Abstract
Depression is a debilitating psychiatric disorder and a growing global public health issue. However, the relationships between microbial infections and depression remains uncertain. A computerized literature search of Medline, ISI Web of Knowledge, PsycINFO, and the Cochrane Library was conducted up to May 2013, and 6362 studies were initially identified for screening. Case-control studies detected biomarker of microorganism were included. Based on inclusion and exclusion criteria, 28 studies were finally included to compare the detection of 16 infectious agents in unipolar depressed patients and healthy controls with a positive incident being defined as a positive biochemical marker of microbial infection. A customized form was used for data extraction. Pooled analysis revealed that the majority of the 16 infectious agents were not significantly associated with depression. However, there were statistically significant associations between depression and infection with Borna disease virus, herpes simplex virus-1, varicella zoster virus, Epstein-Barr virus, and Chlamydophila trachomatis.
Depression is a syndrome characterized by a sad mood exceeding normal sadness or grief, and major depressive disorder (MDD, major depression) has been associated with considerable morbidity and mortality1 and has been projected to become the second-leading cause of disability worldwide by 2020 after ischemic heart disease2. Recent studies have implicated several genes in the development of depression3, although the search for specific genes that confer this risk has been frustrating, as no genetic abnormality has been identified with any certainty4. In addition, several studies have suggested that environmental factors also contribute to an increased risk of depression, including exposure to poverty5, toxic factors6, stress7, and infectious agents8.
