Significance
Cellular powerhouses called mitochondria generate fuel known as adenosine triphosphate, or ATP, to sustain complex life. The capacity of mitochondria to do so depends on a supply of energy from oxidation of energy-rich compounds in foodstuffs to generate a chemical potential difference for hydrogen ions, called the proton motive force (pmf), across the inner mitochondrial membrane. Disruption of this membrane dissipates the pmf, and the cells die for lack of fuel. This event happens, for example, when the concentration of calcium ions inside human mitochondria is increased. The mitochondria respond by opening a pore, water enters, and the mitochondria swell and burst. The molecular identity of the pore is disputed, and we have disproved one proposal.
Keywords: human mitochondria, ATP synthase, permeability transition pore, subunit c, ATP5G
Abstract
The permeability transition in human mitochondria refers to the opening of a nonspecific channel, known as the permeability transition pore (PTP), in the inner membrane. Opening can be triggered by calcium ions, leading to swelling of the organelle, disruption of the inner membrane, and ATP synthesis, followed by cell death. Recent proposals suggest that the pore is associated with the ATP synthase complex and specifically with the ring of c-subunits that constitute the membrane domain of the enzyme’s rotor. The c-subunit is produced from three nuclear genes, ATP5G1, ATP5G2, and ATP5G3, encoding identical copies of the mature protein with different mitochondrial-targeting sequences that are removed during their import into the organelle. To investigate the involvement of the c-subunit in the PTP, we generated a clonal cell, HAP1-A12, from near-haploid human cells, in which ATP5G1, ATP5G2, and ATP5G3 were disrupted. The HAP1-A12 cells are incapable of producing the c-subunit, but they preserve the characteristic properties of the PTP. Therefore, the c-subunit does not provide the PTP. The mitochondria in HAP1-A12 cells assemble a vestigial ATP synthase, with intact F1-catalytic and peripheral stalk domains and the supernumerary subunits e, f, and g, but lacking membrane subunits ATP6 and ATP8. The same vestigial complex plus associated c-subunits was characterized from human 143B ρ0 cells, which cannot make the subunits ATP6 and ATP8, but retain the PTP. Therefore, none of the membrane subunits of the ATP synthase that are involved directly in transmembrane proton translocation is involved in forming the PTP.
The inner membranes of mitochondria contain a high-conductance nonspecific channel, known as the permeability transition pore (or PTP), which opens in response to elevated concentrations of Ca2+ in the mitochondrial matrix (1). Opening of the pore is potentiated by inducers such as phosphate, adenine nucleotide depletion, and thiol oxidants and leads to the swelling of the mitochondria, loss of proton motive force (pmf), disruption of ion homeostasis, and hydrolysis of ATP by the ATP synthase (2). These events have been linked to pathways leading to cell death and to human diseases including cardiac ischemia and muscle dystrophy (3). The opening of the pore can be induced artificially by compounds such as thapsigargin, a noncompetitive inhibitor of the Ca2+-ATPase in the sarcoplasmic and endoplasmic reticula (4), and by ionophores for divalent cations such as ferutinin (5). The opening of the pore can also be inhibited by drugs such as cyclosporin A, mediated via its binding to the prolyl cis-trans isomerase, cyclophilin D, in the mitochondrial matrix (6, 7).
Since the discovery of the mitochondrial permeability transition, many proposals have been made about the protein constituents of the PTP itself, including the ADP/ATP translocase, an abundant component of the inner membranes of mitochondria, and the voltage-dependent anion channel found in the outer membranes of the organelle, but none of these proposals has been established definitively (8, 9). Recently, it has been proposed that the PTP is associated with another abundant component of the inner mitochondrial membrane, the ATP synthase complex (10). The monomeric mammalian ATP synthase is a multiprotein assembly made of 18 different protein subunits (11). The enzyme consists of two major domains, a membrane intrinsic sector and a membrane extrinsic F1 sector, joined together by central and peripheral stalks (11). In mitochondria, the monomeric complexes form dimers via interactions between their membrane domains, and the dimers associate in rows along the edges of the cristae (12, 13). The membrane domain of each monomer contains a rotary motor driven by the pmf produced by respiration. The membrane-embedded part of the rotor is a ring of eight c-subunits in the bovine enzyme (14). Because the sequences of the bovine and human c-subunits are identical, it is reasonable to assume that the human c-ring is identical. In the intact enzyme, the external surface of this ring is in contact with a single ATP6 (or a) subunit (15–18). During ATP synthesis, the c8-ring turns with estimated speeds of up to 300 Hz. The generation of rotation involves the translocation of protons across the membrane domain of the enzyme via a pathway at the interface between the surface of the c-ring and subunit ATP6. The rotational energy of the rotor is transmitted to the catalytic domain by the central stalk, which is a membrane-extrinsic structure attached firmly to the c-ring. The central stalk consists of single copies of the γ-, δ-, and ε-subunits and lies along the central axis of the F1 domain, surrounded by the α3β3-catalytic domain, a hexameric structure of alternating α- and β-subunits. At three of the six α-β interfaces are found the catalytic sites of the enzyme, where ATP is formed from ADP and inorganic phosphate, using energy supplied by rotation (19, 20). In the absence of a proton-motive force, the F1-ATPase inhibitor protein, IF1, prevents the hydrolysis of ATP by binding to one of the catalytic interfaces (21). The main role of the peripheral stalk, a predominantly α-helical structure made from single copies of the oligomycin sensitivity conferral protein (OSCP), subunits b and d, and factor 6 (F6) (22–24) is to link the α3β3 domain to subunit ATP6, which interacts with the two predicted transmembrane α-helices of the b-subunit, so that together the α3β3 domain, the peripheral stalk, and subunit a form the stator of the enzyme (11, 18). The membrane domain of the mammalian ATP synthase also contains six other proteins—e, f, g, DAPIT (diabetes-associated protein in insulin sensitive tissues), 6.8PL (6.8-kDa proteolipid), and ATP8 (or A6L) (25–29). They are known collectively as the “supernumerary” subunits, and with the exception of ATP8, they appear to have no direct role in the synthesis of ATP. They are all localized in the vicinity of the region of interaction between monomers in the dimeric complex. Each is predicted to contain a single transmembrane α-helical span (30). Subunits ATP6 and ATP8 are the only components of the complex that are encoded in the mitochondrial genome (25); all of the other subunits of the human complex are the products of single nuclear genes, except for subunit c, which is encoded by three genes, ATP5G1, ATP5G2, and ATP5G3 (31, 32) (SI Appendix, Fig. S1). Their gene products differ only in the sequences of their N-terminal regions, which direct the protein from the cellular cytoplasm to the inner mitochondrial membrane and are removed by proteolysis to generate the same identical mature c-subunit found assembled in ATP synthase complexes.
