Significance
Cross-cousin marriage (i.e., marriage with the offspring of a parent’s opposite-sex sibling) is the most common preferred marriage arrangement across cultures. Despite intense investigation, the origin and adaptive function of this marriage prescription have not been resolved. An analysis of the fitness consequences of marriages in the Yanomamö—a tribal society in the Amazon—shows that parents and brothers achieve higher fitness outcomes when their respective children and sisters marry more closely related individuals. Meanwhile, the spouses and offspring produced by these unions have lower fitness. These findings suggest that cross-cousin marriage prescriptions and taboos against marrying parallel cousins owe their origin to parent–offspring conflict through parental control of marriage and competition between same-sex siblings.
Keywords: cross-cousin marriage, inbreeding, local mate competition, parent–offspring conflict, Yanomamö
Abstract
Marriage in many traditional societies often concerns the institutionalized exchange of reproductive partners among groups of kin. Such exchanges most often involve cross-cousins—marriage with the child of a parent’s opposite-sex sibling—but it is unclear who benefits from these exchanges. Here we analyze the fitness consequences of marrying relatives among the Yanomamö from the Amazon. When individuals marry close kin, we find that (i) both husbands and wives have slightly lower fertility; (ii) offspring suffer from inbreeding depression; (iii) parents have more grandchildren; and (iv) siblings, especially brothers, benefit when their opposite-sex siblings marry relatives but not when their same-sex siblings do. Therefore, individuals seem to benefit when their children or opposite-sex siblings marry relatives but suffer costs when they, their parents, or same-sex siblings do. These asymmetric fitness outcomes suggest conflicts between parents and offspring and among siblings over optimal mating strategies. Parental control of marriages is reinforced by cultural norms prescribing cross-cousin marriage. We posit that local mate competition combined with parental control over marriages may escalate conflict between same-sex siblings who compete over mates, while simultaneously forging alliances between opposite-sex siblings. If these relationships are carried forward to subsequent generations, they may drive bilateral cross-cousin marriage rules. This study provides insights into the evolutionary importance of how kinship and reciprocity underlie conflicts over who controls mate choice and the origins of cross-cousin marriage prescriptions.
Bonds between married partners establish ties between both extended kin and nonkin who become in-laws (1). Together these relationships form families—the foundation upon which human social structures are built. Several notable anthropologists have depicted marriage as a competition for sexual access to females by male coalitions (2–4) whereas others have viewed it as a reciprocal arrangement between parties whereby offering someone in marriage obligates the receiving family to reciprocate through political support or future spouses (5, 6). In accord with these characterizations, exchanging offspring with close relatives may be a way to ensure that these transactions are eventually reciprocated (7, 8).
Parental Control of Mate Choice Generates Parent–Offspring Conflict
Human mating is unique because parents often exert considerable control over the mate choices of their offspring (9). For example, a cross-cultural survey of hunting and gathering societies found that parents have a strong influence over the marriages of their sons and daughters (10). The basic properties of arranged marriages are (i) the spouses have high exchange value [females are likely to have higher value than males as measured by bride prices, bride service (bride service—sometimes called groom service—is the service rendered by the bridegroom to a bride’s family as a bride price), and “marriage by capture” in egalitarian societies like the Yanomamö] (6); (ii) the individuals doing the arranging and those whose marriages are being arranged are close kin; (iii) the arrangers are usually older and therefore typically have more social and political power; and (iv) the arranger (e.g., parent or sibling) and spouse become in-laws who maintain long-lasting ties that are often driven by contingent reciprocity [i.e., reciprocal altruism (8)] (1, 9, 11). An analysis of hunter-gatherer marriage practices using mitochondrial DNA suggests a long history of marriages based on reciprocity between the families of the spouses (12).
Exchanges of children—especially daughters—by kin groups forms one of the most basic structures of arranged marriages (5). (Marriage “exchanges” are not always reciprocal transactions of offspring and are often exchanges of offspring for political support, bride price, or bride service.) This system is likely to have emerged from a combination of parental control over mate choice and contingent reciprocity (9). If cultural rules and taboos began to exclude exchanges between same-sex siblings, we would arrive at the most widespread and common prescriptive marriage practice described by anthropologists—cross-cousin marriage (5, 13, 14). Cross-cousin marriage is the natural outgrowth of using one’s own [or one’s spouse’s] opposite-sex siblings to exchange children. These practices may start out as mere social patterns and regularities before they achieve a moral character and ultimately become a cultural rule or norm (9).
Parental influence over marriage is expected to generate conflict between the interests of parents and offspring. Trivers’ theory of parent–offspring conflict (15) provides a framework for understanding these tensions. Because mate preferences of offspring may not maximize the fitness of their parents, conflict is expected. For example, children are more likely to value signs of genetic quality of a potential spouse, whereas parents are more likely to value the family background of a potential in-law (16). Parents are also expected to be more tolerant of inbreeding (Inbreeding) than their offspring. These disagreements are the outcome of inclusive fitness (7) differences between parents and offspring and parents are expected to benefit more when their offspring marry relatives (to whom the parents are more closely related). These asymmetric fitness interests are also expected to generate conflict between siblings. This is because parents are equally related to all of their offspring and are therefore expected to value and hence invest in them all equally, whereas offspring are fully related to themselves but only related by a half, or a quarter, to their full and half siblings, respectively (15).
Mate Competition Between Siblings.
Local mate competition (Inbreeding Local Mate Competition) between siblings is common in a variety of taxa (17), and fights to the death over mating can occur between same-sex siblings (18, 19). In many species of insect these conflicts are resolved by the production of female-biased sex ratios (17, 20). In mammals, local mate competition may result in sex-biased dispersal—one sex remains in its natal territory and the other disperses (Inbreeding Sex-Biased Dispersal) (21–23). Sex-biased dispersal provides a way to balance the costs of inbreeding load (24) and local mate competition with the cooperative activities among siblings that lead to local resource enhancement (25).
Once parental control over mating evolved in humans, however, conflicts and cooperation among siblings could also be generated through parent–offspring conflict. This is because marriage exchanges that are under the control of parents are likely to produce uneven fitness benefits across offspring. These unequal outcomes are, in turn, expected to induce sibling competition over spouses. In polygynous and patrilocal societies like the Yanomamö, brothers in particular are expected to vie for wives. At the same time, marriage exchanges may produce benefits for opposite-sex siblings (e.g., a brother receives a wife that is exchanged for his sister). Overall, parental control over marriage generates parent–offspring conflict over mating that can lead to asymmetric fitness consequences for offspring. These discordant outcomes can in turn produce antagonistic interests between same-sex siblings while providing opportunities for opposite-sex siblings to build cooperative alliances.
Predictions.
In this study, we used genealogical data from the Yanomamö, a traditional society of South American horticulturalists, to analyze the effect that spousal relatedness has on the reproductive success of offspring, spouses, parents, and siblings. We test the following predictions (P): P1, offspring of more related parents will have fewer children due to inbreeding depression; P2, spouses who are more closely related will have fewer children due to mechanisms to avoid incest; P3, parents will have more grandchildren when their children marry close relatives because close kin are expected to be more likely to reciprocate promised spousal exchanges and engage in other forms of cooperation; and P4, siblings will have more children when their opposite-sex siblings marry relatives and fewer children when their same-sex siblings do. This is because siblings of the same sex, especially brothers, will compete over marriage partners whereas opposite-sex siblings, especially sisters, will increase opportunities to marry relatives.
The Yanomamö.
At the time these data were collected (Methods, Data Collection), the Yanomamö were a sovereign, indigenous tribal population living in the northern Amazon along the border between Brazil and Venezuela. Until the 1950s there is no record of any sustained contact with a modern western society (11). During the period of N.A.C.’s data collection (1964–1988) the Yanomamö relied on gardens where they grew plantains, bananas, and manioc (26) and also on hunting (27). It is estimated that the Yanomamö numbered approximately 25,000 people across 250 villages during this period (11). The Yanomamö are in many ways an ideal society for answering questions about human evolution because they share many traits that were likely to have been common in ancestral human populations, including polygyny (28), agnatic descent groups (14), patrilocality (29), patrilineality (14) [in the standard cross-cultural sample (SCCS), a widely used worldwide cross-cultural sample, 17% of societies are matrilineal compared with 41% that were classified as patrilineal], lineage exogamy (14), and prescriptive bilateral cross-cousin marriage (30, 31) arranged by older male kin (10). (For primary source ethnographies on Yanomamö economic, social and political life see refs. 2 and 32–35. For additional details on Yanomamö social organization see Inbreeding, Yanomamö Kinship and Politics.)
The Yanomamö practice prescriptive bilateral cross-cousin marriage. This means that males are expected to marry their female cross-cousins (i.e., the daughters of their parent’s opposite-sex siblings). This practice is so embedded in the Yanomamö culture that the words for female cross-cousin and wife are both suaböya and, reciprocally, the words for male cross-cousin and husband are both hearoya (36). If a cross-cousin is not available, an individual is still required to marry someone outside of his or her patrilineage. These rules are often manipulated when it is difficult to find a wife, however, which sometimes leads to conflict. Because prescriptive marriage rules among the Yanomamö require that marriages are between individuals in the same generation, it is common for a father to attempt to manipulate genealogical relationships such as by reclassifying a “niece” as a “sister” so that his sons may now marry her daughters (37). The Yanomamö are normatively patrilocal (the men stay in their natal village and the women move to the village of their husbands), but men who have been promised a wife may have an obligation to perform bride service for their father-in-law although the obligation is less for older males who become polygynous. This bride service, which may last for several years, consists primarily of hunting and providing meat to the household of his father-in-law (36).
Yanomamö marriages are typically arranged either by parents or by the eldest adult male members of local patrilineal descent groups (36). Fathers and brothers exert the most control over the marriages of their respective daughters and sisters whom they attempt to exchange for female cross-cousins (38). Girls are first promised in marriage at a very young age—occasionally at birth—and may be pledged to multiple individuals (2). Young girls marry and begin living with their husbands before or soon after reaching puberty. Members of allied villages are usually reluctant to cede women to their partner villages due to concerns that the latter might not reciprocate as promised (6). “Reciprocal” marriage exchanges are therefore fraught with nervousness on both sides and the parties usually enter into these agreements with caution (37). The marriage patterns observed in Yanomamö villages appear to be the outcomes of strategies that seek to ensure that men will receive brides back in return for the daughters and sisters that they give away. For additional details on Yanomamö marriage see Inbreeding, Yanomamö Marriages.
