Abstract
This study examined the effects of various levels of nitrogen inputs (optimal, insufficient and excessive) and water inputs (optimal, low drought and high drought) to tomato plants (Solanum lycopersicum) on survival and development of an invasive tomato leafminer, Tuta absoluta (Meytick) (Lepidoptera: Gelechiidae). Plant growth i.e. plant height and the number of nodes declined under insufficient or excessive nitrogen treatment. Compared to optimal N, insufficient N treatment decreased leaf N content and increased the carbon/nitrogen ratio (C/N) whereas an excess of N had no effect on both leaf N content and leaf C/N ratio. Sub-optimal nitrogen supplies, water treatments and their interactions, significantly reduced the leafminer survival rate and slowed down its development. Together with the findings from three recent companion studies, we assumed that a combination of changes in nutritional value and chemical defense could explain these observed effects. Furthermore, our findings supported both the “Plant vigor hypothesis” and the “Nitrogen limitation hypothesis”.
Plant-arthropod interactions can be markedly shaped by bottom-up forces1,2,3,4. Phytophagous arthropods need to obtain nutrients from their host plants in order to survive and develop. However, they usually forage in environments where developing plants show contrasted palatability levels in terms of nutritional value (nitrogen richness or water content) as well as toxicity related to defensive traits (through secondary metabolites)1,5. Plant nutritional value and plant defenses are highly variable owing to factors such as plant species, presence of competitors, light, temperature, as well as water and nutrient availability6,7,8,9,10. Such variations in plant quality can lead to trophic cascades such as bottom-up effects on phytophagous arthropods (e.g. on development, fecundity, etc.)2,3,5.
Nitrogen is one of the most commonly applied mineral fertilizers in crop production due to its importance in the composition of plant tissues. Plant feeding insects thus need to transform and utilize the different inorganic forms of nitrogen that occur in plant tissues1,5. Indeed, the development of the lepidopterans has been positively correlated to plant nitrogen content5,7,11,12,13,14,15. By contrast, a shortage of nitrogen input to the plants was shown to impair the performance of herbivore insects, which was termed the “Nitrogen limitation hypothesis”16. The explanation could be that (1) insects need to transform the inorganic nitrogen forms present in plant tissue and/or utilize directly plant-derived amino acids to synthesize structural proteins and enzymes5; (2) nitrogen deficient plants have a higher accumulation of plant allelochemicals which can be toxic to herbivorous insects. For instance, tomato plants subjected to nitrogen limitation accumulate more phenolic compounds in their organs17,18,19,20. Although many studies reported the existence of positive correlations between insect performance and host plant nitrogen content as mentioned above, several studies however have documented negative effects due to high nitrogen content in leaves and undermined the general application of the “Nitrogen limitation hypothesis”15,21,22.
Water is another potentially important abiotic factor which could mediate plant-herbivore insect interactions5,21. Water-availability mediated interactions between herbivorous insects and their host plants are affected by various factors including herbivore feeding specialization, herbivore species and the seasonality of water availability10,23. The plant water content is a useful index of its nutritional value for many lepidopterous larvae5. In addition, drought may influence plant chemical defense level leading to either enhanced or reduced resistance to herbivores7,10. Historically, drought periods have been considered a major factor underlying outbreaks of herbivorous insects24,25,26. However, the consequences of water-availability on plant-insect interactions have been predicted in several hypotheses primarily in the “Plant stress hypothesis” which assumes that stressed plants are better hosts for senescence-feeders24,27 or in the “Plant vigor hypothesis” which suggests that herbivorous insects prefer and perform better on rapidly-growing plants i.e. vigorous ones28. Within a given feeding guild, such as phloem-feeders, aphids do respond positively to intermittently stressed plants but negatively to continuously stressed ones. The “Pulsed stress hypothesis” assumes that the outcomes depend on the occurrence of water stress: continuous or pulsed drought23. In general, insect feeding guild (phloem feeder, leaf miner or leaf chewer) and feeding specialization (contrasting feeding preferences of two Lepidoptera species of the same feeding guild) have been used to explain various herbivore responses to water stressed plants7,10,23.
In this context, we studied how nitrogen and water inputs and/or their interactions may induce potential plant physiological changes, either in terms of nutritional value or chemical defenses and how, in turn, they would affect herbivorous insect survival and development. The tomato plant Solanum lycopersicum L and the herbivorous insect Tuta absoluta (Meytick) (Lepidoptera: Gelechiidae) were used to set up a “nutrient & water input-plant-herbivore insect” testing system to address these questions. The tomato leafminer T. absoluta is a devastating tomato pest from South America which has rapidly invaded the Mediterranean basin since it first appeared in Spain in 200629,30,49. Tuta absoluta adults usually lay eggs on the leaves and stems, and to a lesser extent on fruits. After hatching, young larvae penetrate and enter the plant tissues to feed and develop29. Moreover, leafminers have the most intimate relationships with their host plants and thus are considered the most appropriate to test the hypotheses mentioned above7. Our aims were (1) to test whether the altered nitrogen and water inputs to tomato plants could trigger a bottom-up effect on biological traits of T. absoluta; (2) to evaluate the plant physiological changes that could explain the effects observed on T. absoluta. Attempts to answer these questions may help to provide theoretical support to optimize IPM (Integrated Pest Management) of T. absoluta.
Results
Plant growth
Statistical results on plant growth dataset are summarized in Table 1. Nitrogen and water inputs affected plant height significantly on the three measurement dates (58 days after seeding [DAS 58], Fig. 1; DAS 32 and DAS 40 not shown) whereas effect of nitrogen × water interaction was only found at DAS 58. The number of nodes per plant was affected differently by nitrogen and water inputs and their interaction was significant (Fig. 1).