If the PTP is associated with the ATP synthase complex, it is likely that it will involve one or more of the membrane subunits, and one proposal that has been made is that the c8-ring of the human ATP synthase provides the mitochondrial PTP (33–35). To test this proposal, as described here, we have disrupted ATP5G1, ATP5G2, and ATP5G3 together in a single clone of a near-haploid human cell line and investigated whether the PTP, which is present in the parent HAP1 cells, persists in the mutant cells devoid of subunit c.
Results
Characteristics of Wild-Type HAP1 Cells.
HAP1 cells have a haploid karyotype except for a fragment of chromosome 15, which is located in chromosome 19, and these cells also contain a reciprocal translocation between chromosomes 9 and 22 (36, 37). None of these features affects the structures of the three genes ATP5G1, ATP5G2, and ATP5G3 encoding the three different precursors of the c-subunit of ATP synthase, which are found, respectively, on the single-copy chromosomes 17, 12, and 2. All three genes are transcribed in HAP1 cells, but ATPG3 is expressed predominantly with about 75% of the transcripts arising from this gene (SI Appendix, Fig. S2).
Human Cells Devoid of Subunit c.
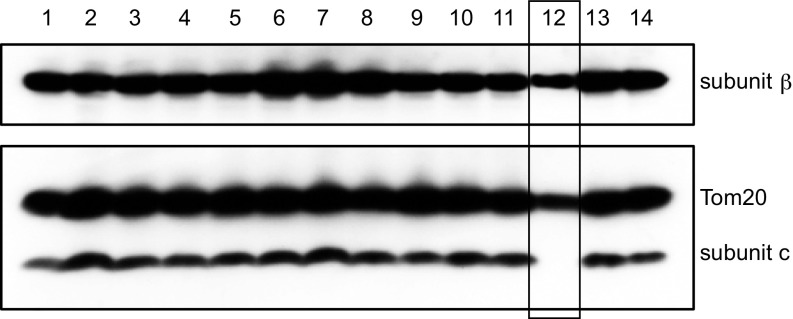
In a clonal HAP1 cell line, the ATP5G1, ATP5G2, and ATP5G3 genes were disrupted. Three pairs of guide RNAs (gRNAs), each pair specific for one of the exons IV in ATP5G1 or ATP5G2, or for exon III in ATP5G3, were introduced together into the cells (SI Appendix, Fig. S1 and Table S1). Clones arising from single cells, identified as having expressed Cas9, were screened for the presence of the c-subunit by Western blotting. By this means, the clone HAP1-A12 was found to be devoid of subunit c (Fig. 1). Analysis of DNA sequences (SI Appendix, Table S2) in regions of the genome of this cell where ATP5G1 and ATP5G2 are found showed that deletions of 140 and 97 bases, respectively, had been introduced into exon IV in ATP5G1 and ATP5G2 (SI Appendix, Fig. S3 A and B). Each deletion had arisen from two gRNAs and nonhomologous end-joining (NHEJ) (38) of the deleted genomic DNA. The deletion in ATP5G1 changed the sequence of the precursor of subunit c immediately before the precursor cleavage site and introduced an unrelated sequence of 18 amino acids, which then terminated, and that in ATP5G2 changed the frame of the coding region in the import sequence, leading to an altered protein sequence four amino acids before the junction with the mature protein, and truncation of the frame-shifted protein 9 amino acids thereafter. Sequencing of ATP5G3 showed that gRNA ATP5G3-1 had been targeted successfully to the gene whereas ATP5G3-2 had not (SI Appendix, Table S1), and a four-base deletion had been introduced into the gene by NHEJ. This deletion changed the frame of the encoded protein in the import precursor region, producing a frame-shifted protein 10 amino acids before the junction between the import sequence and the mature protein, which terminated 14 amino acids later (SI Appendix, Fig. S3C). Thus, none of the three disrupted genes in the HAP1-A12 clone was capable of producing a protein containing any of the sequence of the mature c-subunit.
Fig. 1.
Expression of the c-subunit of human ATP synthase in HAP1 clonal cells following attempted disruption of the genes ATP5G1, ATP5G2, and ATP5G3. Total cell proteins from each clone were fractionated by SDS/PAGE, and the β- and c-subunits of the ATP synthase and Tom20 were detected with antibodies. The results for 14 of the 24 clones that were examined are shown. The A12 clone lacking subunit c is boxed. The signals from the β-subunit and Tom20 are weaker than in other tracks because the HAP1-A12 clone grows slower, and thus the analyzed colony contains fewer cells.
Characteristics of HAP1-A12 Cells.
The codisruption of ATP5G1, ATP5G2, and ATP5G3 and the accompanying removal of the c-subunit slightly diminished the ability of the HAP1-A12 cells to grow relative to the wild-type cells (SI Appendix, Fig. S4A), and it was accompanied by an increase in the copy number of mitochondrial DNA molecules by about 50% (SI Appendix, Fig. S4B). However, the HAP1-A12 cells have a lower respiratory activity (SI Appendix, Fig. S4C), which is sufficient to allow them to maintain a mitochondrial membrane potential in the absence of an active ATP synthase (SI Appendix, Fig. S4 D and E). The mitochondria respire using the substrates glutamate/malate and succinate, confirming the presence of active respiratory complexes. The lower initial tetramethyl rhodamine methyl ester (TMRM) signal for the HAP1-A12 sample suggests that the mitochondria may be less leaky to protons in the absence of the c-subunit, such that a partial membrane potential can be maintained during the preparation. As expected, there was no impact of oligomycin on either respiration or the membrane potential in HAP1-A12 cells.
