling. Development. 2006;133(4):611–619. doi: 10.1242/dev.02232. [DOI] [PubMed] [Google Scholar]
- 23.Narbonne P, Roy R. Caenorhabditis elegans dauers need LKB1/AMPK to ration lipid reserves and ensure long-term survival. Nature. 2009;457(7226):210–214. doi: 10.1038/nature07536. [DOI] [PubMed] [Google Scholar]
- 24.Masse I, Molin L, Billaud M, Solari F. Lifespan and dauer regulation by tissue-specific activities of Caenorhabditis elegans DAF-18. Dev Biol. 2005;286(1):91–101. doi: 10.1016/j.ydbio.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 25.Watanabe S, Yamamoto TG, Kitagawa R. Spindle assembly checkpoint gene mdf-1 regulates germ cell proliferation in response to nutrition signals in C. elegans. EMBO J. 2008;27(7):1085–1096. doi: 10.1038/emboj.2008.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Francis R, Barton MK, Kimble J, Schedl T. gld-1, a tumor suppressor gene required for oocyte development in Caenorhabditis elegans. Genetics. 1995;139(2):579–606. doi: 10.1093/genetics/139.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmed S, Hodgkin J. MRT-2 checkpoint protein is required for germline immortality and telomere replication in C. elegans. Nature. 2000;403(6766):159–164. doi: 10.1038/35003120. [DOI] [PubMed] [Google Scholar]
- 28.Katz DJ, Edwards TM, Reinke V, Kelly WG. A C. elegans LSD1 demethylase contributes to germline immortality by reprogramming epigenetic memory. Cell. 2009;137(2):308–320. doi: 10.1016/j.cell.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meier B, et al. The MRT-1 nuclease is required for DNA crosslink repair and telomerase activity in vivo in Caenorhabditis elegans. EMBO J. 2009;28(22):3549–3563. doi: 10.1038/emboj.2009.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiao Y, et al. Caenorhabditis elegans chromatin-associated proteins SET-2 and ASH-2 are differentially required for histone H3 Lys 4 methylation in embryos and adult germ cells. Proc Natl Acad Sci USA. 2011;108(20):8305–8310. doi: 10.1073/pnas.1019290108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feil R, Fraga MF. Epigenetics and the environment: Emerging patterns and implications. Nat Rev Genet. 2012;13(2):97–109. doi: 10.1038/nrg3142. [DOI] [PubMed] [Google Scholar]
- 32.Briggs SD, et al. Histone H3 lysine 4 methylation is mediated by Set1 and required for cell growth and rDNA silencing in Saccharomyces cerevisiae. Genes Dev. 2001;15(24):3286–3295. doi: 10.1101/gad.940201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dou Y, et al. Regulation of MLL1 H3K4 methyltransferase activity by its core components. Nat Struct Mol Biol. 2006;13(8):713–719. doi: 10.1038/nsmb1128. [DOI] [PubMed] [Google Scholar]
- 34.Steward MM, et al. Molecular regulation of H3K4 trimethylation by ASH2L, a shared subunit of MLL complexes. Nat Struct Mol Biol. 2006;13(9):852–854. doi: 10.1038/nsmb1131. [DOI] [PubMed] [Google Scholar]
- 35.Xu L, Strome S. Depletion of a novel SET-domain protein enhances the sterility of mes-3 and mes-4 mutants of Caenorhabditis elegans. Genetics. 2001;159(3):1019–1029. doi: 10.1093/genetics/159.3.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simonet T, Dulermo R, Schott S, Palladino F. Antagonistic functions of SET-2/SET1 and HPL/HP1 proteins in C. elegans development. Dev Biol. 2007;312(1):367–383. doi: 10.1016/j.ydbio.2007.09.035. [DOI] [PubMed] [Google Scholar]
- 37.Wysocka J, Myers MP, Laherty CD, Eisenman RN, Herr W. Human Sin3 deacetylase and trithorax-related Set1/Ash2 histone H3-K4 methyltransferase are tethered together selectively by the cell-proliferation factor HCF-1. Genes Dev. 2003;17(7):896–911. doi: 10.1101/gad.252103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Christensen J, et al. RBP2 belongs to a family of demethylases, specific for tri-and dimethylated lysine 4 on histone 3. Cell. 2007;128(6):1063–1076. doi: 10.1016/j.cell.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 39.Yoshina S, et al. Identification of a novel ADAMTS9/GON-1 function for protein transport from the ER to the Golgi. Mol Biol Cell. 2012;23(9):1728–1741. doi: 10.1091/mbc.E11-10-0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gwinn DM, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30(2):214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Al-Hakim AK, et al. 14-3-3 cooperates with LKB1 to regulate the activity and localization of QSK and SIK. J Cell Sci. 2005;118(Pt 23):5661–5673. doi: 10.1242/jcs.02670. [DOI] [PubMed] [Google Scholar]