Results
We measured fitness as (i) the total number of children ever born (Table 1 and Tables S1 and S2), (ii) grandchildren (Table 1), (iii) number of children who survived to age 15 y (Table S1), and (iv) number of spouses (Table S2). Table 1 and Table S1 show the impact of spousal relatedness (three groups bracketed by their coefficient of relatedness; Methods) (Fig. S1) on the fitness outcomes of males and females for each of the following relationships: (i) parent’s relatedness on offspring reproduction, (ii) spousal relatedness on the reproduction of their respective husbands or wives, (iii) offspring relatedness on parental reproduction (measured in number of grandchildren produced), and (iv) sibling relatedness on the reproduction of their brothers and sisters.
Table 1.
Fitness outcomes of marrying relatives across three generations
| Consanguinity of marriage | Male reproduction | Female reproduction |
| Parents | Son’s offspring | Daughter’s offspring |
| Low to medium | −, −, ** | −, −, * |
| Low to high | −, −, ** | −, −, ** |
| Medium to high | −, −, | −, −, |
| Overall | , ** | , ** |
| Spouses | Husband’s offspring | Wife’s offspring |
| Low to medium | = −, = −, * | = −, = −, |
| Low to high | = −t = −, | = −t = −, ** |
| Medium to high | , , | = −t = −, |
| Overall | F = 4.71, = 0.009* | , * |
| Offspring | Father’s grandchildren | Mother’s grandchildren |
| Low to medium | , , * | = 4.27 (1.3), , ** |
| Low to high | = 8.61 (2.0), , ** | = 10.64 (1.2), , ** |
| Medium to high | = 3.05 (2.1), , | = 6.37 (1.5), , ** |
| Overall | , ** | , ** |
| Brothers | Brother’s offspring | Sister’s offspring |
| Low to medium | = 0.30 (0.23), = −, ** | = 0.27 (0.23), , |
| Low to high | = −t−, ** | = 0.62 (0.20), , * |
| Medium to high | = −t−, | = 0.35 (0.22), , |
| Overall | , ** | , * |
| Sisters | Brother’s offspring | Sister’s offspring |
| Low to medium | = −t = −, | = 0.29 (0.20), , |
| Low to high | = 1.1 (0.28), , ** | = −t = −, |
| Medium to high | = 1.12 (0.28), , ** | = −t = −, |
| Overall | , ** | , |
is the mean difference between the groups, t is the t statistic, *P <0.01, **P <0.001.
Table S1.
Offspring survival through age 15 y
| Consanguinity of marriage | Male reproduction | Female reproduction |
| Parents | Son’s offspring | Daughter’s offspring |
| Low to Medium | = − t = − , ** | = − t = − , * |
| Low to High | = − t = − , ** | = − t = − , ** |
| Medium to High | = 0.02 (0.26), , | = − t = − , |
| Overall | , ** | ** |
| Spouses | Husband’s offspring | Wife’s offspring |
| Low to Medium | = − t = − , | = 0.08 (0.19), , |
| Low to High | = − t = − , | = − t = − , |
| Medium to High | = 0.22 (0.31), , | = − t = − , |
| Overall | , | , |
is the mean difference between the groups, t is the t statistic, *P <0.01, **P <0.001.
Table S2.
Offspring relatedness to their spouses and the number of offspring and spouses of parents
| Consanguinity of Marriage | Male reproduction | Female reproduction |
| Offspring | Father’s offspring | Mother’s offspring |
| Low to Medium | = 2.1 (0.49), , ** | = 1.02 (0.29), , ** |
| Low to High | = 0.94 (0.48), , | = 0.54 (0.23), , |
| Medium to High | = − = − = 0.049 | = 0.47 (0.30), = − , |
| Overall | , ** | , ** |
| Sons | Father’s wives | Mother’s husbands |
| Overall | , | , |
| Daughters | Father’s wives | Mother’s husbands |
| Overall | , | , |
is the mean difference between the groups, t is the t statistic, *P <0.01, **P <0.001.
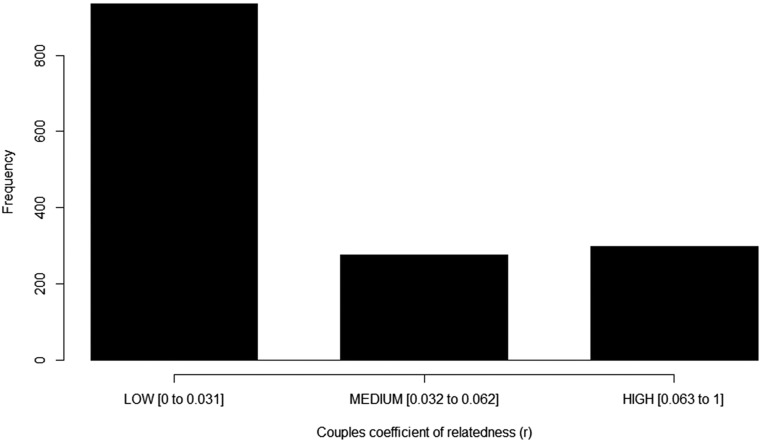
Fig. S1.
Number of couples in each coefficient of relatedness category. These categories roughly equate to third and fourth cousins [Low], second cousins and half first cousins [Medium], and full first cousins, respectively [High]. Couples are related at an average of third cousins (r = 0.032).
P1: Parental Relatedness on Offspring Reproduction.
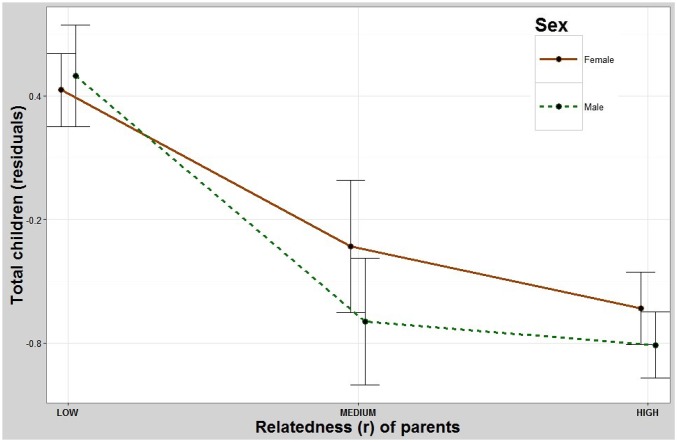
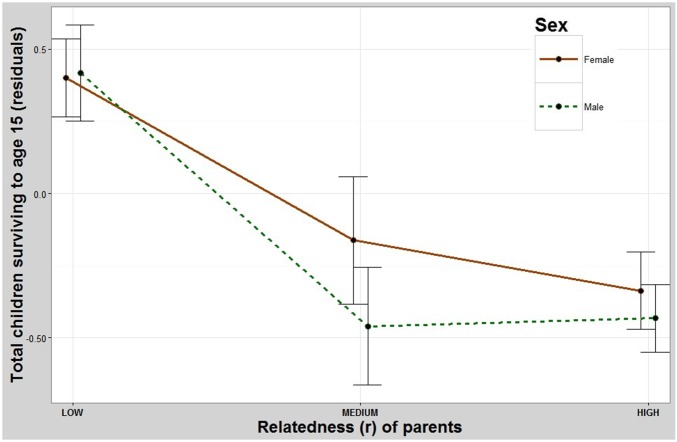
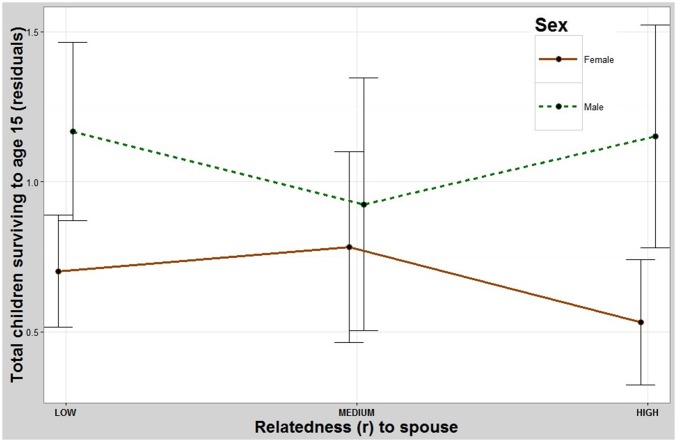
Both sons and daughters of parents who were more closely related had significantly fewer total offspring. Pairwise contrasts showed that the children of the least-related parents had significantly more surviving offspring than both the intermediate-related and most-related groups but showed no significant difference between the intermediate- and most-related groups (Table 1 and Fig. 1). The children of parents who were more closely related also had significantly fewer offspring who survived to age 15 y (Fig. S2 and Table S1).
Fig. 1.
The effect of inbreeding on total fertility. Both sons and daughters of more-related parents have fewer offspring than those whose parents are less related. y axis: residuals (Methods) for total number of children (Fig. S2 shows residuals for survival to age 15 y). x axis: coefficient of relatedness between one’s parents. Error bars: 95% confidence interval (CI).
Fig. S2.
Parent’s relatedness and number of children who survived to age 15 y. Both sons and daughters of more-related parents have fewer offspring that survive to age 15 y than those whose parents are less related. y axis: residuals (Methods) for total number of children who survived to age 15 y. x axis: coefficient of relatedness between parents. Error bars: 95% CI.
P2: Spousal Relatedness on the Reproduction of Husbands and Wives.