Table 1. Factorial ANOVA used to analyze plant traits: (A) Plant height at DAS 32 (32 days after seeding), DAS 40 and DAS 58; (B) Number of nodes at DAS 58. Main factors tested were the “nitrogen” and “water”.
| A - Plant height | |||
|---|---|---|---|
| Source of variation | Df | F | P values |
| DAS 32 | |||
| Nitrogen | 2 | 18.34 | <0.001 |
| Water | 2 | 38.69 | <0.001 |
| Nitrogen × water | 4 | 1.32 | 0.268 |
| DAS 40 | |||
| Nitrogen | 2 | 23.43 | <0.001 |
| Water | 2 | 50.74 | <0.001 |
| Nitrogen × water | 4 | 0.60 | 0.662 |
| DAS 58 | |||
| Nitrogen | 2 | 23.41 | <0.001 |
| Water | 2 | 114.93 | <0.001 |
| Nitrogen × water | 4 | 3.47 | 0.011 |
Figure 1. (A) Plant height (mean ± SEM, n = 12 plants) and (B) Number of nodes (mean ± SEM, n = 12 plants) at 58 days after seeding (DAS 58) for tomato plants treated with different nitrogen and water inputs.
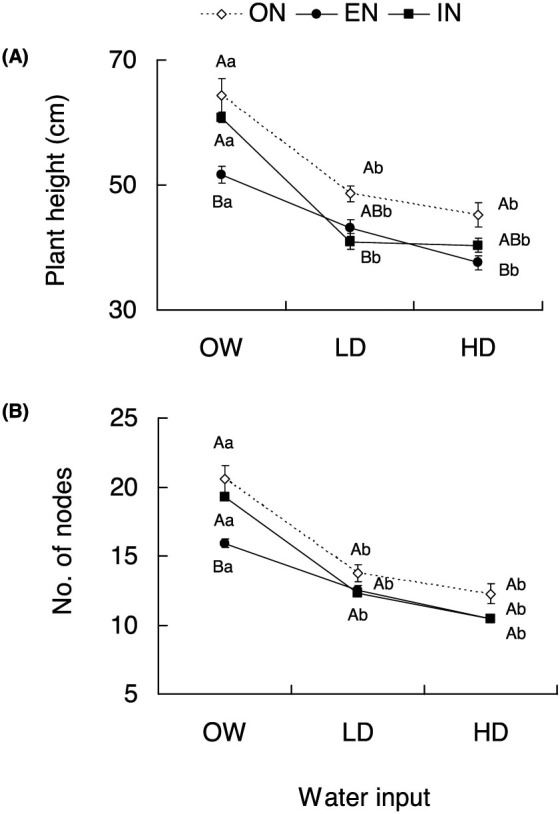
(ON: optimal nitrogen; EN: excessive nitrogen; IN: insufficient nitrogen; OW: optimal water; LD: low drought; HD: high drought). Capital letters indicated the comparison among different nitrogen inputs within a given water input level; Lower case letters indicated the comparison among different water inputs within a given nitrogen input level. Different letters indicate significant difference at P < 0.05.
Plants supplied with the optimal water input increased in height i.e. about 30% higher compared to those subjected to low or high drought. For the optimal water treatment, the plants fertilised with the optimal nitrogen regime attained significantly greater height than those treated with excessive nitrogen input (P < 0.001), but their height was similar to that of those treated with insufficient nitrogen input (P = 0.767). The number of nodes per plant followed a similar pattern as plant height since only excessive nitrogen treatment significantly reduced the number of nodes compared to optimal and insufficient N treatments (both P < 0.001).
Leaf N content and C/N ratio
Leaf N content (dry mass percent) differed significantly among the nitrogen treatments (F2, 135 = 47.556, P < 0.001), but not among water treatments (F2, 135 = 0.230, P = 0.795) (Fig. 2). There was no interaction nitrogen × water on the leaf N content (F4, 135 = 2.392, P = 0.054). The plants treated with optimal nitrogen showed a leaf N content similar to those treated with excessive nitrogen input. Nevertheless the leaf N content was significantly higher than in those treated with insufficient nitrogen input (P < 0.001). Leaf C/N ratio differed significantly among the nitrogen treatments (F2, 135 = 43.487, P < 0.001), but not among the water treatments (F2, 135 = 2.668, P = 0.073) (Fig. 2). There was no interaction nitrogen × water on leaf C/N (F4, 135 = 2.282, P = 0.064). The leaf C/N ratio was similar between optimal and excessive N treatments whereas it was significantly higher on plants treated with insufficient nitrogen input (P < 0.001).
Figure 2. (A) Leaf N content (in dry mass percent, mean ± SEM, n = 6) and (B) Leaf C/N ratio (unit-less, mean ± SEM, n = 6) of tomato leaf before the artificial infestation of T. absoluta.
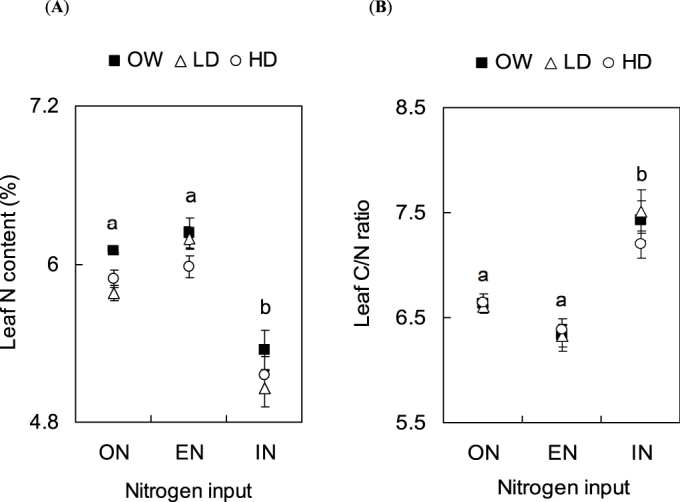
(OW: optimal water; LD: low drought; HD: high drought; ON: optimal nitrogen; EN: excessive nitrogen; IN: insufficient nitrogen). Comparisons were done only among different “nitrogen input” treatments because no effect of “water input” was found. Different Letters indicate significant difference at P < 0.05.