There is a long-standing belief that infectious agents can produce depression8; for example, infections occurring during prenatal and perinatal stages have produced serious neuropsychiatric consequences9, and early cross-sectional studies have demonstrated significantly elevated antibody titers against herpes simplex virus (HSV) in depressed patients10. Therefore, there may be an association between certain infectious agents and the development of depression that could provide a means by which to improve both depression outcomes and prevention.
Despite the substantial number of studies on this subject, contradictory findings have arisen. Early clinical studies supported an association of raised antibody titers against HSV10 and Epstein-Barr virus11 with depression. However, opposing results have also been reported that reveal no significant association between antibodies to HSV12, influenza13, or neurotropic viruses14 with depression. Therefore, this study aims to systematically analyze the current evidence regarding associations between a set of infectious agents and depression.
Results
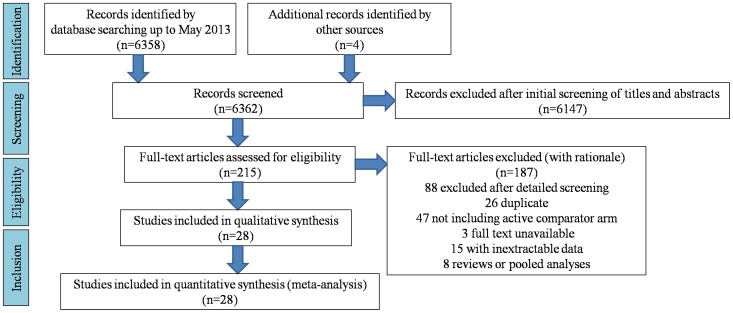
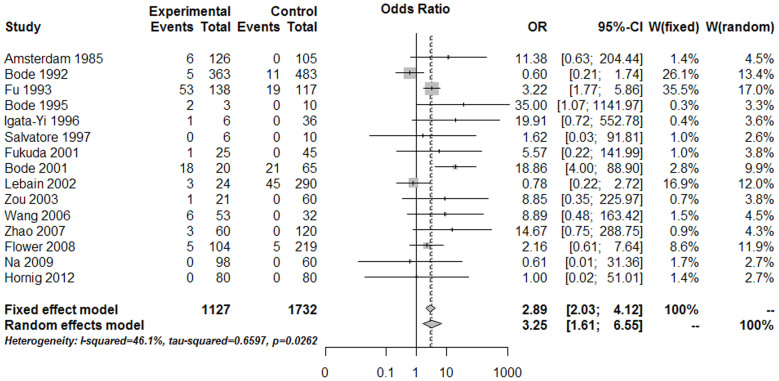
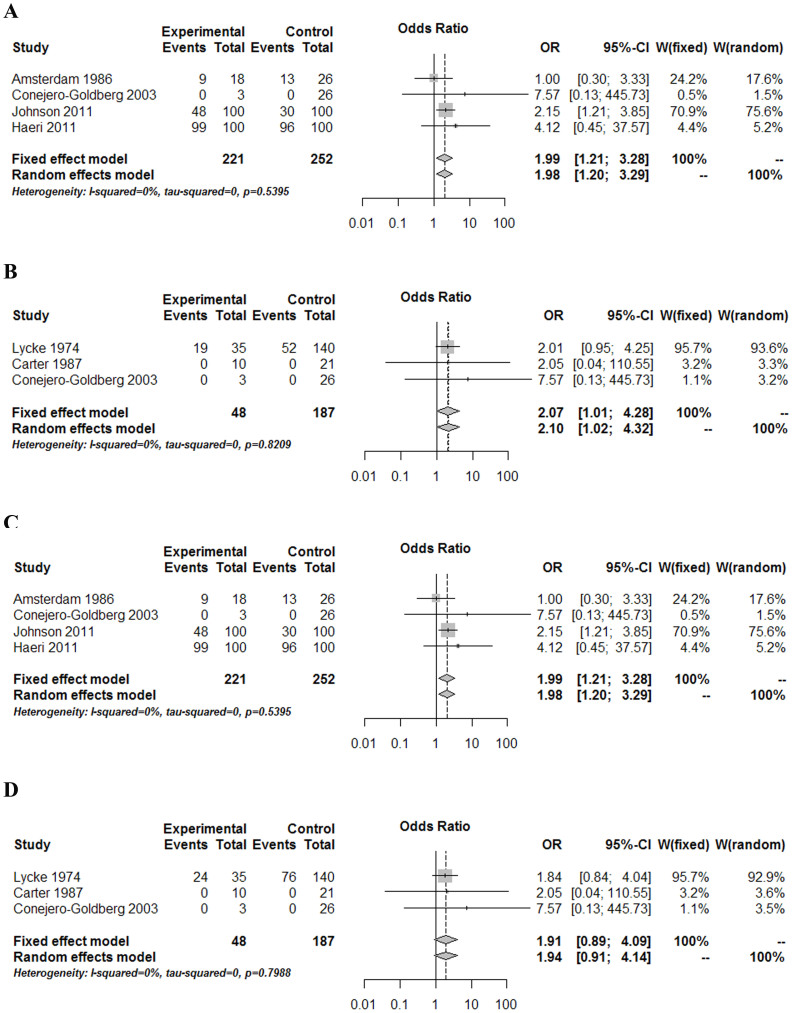
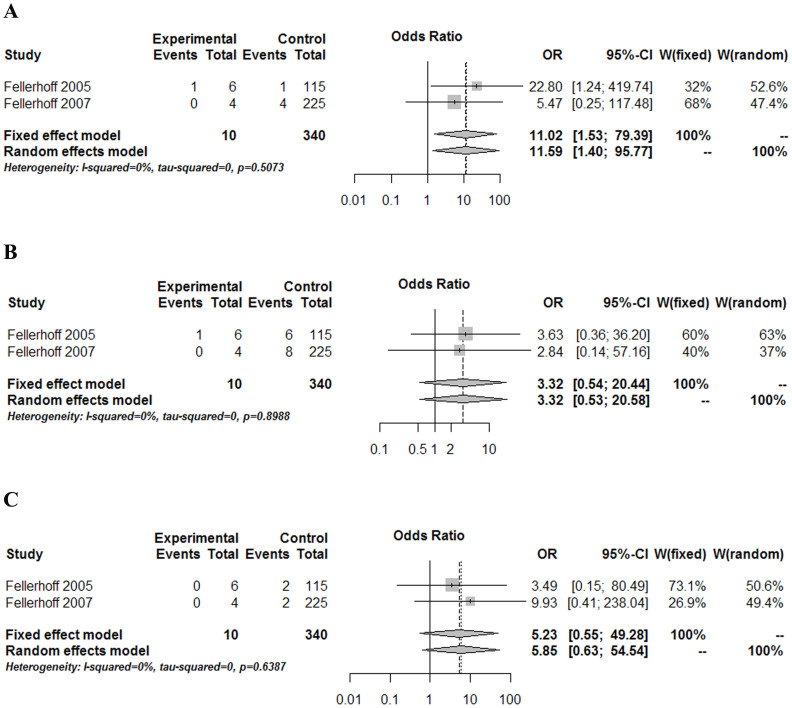
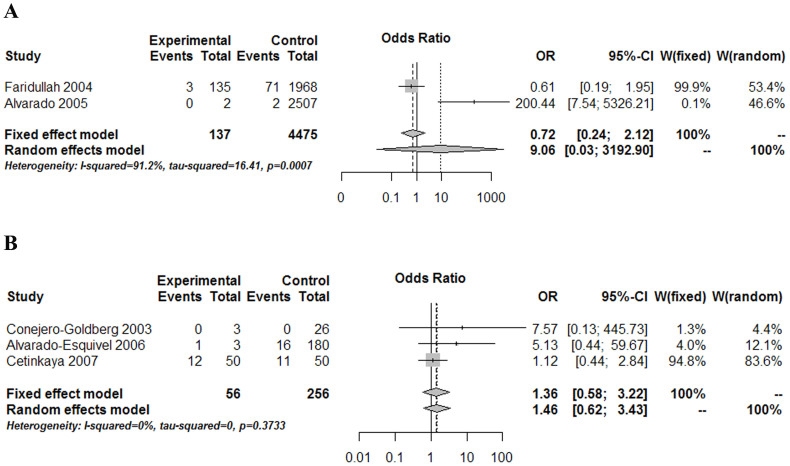
According to aforementioned inclusion and exclusion criteria, 28 studies were finally considered eligible for inclusion in the meta-analysis (Fig. 1, Table 1). Forest plots comparing infections with Borna disease virus (BDV) (Fig. 2), human herpesvirus (HHV, Herpesviridae) (Fig. 3), Chlamydiaceae (Fig. 4), hepatitis B virus (HBV) (Fig. 5A) and Toxoplasma gondii (Fig. 5B) between depressed patients and controls are also provided.