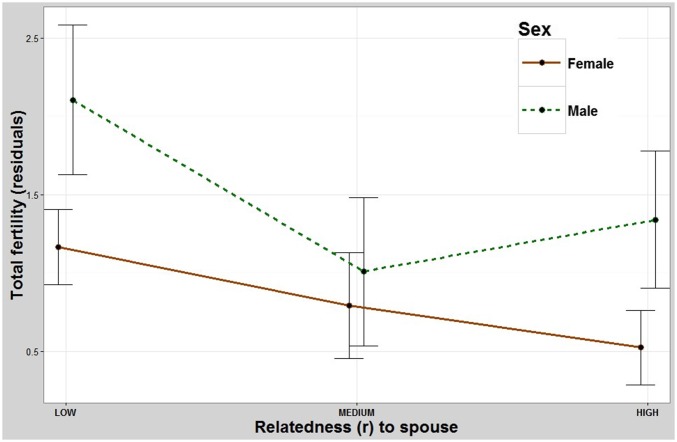
The fitness outcomes of husbands and wives who marry close kin are more ambiguous. A husband’s relatedness to his wife significantly predicted overall differences in reproduction between groups. Pairwise contrasts revealed that husbands who were least related to their wives had significantly fewer offspring than those who were intermediately and most related to their wives but showed no significant difference between the intermediate- and most-related groups (Table 1 and Fig. 2). A wife’s relatedness to her husband also had an overall significant effect on her reproduction. Pairwise contrasts revealed that women who were least related to their husbands did not significantly differ from those who were intermediately related but did show that they have more children than women who were most related to their husbands (Table 1 and Fig. 2). Meanwhile there were no detectable differences between the intermediate- and most-related groups of wives. The impact of marrying relatives on the survival of the couple’s children to age 15 y was also analyzed and did not have any detectable effect on the survival of the offspring of either husbands or wives (Table S1 and Fig. S3).
Fig. 2.
The effect of marrying a relative on total fertility. Relatedness to one’s wife or husband slightly reduces one’s total number of children, especially for females. y axis: residuals (Methods) for total number of children (Fig. S2 shows residual for offspring survival to age 15 y). x axis: mean relatedness to one’s spouse. Error bars: 95% CI.
Fig. S3.
The effect of marrying a relative on the number of children who survive to age 15 y. Relatedness to one’s wife or husband does not significantly predict an individual’s number of children who survive to age 15 y. y axis: residuals (Methods) for total number of children surviving to age 15 y. x axis: mean relatedness to spouse. Error bars: 95% CI.
P3: Offspring Relatedness on Their Parent’s Fitness.
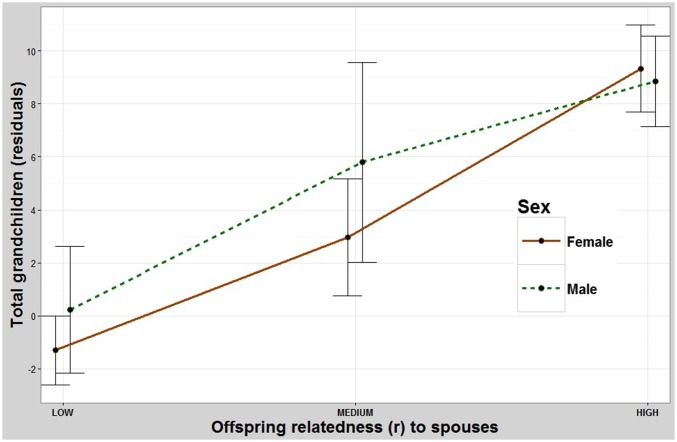
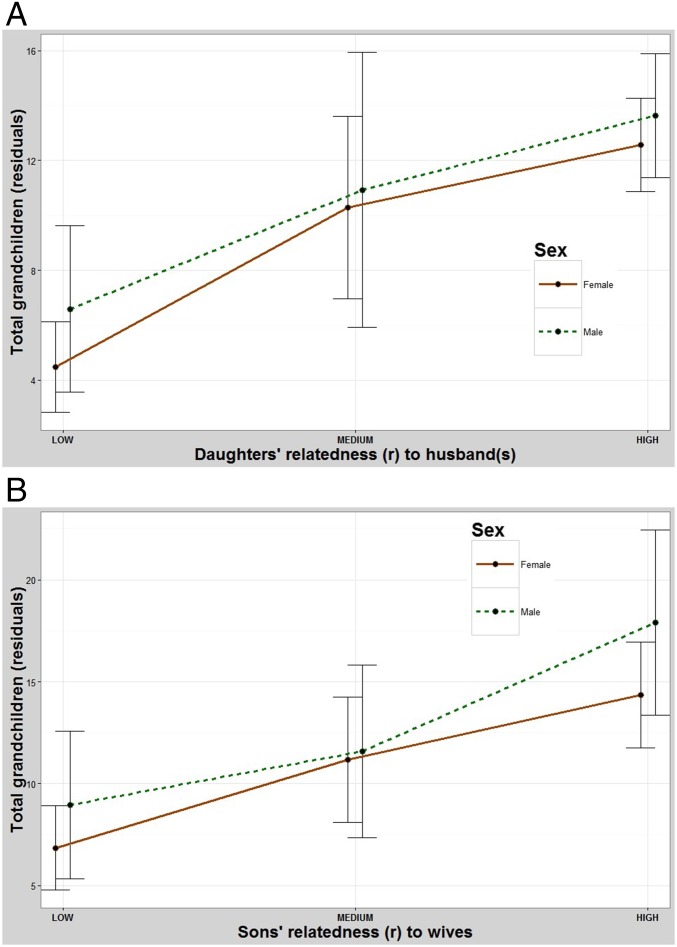
Males have significantly more grandchildren when their children marry more closely related relatives. Pairwise contrasts between the low- and intermediate-relatedness groups and the low- and high-relatedness groups were both significant but the differences between the intermediate- and high-relatedness groups were not (Table 1 and Fig. 3). These fitness benefits do not seem to depend on whether their daughters (ANOVA2,445 F = 4.48, P = 0.012) or their sons (F2,340 = 4.36, P = 0.014) (Fig. S4A) marry relatives. Females also have more grandchildren when their children marry more closely related relatives (Fig. 3) but seem to benefit more when their daughters marry relatives (ANOVA2,525 F =18.2, P < 0.001) than when their sons do (ANOVA2,413 F = 9.88, P < 0.001) (Fig. S4B).
Fig. 3.
The effect of one’s offspring marrying relatives on number of grandchildren produced. Both sexes have more grandchildren when their children marry relatives (Fig. S5 A and B separates this relationship by sons and daughters). y axis: residuals (Methods) for total number of grandchildren. x axis: mean relatedness of all offspring to their spouses. Error bars: 95% CI.
Fig. S4.
(A) The effect of one’s daughters marrying relatives on number of grandchildren produced. Both sexes, but especially females, have more grandchildren when their daughters marry relatives. y axis: residuals (Methods) for total number of grandchildren. x axis: mean relatedness of all daughters to their spouses. Error bars: 95% CI. (B) The effect of one’s sons marrying relatives on number of grandchildren produced. Both sexes have more grandchildren when their sons marry relatives. y axis: residuals (Methods) for total number of grandchildren. x axis: mean relatedness of all sons to their spouses. Error bars: 95% CI.
P4: Sibling Relatedness on the Fitness Outcomes of Brothers and Sisters. Brothers.
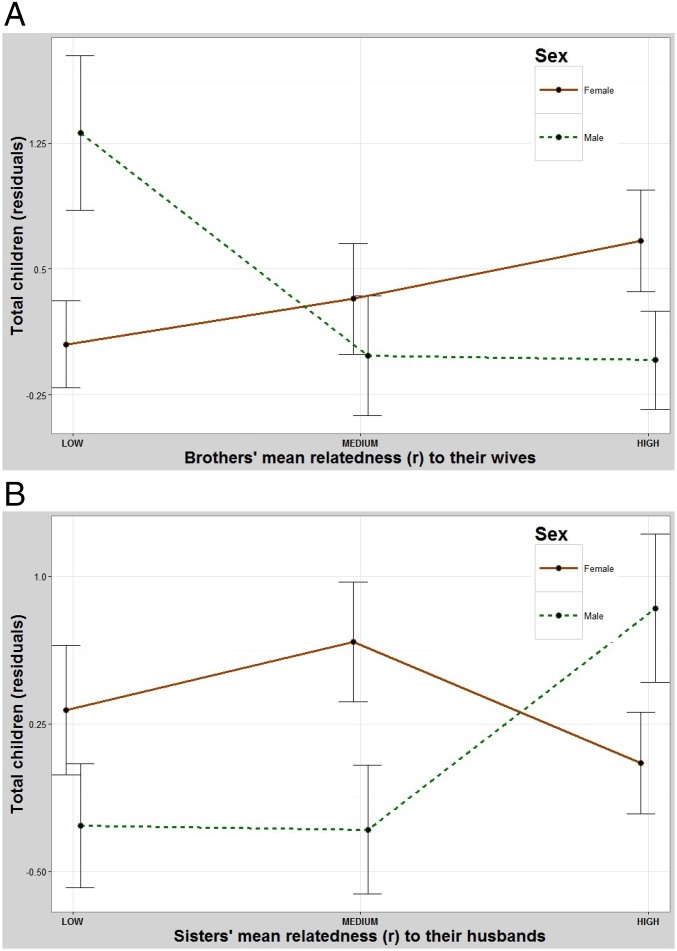
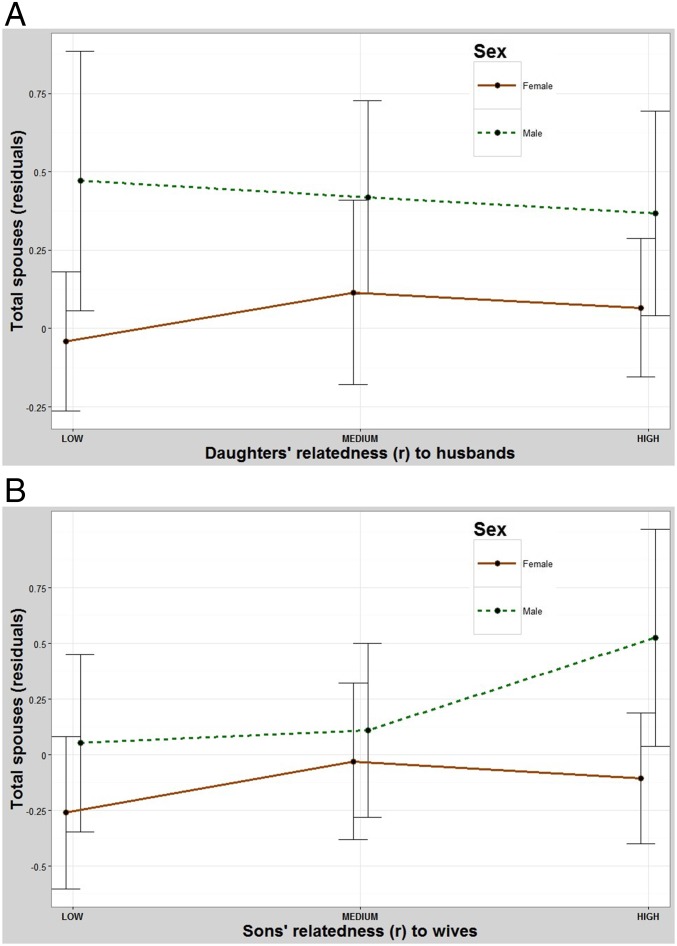
Male reproduction is significantly lower when their brothers marry relatives (Table 1 and Fig. 4A). Pairwise contrasts between the low- and intermediate-relatedness groups and the low- and high-relatedness groups were both significant but the differences between the intermediate- and high-relatedness groups were not. In contrast, males have significantly higher reproduction when their sisters marry relatives (Table 1 and Fig. 4B). A pairwise contrast between the low- and intermediate-relatedness groups was not significant but differences between the low- and high-relatedness groups and the intermediate- and high-relatedness groups were.