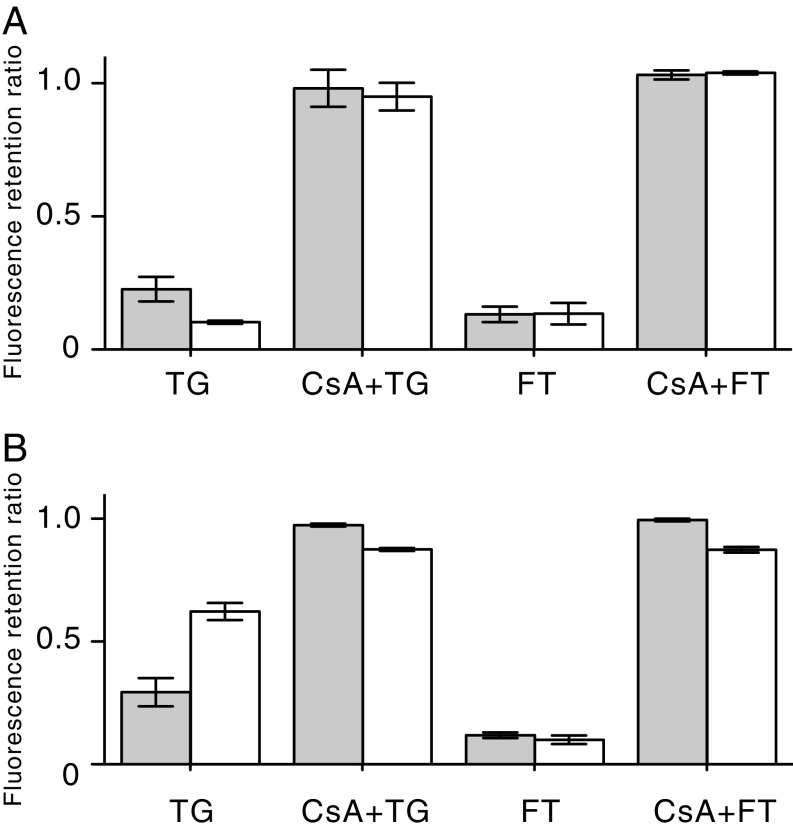
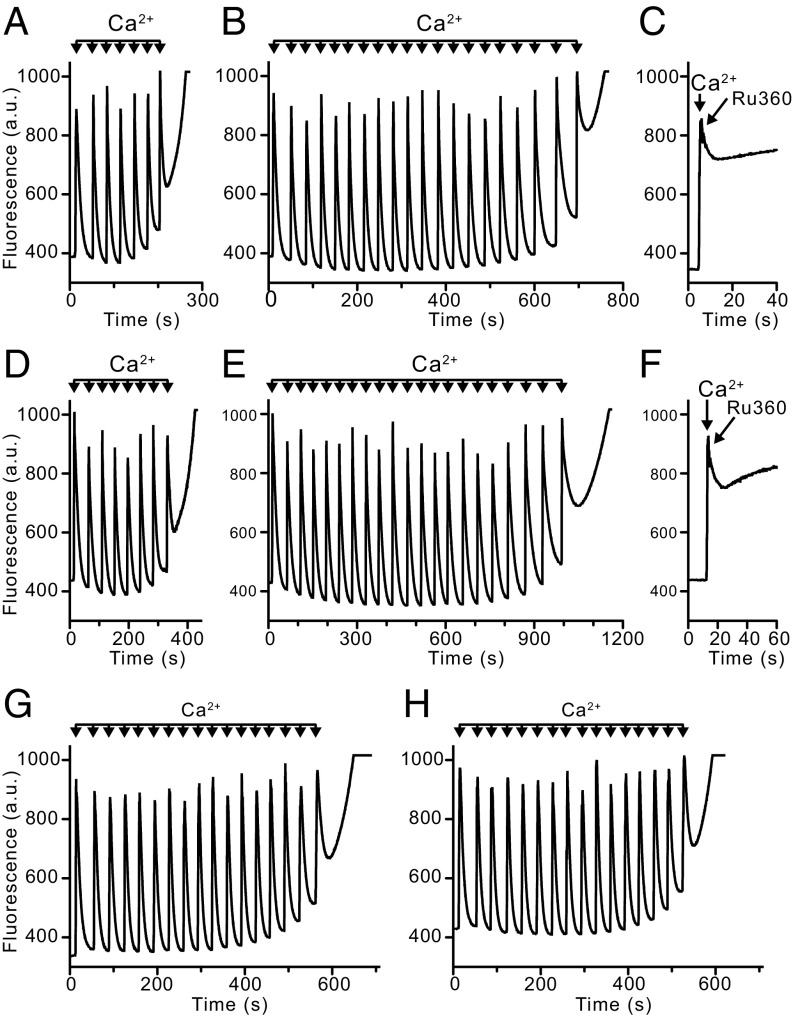
The mitochondria of wild-type HAP1 cells and HAP1-A12 cells were stained to the same extent with both TMRM and calcein (SI Appendix, Fig. S5), allowing treated cells to be normalized with untreated wild-type cells in subsequent experiments. To verify that the mitochondria of HAP1 wild-type cells contain a PTP, the opening of the pore was demonstrated in intact cells in the presence of both thapsigargin and the calcium ionophore ferutinin. For both reagents, the optimum conditions leading to complete opening of the PTP were established (Fig. 2A). With both reagents, the opening of the PTP was prevented by cyclosporin A. Similar results were obtained with HAP1-A12 cells (Fig. 2B). In other experiments with HAP1 and HAP1-A12 cells where their plasma membranes had been permeabilized with digitonin, the responses of the cells to successive pulses of Ca2+ in the absence and presence of cyclosporin A were monitored (Fig. 3 and SI Appendix, Tables S3 and S4). On average, the ratios of the number of calcium pulses required to induce the PTP in the presence and absence of CsA were similar; the values were 2.89 ± 0.62 (n = 9) in wild-type cells and 2.48 ± 0.36 (n = 4) in HAP1-A12 cells. In HAP1-ΔPPIF cells, which lack cyclophilin D, the value was 0.97 ± 0.07 (n = 4) for the clone presented in Fig. 3. Thus, in response to pulses of exogenous Ca2+, there was no significant difference in pore opening in the presence and in the absence of subunit c. As expected, disruption of PPIF (peptidyl-prolyl cis-trans isomerase F, also known as cyclophilin D) had removed the sensitivity of the pore to cyclosporin A in HAP1 cells, and, in both wild-type and HAP1-A12 cells, inhibition of the mitochondrial calcium uniporter immediately after a single calcium injection prevented any further uptake of Ca2+ by mitochondria (Fig. 3 C and F).
Fig. 2.
The opening of the PTP in HAP1 cells. (A) Wild-type cells. (B) HAP1-A12 cells. The cells were stained with both calcein and TMRM and then incubated for 1 h in the presence of either 40 μM thapsigargin (TG) or 25 μM ferutinin (FT). Duplicate samples were incubated first in the presence of 5 μM cyclosporin A (CsA) and then were treated with either thapsigargin or ferutinin. Gray and white columns correspond to the retention ratios for calcein and TMRM, respectively, compared with cells treated with the vehicle DMSO only. All data are mean values ± SDs (n = 4).
Fig. 3.
Calcium-induced opening of the PTP in permeabilized HAP1 cells. (A–C) Wild-type cells. (D–F) HAP1-A12 cells. (G and H) HAP1-ΔPPIF cells. The calcium retention capacity of mitochondria in digitonin-permeabilized cells (20 × 106 cells/mL) was examined in response to pulses of 10 μM CaCl2. Extramitochondrial Ca2+ was measured with calcium green-5N fluorescence (a.u., arbitrary unit). The calcium retention capacity in the absence (A, D, and G) and presence (B, E, and H) of CsA (1 μM). (C and F) Effect on wild-type and HAP1-A12 cells, respectively, of inhibition of the mitochondrial calcium uniporter with Ru360 (0.5 μM) added immediately after a single pulse of Ca2+.