Figure 1. Flow diagram depicting the stages of the systematic review and meta analysis.
The number of studies (n) identified, screened, excluded, and included are detailed.
Table 1. Analysis and Evaluation of Studies Included in the Meta-Analysis.
| Microorganism | Study | Diagnostic criteria | Sample | Technique | Determination | Descriptive statistics | Inferential statistics | Quality | Global OR | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Controls | OR | 95% CI | Weight (%) | S | C | E | |||||||||
| + | − | + | − | (95% CI; P-value) | ||||||||||||
| BDV | Amsterdam JD et al. (1985)49 | DSM-III | SE | IFA | Ab | 6 | 120 | 0 | 105 | 11.38 | 0.63–204.44 | 4.51 | ** | * | 3.25 (1.61–6.55; P = 0.001) | |
| Bode L et al. (1992)17 | DSM-III | SE | IP | Ab | 5 | 358 | 11 | 472 | 0.60 | 0.21–1.74 | 13.38 | * | * | |||
| Fu ZF et al. (1993)15 | DSM-III-R | SE | WB | Ab | 53 | 85 | 19 | 98 | 3.22 | 1.77–5.86 | 16.97 | *** | ** | *** | ||
| Bode L et al. (1995)30 | DSM-III-R | PBMCs | N-RT-PCR, FCM, IF | RNA, Ag, Ab | 2 | 1 | 0 | 10 | 35.00 | 1.07–1141.97 | 3.34 | ** | ** | |||
| Igata-Yi R et al. (1996)50 | DSM-III-R | PBMCs | N-RT-PCR | RNA | 1 | 5 | 0 | 36 | 19.91 | 0.72–552.78 | 3.62 | * | ** | |||
| Salvatore M et al. (1997)51 | DSM-III-R | BR | N-RT-PCR | RNA | 0 | 6 | 0 | 10 | 1.62 | 0.03–91.81 | 2.60 | * | * | |||
| Fukuda K et al. (2001)52 | DSM-IV | PL | WB, ECLIA, IF | Ab | 1 | 24 | 0 | 45 | 5.57 | 0.22–141.99 | 3.77 | *** | ** | ** | ||
| Bode L et al. (2001)29 | DSM-III-R | PL | ELISA | CIC | 18 | 2 | 21 | 44 | 18.86 | 4.00–88.90 | 9.94 | ** | * | |||
| Lebain P et al. (2002)53 | DSM-IV | SE | IFA | Ab | 3 | 21 | 45 | 245 | 0.78 | 0.22–2.72 | 11.98 | *** | *** | |||
| Zou Dezhi et al. (2003)54 | CCMD-3 | PBMCs | RT-qPCR | RNA | 1 | 20 | 0 | 60 | 8.85 | 0.35–225.97 | 3.77 | ** | ** | |||
| Wang Zhenhai et al. (2006)55 | CCMD-3 | PBMCs | RT-qPCR | RNA | 6 | 47 | 0 | 32 | 8.89 | 0.48–163.42 | 4.46 | *** | ** | |||
| Zhao Libo et al. (2007)56 | CCMD-3 | PBMCs | RT-qPCR | RNA | 3 | 57 | 0 | 120 | 14.67 | 0.75–288.75 | 4.30 | *** | * | ** | ||
| Flower RL et al. (2008)57 | DSM-IV | PL | ELISA | Ab | 5 | 99 | 5 | 214 | 2.16 | 0.61–7.64 | 11.90 | ** | ** | |||
| Na KS et al. (2009)28 | DSM-IV | PBMCs | IFA, RT-qPCR | Ab, RNA | 0 | 98 | 0 | 60 | 0.61 | 0.01–31.36 | 2.73 | ** | * | |||
| Hornig M et al. (2012)16 | DSM-IV | BL | RT-qPCR | RNA | 0 | 80 | 0 | 80 | 1.00 | 0.02–51.01 | 2.73 | **** | ** | *** | ||
| HSV-1 (HHV-1) | Pokorny AD et al. (1973)12 | DSM-I | SE | CF | Ab | 54 | 8 | 35 | 3 | 0.58 | 0.14–2.33 | 55.45 | *** | ** | 2.26 (1.08–4.71; P = 0.030) | |
| Lycke E et al. (1974)18 | DSM-I | SE | CF | Ab | 31 | 4 | 89 | 51 | 4.44 | 1.48–13.30 | 40.28 | ** | * | ** | ||