T. absoluta survival
Both water and nitrogen inputs significantly affected T. absoluta survival from egg to pupa or to adult (nitrogen: χ2 = 23.89, df = 2, P < 0.001; water: χ2 = 17.68, df = 2, P < 0.001; interaction: χ2 = 4.71, df = 4, P = 0.318) (Table 2). Tuta absoluta survival also showed various, different responses to nitrogen and water inputs between both pupa and adult stages (χ2 = 12.96, df = 1, P < 0.001). For the optimal nitrogen input, T. absoluta had a significantly lower survival rate (proportion of eggs reaching pupa or adult stages) when the plants suffered high drought (ON-OW vs. ON-HD, Table 2). For the excessive nitrogen input, the pattern was similar to those observed for optimal nitrogen. However, significantly fewer T. absoluta reached adulthood when the plants received optimal water input (EN-OW vs. ON-OW, Table 2). For the insufficient nitrogen input, the rate of T. absoluta survival significantly declined (for both pupae and adults) in all water treatments when compared to the ON-OW condition (ON-OW vs. IN-treatments, Table 2).
Table 2. Survival proportions of T. absoluta individuals reaching pupae (from egg to pupa) or adults (from egg to adult) when feeding on tomato plants supplied with various nitrogen and water inputs (ON: optimal nitrogen; EN: excessive nitrogen; IN: insufficient nitrogen; OW: optimal water; LD: low drought; HD: high drought). ON-OW was considered as control group and other treatments were compared to this control group. * P < 0.05, ** P < 0.01, *** P < 0.001 (significantly different from values of individuals developing on plants subjected to optimal nitrogen [ON] and optimal water [OW], in italics) (permuted Fisher exact test).
| Nitrogen input | Water input | Proportion of individuals reaching pupae | Proportion of individuals reaching adults |
|---|---|---|---|
| ON | OW | 0.70 | 0.57 |
| ON | LD | 0.58 | 0.44 |
| ON | HD | 0.51 ** | 0.39 * |
| EN | OW | 0.58 | 0.42 * |
| EN | LD | 0.60 | 0.44 |
| EN | HD | 0.43 *** | 0.36 ** |
| IN | OW | 0.54 * | 0.39 * |
| IN | LD | 0.38 *** | 0.26 *** |
| IN | HD | 0.28 *** | 0.22 *** |
T. absoluta development
Pupal weight
Both nitrogen and water inputs and their interaction had significant effect on T. absoluta pupal weight (Fig. 3A, nitrogen: F2, 399 = 66.851, P <0.001; water: F2, 399 = 13.815, P < 0.001; interaction: F4, 399 = 5.583, P < 0.001). For both the optimal water input and the low drought, pupal weight was significantly higher under optimal nitrogen input than under excessive or insufficient nitrogen inputs (all P < 0.05). However, in the high drought treatment, pupal weight was equally low at the three nitrogen levels (IN vs. ON: P = 0.460; EN vs. ON: P = 0.500).
Figure 3. (A) Pupal weight (mean ± SEM, n = 25–63); (B) Development time (mean ± SEM, n = 25–63) from egg to pupa; (C) Development time (mean ± SEM, n = 20–51) from egg to adult of T. absoluta feeding on tomato plants with different nitrogen and water inputs.
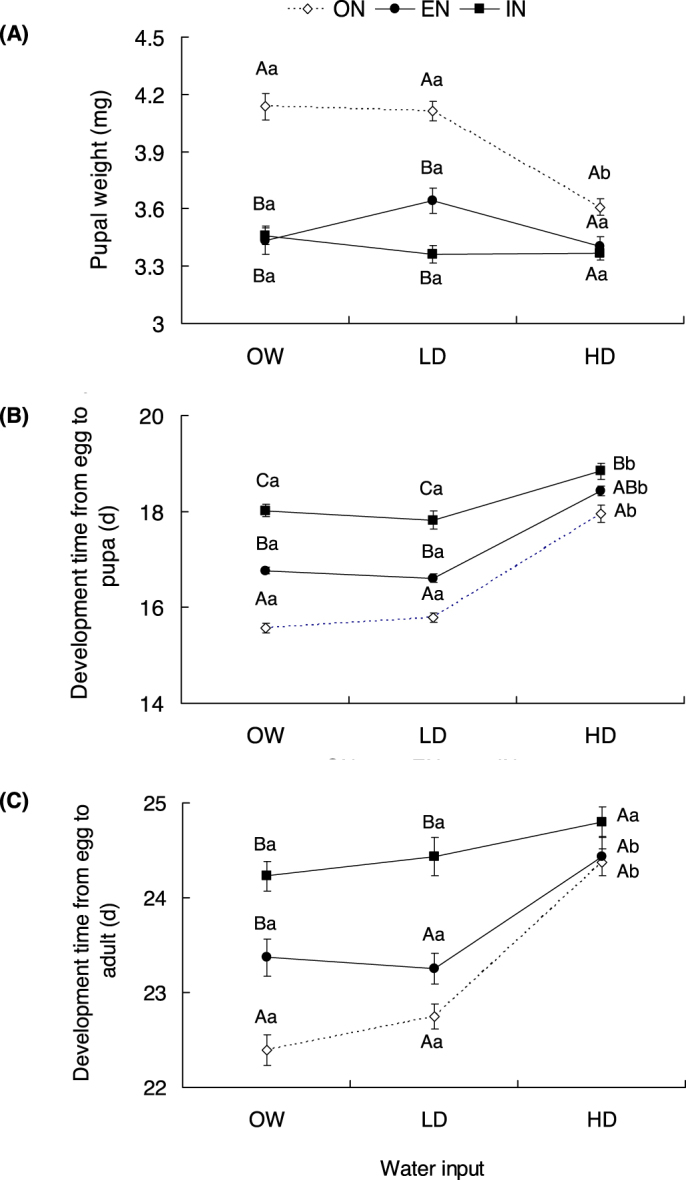
(ON: optimal nitrogen; EN: excessive nitrogen; IN: insufficient nitrogen; OW: optimal water; LD: low drought; HD: high drought). Capital letters indicate the comparison among different nitrogen inputs at a given water input level; Lower case letters indicate the comparison among different water inputs at a given nitrogen input level. Different Letters indicate significant difference at P < 0.05.
Development time from egg to pupa or to adult
Both nitrogen and water and their interaction significantly affected T. absoluta development time (Fig. 3B “from egg to pupa”: nitrogen: F2, 399 = 109.96, P < 0.001; water: F2, 399 = 118.72, P < 0.001; interaction: F4, 399 = 6.172, P < 0.001; Fig. 3C “from egg to adult”: nitrogen: F2, 299 = 33.059, P < 0.001; water: F2, 299 = 33.126, P < 0.001; interaction: F4, 299 = 3.328, P = 0.011).