Characterization of the Vestigial ATP Synthase in HAP1-A12 and ρ0 Cells.
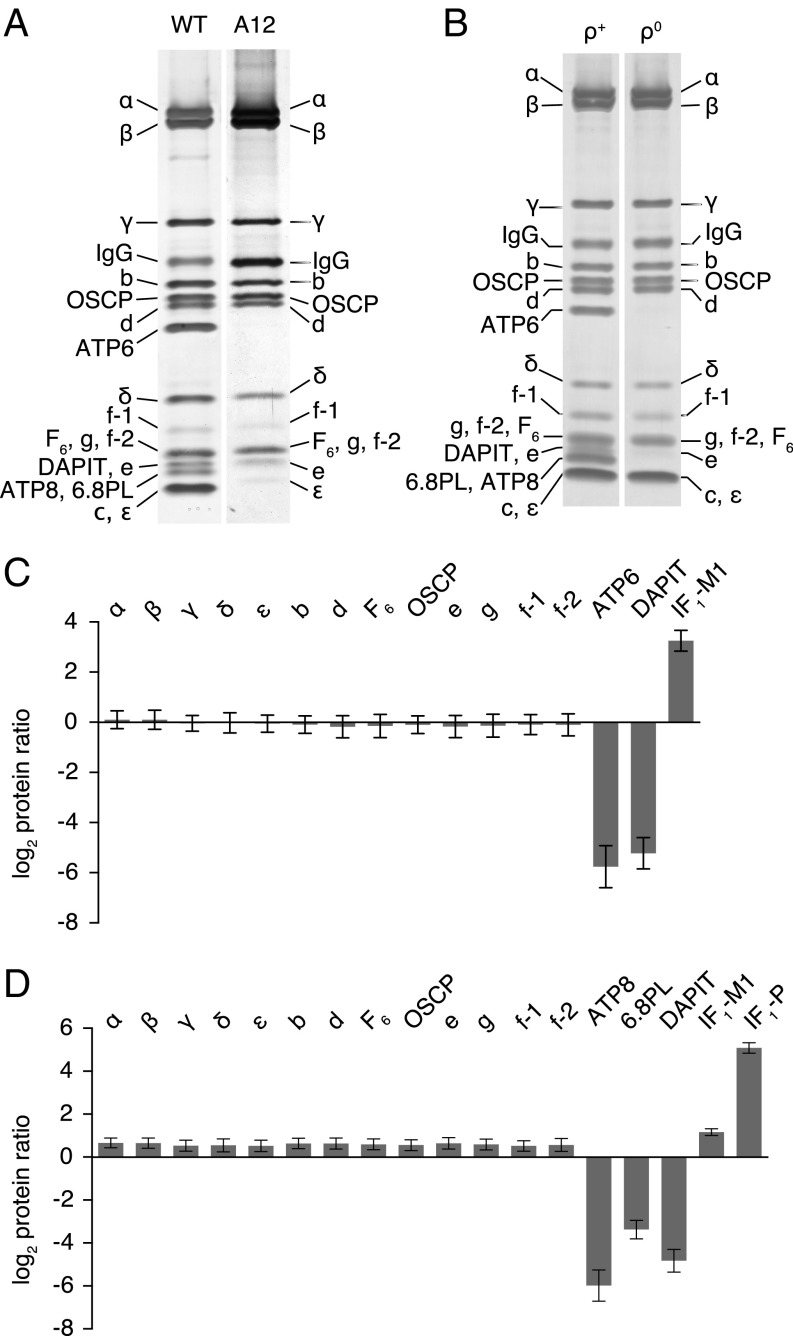
Despite the significant effect of the removal of subunit c on cellular respiration, the mitochondria of HAP1-A12 cells retain an assembled vestigial ATP synthase complex. This complex, examined by SDS/PAGE analysis (Fig. 4A) and by quantitative mass spectrometry of the subunits of the purified complex (Fig. 4; SI Appendix, Fig. S6; Datasets S1 and S2), contained a complete complement of subunits of the F1-catalytic domain (subunits α, β, γ, δ, and ε) and the peripheral stalk (the OSCP and subunits b, d, and F6). It also retained the supernumerary membrane subunits e, f, and g, but lacked DAPIT and the mitochondrially encoded subunit ATP6. The analyses suggested also that subunits ATP8 and 6.8PL were absent from the vestigial complex (Fig. 4A). In contrast, the abundance of one mature form of IF1, IF1-M1 (SI Appendix, Fig. S7), found in association with the vestigial complex, had increased by 7- to 13-fold relative to the wild-type complex.
Fig. 4.
Effects of the deletion of the c-subunit of human ATP synthase in HAP1-A12 cells and of the removal of ATP6 and ATP8 in ρ0 cells. (A) Impact of removal of c-subunits on the subunit composition of the vestigial ATP synthase complex. The complex was purified from mitoplasts derived from wild-type (WT) HAP1 and HAP1-A12 cells and analyzed by SDS/PAGE. (B) ATP synthase and the vestigial complex purified from mitoplasts from 143B ρ+ and ρ0 cells and analyzed by SDS/PAGE. (C and D) Relative abundance of subunits of ATP synthase and two forms of the ATPase inhibitor protein, IF1-M1 and IF1-P (SI Appendix, Fig. S7). The complex and residual complex were purified from a 1:1 mixture of SILAC-labeled wild-type HAP1 cells and HAP1-A12 cells (C) or 143B ρ+ and ρ0 cells (D), and tryptic peptides were analyzed by quantitative mass spectrometry. The experiments were performed twice with reciprocal protein labeling. The bars represent median values of both relative abundance ratios determined for proteins identified in the complementary SILAC-labeling experiments. Error bars show the range of the two values. The proteins in A and B were stained with silver. Subunits were identified by mass spectrometric analysis of tryptic digests of bands from a duplicate gel stained with Coomassie blue dye. The histograms are derived from the data in SI Appendix, Fig. S6, and Datasets S1–S4. Isoforms 1 and 2 of the f-subunit (f-1 and f-2) (SI Appendix, Fig. S7) were identified in human cells.