| Carter et al. (1987)35 | DSM-IV | BR | HIB | DNA | 0 | 10 | 0 | 21 | 2.05 | 0.04–110.55 | 3.15 | **** | * | |||
| Conejero-Goldberg C et al. (2003)23 | DSM-IV | BR | N-PCR, HIB | DNA | 0 | 3 | 0 | 26 | 7.57 | 0.13–445.73 | 1.12 | *** | * | |||
| HSV-2 (HHV-2) | Conejero-Goldberg C et al. (2003)23 | DSM-IV | BR | N-PCR, HIB | DNA | 0 | 3 | 0 | 26 | 7.57 | 0.13–445.73 | *** | * | P = 0.330 | ||
| VZV (HHV-3) | Lycke E et al. (1974)18 | DSM-I | SE | CF | Ab | 19 | 16 | 52 | 88 | 2.01 | 0.95–4.25 | 95.7 | ** | * | ** | 2.07 (1.01–4.28; P = 0.048) |
| Carter et al. (1987)35 | DSM-III | BR | HIB | DNA | 0 | 10 | 0 | 21 | 2.05 | 0.04–110.55 | 3.2 | **** | * | |||
| Conejero-Goldberg C et al. (2003)23 | DSM-IV | BR | HIB | DNA | 0 | 3 | 0 | 26 | 7.57 | 0.13–445.73 | 1.1 | *** | * | |||
| EBV (HHV-4) | Amsterdam JD et al. (1986)38 | DSM-IV | SE | IF | Ab | 9 | 9 | 13 | 13 | 1.00 | 0.30–3.33 | 24.18 | *** | ** | *** | 1.99 (1.21–3.28; P = 0.007) |
| Conejero-Goldberg C et al. (2003)23 | DSM-IV | BR | N-PCR, HIB | DNA | 0 | 3 | 0 | 26 | 7.57 | 0.13–445.73 | 0.51 | *** | * | |||
| Johnson N et al. (2011)20 | EPDS | SE | ? | Ab | 48 | 52 | 30 | 70 | 2.15 | 1.21–3.85 | 70.94 | ** | * | ** | ||
| Haeri S et al. (2011)19 | DSM-IV | SE | ELISA | Ab | 99 | 1 | 96 | 4 | 4.13 | 0.45–37.57 | 4.37 | ** | * | * | ||
| CMV (HHV-5) | Lycke E et al. (1974)18 | DSM-I | SE | CF | Ab | 24 | 11 | 76 | 64 | 1.84 | 0.84–4.04 | 95.68 | ** | * | ** | 1.91 (0.89–4.09; P = 0.096) |
| Carter et al. (1987)35 | DSM-IV | BR | HIB | DNA | 0 | 10 | 0 | 21 | 2.05 | 0.04–110.55 | 3.19 | **** | * | |||
| Conejero-Goldberg C et al. (2003)23 | DSM-IV | BR | N-PCR, HIB | DNA | 0 | 3 | 0 | 26 | 7.57 | 0.13–445.73 | 1.13 | *** | * | |||
| HHV-6 | Conejero-Goldberg C et al. (2003)23 | DSM-IV | BR | N-PCR | DNA | 0 | 3 | 1 | 25 | 2.43 | 0.08–72.05 | *** | * | P = 0.608 | ||
| C. trachomatis | Fellerhoff B et al. (2005)21 | DSM-IV | BL | PCR | DNA | 1 | 5 | 1 | 114 | 22.80 | 1.24–419.74 | 32.04 | ** | ** | ** | 11.02 (1.53–79.39; P = 0.017) |
| Fellerhoff B et al. (2007)22 | DSM-IV | BL | PCR | DNA | 0 | 4 | 4 | 221 | 5.47 | 0.25–117.48 | 67.96 | ** | ** | ** | ||
| C. pneumoniae | Fellerhoff B et al. (2005)21 | DSM-IV | BL | PCR | DNA | 1 | 5 | 6 | 109 | 3.63 | 0.36–36.20 | 59.96 | ** | ** | ** | 3.32 (0.54–20.44; P = 0.196) |
| Fellerhoff B et al. (2007)22 | DSM-IV | BL | PCR | DNA | 0 | 4 | 8 | 217 | 2.84 | 0.14–57.16 | 40.04 | ** | ** | ** | ||
| C. psittaci | Fellerhoff B et al. (2005)21 | DSM-IV | BL | PCR | DNA | 0 | 6 | 2 | 113 | 3.49 | 0.15–80.49 | 73.07 | ** | ** | ** | 5.23 (0.55–49.28; P = 0.149) |
| Fellerhoff B et al. (2007)22 | DSM-IV | BL | PCR | DNA | 0 | 4 | 2 | 223 | 9.93 | 0.41–238.04 | 26.93 | ** | ** | ** | ||