Under the optimal water and the low drought treatments, the development time from egg to pupa was significantly extended when the plants received either excessive or insufficient nitrogen inputs (all P < 0.05, Fig. 3B). Under high drought, the development time was only prolonged significantly by the insufficient nitrogen input (P = 0.017). Besides, the development time from egg to pupa increased on the plants subjected to high drought at any nitrogen input level (all P < 0.05, Fig. 3B).
Under the optimal water input, the development time from egg to adult was significantly extended when the plants were under excessive or insufficient nitrogen inputs (all P < 0.05, Fig. 3C). Under the low drought treatment, this development time was only significantly delayed by the insufficient nitrogen input (P < 0.001, Fig. 3C). Under high drought, there was no difference in development time among the three nitrogen input levels (IN vs. ON: P = 0.950; EN vs. ON: P = 0.980). For optimal and excessive nitrogen treatment, the delay in the development time from egg to adult was significantly prolonged under high drought (all P < 0.05).
Relationship between T. absoluta development and leaf N content
Table 3 shows the regression relationship between leaf N content and T. absoluta development i.e. development time either from egg to pupa or from egg to adult and pupal weight. The development time was negatively correlated to leaf N content and pupal weight was positively correlated to leaf N content. The degree of linear correlation and the correlation coefficient (R2) varied depending on the water treatments. The linear correlation was stronger in the case of optimal water input than in that of low or high drought for the three parameters.
Table 3. Linear correlation between (1) development time of T. absoluta from egg to pupa, (2) development time of T. absoluta from egg to adult or (3) T. absoluta pupal weight and plant leaf N content under various water inputs to the plants (OW: optimal water; LD: low drought; HD: high drought).
| OW | LD | HD | |
|---|---|---|---|
| Development time from egg to pupa | y = −2.50x + 31.17 | y = −2.09x + 28.18 | y = −0.46x + 20.99 |
| R2 = 0.715 | R2 = 0.667 | R2 = 0.080 | |
| Development time from egg to adult | y = −2.09x + 35.48 | y = −2.20x + 35.63 | y = −0.39x + 26.80 |
| R2 = 0.735 | R2 = 0.630 | R2 = 0.099 | |
| Pupal weight | y = 0.74x − 0.40 | y = 0.62x + 0.41 | y = 0.17x + 2.54 |
| R2 = 0.624 | R2 = 0.372 | R2 = 0.174 |
Discussion
Our data demonstrated that variation of nitrogen and water inputs to tomato plants significantly affected some biological traits of T. absoluta. Firstly, nitrogen limitation and drought reduced the survival rate from egg to pupa or adult stages. Secondly, nitrogen limitation and drought reduced pupal weight and slowed down the development of T. absoluta. These results therefore fulfill the first aim of this paper by making it clear that altered nitrogen and water inputs to tomato plants do trigger a bottom-up effect on the biological traits of T. absoluta. Thus, two questions remain to be answered: (i) what are the physiological changes in the host plants that could explain the effects on the biological traits of T. absoluta? (ii) Did our results support or refute the “Nitrogen limitation hypothesis”, the “Plant stress hypothesis” and the “Plant vigor hypothesis”?
In this section we will discuss how the water/nitrogen treatments influenced plant physiology and in turn affected the survival and development of T. absoluta. Drought was seen to negatively affect T. absoluta survival and development. In low or high drought treatments, the foliage tended to wilt thus probably becoming less edible for Lepidoptera larvae10. Here, T. absoluta showed a lower survival rate when fed on the host plants subjected to drought. We assumed that larvae faced difficulty in obtaining enough water for optimal development thus causing a slowdown in their development. In addition, water not only acted as an indispensable ingredient for herbivore metabolism but also mediated nitrogen availability for plants23. It was generally agreed that nitrogen and water content are positively correlated31. T. absoluta larvae therefore may suffer a deficit of N-based nutrient in the plants subjected to drought.
Insufficient nitrogen input to the tomato plants triggered negative bottom-up effects on T. absoluta which was consistent with the “Nitrogen limitation hypothesis”16. We may infer that T. aboluta larvae could be affected by changes in nutritional value and chemical defense in tomato plants. Firstly, N-limited tomato plants might have lower leaf nutritional value for T. absoluta larvae. Indeed, nitrogen was considered as a limiting nutrient factor for the growth of many Lepidoptera herbivores because larvae may experience lack of organic nitrogen, i.e. specific protein and/or amino acids, leading to reduced or impaired metabolism during their critical growth period and even to premature death13,14,32. Moreover, concentration of non-nitrogeneous compounds in leaves could also be negatively affected by insufficient nitrogen input, such as starch in tomato leaves as well as soluble carbohydrate in young tomato leaves20. T. absoluta suffered a higher mortality rate during the larvae stage. In addition, lower leaf nutritional value may influence the feeding behaviour of the larvae5; in our study, we observed T. absoluta had a longer development time i.e. delayed development. One possible explanation could be that larvae must compensate for the insufficient N-based food by increasing daily food intake or switching foraging activity toward N-richer plant organs such as young leaves or buds33,34,35. In our study, T. absoluta larvae were confined in a mesh cage with only one leaf as food so they could not forage other areas richer in nitrogen. Therefore, the larvae development might be slower due to sub-optimal food quality and/or quantity e.g. fewer N-based nutrients.
Secondly, N-limited tomato plants could have higher levels of chemical defense against T. absoluta. GDBH (Growth Differentiation Balance Hypothesis) predicts that moderate growth limitations caused by external factors such as low nutrient availability will result in an accumulation of carbohydrates and, subsequently, in increased concentrations of constitutive secondary compounds36. Indeed, we assumed that the N-limited tomato plants produced more insect-defensive soluble phenolics (C-based defensive compounds) i.e. chlorogenic acid, rutin, kaempferol-rutinoside since their concentration is positively correlated with C/N ratio in tomato leaves7,18,19,20,37. Moreover, the concentration of constitutive tomatine, a key N-based glycoalkaloid defensive compound in tomato, is also positively correlated with leaf C/N ratio20.