The mitochondria from human 143B ρ0 cells also retain a vestigial ATP synthase complex resembling the vestigial enzyme complex in HAP1-A12 cells (Fig. 4; SI Appendix, Fig. S6; Datasets S3 and S4). This complex also has a full complement of subunits in the catalytic F1 and peripheral stalk domains, plus supernumerary subunits e, f, and g. The relative abundance of proteins in mitoplasts of HAP1-A12 cells and 143B ρ0 cells were also determined (SI Appendix, Fig. S6 and Datasets S5–S8). The levels of DAPIT and 6.8PL were found to have diminished in mitoplasts of 143B ρ0 cells relative to wild-type cells (SI Appendix, Fig. S6, and Dataset S7), and still more in the purified complex (SI Appendix, Fig. S6, and Dataset S3), implying that they are weakly associated with the complex and partially lost during its extraction and purification. The relative abundance of the IF1-M1 form of the inhibitor protein (SI Appendix, Fig. S7A) associated with the vestigial complex increased slightly. However, in ρ0 cells the mitochondrial import precursor of the inhibitor protein, IF1-P (SI Appendix, Fig. S7A), was increased by 34-fold relative to the parent cells. The presence of IF1-P in the mitochondria of ρ0 cells has been noted before (39). These mitochondria also have an active PTP, as reported before (40) and confirmed here, where opening could be stimulated by either ferutinin or thapsigargin and inhibited by cyclosporin A (SI Appendix, Fig. S8). Therefore, these experiments with HAP1-A12 and 143B ρ0 cells together show that subunit c, and also ATP6 and ATP8, are not involved in forming the PTP. An additional more tentative conclusion is that subunits DAPIT and 6.8PL of the ATP synthase are also not part of the PTP.
Oligomeric State of Vestigial ATP Synthases.
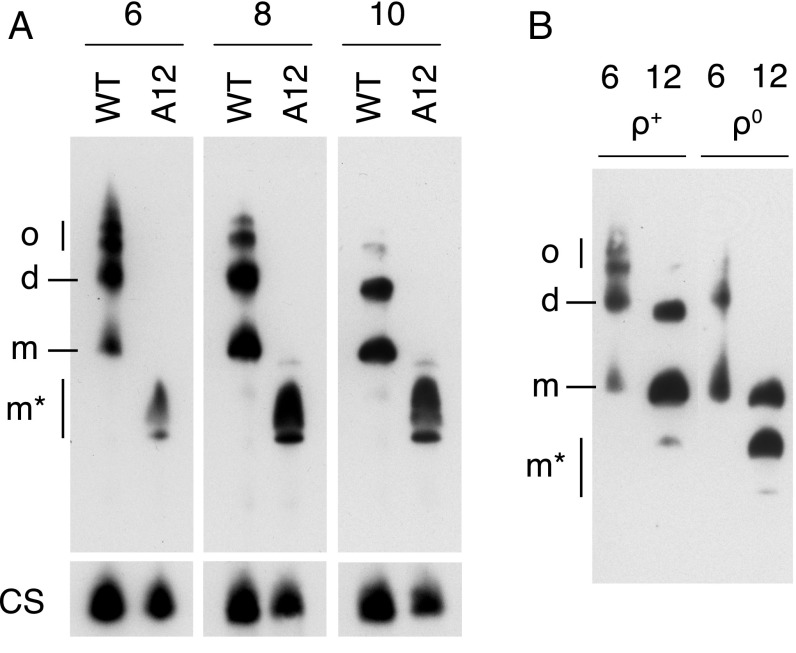
The PTP has been proposed to be associated with dimers, and not with monomers of the ATP synthase complex (10, 41). Therefore, the oligomeric states of the vestigial complexes in HAP1-A12 and 143B ρ0 cells were investigated by extraction of the complexes with digitonin and native gel electrophoresis of the extract (Fig. 5). Under the conditions used for the extraction of the complexes from mitoplasts from HAP1-A12 cells for native gel analysis, the vestigial ATP synthase contains two or three related subcomplexes with a smaller apparent molecular weight than the intact monomeric ATP synthase complex (Fig. 5A). Thus, this gel seemed to indicate that the “complex” from HAP1-A12 cells is a mixture of related complexes. This conclusion is not supported by the stable isotope labeling in cell culture (SILAC) data, which strongly indicate a single complex (Fig. 4) and that therefore the multiple bands on the native gel are artifactual. It is likely that the removal of the c-ring and accompanying loss of subunits ATP6 and ATP8 destabilizes the dimerization interface, which probably relies on subunits e and g and may also depend on supernumerary subunits f, DAPIT, and 6.8PL (17, 18). It is also possible that the vestigial complex remains dimeric in the membrane and that it is destabilized (monomerized) artifactually by the extraction process. The dependency of the oligomeric state of the ATP synthases and vestigial complexes on conditions for their extraction from mitoplast is illustrated by the analysis of the vestigial complex from 143B ρ0 cells (Fig. 5B). At the lower concentration of digitonin, the extract contains species corresponding to dimers and monomers of the enzyme, and at the higher concentration of digitonin, to monomers and smaller subcomplexes.
Fig. 5.
Oligomeric state of ATP synthase in HAP1 and 143B ρ+ cells and of vestigial ATP synthase complexes in HAP1-A12 and 143B ρ0 cells. Mitoplasts were extracted with various concentrations of digitonin/protein (wt/wt) indicated above each lane. The ATP synthase and vestigial complexes were analyzed by blue native-PAGE, and detected by Western blotting with an antibody against the (A) α- or (B) β-subunit. (A and B) Analysis of, respectively, HAP1 wild-type (WT) and HAP1-A12 cells, and 143B ρ+ and ρ0 cells. In A, citrate synthase (CS) was used as a loading control. d, dimers; m, monomers; m*, other complexes of unknown oligomeric state; o, oligomers.
Discussion
The experiments described above with HAP1-A12 cells show conclusively by three independent assays of the PTP that, even when the c-protein is absent from their mitochondria, the cells retain a PTP. In intact cells, the PTP opens characteristically in response to treatment with either thapsigargin or ferutinin and, in permeabilized cells, in response to elevated concentrations of extramitochondrial Ca2+. In all of these assays, opening of the pore can be inhibited by cyclosporin A. Therefore, the c-ring is not an essential component of the PTP.