| HBV | Faridullah Shah et al. (2004)58 | DSM-IV | BL | ELISA | Ag | 3 | 132 | 71 | 1897 | 0.61 | 0.19–1.95 | 53.4 | ** | ** | 9.06 (0.026–3192.90; P = 0.461) | |
| Alvarado Esquivel C (2005)59 | DSM-IV | SE | ELISA | Ag, Ab | 0 | 2 | 2 | 2505 | 200.44 | 7.54–5326.21 | 46.6 | ** | ** | |||
| T. gondii | Conejero-Goldberg C et al. (2003)23 | DSM-IV | BR | N-PCR | DNA | 0 | 3 | 0 | 26 | 7.57 | 0.13–445.73 | 1.28 | *** | * | 1.36 (0.58–3.22; P = 0.483) | |
| Alvarado-Esquivel C et al. (2006)60 | DSM-IV | SE | ELISA | Ab | 1 | 2 | 16 | 164 | 5.13 | 0.44–59.67 | 3.96 | *** | ** | * | ||
| Cetinkaya Z et al. (2007)61 | DSM-IV | SE | ELISA | Ab | 12 | 38 | 11 | 39 | 1.12 | 0.44–2.84 | 94.76 | ** | * | ** | ||
| HCV | Faridullah Shah et al. (2004)58 | DSM-IV | BL | ELISA | Ab | 31 | 104 | 124 | 1844 | 4.43 | 2.85–6.89 | ** | ** | P < 0.001 | ||
| JCV | Carter et al. (1987)35 | DSM-IV | BR | HIB | DNA | 0 | 10 | 0 | 21 | 2.00 | 0.11–35.09 | **** | * | P = 1.000 | ||
| BKV | Carter et al. (1987)35 | DSM-IV | BR | HIB | DNA | 0 | 10 | 0 | 21 | 2.00 | 0.11–35.09 | **** | * | P = 1.000 | ||
| Measles virus | Lycke E et al. (1974)18 | DSM-I | SE | CF | Ab | 14 | 21 | 84 | 56 | 0.44 | 0.21–0.95 | ** | * | ** | P = 0.033 | |
Abbreviations: EPDS, Edinburgh Depression Scale; DSM, Diagnostic and Statistical Manual of Mental Disorders; CCMD-3, Classification and Diagnostic Criteria of Mental Disorders in China; HHV-1(HSV-1), herpes simplex virus-1; HHV-2 (HSV-2), herpes simplex virus-2; HHV-3 (VZV), varicella zoster virus; HHV-4 (EBV), Epstein-Barr virus; HHV-5 (CMV), cytomegalovirus; HHV-6, roseolovirus; HBV, hepatitis B virus; BDV, Borna disease virus; C. trachomatis, Chlamydophila trachomatis, C. pneumoniae, Chlamydophila pneumoniae; C. psittaci, Chlamydophila psittaci; T. gondii, Toxoplasma gondii; HCV, hepatitis C virus; JCV, John Cunningham virus; BKV, BK virus; SE, serum; PL, plasma; BR, brain biopsy, BL, blood; PBMCs, peripheral blood mononuclear cells; PCR, polymerase chain reaction; N-PCR, nested-polymerase chain reaction; RT-PCR, reverse transcriptase polymerase chain reaction; N-RT-PCR, nested reverse transcription polymerase chain reaction; RT-qPCR, real-time reverse transcriptase polymerase chain reaction; HIB, hybridization; ELISA, enzyme-linked immunosorbent assay; WB, Western blot; ECLIA, electrochemiluminescence immunoassay; FCM, flow cytometric analysis; EIA, enzyme immunoassay; IF, immunofluorescence; IFA, immunofluorescence assay; IP, immunoprecipitation; CF, complement fixation antibodies; NT, neutralizing antibodies; ?, unknown; DNA, deoxyribonucleic acid; RNA, ribonucleic acid; CIC, circulating immune complexes; Ag, antigen; Ab, antibody; +, positive; −, negative; S, Selection; C, Comparability; E, Exposure.