Excessive nitrogen input to the tomato plants also triggered negative bottom-up effects on T. absoluta. This study reported for the first time that excessive nitrogen input slowed down the development of a lepidoptera herbivore and induced a lower pupal mass. The negative effects on T. absoluta could only be attributed to higher chemical defense because the leaf nutritional value was similar between optimal and excessive nitrogen treatments; leaf N content and leaf C/N ratio are equal. We assumed that plants excessively supplied with nitrogen could allocate their surplus of nutrient resources to the synthesizing of N-containing compounds such as glycoalkaloids which may deter the insect metabolism and thus slow down their development. For example, Johnson et al.38 observed on N2-fixing plant (lupines) that nitrogen-rich plants may benefit from faster and higher alkaloid induction than nitrogen-limited plants under herbivore attack.
To conclude, we suggest that the negative impact of insufficient nitrogen treatment on T. absoluta survival and development might be due to both the leaf nutritional value decrease and the leaf chemical defense increase. Furthermore, the negative impact of excessive nitrogen treatment could only be linked to higher chemical defense levels. Finally, there is an interaction between both factors on T. absoluta development showing that either factor is limiting with regard to the life-history parameters examined (Fig. 3A: “nitrogen” as a limiting factor for pupal weight; Fig. 3B, 3C: “water” as a limiting factor for development time from egg to pupa or to adult).
Two main hypotheses have so far been acknowledged to explain the effects of plant growth related stresses on insect herbivores, namely the “Plant stress hypothesis” and the “Plant vigor hypothesis”. The former assumes that stressed plants serve as a more suitable host for herbivore insects because of increased nutritional value i.e. amino acid and reduced plant defensive compound syntheses25,32. Larsson39 modified this hypothesis and emphasized the idea that the effects of stressed plants on herbivore performances greatly depend on the insect feeding strategy, such as leaf mining, leaf chewing or sap feeding. The latter theory, an opposing one called the “Plant vigor hypothesis”, predicted that the best plant hosts for insect herbivores are vigorous plants28. Our findings support the second hypothesis since T. absoluta survived better and developed faster on the tomato plants with better growth status e.g. a larger plant growth rate in terms of height and node numbers (Fig. 1). By contrast, the plants subjected to non-optimal nitrogen and drought treatments were less suitable hosts for T. absoluta; larvae showed a lower survival rate and sub-optimal development compared to those developing on plants under optimal treatment.
Among the various stress types, the role of nitrogen limitation has received important attention in predicting plant-herbivore insect interactions. Our data also supported the “Nitrogen limitation hypothesis” since insufficient nitrogen input significantly lowered leaf N content (see Fig. 2) which in turn compromised the survival and development performances of T. absoluta. In particular, T. absoluta development was found to be negatively correlated to the leaf N content (see Table 3). The leafminer needed more time to develop in order to compensate for the nitrogen deficiency; despite longer development time, optimal pupal weight was not reached.
In conclusion, various nitrogen and water inputs to tomato plants affected the biological traits of the leafminer T. absoluta and these effects were probably due to a combination of lower plant nutritional value and higher chemical defenses. Our findings support both the “Plant vigor hypothesis” and the “Nitrogen limitation hypothesis” which are well reported for Lepidoptera pests. The manipulation of fertilizing and irrigating regimes may help to optimize agricultural practices by promoting negative effects on herbivorous insects. This could actually be achieved with little damage to plants and negligible crop yield losses40,41,42. Our findings show that sub-optimal nitrogen input, excessive or insufficient, and drought to tomato plants are unfavorable to T. absoluta but also appear unfavorable to plants resulting in lower growing status. Future work should aim at quantifying the trade-off between the negative impact on herbivorous pests and plant yield, as well as including arthropods from the higher trophic level i.e. natural enemies.
Methods
Biological material
Tomato plants cv. Marmande were grown from seeds in a climatic chamber (12 h light, 24 ± 1°C, 65 ± 5% RH) in cubic plastic pots (7 × 7 × 6.5 cm). The plants were then grown under laboratory conditions (16 h light, 24 ± 1°C, 65 ± 5% RH). At DAS 8 (Days After Seeding) the seedings were washed to remove soil and then transferred to new pots containing limestone grains (Perlite Italiana srl, Corsico, Italy) (see schedule Fig. 4). At DAS 24, the plants were transferred to larger pots (diameter: 10 cm, height: 9 cm) filled with the same substrate.
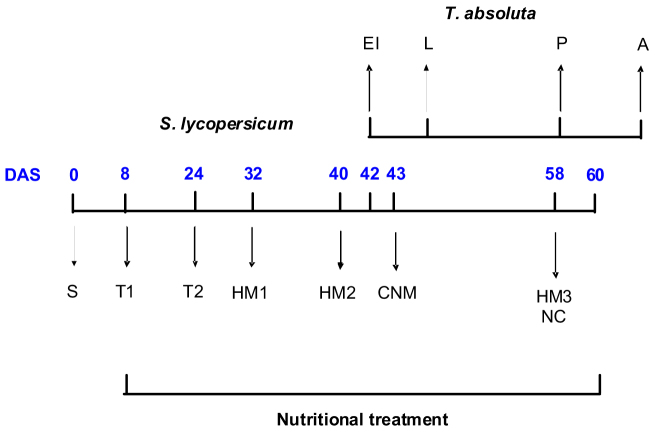
Figure 4. Sampling protocol schedule including plant growth (S: plant seeding; T1/T2: routine plants transfer), T. absoluta infestation and development throughout the plant development stages (EI: egg incubation on plant leaves; L: larvae; P: pupae; A: adults) and plant sampling events (HM1, HM2 and HM3: 1st, 2nd and 3rd plant height measurement, respectively; CNM: carbon/nitrogen content measurement in tomato leaves before T. absoluta larvae infestation; NC: node counting of tomato plants); DAS: days after seeding; Nutritional treatments lasted from DAS 8 to DAS 60 (also see Figure 1).