Even before these experiments were carried out, a number of features of human c8-rings were apparent that seemed to be incompatible with the presence in the rings of a nonspecific aqueous channel capable of passing hydrophilic molecules with a molecular weight up to 1,500 Da (1, 42), as a description of the ring illustrates. The sequence of the identical human and bovine mature c-subunits is 75 amino acids long. In the structure of the bovine c-ring (14), residues 4–37 and 44–74 of each c-subunit are folded into two antiparallel α-helices, arranged, respectively, in inner and outer rings. The two α-helices in each subunit are joined by extended polar loops from residues 38 to 43 in the lipid head group region on the matrix side of the inner membrane. Each loop region contains an arginine residue at position 38 and a fully trimethylated lysine residue at position 43 (43). The N-terminal regions extend toward the space between the inner and outer membranes of the organelle, and each N-terminal region has aspartic acid residues at positions 1 and 3 and an unmodified lysine at position 7. Residues in the polar loops and the N-terminal extensions attract cardiolipin molecules selectively over phospholipids (44). With the exception of glutamate-58 on the outer surface of the ring, which plays a central role in the pathway for membrane translocation of protons, the outer surface of the ring is almost entirely hydrophobic, in keeping with its contact with the hydrophobic environment of the inner mitochondrial membrane. The inner surface of the ring is also almost entirely hydrophobic, with the exception of an accessible threonine residue at position 27 (SI Appendix, Fig. S9), and it is likely that the central cavity of the mitochondrial c-ring is occupied by lipids, as demonstrated in the related spinach chloroplast c14-rings and the c-ring from Escherichia coli (45, 46).
A second structural feature that tends to argue against the c-ring functioning as the PTP is that, in the intact enzyme, access to the central cavity of the c-ring from the matrix side of the membrane is impeded, and possibly completely blocked, by the association of the foot of the central stalk with the c-ring (14) (SI Appendix, Fig. S9). At its narrowest point, approximately in the middle of the lipid bilayer, the cavity has a diameter of about 4 Å, widening in both directions to about 12 Å at the external surfaces. The interaction between the c-ring and the central stalk provides a crucial connection between the membrane intrinsic and membrane extrinsic parts of the rotor of the ATP synthase that has to be sufficiently robust to resist the rotational torque of the motor turning at up to 300 Hz. In the structure of the bovine F1-c8 complex, the interacting region involves loop regions from five of the c-subunits and regions of the γ-, δ-, and ε-subunits (SI Appendix, Fig. S9). The interface is extensive and has a buried surface area of greater than 790 Å2. However, the positions of amino-acid side chains in the interface are uncertain, and for that reason they have not been introduced into the structural model. If they were, it is likely that most or all of the small apparent crevice in the interface region leading to the central cavity would be blocked entirely.
One possible way of exposing the central cavity would be to release the central stalk and α3β3-domain, leaving the intact membrane domain with a central cavity freely accessible from both sides of the membrane. In the related V-ATPases, such a physiological mechanism exists as a way of regulating the activity of the enzyme, and a free Vo membrane domain containing the equivalent of the c-ring is produced by the dissociation of the V1 catalytic domain (47, 48). However, a similar mechanism has not been demonstrated in the mitochondrial F-ATP synthases, and the hydrolytic activity of the enzyme is regulated by a different mechanism involving the inhibitor protein, IF1 (21). In the purified enzyme and in everted inner membrane vesicles, the interface between the F1 and membrane domains is resistant to dissociation, for example, by mild detergents and mild chaotropes or by elevated salt concentrations. The only known biochemical way of removing the catalytic domain from membranes so as to leave the membrane domain intact is to dissociate the membrane extrinsic proteins with strong chaotropes such as sodium bromide or guanidinium hydrochloride, leaving the membrane domain of the enzyme intact and protected by the lipid bilayer (49). The only patho-physiological circumstance where isolated human c-rings have been observed is in insoluble storage bodies derived from lysosomes associated with ceroid lipofuscinosis, or Batten’s disease (50). Here, the accumulation of c-rings in the storage bodies appears to be derived from defects in a lysosomal pathway for degradation of the c-protein (51).
A somewhat surprising corollary of the demonstration of the PTP in HAP1-A12 cells is that, in the absence of subunit c, a vestigial ATPase complex containing the catalytic and peripheral stalk domains and supernumerary subunits e, f, and g is still assembled in the mitochondria (Fig. 4). However, the vestigial complex and the mitoplasts from which it is derived also lack subunit ATP6. Therefore, neither the proton translocation pathway at the interface of the c-ring and ATP6, nor ATP6 itself, is associated with the PTP. The presence of the PTP in ρ0 cells, which lack mitochondrial DNA and the encoded ATP6 and ATP8 subunits, has been noted before (40), and the vestigial F-ATPase complexes from HAP1-A12 cells and 143B ρ0 cells are remarkably similar, except that the latter contains additionally the c-subunit (presumably organized in the c8-ring). Thus, the c-ring is not an absolute requirement for the assembly of either the F1 or peripheral stalk domains.
Both vestigial complexes also probably lack the supernumerary subunits DAPIT and 6.8PL, and therefore they are unlikely to be components of the PTP either. Thus, if the human PTP is associated with the ATP synthase complex, the most likely components available to form the pore are any or all of the remaining membrane subunits, b, e, f, and g. If such a pore complex exists, it may not contain any associated F1 subunits, and so the investigation of the possible involvement of these subunits in pore formation will require the application of specific antibodies and gene disruption approaches to each of the membrane-specific subunits of the ATP synthase.
The ATP synthase complex contains a single copy of subunit b with two transmembrane α-helices and is assumed to contain single copies of subunits e, f, and g, each predicted to have a single transmembrane α-helix. Recent cryo-electron microscopy analyses of the ATP synthase complexes suggest that at least subunits e and g are involved in the interface between monomers of the ATP synthase in the dimeric complexes (17, 18). The gene disruption strategy followed for the c-subunit provides a possible approach toward resolving the issue of whether any or all of these subunits are involved in forming the PTP.
Materials and Methods
Human HAP1 and 143B cells were grown under standard conditions. Oxygen consumption rate was measured in a Seahorse XFc24 instrument. The mitochondrial membrane potential of HAP1 cells was monitored in digitonin-permeabilized cells. The levels of transcripts from the ATPG1, ATPG2, and ATPG3 genes were estimated by quantitative PCR. The same three genes were disrupted in HAP1 cells by CRISPR-Cas9 technology (52). The ρ0 cells devoid of mtDNA were obtained from 143B cells by culturing them in the presence of ethidium bromide. ATP synthase was purified from mitoplasts by immunocapture. Stable isotopes were introduced into proteins by SILAC. Labeled proteins were quantitated by mass spectrometry.
The assays of the opening of the PTP were based on the triggering of opening of the pore by three independent methods. In intact human cells, pore opening was induced by thapsigargin (4) or ferutinin (5), and, in cells where the plasma membrane had been permeabilized with digitonin, by examination of the capacity of the mitochondria to retain Ca2+ introduced exogenously (53).
For full details of all these processes, see SI Appendix.
Supplementary Material
Acknowledgments
This work was supported by the Medical Research Council (MRC) of the United Kingdom by Grant MC_U1065663150 and by Programme Grant MR/M009858/1 (to J.E.W.). H.C.F. received an MRC PhD studentship.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1702357114/-/DCSupplemental.