Fig. 4.
(A) The effect of one’s brothers marrying relatives on total fertility. Males have significantly fewer children when their brothers marry relatives and females have slightly more children when their brothers marry relatives. y axis: residuals (Methods) for total number of children. x axis: mean relatedness of one’s brothers (maternal and paternal) to their spouses. Error bars: 95% CI. (B) The effect of one’s sisters marrying relatives on total fertility. Males have more children when their sisters marry relatives and females are largely unaffected by whom their sisters marry. y axis: residuals (Methods) for total number of children. x axis: mean relatedness of one’s sisters (maternal and paternal) to their spouses. Error bars: 95% CI.
Sisters.
The fitness of females increases slightly when their brothers marry relatives (Table 1 and Fig. 4A). Pairwise contrasts between the low- and intermediate-relatedness groups and the intermediate- and high-relateness groups were not significant but the difference between the low- and high-relatedness groups was. Meanwhile, females are largely unaffected by the relatedness of their sisters to their spouses (Table 1 and Fig. 4B). Pairwise contrasts between the low- and intermediate-relatedness groups and the low- and high-relatedness groups were not significant, but the difference between the intermediate- and high-relatedness groups was.
Discussion
To better understand the fitness consequences of consanguineous marriage, we examined the effects of marrying relatives on the reproduction of members of the nuclear family: offspring, spouses, parents, and siblings. We found that (i) children suffer from inbreeding depression when their parents are closely related, (ii) the reproduction of wives is slightly lower whereas the effects on husbands are equivocal when they marry close kin, (iii) parents have more grandchildren when their children marry relatives, and (iv) brothers have more children when their sisters marry relatives and fewer children when their brothers do whereas sisters benefit slightly when their brothers marry kin and are unaffected when their sisters do. These results suggest that parents benefit from exchanging their daughters with close relatives. Overall, the asymmetric fitness costs and benefits reported here provide evidence of an evolutionary conflict over mate choice between parents and their offspring and among brothers.
Parent–Offspring Conflict over Marriage.
Differences in optimal fitness outcomes between parents and offspring are expected to cause conflict over marriage preferences (15). The asymmetric fitness costs and benefits of marrying relatives among the Yanomamö support this prediction and suggest that parents should prefer that their offspring marry more closely related kin than the spouses themselves or the offspring would prefer. But how exactly are parents benefiting? Because of high male reproductive skew among the Yanomamö, it is possible that fathers might actively desire to exchange their daughters for wives for themselves—a particular risk in polygynous societies. However, the Yanomamö have a generational rule that forbids males from marrying females in the descending generation (36). Although such marriages may occasionally occur, our results do not indicate that individuals obtain more spouses when either their daughters (Fig. S5A) or sons (Fig. S5B) marry relatives. If fathers were preferentially exchanging daughters with relatives to obtain additional spouses for themselves, we would expect to see a positive association between their numbers of wives and the relatedness of their daughters to their sons in-law. Instead, parents appear to be exchanging their daughters for daughters in-law. In other words, exchanging daughters with relatives enables parents to obtain more brides for their sons, which in turn seems to generate more grandchildren.
Fig. S5.
(A) The effect of one’s daughters marrying relatives on number of spouses. Neither males nor females have more spouses when their daughters marry relatives. This suggests that fathers do not exchange their daughters for wives for themselves. y axis: residuals (Methods) for total number of spouses. x axis: mean relatedness of all daughters to their husbands. Error bars: 95% CI. (B) The effect of one’s sons marrying relatives on number of spouses. Neither males nor females have more spouses when their sons marry relatives. y axis: residuals (Methods) for total number of spouses. x axis: mean relatedness of all sons to their wives. Error bars: 95% CI.
The Origins of Cross-Cousin Marriage.
Preferentially exchanging spouses with close relatives may be driven by contingent reciprocity. One of the biggest problems that individuals face when engaged in high-stakes exchanges, which are often separated by extended time lags, is how to accurately assess the risk of defection by the other party (8). This is particularly important when giving a daughter away in exchange for the mere promise of a future bride who is either not yet born or very young. Therefore, parents may attempt to hedge the risk of the other party defecting by preferentially arranging marriage exchanges with close relatives (7). From the parents’ point of view, kin marriages have the additional benefit of strengthening kin networks (e.g., kin marriages increase overall within-family relatedness) (15, 36). Although these benefits may help to explain the frequency of consanguineous marriages, they do not account for why cross-cousin marriage (marriage between the offspring of opposite-sex siblings), in particular, is so much more common than parallel-cousin marriage (marriage between the offspring of same-sex siblings) across cultures (14, 30).
Traditionally, hypotheses for cross-cousin marriage prescriptions have fallen into two basic camps—inbreeding avoidance and mate exchange. Alexander (39) was the first to suggest that parallel-cousin marriages were often prohibited because of the possibility that same-sex siblings might share (sexually) each other’s spouse(s), which increases the risk that putative parallel cousins are actually half siblings. In this interpretation parallel-cousin marriage taboos function to lower the risks of inbreeding. Meanwhile, Levi-Strauss (5) and others have argued that an important function of marriage is to form alliances between groups and that cross-cousins are usually from different lineages (e.g., matrilines or patrilines). Proponents of this “mate exchange hypothesis” argue that cross-cousin marriage promotes lineage exogamy and functions to build alliances between descent groups (40). More recently, however, Chapais (9) proposed a phylogenetic explanation of the mate exchange hypothesis in which male philopatry and female dispersal (Inbreeding, Sex-Biased Dispersal) combined with the practice of exogamy created a structural bias for marrying cross-cousins. According to this hypothesis, cross-cousin marriage is expected to naturally emerge in the presence of pair bonding, patrilocality, exogamy, parental control of children, and daughter or sister exchange (9). Whereas the mate exchange hypothesis provides a functional explanation for cross-cousin marriage, “the phylogenetic hypothesis” invokes evolutionary constraints and suggests that it was lineage exogamy that promoted cross-cousin marriage rather than the other way around.
Although our results are broadly consistent with both mate exchange and a phylogenetic explanation, they also suggest that mate competition and cooperation among siblings may play an important role in the origin and frequency of cross-cousin marriage systems cross-culturally. Patrilocality can foster mate competition between brothers who often reside together in adulthood and mutually beneficial relationships between brothers and sisters who usually do not live together as adults and therefore do not compete over resources. Meanwhile, polygyny can exacerbate same-sex competition for mates such that the conditions favoring the formation of male–male alliances may be less common than those favoring alliances between sisters or opposite-sex siblings (41). For example, social groups of polygynous mountain gorillas (Gorilla beringei beringei) often contain sexually mature siblings of both sexes (42, 43) and brothers have been observed to engage in more aggressive interactions and fewer affiliative behaviors, compared with mixed-sex or sister dyads (44). Interestingly, these observations seem to suggest that alliances between opposite-sex siblings and conflicts between brothers could develop in the absence of parental control over mate choice. However, when marriages are arranged by parents (e.g., daughter exchange instead of sister exchange), the bond between the parents of the spouses is the critical factor, and this can generate cross-cousin marriage (i.e., the exchange of daughters between opposite-sex siblings). Although these results do not mean that cross-cousin marriage prescriptions are the necessary result of mate competition between brothers, they do suggest that mate competition between brothers could generate strong biases for cross-cousin marriage independently of patrilocality (Inbreeding, Sex-Biased Dispersal) and lineage exogamy.
Among the Yanomamö, both males and females have more children when their opposite-sex siblings marry close kin. Males, on the other hand, endure reduced fitness whereas females are largely unaffected by the consanguinity of their same-sex siblings (Fig. 4 A and B). Because parents can influence the marriages of their children, these competitive and cooperative relationships can bleed into the next generation and eventually become embedded in the rules governing the marriage exchange system. Chapais (9) has argued that these patterns, which often begin as mere social regularities, can over time acquire a formal status and ultimately become codified in the culture as social norms prescribing that only the offspring of opposite-sex siblings are permitted to marry. It should be noted that these same patterns are also consistent with the phylogenetic hypothesis (9) although the causation is different. The phylogenetic constraints interpretation of our results is that cross-cousin marriage generates mate competition between siblings, whereas the “local mate competition hypothesis” suggests the opposite—sibling competition drives cross-cousin marriage prescriptions.