The T. absoluta colony was maintained in regular chambers using tomato plants (25 ± 1°C, RH 70 ± 10%, 16 h light). It was kept in cages measuring 55 × 75 × 80 cm and containing tomato plants (as described by Biondi et al.43). Food (honey) and water were provided ad libitum to adults in rearing cages. Newly-oviposited T. absoluta eggs (≤24 h) were used to infest the plants. To obtain these eggs, we used the method described by Chailleux et al.44. Ten couples of T. absoluta adults were placed inside a plastic tube containing a fresh tomato leaf to enable mating and oviposition.
Nitrogen and water input
At DAS 8, nine treatments were initiated on the potted seedlings using a fully crossed design with three nitrogen inputs (ON: optimal nitrogen; EN: excessive nitrogen; IN: insufficient nitrogen) and three water inputs (OW: optimal water; LD: low drought; HD: high drought) (see Fig. 5).
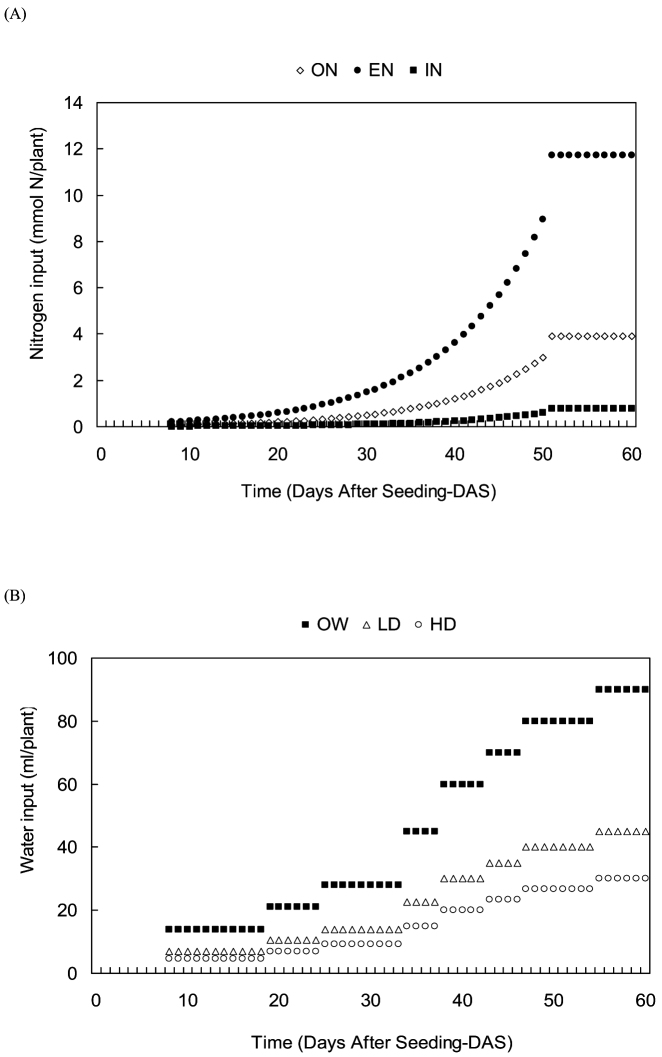
Figure 5. (A) Amount of daily nitrogen input (mmol) per pot throughout the plant growth period; (B) Input of water volume (ml of solution) per pot throughout the growth period.
The amounts of nutrient inputs and volumes of feeding solution supplied to plants increased daily depending on the plant development stage. Indeed, the amount of nitrogen taken by plants was controlled by the increase in their daily dry mass45,46. Moreover, when growth reached steady-state i.e. exponential growth phase, plant N concentration remained constant45. We therefore calculated the daily amounts of nitrogen required to produce optimal dry mass during approximately 52 days of growth (ON treatment). To proceed, we used data of tomato growth previously obtained in our laboratory conditions47. We used 3 stock solutions to provide nitrate, phosphate and sulphate salts independently (+ Fe & micronutrients). The following concentrations were used (in M: NO3 = 1; H2PO4 = 0.21; SO4 = 0.055; K = 0.641 Ca = 0.215; Mg = 0.114). Kanieltra trace elements (Hydro Azote, France) were given as well as formula 6 Fe and EDTA-Fe at the following concentrations (in μM in the SO42− stock solution: Mo 20; Mn 815; Zn 227; Cu 33; B 1444; Fe 3760). To differentiate N inputs, three different doses, namely vs (ON), 3vs (EN) and vs/5 (IN) of the nitrate stock solution were used daily to fertilize the plants. These doses were added to the water intake (see below) of each pot in order to set the optimal, excessive and insufficient nitrogen inputs, respectively (Fig. 5A).
We applied a “step increase” pattern to daily water inputs (Fig. 5B). Optimal daily water input (hereafter named “v” in volume) was determined as the amount capable of fully-saturating the perlite substrate without visible drainage, i.e. field capacity. Based on v, complementary volumes of v/2 and v/3 were applied daily to set the low and high drought treatments per plant respectively. All nutrient solutions were adjusted to pH 5.5 by using H2SO4 (0.2 M).
Sampling protocols
Figure 2 shows the schedule of all the events recorded during the experiment with regard to leaf sampling and the period of pest artificial infestation on plants.
Plant infestation
At DAS 42 (see Fig. 4), one leaf, the 3rd fully-developed leaf (with 5 leaflets) from the top, from each plant was selected to be infested with one newly-oviposited T. absoluta egg (≤24 h). The eggs were checked daily until larvae hatched. If an egg failed to hatch, a newly-hatched larva (<6 h old) was released on the leaf. To avoid larvae escaping, each infested leaf was then bagged with a nylon mesh (0.2 mm, 30 × 24 cm).
Biological traits of T. absoluta
Survival and development time were recorded for each insect. After pupation, all the pupae (pupated within 48 h) were weighed individually and then kept in a small petri dish (4.2 cm in diameter) until the adult emerged.
Leaf sampling and plant measurements
Plant height was measured individually on three dates (DAS 32, 40, 58). For each plant, the node number was counted at DAS 58. To assess the effects of the treatments on leaf nitrogen and carbon contents, we randomly selected six plants from each treatment and sampled their 4th fully-developed leaf from the top. Leaf samples were taken at DAS 43 before T. absoluta larvae infestation and were harvested and dried at 60°C for 72 h.