- 42.Wang W, Shakes DC. Expression patterns and transcript processing of ftt-1 and ftt-2, two C. elegans 14-3-3 homologues. J Mol Biol. 1997;268(3):619–630. doi: 10.1006/jmbi.1997.1002. [DOI] [PubMed] [Google Scholar]
- 43.Morton DG, et al. The Caenorhabditis elegans par-5 gene encodes a 14-3-3 protein required for cellular asymmetry in the early embryo. Dev Biol. 2002;241(1):47–58. doi: 10.1006/dbio.2001.0489. [DOI] [PubMed] [Google Scholar]
- 44.Weaver IC, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7(8):847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 45.Siklenka K, et al. Disruption of histone methylation in developing sperm impairs offspring health transgenerationally. Science. 2015;350(6261):aab2006. doi: 10.1126/science.aab2006. [DOI] [PubMed] [Google Scholar]
- 46.Cui M, Cohen ML, Teng C, Han M. The tumor suppressor Rb critically regulates starvation-induced stress response in C. elegans. Curr Biol. 2013;23(11):975–980. doi: 10.1016/j.cub.2013.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maxwell CS, et al. Pol II docking and pausing at growth and stress genes in C. elegans. Cell Reports. 2014;6(3):455–466. doi: 10.1016/j.celrep.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baugh LR, Demodena J, Sternberg PW. RNA Pol II accumulates at promoters of growth genes during developmental arrest. Science. 2009;324(5923):92–94. doi: 10.1126/science.1169628. [DOI] [PubMed] [Google Scholar]
- 49.Levine M. Paused RNA polymerase II as a developmental checkpoint. Cell. 2011;145(4):502–511. doi: 10.1016/j.cell.2011.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bowman EA, Bowman CR, Ahn JH, Kelly WG. Phosphorylation of RNA polymerase II is independent of P-TEFb in the C. elegans germline. Development. 2013;140(17):3703–3713. doi: 10.1242/dev.095778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fraser NW, Sehgal PB, Darnell JE. DRB-induced premature termination of late adenovirus transcription. Nature. 1978;272(5654):590–593. doi: 10.1038/272590a0. [DOI] [PubMed] [Google Scholar]
- 52.Yankulov K, Yamashita K, Roy R, Egly JM, Bentley DL. The transcriptional elongation inhibitor 5,6-dichloro-1-beta-D-ribofuranosylbenzimidazole inhibits transcription factor IIH-associated protein kinase. J Biol Chem. 1995;270(41):23922–23925. doi: 10.1074/jbc.270.41.23922. [DOI] [PubMed] [Google Scholar]
- 53.Greer EL, et al. Transgenerational epigenetic inheritance of longevity in Caenorhabditis elegans. Nature. 2011;479(7373):365–371. doi: 10.1038/nature10572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Greer EL, et al. A histone methylation network regulates transgenerational epigenetic memory in C. elegans. Cell Reports. 2014;7(1):113–126. doi: 10.1016/j.celrep.2014.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77(1):71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee BH, Ashrafi K. A TRPV channel modulates C. elegans neurosecretion, larval starvation survival, and adult lifespan. PLoS Genet. 2008;4(10):e1000213. doi: 10.1371/journal.pgen.1000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang X, Zabinsky R, Teng Y, Cui M, Han M. microRNAs play critical roles in the survival and recovery of Caenorhabditis elegans from starvation-induced L1 diapause. Proc Natl Acad Sci USA. 2011;108(44):17997–18002. doi: 10.1073/pnas.1105982108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Montgomery MK, Xu S, Fire A. RNA as a target of double-stranded RNA-mediated genetic interference in Caenorhabditis elegans. Proc Natl Acad Sci USA. 1998;95(26):15502–15507. doi: 10.1073/pnas.95.26.15502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goodyer W, et al. HTP-3 links DSB formation with homolog pairing and crossing over during C. elegans meiosis. Dev Cell. 2008;14(2):263–274. doi: 10.1016/j.devcel.2007.11.016. [DOI] [PubMed] [Google Scholar]