If the local mate competition hypothesis is true and competition between siblings plays an important role in the origin of cross-cousin marriage prescriptions, then we should expect to see parallel-cousin marriages more often when mate competition between same-sex siblings is low. Almost all parallel-cousin marriages noted in the cross-cultural record are exchanges of offspring between brothers (i.e., patrilateral parallel-cousin marriage) and most of these occur in or near the Middle East (30, 45). Bedouin men, for instance, are presumed to possess marital rights over the daughters of their paternal uncles (46). Historically, explanations for this type of arrangement have focused on males inheriting resources in these societies and the importance of retaining property within families (40). Perhaps, however, these inheritance rules serve to strengthen bonds between brothers and reduce mate competition, thereby enabling enough trust to allow them to exchange their daughters. There may also be something about challenging desert environments or pastoralist societies (e.g., goat herding) in general that reduces mate competition and cultivates alliances between brothers. For example, camel raiding and the defense of herds among Bedouin pastoralists has been seen as important in the formation and preservation of alliances and central to the preservation of the culture of these kin-based societies (47). The need to defend and tend to herds can increase the benefits of cooperation between males. It is important to note, however, that a reduction in the intensity of mate competition between brothers in parallel-cousin marriage systems does not address causation and is compatible with both the “phylogenetic constraints” and local mate competition hypotheses. Which sex disperses (Inbreeding, Sex-Biased Dispersal) is also likely to affect mate competition and resource competition among siblings (48), so we should expect to see more competition between maternal kin and less conflict between paternal kin (49) in matrilocal societies. Female philopatry and high male reproductive skew (e.g., polygyny) are expected to expected to impact mate competition among siblings oppositely, however, so these interactions can be complicated.
Conclusion
In summary, marriages between close kin (i) significantly reduce the reproductive success of offspring that result from these unions, (ii) impose a slightly negative effect on the reproductive success of husbands and wives, and (iii) provide a substantial reproductive benefit to both parents and opposite-sex siblings. Together, these results suggest an important role of both parent–offspring conflict and sibling competition over mate choice among the Yanomamö. These results have broad implications for understanding the prevalence and origins of marriage systems across cultures. Our findings may also reveal new ways to understand within-family conflicts over mate choice. Overall, the important role that Yanomamö parents play in the marriages of their children, combined with asymmetric fitness outcomes between parents and children, suggests that individual mate preferences (especially those of daughters) may be less important than those of parents (2, 10, 50) in generating marriage prescriptions. In the Yanomamö, at least, cross-cousin marriage may be a partial resolution to mate competition between coresident brothers.
Methods
Data Collection.
This study is based on data collected by Napoleon A. Chagnon (N.A.C.) between 1964 and 1988, ranging over a period of 50 nonconsecutive months from interviews of almost 2,000 individuals in 60 villages. Information was obtained for 4,158 individuals and 1,507 marriages. The genealogical data used in this study include data on all marriages, including information on the spouses, their children, their siblings, and their parents (2). There were approximately 25,000 Yanomamö living in this area at the time these data were collected (11). The ways in which spouses were related could often be traced through multiple common ancestors and these complex relationships were determined using the KINDEMCOM program. The relatedness values between the spouses are coefficients of relatedness (r) (the probability that any two individuals share two alleles that are identical by descent from a common ancestor) (51) and were derived from the genealogy.
The Yanomamö do not have a written language, so the genealogical data are reliant upon information gathered through oral interviews. The genealogies range from a depth of 1 to as many as 10 generations. The accuracy of the estimates of relatedness between spouses depends on the number of generations ancestral to the couple that are known, whereas the completeness of reproductive histories depends on the age of the individuals at the time of the census. To improve the accuracy of the analysis, we excluded individuals if either of their parents was not known and because the youngest male to reproduce was 17 y old and the youngest female was 14 y old, we excluded all males and females younger than these ages. In the end there were 1,599 individuals (770 males and 829 females) for which there was enough information to obtain reliable measures of spousal relatedness and estimates of fitness. This project was approved on April 28, 2016, by the University of Missouri Institutional Review Board (project no. 2001639).
Analysis.
We used R version 3.2.2 for all analyses. The dependent variables used in this study were age and year of birth adjusted estimates of an individual’s (i) number of children, (ii) number of grandchildren, (iii) number of children who survived to age 15 y, and (iv) number of spouses. All of the dependent variables showed signs of overdispersion and an excess of zeroes so a zero-inflated negative binomial (ZINBI) generalized linear regression model was used.
The sample was first divided into dead (N = 743) and living (N = 856) individuals over the ages of 17 y and 13 y for males and females, respectively. For individuals who were alive at the time of the last interview the residuals for males and for females were obtained separately. This was done because of the high reproductive skew and different life history patterns of Yanomamö men and women: Male variance in reproduction = 10.34 (N = 2,268) is higher than female variance = 6.0 (N = 1,890). The age of the oldest female to give birth was 52 y but 99.5% of all female births were to mothers who were 45 y old or younger. Therefore, all females over 45 y old were given the truncated age of 45 y so that all females in our sample had adjusted ages that were between 14 y and 45 y old. The oldest male to father a child was 81 y but 99.5% of births were to fathers who were 72 y of age or younger. Therefore, all males over the age of 72 y were given the truncated age of 72 y so that all males in or sample had adjusted ages between 17 y and 72 y old.
Using these adjusted ages, we then used a retrospective technique to estimate the completion of these key fitness traits had these males and females lived to senescence (52). Year of birth, age, and age squared were all found to have strong effects on fitness consequences so we entered these three variables into our model. Age squared was included to account for quadratic relationships (e.g., fitness returns may decline beyond a certain age). Year of birth was used to control for cohort effects (i.e., certain generations or time periods when individuals reproduced more) and because it provided an estimate of the maximum amount of time that any additional data (e.g., children, grandchildren, spouses, mean relatedness to spouses) could have been collected on any particular individual. For example, an individual born in 1950 and censused in 1974 may have been 24 y old the last time N.A.C. actually saw and recorded data on him but life history or spousal relatedness information on this individual may have been updated up until the last census in 1988.
For males the model revealed a strong and positive impact of all covariates—age, age squared, and year of birth—on an individual’s total fertility (McFadden’s pseudo- = 0.49, N = 403), offspring who survived to age 15 y (McFadden’s pseudo- = 0.49, N = 403), number of grandchildren (McFadden’s pseudo-R2 = 0.69, N = 403), and number of spouses (McFadden’s pseudo- = 0.27, N = 403). For females the model also showed a strong and positive impact of age, age squared, and year of birth on an individual’s total fertility (McFadden’s pseudo- = 0.50, N = 453), offspring who survived to age 15 y (McFadden’s pseudo- = 0.49, N = 453), number of grandchildren (McFadden’s pseudo- = 0.70, N = 453), and spouses (McFadden’s pseudo- = 0.14, N = 453) for all individuals who were still alive at the time of the last census. Using these three covariates also produced the lowest adjusted and unadjusted Akaike’s information criterion scores for both males and females. Therefore, a ZINBI generalized linear model was used to regress year of birth, age, and age squared on each of the dependent variables and these residuals were saved and used to estimate fitness for all individuals who were alive at the time of the last interview for all subsequent analyses. Dead subjects were presumed to have complete life histories, so their fitness (i.e., total number of children, children surviving to age 15 y, grandchildren, and spouses) was fitted to a model in which year of birth was entered as a predictor to correct for birth cohort effects on fitness residuals. These residuals for living and dead individuals were saved and were used as our estimate of fitness.
Finally, individuals were categorized into three groups based on their mean coefficient of relatedness to their spouse(s)—[Low] = 0–0.031, [Medium] = 0.032–0.062, and [High] = 0.063–1. These categories are meaningful as composite categories of cousin relatedness in a society with high reproductive skew and where half-cousin marriages are much more common than full-cousin marriages. These categories roughly equate to third and fourth cousins [Low], second cousins and half-first cousins [Medium], and full first cousins [High] and have enough individuals in each group so that meaningful statistical comparisons between all categories can be made (Fig. S1).
Data Archival.
The data used in all these analyses can be found in Dataset S1. For queries on any of these results or analyses please contact Robert Lynch at robertflynch@gmail.com.
Inbreeding
Charles Darwin conducted the first rigorous studies of inbreeding (53–56). His results supported the hypothesis that breeding with close relatives reduces the fitness of progeny (“inbreeding depression”). Researchers since Darwin have confirmed the negative fitness outcomes caused by inbreeding in many plant and animal species (56–58) Inbreeding depression results from closely related parents both transferring deleterious recessive alleles, increased homozygosity, and vulnerability to pathogens (59–62). On the other hand, this is a citation to an earlier citation in the main text: ref. 2.
Too much outbreeding, however, can also have negative effects on fitness (63). When intermediate phenotypes are selected against (64), or when locally adapted gene complexes have undergone positive selection (65), outbreeding is no longer beneficial. Although both inbreeding and outbreeding can affect the fitness of offspring, the costs of inbreeding are usually more immediate and severe, whereas the costs of outbreeding tend to accrue slowly and accumulate across several generations. Because both hybrid vigor and outbreeding depression can occur simultaneously and have opposite effects on fitness, there is often an “optimal” level of inbreeding at which fitness peaks (66, 67).
The mechanisms by which organisms achieve an ideal balance between inbreeding and outbreeding have received a great deal of attention from researchers (68–70). The most common mechanism among mammals and birds is sex-biased dispersal, where one sex disperses from the natal group upon reaching sexual maturity (21, 71). Humans are distinct among primates in that either sex may disperse or remain in its natal group (1). This pattern renders the ability to recognize the relatedness of potential mates critical and humans exhibit the most extensive pattern of kin recognition of any known species (9). Incest avoidance in humans is now understood on both a functional and a mechanistic level (39, 72–76). Whereas functional explanations tend to cite the genetic disadvantages of inbreeding (63), mechanistic explanations for how opposite-sex relatives recognize one another usually cite the duration and timing of coresidence and cues assessing the interaction of a potential relative with one’s biological mother around birth (72, 76).