Asterisks indicate the level of study quality from low quality (*) to (****) high quality.
Figure 2. Forest plot of studies comparing Borna disease virus (BDV) infection between depressed patients and healthy controls.
BDV infection was shown to have a statistically significant association with depression.
Figure 3. Forest plot of studies comparing human herpes virus (HHV) infections between depressed patients and healthy controls.
(A) herpes simplex virus-1 (HSV-1, HHV-1), (B) varciella zoster virus (VZV, HHV-3), (C) Epstein-Barr virus (EBV, HHV-4), and (D) cytomegalovirus (CMV, HHV-5). Only HSV-1, VZV, EBV infection was shown to have a statistically significant association with depression.
Figure 4. Forest plot of studies comparing Chlamydiaceae infections between depressed patients and healthy controls.
(A) C. trachomatis, (B) C. pneumoniae, and (C) C. psittaci. Only C. trachomatis infection was shown to have a statistically significant association with depression.
Figure 5. Forest plot of studies comparing two other microorganisms infections between depressed patients and healthy controls.
(A) hepatitis B virus, (B) Toxoplasma gondii. Neither of them infection was shown to have a statistically significant association with depression.
BDV
BDV is the most widely-studied infectious agent associated with depression. A total of 15 studies were included, two of which stood out as those of highest weight15 and quality16 (Fig. 2, Table 1). The narrowest CI 95% range study was a Bode17 study on account of the large amount of participants included. The estimated combined OR was 3.25 (CI 95%: 1.61–6.55; P = 0.001), indicating a statistically significant association between BDV infection and depression. After performing heterogeneity testing between these studies, the resulting χ2exp = 25.96 (14 degrees of freedom, P = 0.026) indicated a trend for significant heterogeneity among OR values obtained from different studies. This finding is corroborated by a I2 coefficient of 46.1% (CI 95%: 0.02–0.71), which indicates that 46.1% of the variability between the OR values obtained from different studies was due to a moderate level of heterogeneity between them (<75%). Using meta-regression, we found that the technique used to detect BDV infection did not influence the OR value (P = 0.494). Thus, the OR values of the studies that detected RNA from blood or PBMCs (OR = 8.55, CI 95%: 2.48–29.44) were not significantly different from the OR values obtained in studies in which serum antibodies were detected (OR = 2.39, CI 95%: 1.01–5.66). Begg's and Egger's tests were both performed, and the findings therefrom were not significant (P = 0.805 and P = 0.297, respectively), indicating no publication bias.
HHV
Six HHVs – herpes simplex virus-1 (HSV-1, HHV-1), herpes simplex virus-2 (HSV-2, HHV-2), varicella zoster virus (VZV, HHV-3), Epstein-Barr virus (EBV, HHV-4), cytomegalovirus (CMV, HHV-5), and roseolovirus (HHV-6) – were analyzed in the current study (Fig. 3). As to HSV-1, significant differences were found (P = 0.030) from the analysis of four studies that compared HSV-1 infection in depressed patients and healthy controls (Fig. 3A). The Pokorny et al.12 study was assigned the highest weight. When heterogeneity tests were performed, a χ2exp = 5.47 was obtained with three degrees of freedom and a P = 0.140, indicating that the differences between the studies can be attributed to randomness. This finding was further corroborated by an I2 coefficient of 45.2% (CI 95%: 0–0.82), indicating that there was not substantial inconsistency. The Begg's test result was not significant (P = 1.000), indicating no publication bias. A similar result was obtained after Egger's test (P = 0.986). With respect to VZV, significant differences were found (OR = 2.07; CI 95%: 1.01–4.28; P = 0.048) from the analysis of three low-quality studies that compared VZV infection in depressed patients and healthy controls (Fig. 3B). The Lycke et al.18 study was assigned the highest weight and relative high-quality. After heterogeneity tests, a value of χ2exp = 0.39 with two degrees of freedom, and P = 0.821, suggested that the differences found between the studies could be due to randomness. This finding was also corroborated by an I2 coefficient of 0.0% (CI 95%: 0–0.47), indicating that no OR variability was due to study heterogeneity. Both Begg's and Egger's tests were not significant (P = 0.117 and P = 0.500, respectively), indicating that there was no publishing bias. With regard to EBV, four studies compared EBV infection in depressed patients and healthy controls (Fig. 3C). The studies were heterogeneous, and the most relevant ones were those by Haeri19 and Johnson20 due to their larger sample sizes. When all four studies were combined, an overall OR of 1.99 (CI 95%: 1.21–3.28; P = 0.007) was obtained for the association between EBV and depression. Therefore, depression was 1.99 times more frequent in individuals displaying the EBV marker than in individuals who did not. When only studies that used serum antibodies as the EBV infection marker were included, the result remained statistically significant with an OR of 1.96 (CI 95%: 1.18–3.24; P = 0.009). When heterogeneity analysis was conducted, a χ2exp = 2.16 was obtained with three degrees of freedom and a P = 0.540, indicating that the differences between the studies could be due to randomness. This finding was also corroborated by an I2 coefficient of 0.0% (CI 95%: 0–0.79), indicating that no OR variability was due to study heterogeneity. There was no evident publishing bias, as the Begg's (P = 0.497) and Egger's (P = 0.730) tests were not significant.