References
- 1.Haworth RA, Hunter DR. The Ca2+-induced membrane transition in mitochondria. II. Nature of the Ca2+ trigger site. Arch Biochem Biophys. 1979;195(2):460–467. doi: 10.1016/0003-9861(79)90372-2. [DOI] [PubMed] [Google Scholar]
- 2.Zoratti M, Szabò I. The mitochondrial permeability transition. Biochim Biophys Acta. 1995;1241(2):139–176. doi: 10.1016/0304-4157(95)00003-a. [DOI] [PubMed] [Google Scholar]
- 3.Rasola A, Bernardi P. The mitochondrial permeability transition pore and its involvement in cell death and in disease pathogenesis. Apoptosis. 2007;12(5):815–833. doi: 10.1007/s10495-007-0723-y. [DOI] [PubMed] [Google Scholar]
- 4.Korge P, Weiss JN. Thapsigargin directly induces the mitochondrial permeability transition. Eur J Biochem. 1999;265(1):273–280. doi: 10.1046/j.1432-1327.1999.00724.x. [DOI] [PubMed] [Google Scholar]
- 5.Abramov AY, Duchen MR. Actions of ionomycin, 4-BrA23187 and a novel electrogenic Ca2+ ionophore on mitochondria in intact cells. Cell Calcium. 2003;33(2):101–112. doi: 10.1016/s0143-4160(02)00203-8. [DOI] [PubMed] [Google Scholar]
- 6.Crompton M, Ellinger H, Costi A. Inhibition by cyclosporin A of a Ca2+-dependent pore in heart mitochondria activated by inorganic phosphate and oxidative stress. Biochem J. 1988;255(1):357–360. [PMC free article] [PubMed] [Google Scholar]
- 7.Elrod JW, Molkentin JD. Physiologic functions of cyclophilin D and the mitochondrial permeability transition pore. Circ J. 2013;77(5):1111–1122. doi: 10.1253/circj.cj-13-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kokoszka JE, et al. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature. 2004;427(6973):461–465. doi: 10.1038/nature02229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baines CP, Kaiser RA, Sheiko T, Craigen WJ, Molkentin JD. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat Cell Biol. 2007;9(5):550–555. doi: 10.1038/ncb1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giorgio V, et al. Dimers of mitochondrial ATP synthase form the permeability transition pore. Proc Natl Acad Sci USA. 2013;110(15):5887–5892. doi: 10.1073/pnas.1217823110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walker JE. The ATP synthase: The understood, the uncertain and the unknown. Biochem Soc Trans. 2013;41(1):1–16. doi: 10.1042/BST20110773. [DOI] [PubMed] [Google Scholar]
- 12.Dudkina NV, Heinemeyer J, Keegstra W, Boekema EJ, Braun HP. Structure of dimeric ATP synthase from mitochondria: An angular association of monomers induces the strong curvature of the inner membrane. FEBS Lett. 2005;579(25):5769–5772. doi: 10.1016/j.febslet.2005.09.065. [DOI] [PubMed] [Google Scholar]
- 13.Strauss M, Hofhaus G, Schröder RR, Kühlbrandt W. Dimer ribbons of ATP synthase shape the inner mitochondrial membrane. EMBO J. 2008;27(7):1154–1160. doi: 10.1038/emboj.2008.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watt IN, Montgomery MG, Runswick MJ, Leslie AGW, Walker JE. Bioenergetic cost of making an adenosine triphosphate molecule in animal mitochondria. Proc Natl Acad Sci USA. 2010;107(39):16823–16827. doi: 10.1073/pnas.1011099107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou A, et al. Structure and conformational states of the bovine mitochondrial ATP synthase by cryo-EM. eLife. 2015;4:e10180. doi: 10.7554/eLife.10180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morales-Rios E, Montgomery MG, Leslie AGW, Walker JE. Structure of ATP synthase from Paracoccus denitrificans determined by X-ray crystallography at 4.0 Å resolution. Proc Natl Acad Sci USA. 2015;112(43):13231–13236. doi: 10.1073/pnas.1517542112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hahn A, et al. Structure of a complete ATP synthase dimer reveals the molecular basis of inner mitochondrial membrane morphology. Mol Cell. 2016;63(3):445–456. doi: 10.1016/j.molcel.2016.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vinothkumar KR, Montgomery MG, Liu S, Walker JE. Structure of the mitochondrial ATP synthase from Pichia angusta determined by electron cryo-microscopy. Proc Natl Acad Sci USA. 2016;113(45):12709–12714. doi: 10.1073/pnas.1615902113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abrahams JP, Leslie AG, Lutter R, Walker JE. Structure at 2.8 Å resolution of F1-ATPase from bovine heart mitochondria. Nature. 1994;370(6491):621–628. doi: 10.1038/370621a0. [DOI] [PubMed] [Google Scholar]
- 20.Bason JV, Montgomery MG, Leslie AGW, Walker JE. How release of phosphate from mammalian F1-ATPase generates a rotary substep. Proc Natl Acad Sci USA. 2015;112(19):6009–6014. doi: 10.1073/pnas.1506465112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gledhill JR, Montgomery MG, Leslie AGW, Walker JE. How the regulatory protein, IF1, inhibits F1-ATPase from bovine mitochondria. Proc Natl Acad Sci USA. 2007;104(40):15671–15676. doi: 10.1073/pnas.0707326104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collinson IR, et al. ATP synthase from bovine heart mitochondria. In vitro assembly of a stalk complex in the presence of F1-ATPase and in its absence. J Mol Biol. 1994;242(4):408–421. doi: 10.1006/jmbi.1994.1591. [DOI] [PubMed] [Google Scholar]
- 23.Dickson VK, Silvester JA, Fearnley IM, Leslie AGW, Walker JE. On the structure of the stator of the mitochondrial ATP synthase. EMBO J. 2006;25(12):2911–2918. doi: 10.1038/sj.emboj.7601177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rees DM, Leslie AGW, Walker JE. The structure of the membrane extrinsic region of bovine ATP synthase. Proc Natl Acad Sci USA. 2009;106(51):21597–21601. doi: 10.1073/pnas.0910365106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fearnley IM, Walker JE. Two overlapping genes in bovine mitochondrial DNA encode membrane components of ATP synthase. EMBO J. 1986;5(8):2003–2008. doi: 10.1002/j.1460-2075.1986.tb04456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walker JE, Lutter R, Dupuis A, Runswick MJ. Identification of the subunits of F1Fo-ATPase from bovine heart mitochondria. Biochemistry. 1991;30(22):5369–5378. doi: 10.1021/bi00236a007. [DOI] [PubMed] [Google Scholar]
- 27.Collinson IR, et al. Fo membrane domain of ATP synthase from bovine heart mitochondria: Purification, subunit composition, and reconstitution with F1-ATPase. Biochemistry. 1994;33(25):7971–7978. doi: 10.1021/bi00191a026. [DOI] [PubMed] [Google Scholar]