Studies of optimal inbreeding in humans have produced mixed results. The analysis of an Icelandic genealogy, for example, showed that reproductive success was highest for couples who were related as third and fourth cousins [coefficient of relatedness (r) between 0.0039 and 0.0625] (77). Walker and Bailey (78) examined how reproductive success varied with degree of inbreeding across different types of societies. Among foragers the number of surviving children was highest when couples were related as second and third cousins (r between 0.0312 and 0.1249) but dropped rapidly at higher coefficients of relatedness. Among traditional nonforagers (i.e., horticulturalists, pastoralists, agriculturalists), however, fitness increased linearly with relatedness, and the most related couples (r > 0.125) had the most surviving children. Although closely related couples do not necessarily have reduced fertility, most research suggests that their offspring do (60, 73) and mortality rates for the offspring of first cousins is about 3.5% higher than for the offspring of unrelated couples (79), as well as a 4.4% increase in prereproductive mortality (e.g., still births) compared with population means (80). Other studies have shown higher infant mortality rates for offspring (81) and higher divorce rates and lower fertility (82) for the spouses of patrilateral parallel first-cousin marriages.
Despite these negative fitness outcomes, many traditional societies, and some modern ones in the Middle East, India, Pakistan, and Bangladesh (83), exhibit relatively high levels of consanguineous marriage (53), and unions between first or second cousins are estimated to have comprised over 80% of marriages in human prehistory (79). Furthermore, average coefficients of relatedness (r) (51) between spouses in traditional societies are far higher than models of optimal inbreeding predict (84).
Local Mate Competition.
Same-sex siblings often compete for mates. Fights to the death are common between parasitic wasp brothers as they compete for mates (18) and between honey bee sisters when they compete to head the original colony after it divides and swarms (19). When inbreeding is high and females have direct control of the sex ratio of their offspring (20, 85–88), conflicts between siblings can be resolved by the production of female-biased sex ratios because inbreeding is less costly to parents than it is to offspring (17). In mammals, competition between same-sex siblings over mates may affect dispersal patterns (89, 90). For example, when mate competition and relatedness are both high, same-sex siblings may elect to either breed cooperatively or compete (91). Under these conditions altruism toward kin and local mate competition are often in conflict because altruism toward one sibling (e.g., foregoing reproduction by cooperative breeding) is less advantageous if it comes at a cost to another sibling (92). To put it another way, there is no point in being altruistic toward a brother if his increased fitness is costly to a different brother (93). In humans, competition over mates and resources has been shown to drive both sex-biased investment (94) and dispersal patterns (95).
Sex-Biased Dispersal.
Kin competition and cooperation over mates and resources interact with inbreeding to affect which sex is more likely to disperse (22, 90, 96). Greenwood (21) was the first to suggest that the direction of dispersal bias is linked to the mating system. He argued that male–male competition for resources combined with high male investment in defending resources favors monogamy, patrilineal social organization, and female dispersal (male philopatry)—a pattern typical in birds—whereas male–male competition for mates together with low male investment in resources favors polygyny, matrilineal social organization, and male dispersal (female philopatry)—a pattern commonly seen in mammals. In other words, sex-biased dispersal is generally governed by the defense of resources in birds and by the defense of mates in mammals (97).
Researchers trying to explain sex-biased dispersal patterns across species have built on this work and evolutionary models now typically include inbreeding avoidance, local resource competition (48), local mate competition (17, 89, 98), and local resource enhancement (99). The benefits of sex-biased dispersal, which include a reduced risk of inbreeding (23, 24, 71), lower intersexual mate competition, and reduced resource competition with relatives of both sexes (48, 100), must be balanced against cooperative activities among kin that lead to local resource enhancement (25). An experimental study of root voles (Microtus oeconomus), for example, found that males without sisters emigrated more often than males with sisters (101), suggesting that there are benefits of living near opposite-sex siblings. In contrast, Bollinger et al. (102) found in the meadow vole (Microtus pennsylvanicus) that both sexes dispersed more often from sibling groups than they did from nonsibling groups and that sibling males were more likely to disperse than nonsibling males, which indicates that there are costs associated with residing near siblings. Together these studies show that inbreeding, local mate competition, local resource competition, and local resource enhancement interact to affect dispersal in complex ways.
In general, however, cooperation and conflict between siblings play an important role in the evolution of sex-biased dispersal (21, 97). Individuals are expected to balance the costs of inbreeding (23, 24, 71) and competition with relatives over mates (48, 100) and resources with the benefits of cooperative activities among kin (25). When parents can influence offspring mate choice, as in humans, these relationships may generate cultural norms and taboos over who is permitted to marry and which sex disperses (9, 103).
Yanomamö Kinship and Politics.
Although the Yanomamö live in environments that are ecologically different (horticulturalists in South America) from the East African savannas in which our hominin ancestors evolved, they share a political organization and social structure that are similar to what many anthropologists expect were common for individuals living in the environment of evolutionary adaptedness (EEA) (11, 104). Many aspects of the social environments in which the Yanomamö live also contain features (e.g., cousin marriage) that our ancestors are likely to have shared. For an adaptation to solve a specific environmental problem it needs only to interact in a consistent way with the specific features of the environment in which it evolved. In other words, not all aspects of an environment need to be identical to a presumed EEA to make meaningful inferences about adaptive outcomes. Behavioral ecologists refer to these features as an adaptively relevant environment (ARE) (105). Yanomamö social and political organizations share many of these characteristics with those of our distant ancestors; hence fitness measures in the Yanomamö are relevant to understanding the evolution of marriage practices, sexual selection, and mate choice in humans.
Average coefficients of relatedness between Yanomamö spouses (r = 0.031) are similar to those observed in many other traditional societies (79), including several other horticulturalist groups (78). They may also be similar to what population geneticists estimate was common throughout much of human evolution. If, for example, we assume an effective population size of human ancestors living 1.2 Mya was 18,500 (106), then simulations based on current genetic population structure and heterozygosity predict that mean spousal coefficients of relatedness will vary from 0.17 (between first cousins and half siblings) at the out-of-Africa bottleneck 70 kya to 0.016 (between second and third cousins) in Africa before the expansion out of Africa (107). These estimates will vary, depending on various assumptions including recombination rates, effective population sizes, population structure, and population boom and bust cycles. Despite this uncertainty, our analysis shows that the mean relatedness of Yanomamö spouses (r = 0.031) is right in the middle of these estimates.
Yanomamö villages are on average around 100 individuals (ranging between 25 and 400), which is similar in size to what many anthropologists and geneticists believe was common throughout much of our evolutionary history (108). When villages fission they usually do so along lines of kinship, such that all of the individuals in each of the new villages are on average more related to each other than they were to members of the original village (38). There is a push and pull to achieving the proper balance between group size and vulnerability to attack. Although most individuals prefer to live in smaller villages because internal conflicts (often over women) increase in proportion to the village size, smaller villages are increasingly at risk for being attacked and villages that fall below a threshold of 40 are usually unsustainable (11). Villages are typically led by a headman and status differentials between men are in large part determined by an individual’s number of close kin, especially patrilineal kin who are residents of the same village, and culturally valued accomplishments such as prowess in intervillage raiding (2).
Yanomamö Marriages.
Yanomamö marriages are political events that have strong impacts on relationships between groups of adult men both within and between villages (11). Indeed, much of the internal cohesion of Yanomamö villages depends on the bonds of marriage and kinship (2). The political alliances between Yanomamö villages are also based on kinship and are therefore strongly influenced by the reciprocal marriage arrangements between groups, and the exchange of women between independent villages ties them together (2).
Competition over wives is a major source of conflict among the Yanomamö. Polygyny results in high reproductive skew (e.g., great reproductive success of a few powerful males) and this can have a large impact on marriage exchanges. When there are more competing lineages (fewer highly successful patrilines) and a young woman is ready for marriage, the temptation to defect is higher and long-term betrothal promises are often subverted by short-term opportunities that can lead to conflicts (37). In addition to obtaining wives by exchanging them with other patrilineages, men may also acquire wives by force. They may steal women from other villages in raids or, if they are powerful enough, take a woman from a coresident man. In militarily strong lowland villages an average of 17% of married women have been abducted compared with 11% in weaker upland villages (see ref. 36, p. 87 and ref. 109, pp. 78–79 on 12 marital abductions among the Xiliana Yanomamö subgroup).
Kinship networks also affect an individual’s chances of acquiring mates. Male Yanomamö who have more ancestral-generation and same-generation matrilateral kin have more potential mates (2). This is because having powerful elder-generation allies (those who arrange the marriages) improves one’s chances of securing a wife, whereas a paucity of matrilateral kin (e.g., when one’s mother has been abducted or has come from a distant village) reduces the availability of marriageable matrilateral kin (e.g., mother’s brother’s daughters) (37). Because of bride service and frequent village fissioning, females may have nearly as many close relatives in their village as males, however.
Although Yanomamö politics considerably restrict the ability of women (and to a lesser extent, younger and less powerful men) to exercise choice in selecting a mate, older women are not entirely powerless. Although they risk being severely punished, women may commit adultery or in other ways try to make life so miserable for their husband that they provoke him into abandoning them (36). Because all marriages have important political implications, conflicts of interest in any marriage are common. As a consequence, a woman’s success in making a satisfactory marriage for herself or her daughters depends on the same types of skills that determine male success in politics: her ability to manipulate social situations to her own advantage and her ability to draw support from older men in her patrilineage (11).
Supplementary Material
Acknowledgments
We thank Robin Fox and Bernard Chapais for their useful comments on this article. N.A.C. was supported by National Science Foundation (NSF) Grant BCS-1461532 and R.H. was supported by NSF Grant BCS-1461504.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1618655114/-/DCSupplemental.