Leaf N content and C/N ratio measurement
To analyse the nitrogen and carbon contents (dry mass percent), the dried leaf samples were ground into fine powder using a ball mill MM301 (Retsch, Germany) and stored individually in small tubes. Leaf nitrogen and carbon contents were measured with an elemental analyser (Flash EA1112 Series, ThermoFinnigan, Milan, Italy) at INRA-PSH Avignon. The leaf C/N ratio was calculated from the mass data (in g).
Data analyses
We performed two-way ANOVA, followed by Tukey's post hoc test for multiple comparisons when necessary, in order to determine the effects of nitrogen and water inputs on plant height, number of nodes per plant, leaf N content, leaf C/N ratio, T. absoluta pupal weight, development time from egg to pupa and from egg to adult. Shapiro and Bartlett tests were used to assess variance homogeneity and normality, respectively. In addition, survival of T. absoluta was fitted to a log-linear model (using “water”, “nitrogen” and “stage” as factors), and further analyzed with permuted Fisher exact test. In order to test the “Nitrogen limitation hypothesis”, insufficient and optimal nitrogen data were used to test the relationships between leaf N content and T. absoluta development traits i.e. development time and pupal weight by using regression analysis. The excessive nitrogen dataset was not included in this analysis because nitrogen provided in excess may induce various effects (notably negative ones) beyond the actual “nitrogen concentration - plant growth” relationship17. All these data were analysed using the R software48.
Acknowledgments
This work was supported by funds from the Environment and Agronomy department of INRA and from the Chinese government (PhD fellowship to P.H.). We thank Philippe Bearez for technical assistance and Antonio Biondi for comments on an earlier version of the manuscript.
Footnotes
The authors declare no competing financial interests.
Author Contributions P.H., A.L.V. and N.D. scoped and designed the study; P.H., A.L.V. and E.A.D. performed the experiments and analyzed data; P.H., A.L.V., J.L.B. and N.D. interpreted results and wrote the manuscript.
References
- Mattson W. J. Herbivory in relation to plant nitrogen content. Ann. Rev. Ecol. 11, 119–161 (1980). [Google Scholar]
- Hunter M. D. & Price P. W. Playing chutes and ladders: heterogeneity and the relative roles of bottom-up and top-down forces in natural communities. Ecology 73, 724–732 (1992). [Google Scholar]
- Hunter M. D. Multiple approaches to estimating the relative importance of top-down and bottom-up forces on insect populations: experiments, life tables, and time-series analysis. Basic. Appl. Ecol. 2, 295–309 (2001). [Google Scholar]
- Chen Y. G., Olson D. M. & Ruberson J. R. Effects of nitrogen fertilization on tritrophic interactions. Arthropod-Plant Inte. 4, 81–94 (2010). [Google Scholar]
- Schoonhoven L. M., van Loon J. J. A. & Dicke M. Insect-plant biology. (Oxford University Press, Oxford, 2005). [Google Scholar]
- Mouttet R., Kaplan I., Bearez P., Amiens-Desneux E. & Desneux N. Spatiotemporal patterns of induced resistance and susceptibility linking diverse plant parasites. Oecologia 173, 1379–1386 (2013). [DOI] [PubMed] [Google Scholar]
- Inbar M., Doostdar H. & Mayer R. Suitability of stressed and vigorous plants to various insect herbivores. Oikos 94, 228–235 (2001). [Google Scholar]
- Turtola S. et al. Clone-specifi c responses in leaf phenolics of willows exposed to enhanced UVB radiation and drought stress. Global. Change Biol. 11, 1655–1663 (2005). [Google Scholar]
- Mody K., Unsicker S. B. & Linsenmair K. E. Fitness related diet-mixing by intraspecific host-plant-switching of specialist insect herbivores. Ecology 88, 1012–1020 (2007). [DOI] [PubMed] [Google Scholar]
- Gutbrodt B., Mody K. & Dorn S. Drought changes plant chemistry and causes contrasting responses in lepidopteran herbivores. Oikos 120, 1732–1740 (2011). [Google Scholar]
- Cates R. G., Henderson C. B. & Redak R. A. Responses of the western spruce budworm to varying levels of nitrogen and terpenes. Oecologia 73, 312–316 (1987). [DOI] [PubMed] [Google Scholar]
- Estiarte M., Filella I., Serra J. & Peñuelas J. Effects of nutrient and water stress on leaf phenolic content of peppers and susceptibility to generalist herbivore Helicoverpa armigera (Hubner). Oecologia 99, 387–391 (1994). [DOI] [PubMed] [Google Scholar]
- Hunter M. D. & McNeil J. N. Host-plant quality influences diapause and voltinism in a polyphagous insect herbivore. Ecology 78, 977–986 (1997). [Google Scholar]
- Grundel R., Pavlovic N. B. & Sulzman C. L. The effect of canopy cover and seasonal change on host plant quality for the endangered Karner blue butterfly (Lycaeides melissa samuelis). Oecologia 114, 243–250 (1998). [DOI] [PubMed] [Google Scholar]
- Fischer K. & Fiedler K. Response of the copper butterfly Lycaena tityrus to increased leaf nitrogen in natural food plants: evidence against the nitrogen limitation hypothesis. Oecologia 124, 235–41 (2000). [DOI] [PubMed] [Google Scholar]
- White T. C. R. The inadequate environment: nitrogen and the abundance of animals (Springer, Berlin, 1993). [Google Scholar]
- Le Bot J., Bénard C., Robin C., Bourgaud F. & Adamowicz S. The "trade-off" between synthesis of primary and secondary compounds in young tomato leaves is altered by nitrate nutrition: experimental evidence and model consistency. J. Exp. Bot. 60, 4301–4314 (2009). [DOI] [PubMed] [Google Scholar]
- Larbat R. et al. Influence of repeated short-term nitrogen limitations on leaf phenolics metabolism in tomato. Phytochemistry 77, 119–128 (2012). [DOI] [PubMed] [Google Scholar]