With respect to the other HHVs, no significant differences were found when comparing infection with HSV-2, CMV, or HHV-6 in depressed patients and healthy controls. As to CMV, three studies compared CMV infection by means of antibody or viral DNA in depressed patients and healthy controls (Fig. 3D). The weight of the studies performed in 197418 superceeded the other two studies, as it had the largest sample size and highest quality. Significant OR values were not found for any of the studies nor for the combined OR (OR = 1.91; CI 95%: 0.89–4.09; P = 0.096). When heterogeneity tests were performed, a χ2exp = 0.45 was obtained with two degrees of freedom and a P = 0.799, indicating that the differences between the studies can be attributed to randomness. This finding was also corroborated by an I2 coefficient of 0.0% (CI 95%: 0–0.54), indicating that no OR variability was due to study heterogeneity. The Begg's test result was not significant (P = 0.117), indicating no publication bias. A similar result was obtained after Egger's test (P = 0.46).
Chlamydiaceae
Two studies of similar quality by Fellerhoff et al.21,22 were included and used blood samples to detect the DNA of three Chlamydiaceae species (i.e., C. trachomatis, C. pneumoniae, and C. psittaci) through polymerase chain reaction (PCR) (Fig. 4). A significant association was found for C. trachomatis with a wide 95% confidence interval (OR = 11.02; CI 95%: 1.53–79.39; P = 0.017; Fig. 4A). However, in the cases of C. pneumoniae (OR = 3.32; CI 95%: 0.54–20.44; P = 0.196; Fig. 4B) and C. psittaci (OR = 5.23; CI 95%: 0.55–49.28; P = 0.149; Fig. 4C), no significant association was observed between infection with these two Chlamydiaceae species and depression.
Other infectious agents
As to hepatitis B virus (HBV), no significant differences were found (OR = 9.06; CI 95%: 0.026–3192.90; P = 0.461) from the analysis of two low-quality studies that compared HBV infection in depressed patients and healthy controls (Fig. 5A, Table 1). With regard to T. gondii, three studies focused on the possible relationship between T. gondii infection and depression by comparing the detection of infection markers in samples of depressed patients and healthy controls (Fig. 5B, Table 1). Conejero-Goldberg et al.23 was limited to post-mortem brain tissue samples, and therefore, included a smaller number of cases and controls; this study was also of lower quality. After combining the different studies, no significant association was found between T. gondii parasitization and depression (OR = 1.36; CI 95%: 0.58–3.22; P = 0.483). With respect to the remaining infectious agents, results from the individual studies that analyzed the relationships between depression and infection with the hepatitis C virus (HCV), the human polyoma virus John Cunningham virus (JCV), the papovavirus BK virus (BKV), and the measles virus are also provided (Table 1).
Discussion
From the 28 included studies in this systematic review and meta-analysis, 16 infectious agents were discovered in both depressed patients and healthy controls. The majority of included studies focused on BDV, the HHV family, the Chlamydiaceae family, T. gondii, and HBV. Scattered reports on HCV, JCV, BKV, and measles virus were also included.
Pooled analysis revealed that the majority of the 16 infectious agents were not significantly associated with depression. However, there were statistically significant associations between depression and infection with BDV, HSV-1, VZV, EBV, and C. trachomatis.
BDV, a neurotropic, non-cytolytic, non-segmented, negative-stranded non-retroviral RNA virus24, infects a variety of animal species, including primates25 and tree shrews26. BDV displays a predilection for infecting the limbic system and hippocampus27, two brain regions which play important roles in affective disorders such as depression. Researchers have attempted to clarify the relationship between BDV infection and depression in numerous studies using different populations and diverse techniques; nonetheless, the relationship between BDV infection and depression remains controversial. For instance, Na28 and Hornig16 obtained negative results from RT-qPCR of peripheral blood mononuclear cells (PBMCs) and blood; however, persistent BDV seropositivity has been repeatedly demonstrated in a high percentage of depression patients15,29,30. Through our pooled analysis, we found that individuals who suffer from depression are 3.25 times more likely to be infected by BDV. This finding indicates that a statistically significant association between BDV infection and depression exists and requires further investigation.