References
- 1.Hill KR, et al. Co-residence patterns in hunter-gatherer societies show unique human social structure. Science. 2011;331(6022):1286–1289. doi: 10.1126/science.1199071. [DOI] [PubMed] [Google Scholar]
- 2.Chagnon NA. Studying the Yanomamö. Holt Rinehart & Winston; New York: 1974. [Google Scholar]
- 3.Irons W. Why lineage exogamy. In: Alexander RD, Tinkle DW, editors. Natural Selection and Social Behavior. Chiron; New York: 1981. pp. 476–489. [Google Scholar]
- 4.Flinn MV. Evolutionary anthropology of the human family. In: Salmon CA, Shackelford TK, editors. Oxford Handbook of Evolutionary Family Psychology. Oxford University Press; New York: 2011. pp. 12–32. [Google Scholar]
- 5.Lévi-Strauss C. The Elementary Structures of Kinship. Beacon; Boston: 1949. [Google Scholar]
- 6.Chagnon N. Kin selection theory, kinship, marriage, and fitness among the Yanomamo Indians. In: Salmon CA, Shackelford TK, editors. Sociobiology: Beyond Nature/Nurture. Oxford University Press; New York: 1980. pp. 545–571. [Google Scholar]
- 7.Hamilton WD. The genetical evolution of social behaviour. II. J Theor Biol. 1964;7(1):17–52. doi: 10.1016/0022-5193(64)90039-6. [DOI] [PubMed] [Google Scholar]
- 8.Trivers RL. The evolution of reciprocal altruism. Q Rev Biol. 1971;46(1):35–57. [Google Scholar]
- 9.Chapais B. Complex kinship patterns as evolutionary constructions, and the origins of sociocultural universals. Curr Anthropol. 2014;55(6):751–783. [Google Scholar]
- 10.Apostolou M. Sexual selection under parental choice: The role of parents in the evolution of human mating. Evol Hum Behav. 2007;28(6):403–409. [Google Scholar]
- 11.Chagnon NA. Noble Savages: My Life Among Two Dangerous Tribes–the Yanomamo and the Anthropologists. Simon and Schuster; New York: 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walker RS, Hill KR, Flinn MV, Ellsworth RM. Evolutionary history of hunter-gatherer marriage practices. PLoS One. 2011;6(4):e19066. doi: 10.1371/journal.pone.0019066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fox R. Kinship and Marriage: An Anthropological Perspective. Vol 50 Cambridge Univ Press; Cambridge, UK: 1967. [Google Scholar]
- 14.Murdock GP, White DR. Standard cross-cultural sample. Ethnology. 1969;8(4):329–369. [Google Scholar]
- 15.Trivers RL. Parent-offspring conflict. Am Zool. 1974;14(1):249–264. [Google Scholar]
- 16.Apostolou M. Parent-offspring conflict over mating: The case of family background. Evol Psychol. 2008;6(3) [PubMed] [Google Scholar]
- 17.Hamilton WD. Extraordinary sex ratios. Science. 1967;156(3774):477–488. doi: 10.1126/science.156.3774.477. [DOI] [PubMed] [Google Scholar]
- 18.Hamilton W. In: Wingless and fighting males. Sexual Selection and Reproductive Competition in Insects. Blum E, editor. Academic Press; NY: 1979. pp. 167–200. [Google Scholar]
- 19.Seeley TD. Honeybee Ecology: A Study of Adaptation in Social Life. Princeton Univ Press; Princeton: 2014. [Google Scholar]
- 20.West S, Herre E. Partial local mate competition and the sex ratio: A study on non-pollinating fig wasps. J Evol Biol. 1998;11(5):531–548. [Google Scholar]
- 21.Greenwood PJ. Mating systems, philopatry and dispersal in birds and mammals. Anim Behav. 1980;28(4):1140–1162. [Google Scholar]
- 22.Pusey AE. Sex-biased dispersal and inbreeding avoidance in birds and mammals. Trends Ecol Evol. 1987;2(10):295–299. doi: 10.1016/0169-5347(87)90081-4. [DOI] [PubMed] [Google Scholar]
- 23.Perrin N, Mazalov V. Local competition, inbreeding, and the evolution of sex-biased dispersal. Am Nat. 2000;155(1):116–127. doi: 10.1086/303296. [DOI] [PubMed] [Google Scholar]
- 24.Cockburn A, Scott MP, Scotts DJ. Inbreeding avoidance and male-biased natal dispersal in Antechinus spp. (Marsupialia: Dasyuridae) Anim Behav. 1985;33(3):908–915. [Google Scholar]
- 25.Perrin N, Lehmann L. Is sociality driven by the costs of dispersal or the benefits of philopatry? A role for kin-discrimination mechanisms. Am Nat. 2001;158(5):471–483. doi: 10.1086/323114. [DOI] [PubMed] [Google Scholar]
- 26.Hames R. Monoculture, polyculture, and polyvariety in tropical forest Swidden cultivation. Hum Ecol. 1983;11(1):13–34. [Google Scholar]
- 27.Hames R. A comparison of the efficiencies of the shotgun and the bow in neotropical forest hunting. Hum Ecol. 1979;7(3):219–252. [Google Scholar]
- 28.Marlowe FW. The mating system of foragers in the standard cross-cultural sample. Cross Cult Res. 2003;37(3):282–306. [Google Scholar]
- 29.Ember M, Ember CR. The conditions favoring matrilocal versus patrilocal residence. Am Anthropol. 1971;73(3):571–594. [Google Scholar]
- 30.Murdock GP. World ethnographic sample. Am Anthropol. 1957;59(4):664–687. [Google Scholar]
- 31.Sawyer J, LeVine RA. Cultural dimensions: A factor analysis of the world ethnographic sample. Am Anthropol. 1966;68(3):708–731. [Google Scholar]
- 32.Chagnon NA. 1966. Yanomamo Warfare, Social Organization and Marriage Alliances PhD Dissertation. University Michigan. University Microfilms, Ann Arbor, MI.
- 33.Chagnon NA. Yanomamö: The Fierce People. Holt Rinehart and Winston; New York: 1968. [Google Scholar]
- 34.Lizot J. 1976. The Yanomami in the Face of Ethnocide, IWGIA Document 22 (ERIC, Copenhagen)
- 35.Lizot J. Tales of the Yanomami: Daily Life in the Venezuelan Forest. No 55 Cambridge Univ Press; Cambridge, UK: 1991. [Google Scholar]
- 36.Chagnon NA. Yanomamo: The Fierce People. Henry Holt; New York: 1983. [Google Scholar]
- 37.Chagnon NA. 1982. Sociodemographic attributes of nepotism in tribal populations. Current Problems in Sociobiology, eds Kings College Sociobiology Group (Cambridge Univ Press, Cambridge, UK), pp 291–318. [Google Scholar]
- 38.Chagnon NA. Mate competition, favoring close kin, and village fissioning among the Yanomamo Indians. In: Chagnon N, Irons W, editors. Evolutionary Biology and Human Social Behavior. Duxbury; Duxbury, MA: 1979. pp. 112–145. [Google Scholar]
- 39.Alexander RD. Natural selection and the analysis of human sociality. In: Goulden CE, editor. Changing Scenes in Natural Sciences. Academy of Natural Sciences; Philadelphia: 1977. pp. 283–337. [Google Scholar]
- 40.Flinn MV, Low BS. Ecological Aspects of Social Evolution. 1986. Resource distribution, social competition, and mating patterns in human societies; pp. 217–243. [Google Scholar]
- 41.Chapais B. Alliances as a means of competition in primates: Evolutionary, developmental, and cognitive aspects. Am J Phys Anthropol. 1995;38(S21):115–136. [Google Scholar]
- 42.Fossey D. Gorillas in the Mist. Houghton Mifflin Harcourt; Boston: 2000. [Google Scholar]
- 43.Stoinski TS, et al. Proximate factors influencing dispersal decisions in male mountain gorillas, Gorilla beringei beringei. Anim Behav. 2009;77(5):1155–1164. [Google Scholar]
- 44.Rosenbaum S. 2014. Who’s your daddy? The causes and consequences of male-immature relationships in wild mountain gorillas (Gorilla beringei beringei). PhD dissertation (University of California, Los Angeles)
- 45.Korotayev A. Parallel-cousin (FBD) marriage, Islamization, and Arabization. Ethnology. 2000;39(4):395–407. [Google Scholar]
- 46.Murphy RF, Kasdan L. The structure of parallel cousin marriage. Am Anthropol. 1959;61(1):17–29. [Google Scholar]
- 47.Sweet LE. Camel raiding of north Arabian Bedouin: A mechanism of ecological adaptation. Am Anthropol. 1965;67(5):1132–1150. [Google Scholar]
- 48.Clark AB. Sex ratio and local resource competition in a prosimian primate. Science. 1978;201(4351):163–165. doi: 10.1126/science.201.4351.163. [DOI] [PubMed] [Google Scholar]
- 49.Silk JB. Kin selection in primate groups. Int J Primatol. 2002;23(4):849–875. [Google Scholar]
- 50.Apostolou M. Sexual selection under parental choice in agropastoral societies. Evol Hum Behav. 2010;31(1):39–47. [Google Scholar]
- 51.Wright S. Coefficients of inbreeding and relationship. Am Nat. 1922;56(645):330–338. [Google Scholar]
- 52.Strassmann BI, Gillespie B. How to measure reproductive success? Am J Hum Biol. 2003;15(3):361–369. doi: 10.1002/ajhb.10154. [DOI] [PubMed] [Google Scholar]
- 53.Darwin C. The Various Contrivances by Which Orchids are Fertilised by Insects. John Murray; London: 1888. [Google Scholar]
- 54.Darwin C. The Effects of Cross and Self Fertilisation in the Vegetable Kingdom. John Murray; London: 1876. [Google Scholar]
- 55.Darwin C. The Different Forms of Flowers on Plants of the Same Species. John Murray; London: 1877. [Google Scholar]
- 56.Ritland K. Inferences about inbreeding depression based on changes of the inbreeding coefficient. Evolution. 1990;44(5):1230–1241. doi: 10.1111/j.1558-5646.1990.tb05227.x. [DOI] [PubMed] [Google Scholar]
- 57.Richards CM, Church S, McCauley DE. The influence of population size and isolation on gene flow by pollen in Silene alba. Evolution. 1999;53(1):63–73. doi: 10.1111/j.1558-5646.1999.tb05333.x. [DOI] [PubMed] [Google Scholar]