- Larbat R., Le. Bot J., Bourgaud F., Robin C. & Adamowicz S. Organ-specific responses of tomato growth and phenolic metabolism to nitrate limitation. Plant Biology 14, 760–769 (2012). [DOI] [PubMed] [Google Scholar]
- Royer M., Larbat R., Le Bot J., Adamowicz S. & Robin C. Is the C:N ratio a reliable indicator of C allocation to primary and defence-related metabolisms in tomato? Phytochemistry 88, 25–33 (2013). [DOI] [PubMed] [Google Scholar]
- Scriber J. M. Chemical ecology of insects. (Chapman & Hall, London, 1984). [Google Scholar]
- Joern A. & Behmer S. T. Impact of diet quality on demographic attributes in adult grasshoppers and the nitrogen limitation hypothesis. Ecol. Entomol. 23, 174–184 (1998). [Google Scholar]
- Huberty A. F. & Denno R. F. Plant water stress and its consequences for herbivorous insects: a new synthesis. Ecology 85, 1383–1398 (2004). [Google Scholar]
- White T. C. R. An index to measure weather-induced stress of trees associated with outbreaks of psyllids in Australia. Ecology 50, 905–909 (1969). [Google Scholar]
- Mattson W. J. & Haack R. A. The role of drought in outbreaks of plant-eating insects. BioScience 37, 110 (1987). [Google Scholar]
- Mattson W. J. & Haack R. A. Insect outbreaks. (Academic Press, New York, 1987). [Google Scholar]
- White T. C. R. Plant vigour versus plant stress: a false dichotomy. Oikos 118, 807–808 (2009). [Google Scholar]
- Price P. W. The plant vigor hypothesis and herbivore attack. Oikos 62, 244–251 (1991). [Google Scholar]
- Desneux N. et al. Biological invasion of European tomato crops by Tuta absoluta: ecology, geographic expansion and prospects for biological control. J. Pest Sci. 83, 197–215 (2010). [Google Scholar]
- Desneux N., Luna M. G., Guillemaud T. & Urbaneja A. The invasive South American tomato pinworm, Tuta absoluta, continues to spread in Afro-Eurasia and beyond - the new threat to tomato world production. J. Pest Sci. 84, 403–408 (2011). [Google Scholar]
- Cárdenas-Navarro R., Adamowicz S. & Robin P. Nitrate accumulation in plants: a role for water. J. Exp. Bot. 50, 613–624 (1999). [Google Scholar]
- White T. C. R. The availability of invertebrate herbivores in relation to the availability of nitrogen in stressed food plants. Oecologia 63, 90–105 (1984). [DOI] [PubMed] [Google Scholar]
- Slansky F. & Wheeler G. S. Caterpillars' compensatory feeding response to diluted nutrients leads to toxic allelochemical dose. Entomol. Exp. Appl. 65, 171–186 (1992). [Google Scholar]
- Ravenscroft N. O. M. The ecology of the chequered skipper butterfly Carterocephalus palaemon in Scotland. 2. Food plant quality and population range. J. Appl. Ecol. 31, 623–630 (1994). [Google Scholar]
- Obermaier E. & Zwölfer H. Plant quality or quantity? Host exploitation strategies in three Chrysomelidae species associated with Asteraceae host plants. Entomol. Exp. Appl. 92, 165–177 (1999). [Google Scholar]
- Herms D. A. & Mattson W. J. The dilemma of plants: to grow or defend. Q. Rev. Biol 67, 283–335 (1992). [Google Scholar]
- Stout M. J., Brovont R. A. & Duffey S. S. Effect of nitrogen availability on expression of constitutive and inducible chemical defenses in tomato, Lycopersicon esculentum. J. Chem. Ecol. 24, 945–963 (1998). [Google Scholar]
- Johnson N. D., Kidney L. P. & Bentley B. L. Short-term induction of alkaloid production in lupines: Differences between N2-fixing and nitrogen-limited plants. J. Chem. Ecol. 15, 2425–2433 (1989). [DOI] [PubMed] [Google Scholar]
- Larsson S. Stressful times for the plants stress-insect performance hypothesis. Oikos 56, 277–283 (1989). [Google Scholar]
- Chau A., Heinz K. M. & Davies, Jr F. T. Influences of fertilization on Aphis gossypii and insecticide usage. J. Appl. Entomol. 129, 89–97 (2005). [Google Scholar]
- Chau A., Heinz K. M. & Davies, Jr F. T. Influences of fertilization on population abundance, distribution, and control of Frankliniella occidentalis on chrysanthemum. Entomol. Exp. Appl. 117, 27–39 (2005). [Google Scholar]
- Chow A., Chau A. & Heinz K. M. Reducing fertilization: a management tactic against western flower thrips on roses. J. Appl. Entomol. 136, 520–529 (2012). [Google Scholar]
- Biondi A., Desneux N., Amiens-Desneux E., Siscaro G. & Zappalà L. Biology and developmental strategies of the palearctic parasitoid Bracon nigricans (Hymenoptera: Braconidae) on the neotropical moth Tuta absoluta (Lepidoptera: Gelechiidae). J. Econ. Entomol. 106, 1638–1647 (2013). [DOI] [PubMed] [Google Scholar]
- Chailleux A., Desneux N., Seguret J., Do Thi Khanh H., Maignet P. & Tabone E. Assessing European Egg Parasitoids as a Mean of Controlling the Invasive South American Tomato Pinworm Tuta absoluta. PLoS ONE 7, e48068. 10.1371/journal.pone.0048068. (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingestad T. & Agren G. I. Plant nutrition and growth - Basic principles. Plant Soil 168, 15–20 (1995). [Google Scholar]
- Le Bot J., Adamowicz S. & Robin P. Modelling plant nutrition of horticultural crops: a review. Sci Hortic 74, 47–52 (1998). [Google Scholar]
- Adamowicz S. & Le Bot J. Altering young tomato plant growth by nitrate and CO2 preserves the proportionate relation linking long-term organic-nitrogen accumulation to intercepted radiation. New Phytol. 180, 663–672 (2008). [DOI] [PubMed] [Google Scholar]
- R Core Team. The R Project for Statistical Computing, Vienna, Austria. http://www.R-project.org/ (2009).
- Zappalà L. et al. Natural enemies of the South American moth, Tuta absoluta, in Europe, North Africa and Middle-East, and their potential use in pest control strategies. J. Pest Sci. 86, 635–647 (2013). [Google Scholar]