- 28.Chen R, Runswick MJ, Carroll J, Fearnley IM, Walker JE. Association of two proteolipids of unknown function with ATP synthase from bovine heart mitochondria. FEBS Lett. 2007;581(17):3145–3148. doi: 10.1016/j.febslet.2007.05.079. [DOI] [PubMed] [Google Scholar]
- 29.Meyer B, Wittig I, Trifilieff E, Karas M, Schägger H. Identification of two proteins associated with mammalian ATP synthase. Mol Cell Proteomics. 2007;6(10):1690–1699. doi: 10.1074/mcp.M700097-MCP200. [DOI] [PubMed] [Google Scholar]
- 30.Lee J, et al. Organization of subunits in the membrane domain of the bovine F-ATPase revealed by covalent cross-linking. J Biol Chem. 2015;290(21):13308–13320. doi: 10.1074/jbc.M115.645283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dyer MR, Walker JE. Sequences of members of the human gene family for the c subunit of mitochondrial ATP synthase. Biochem J. 1993;293(Pt 1):51–64. doi: 10.1042/bj2930051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yan WL, Lerner TJ, Haines JL, Gusella JF. Sequence analysis and mapping of a novel human mitochondrial ATP synthase subunit 9 cDNA (ATP5G3) Genomics. 1994;24(2):375–377. doi: 10.1006/geno.1994.1631. [DOI] [PubMed] [Google Scholar]
- 33.Bonora M, et al. Role of the c subunit of the FO ATP synthase in mitochondrial permeability transition. Cell Cycle. 2013;12(4):674–683. doi: 10.4161/cc.23599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Azarashvili T, et al. Potential role of subunit c of FoF1-ATPase and subunit c of storage body in the mitochondrial permeability transition. Effect of the phosphorylation status of subunit c on pore opening. Cell Calcium. 2014;55(2):69–77. doi: 10.1016/j.ceca.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 35.Alavian KN, et al. An uncoupling channel within the c-subunit ring of the F1FO ATP synthase is the mitochondrial permeability transition pore. Proc Natl Acad Sci USA. 2014;111(29):10580–10585. doi: 10.1073/pnas.1401591111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carette JE, et al. Ebola virus entry requires the cholesterol transporter Niemann-Pick C1. Nature. 2011;477(7364):340–343. doi: 10.1038/nature10348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Essletzbichler P, et al. Megabase-scale deletion using CRISPR/Cas9 to generate a fully haploid human cell line. Genome Res. 2014;24(12):2059–2065. doi: 10.1101/gr.177220.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lieber MR. The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway. Annu Rev Biochem. 2010;79:181–211. doi: 10.1146/annurev.biochem.052308.093131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wittig I, et al. Assembly and oligomerization of human ATP synthase lacking mitochondrial subunits a and A6L. Biochim Biophys Acta. 2010;1797(6-7):1004–1011. doi: 10.1016/j.bbabio.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 40.Masgras I, Rasola A, Bernardi P. Induction of the permeability transition pore in cells depleted of mitochondrial DNA. Biochim Biophys Acta. 2012;1817(10):1860–1866. doi: 10.1016/j.bbabio.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 41.Carraro M, et al. Channel formation by yeast F-ATP synthase and the role of dimerization in the mitochondrial permeability transition. J Biol Chem. 2014;289(23):15980–15985. doi: 10.1074/jbc.C114.559633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vercesi AE. Dissociation of NAD(P)+-stimulated mitochondrial Ca2+ efflux from swelling and membrane damage. Arch Biochem Biophys. 1984;232(1):86–91. doi: 10.1016/0003-9861(84)90523-x. [DOI] [PubMed] [Google Scholar]
- 43.Walpole TB, et al. Conservation of complete trimethylation of lysine-43 in the rotor ring of c-subunits of metazoan adenosine triphosphate (ATP) synthases. Mol Cell Proteomics. 2015;14(4):828–840. doi: 10.1074/mcp.M114.047456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duncan AL, Robinson AJ, Walker JE. Cardiolipin binds selectively but transiently to conserved lysine residues in the rotor of metazoan ATP synthases. Proc Natl Acad Sci USA. 2016;113(31):8687–8692. doi: 10.1073/pnas.1608396113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oberfeld B, Brunner J, Dimroth P. Phospholipids occupy the internal lumen of the c ring of the ATP synthase of Escherichia coli. Biochemistry. 2006;45(6):1841–1851. doi: 10.1021/bi052304+. [DOI] [PubMed] [Google Scholar]
- 46.Schmidt C, et al. Comparative cross-linking and mass spectrometry of an intact F-type ATPase suggest a role for phosphorylation. Nat Commun. 2013;4:1985. doi: 10.1038/ncomms2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kane PM. Disassembly and reassembly of the yeast vacuolar H+-ATPase in vivo. J Biol Chem. 1995;270(28):17025–17032. [PubMed] [Google Scholar]
- 48.Sumner JP, et al. Regulation of plasma membrane V-ATPase activity by dissociation of peripheral subunits. J Biol Chem. 1995;270(10):5649–5653. doi: 10.1074/jbc.270.10.5649. [DOI] [PubMed] [Google Scholar]
- 49.Collinson IR, Fearnley IM, Skehel JM, Runswick MJ, Walker JE. ATP synthase from bovine heart mitochondria: Identification by proteolysis of sites in Fo exposed by removal of F1 and the oligomycin-sensitivity conferral protein. Biochem J. 1994;303(Pt 2):639–645. doi: 10.1042/bj3030639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Palmer DN, et al. Mitochondrial ATP synthase subunit c storage in the ceroid-lipofuscinoses (Batten disease) Am J Med Genet. 1992;42(4):561–567. doi: 10.1002/ajmg.1320420428. [DOI] [PubMed] [Google Scholar]
- 51.Ezaki J, Takeda-Ezaki M, Kominami E. Tripeptidyl peptidase I, the late infantile neuronal ceroid lipofuscinosis gene product, initiates the lysosomal degradation of subunit c of ATP synthase. J Biochem. 2000;128(3):509–516. doi: 10.1093/oxfordjournals.jbchem.a022781. [DOI] [PubMed] [Google Scholar]
- 52.Ran FA, et al. Genome engineering using the CRISPR-Cas9 system. Nat Protoc. 2013;8(11):2281–2308. doi: 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murphy AN, Bredesen DE, Cortopassi G, Wang E, Fiskum G. Bcl-2 potentiates the maximal calcium uptake capacity of neural cell mitochondria. Proc Natl Acad Sci USA. 1996;93(18):9893–9898. doi: 10.1073/pnas.93.18.9893. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.