- 58.Herlihy CR, Eckert CG. Genetic cost of reproductive assurance in a self-fertilizing plant. Nature. 2002;416(6878):320–323. doi: 10.1038/416320a. [DOI] [PubMed] [Google Scholar]
- 59.Tooby J. Pathogens, polymorphism, and the evolution of sex. J Theor Biol. 1982;97(4):557–576. doi: 10.1016/0022-5193(82)90358-7. [DOI] [PubMed] [Google Scholar]
- 60.Bittles AH, Neel JV. The costs of human inbreeding and their implications for variations at the DNA level. Nat Genet. 1994;8(2):117–121. doi: 10.1038/ng1094-117. [DOI] [PubMed] [Google Scholar]
- 61.Penn DJ, Potts WK. The evolution of mating preferences and major histocompatibility complex genes. Am Nat. 1999;153(2):145–164. doi: 10.1086/303166. [DOI] [PubMed] [Google Scholar]
- 62.Charlesworth D, Willis JH. The genetics of inbreeding depression. Nat Rev Genet. 2009;10(11):783–796. doi: 10.1038/nrg2664. [DOI] [PubMed] [Google Scholar]
- 63.Leimu R, Fischer M. Between-population outbreeding affects plant defence. PLoS One. 2010;5(9):e12614. doi: 10.1371/journal.pone.0012614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lynch M. The genetic interpretation of inbreeding depression and outbreeding depression. Evolution. 1991;45(3):622–629. doi: 10.1111/j.1558-5646.1991.tb04333.x. [DOI] [PubMed] [Google Scholar]
- 65.Leimu R, Fischer M. A meta-analysis of local adaptation in plants. PLoS One. 2008;3(12):e4010. doi: 10.1371/journal.pone.0004010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Waser NM, Price MV. Optimal outcrossing in Ipomopsis aggregata: Seed set and offspring fitness. Evolution. 1989;43(5):1097–1109. doi: 10.1111/j.1558-5646.1989.tb02554.x. [DOI] [PubMed] [Google Scholar]
- 67.Fischer M, Matthies D. Mating structure and inbreeding and outbreeding depression in the rare plant Gentianella germanica (Gentianaceae) Am J Bot. 1997;84(12):1685–1685. [PubMed] [Google Scholar]
- 68.Bateson P. Mate Choice. Cambridge University Press; Cambridge, UK: 1983. Optimal outbreeding; pp. 257–277. [Google Scholar]
- 69.Waser NM. Population structure, optimal outbreeding, and assortative mating in angiosperms. In: Thornhill NW, editor. The Natural History of Inbreeding and Outbreeding. University of Chicago Press; Chicago: 1993. pp. 173–199. [Google Scholar]
- 70.Puurtinen M. Mate choice for optimal (k) inbreeding. Evolution. 2011;65(5):1501–1505. doi: 10.1111/j.1558-5646.2010.01217.x. [DOI] [PubMed] [Google Scholar]
- 71.Pusey AE. Primate Societies. University of Chicago Press; Chicago: 1987. Dispersal and philopatry; pp. 250–266. [Google Scholar]
- 72.Westermarck E. The History of Human Marriage. Vol 2 Macmillan; London: 1921. [Google Scholar]
- 73.Bittles AH, Mason WM, Greene J, Rao NA. Reproductive behavior and health in consanguineous marriages. Science. 1991;252(5007):789–794. doi: 10.1126/science.2028254. [DOI] [PubMed] [Google Scholar]
- 74.Brown DE. Human Universals. McGraw-Hill; New York: 1991. [Google Scholar]
- 75.Harris M. Culture, People, Nature: An Introduction to General Anthropology. Allyn & Bacon; Needham Heights, MA: 1997. [Google Scholar]
- 76.Lieberman D, Tooby J, Cosmides L. The architecture of human kin detection. Nature. 2007;445(7129):727–731. doi: 10.1038/nature05510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Helgason A, Pálsson S, Guðbjartsson DF, Stefánsson K, Kristjánsson T. An association between the kinship and fertility of human couples. Science. 2008;319(5864):813–816. doi: 10.1126/science.1150232. [DOI] [PubMed] [Google Scholar]
- 78.Walker RS, Bailey DH. Marrying kin in small-scale societies. Am J Hum Biol. 2014;26(3):384–388. doi: 10.1002/ajhb.22527. [DOI] [PubMed] [Google Scholar]
- 79.Bittles AH, Black M. Consanguinity, human evolution, and complex diseases. Proc Natl Acad Sci USA. 2010;107(Suppl 1):1779–1786. doi: 10.1073/pnas.0906079106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bennett RL. Genetic counseling and screening of consanguineous couples and their offspring: Recommendations of the National Society of Genetic Counselors. J Genet Couns. 2002;11(2):97–119. doi: 10.1023/A:1014593404915. [DOI] [PubMed] [Google Scholar]
- 81.Joseph SE. Kissing cousins. Curr Anthropol. 2007;48(5):756–764. [Google Scholar]
- 82.McCabe J. FBD marriage: Further support for the Westermarck hypothesis of the incest taboo. Am Anthropol. 1983;85(1):50–69. [Google Scholar]
- 83.Bittles AH. 2015. The prevalence and outcomes of consanguineous marriage in contemporary societies. Cousin Marriages: Between Tradition, Genetic Risk and Cultural Change, New York and Oxford: Berghahn Books, 33–45. eds Shaw A, Raz A (Berghahn Books, New York), pp 33–45.
- 84.Fox R. Marry in or die out: Optimal inbreeding and the meaning of mediogamy. In: Maryanski A, Machalek R, Turner JH, editors. Handbook on Evolution and Society: Toward an Evolutionary Social Science. Routledge; Abingdon, UK: 2015. pp. 138–162. [Google Scholar]
- 85.Herre EA. Sex ratio adjustment in fig wasps. Science. 1985;228(4701):896–898. doi: 10.1126/science.228.4701.896. [DOI] [PubMed] [Google Scholar]
- 86.Frank SA. Hierarchical selection theory and sex ratios. II. On applying the theory, and a test with fig wasps. Evolution. 1985;39(5):949–964. doi: 10.1111/j.1558-5646.1985.tb00440.x. [DOI] [PubMed] [Google Scholar]
- 87.Godfray HCJ. Parasitoids: Behavioral and Evolutionary Ecology. Princeton Univ Press; Princeton: 1994. [Google Scholar]
- 88.Godfray H, Cook J. Mating systems of parasitoid wasps. In: Choe JC, Crespi BJ, editors. The Evolution of Mating Systems in Insects and Arachnids. Cambridge Univ Press; Cambridge, UK: 1997. pp. 211–235. [Google Scholar]
- 89.Dobson FS. Competition for mates and predominant juvenile male dispersal in mammals. Anim Behav. 1982;30(4):1183–1192. [Google Scholar]
- 90.Perrin N, Mazalov V. Dispersal and inbreeding avoidance. Am Nat. 1999;154(3):282–292. doi: 10.1086/303236. [DOI] [PubMed] [Google Scholar]
- 91.West SA, Pen I, Griffin AS. Cooperation and competition between relatives. Science. 2002;296(5565):72–75. doi: 10.1126/science.1065507. [DOI] [PubMed] [Google Scholar]
- 92.Griffin AS, West SA. Kin selection: Fact and fiction. Trends Ecol Evol. 2002;17(1):15–21. [Google Scholar]
- 93.West SA, Murray MG, Machado CA, Griffin AS, Herre EA. Testing Hamilton’s rule with competition between relatives. Nature. 2001;409(6819):510–513. doi: 10.1038/35054057. [DOI] [PubMed] [Google Scholar]
- 94.Cronk L. Preferential parental investment in daughters over sons. Hum Nat. 1991;2(4):387–417. doi: 10.1007/BF02692198. [DOI] [PubMed] [Google Scholar]
- 95.Beise J, Voland E. Intrafamilial resource competition and mate competition shaped social-group-specific natal dispersal in the 18th and 19th century Krummhörn population. Am J Hum Biol. 2008;20(3):325–336. doi: 10.1002/ajhb.20730. [DOI] [PubMed] [Google Scholar]
- 96.Gandon S. Kin competition, the cost of inbreeding and the evolution of dispersal. J Theor Biol. 1999;200(4):345–364. doi: 10.1006/jtbi.1999.0994. [DOI] [PubMed] [Google Scholar]
- 97.Dobson FS. The enduring question of sex-biased dispersal: Paul J. Greenwood’s (1980) seminal contribution. Anim Behav. 2013;85(2):299–304. [Google Scholar]
- 98.Moore J, Ali R. Are dispersal and inbreeding avoidance related? Anim Behav. 1984;32(1):94–112. [Google Scholar]
- 99.Schwarz MP. Local resource enhancement and sex ratios in a primitively social bee. Nature. 1988;331:346–348. [Google Scholar]
- 100.Hamilton W, May RM. Dispersal in stable habitats. Nature. 1977;269:578–581. [Google Scholar]
- 101.Le Galliard J, Gundersen G, Steen H. Mother–offspring interactions do not affect natal dispersal in a small rodent. Behav Ecol. 2007;18(4):665–673. [Google Scholar]
- 102.Bollinger EK, Harper SJ, Barrett GW. Inbreeding avoidance increases dispersal movements of the meadow vole. Ecology. 1993;74(4):1153–1156. [Google Scholar]
- 103.Apostolou M. Sexual selection under parental choice: Evidence from sixteen historical societies. Evol Psychol. 2012;10(3) [PubMed] [Google Scholar]
- 104.Bowlby J. 1969. Attachment, Attachment and Loss (Basic Books, New York), Vol 1.
- 105.Irons W. Adaptively relevant environments versus the environment of evolutionary adaptedness. Evol Anthropol Issues News Rev. 1998;6(6):194–204. [Google Scholar]
- 106.Huff CD, Xing J, Rogers AR, Witherspoon D, Jorde LB. Mobile elements reveal small population size in the ancient ancestors of Homo sapiens. Proc Natl Acad Sci USA. 2010;107(5):2147–2152. doi: 10.1073/pnas.0909000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schaffner SF. Calibrating a coalescent simulation of human genome sequence variation. Genome Res. 2005;15(11):1576–1583. doi: 10.1101/gr.3709305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dunbar RI. Neocortex size as a constraint on group size in primates. J Hum Evol. 1992;22(6):469–493. [Google Scholar]
- 109.Peters JF. Polyandry among the Yanomama Shirishana revisited. J Comp Fam Stud. 1982;13(1):89–95. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.