HSV-1 research with respect to depression has been typically descriptive in nature and has been based on two approaches: (i) serological tests showing antibody prevalence in depressed patients and healthy controls, and (ii) brain tissue sampling to ascertain whether the presence of HSV-1 in brain cells is associated with depression. These previous studies, save one18, have shown no conclusive findings31,32. In this study, a significant association between HSV-1 infection and depression was found. This may be due to the limitations inherent in the human brain tissue studies used in this meta-analysis. For example, after HSV-1 infection, various brain areas may have differing, scant distributions of HSV-1 particles, and with current techniques, it is difficult to detect small quantities of HSV-1 particles. Additionally, post-biopsy brain tissue processing may have affected the viral findings.
Regarding VZV, chickenpox in children has been shown to produce neurological damage in addition to viral encephalitis33. Furthermore, major depression has been associated with a marked decline in VZV-specific cellular immunity34. These previous studies23,35, the lack of association between VZV infection and depression observed here may partly be due to the use of brain tissue for viral DNA detection, which has provided negative results in all participants assessed by this method. Therefore, detection of different tissue samples by various methods should be performed.
Several studies have focused on the relationship between EBV and depression31,36,37. Amsterdam et al.38 showed no association between unrecognized chronic active EBV infection and depression; moreover, Conejero-Goldberg et al.23 also failed to demonstrate an association between EBV infection and depression. However, the titers of antibodies to EBV have been shown to be significantly higher in psychiatric patients than controls31. Most notably, antibodies to EBV viral capsid antigen (EBV-VCA) have been detected in the cerebrospinal fluid (CSF) of psychiatric patients31. In addition, a direct association between EBV reactivation and maternal depression has been found among women undergoing first-trimester genetic screening19. With respect to the current study, the significant association between EBV infection and depression reported here is strongly dependent on the association reported by Johnson (n = 200)20 and Haeri (n = 200)19, which were the largest EBV studies by sample size as compared to the aforementioned studies by Amsterdam et al.38 (n = 44) and Conejero-Goldberg et al.23 (n = 29) (Table 1). These findings suggest that smaller sample-sized studies tend to produce negative results, and identifying a significant association between EBV infection and depression may require a sufficiently large sample size.
Only one study explored the association between HSV-2 infection and depression, which failed to show a significant association between the presence of HSV-2 DNA in brain tissue and depression. Although there were no apparent differences in the mean HSV-2 lgG antibody titers between depressed patients and healthy controls in the current study32, previous research has shown that maternal exposure to HSV-2 is associated with an increased risk for psychosis among adult offspring9. This fact may be attributed to the heightened risk of HSV-2 transmission to infants exposed to asymptomatic shedding at delivery39. These previous findings suggest that HSV-2 infection at childbirth may play a role in the later development of depression; thus, further investigation on this issue is required.
CMV is a ubiquitous virus, with an especially high prevalence in lower socioeconomic status populations18. Individuals with higher CMV-specific antibody titres are more likely to be depressed and experience more overall psychological morbidity, suggesting that symptoms of depression may induce CMV reactivation40. Our negative result may be attributed to the use of brain tissue samples for the detection of viral DNA that may have been degraded.
One out of five depressed patients carry C. trachomatis DNA, which far exceeds the prevalence in healthy individuals, and indicates a significant association between C. trachomatis infection and depression. Interestingly, C. trachomatis produces a sexually transmitted disease (STI) as opposed to C. pneumoniae and C. psittaci, which both produce pneumonia41. Although the causal nature of the association between increased depressive symptoms and STIs has not been discerned, sexually risky behaviors may be related to dispositional characteristics, such as impulsiveness, sensation seeking, or a desire for unconventionality, that can be associated with substance abuse and depression42.
This systematic review and meta-analysis showed no significant association between HBV infection and depression. Several studies have reported that mentally ill patients have a higher prevalence of HBV infection43,44. Unfortunately, few studies have directly examined HBV infection among depression patients with comparable healthy controls. Therefore, future research should focus on the possible relationship between HBV infection and depression.
T. gondii is an obligate intracellular protozoan parasite infecting one-third of the world population and dormantly resides in the brains of immunocompetent hosts45. Higher T. gondii immunoglobulin G titers in pregnant women have been associated with depression during pregnancy46, and subclinical reactivation of T. gondii or immune responses to T. gondii have been shown worsen mood in pregnant women. From one case report, the response to antidepressant treatment improved only after adequate treatment for toxoplasma47. Nonetheless, according to the data obtained in this meta-analyses, no significant relationship exists between T. gondii seropositivity and recurrent mood disorder45.
Finally, four viruses (HCV, JCV, BKV, and measles virus) were analyzed to determine their possible association with depression. Only two, HCV and measles virus, were shown to have a statistically significant association with depression (P < 0.05). However, as these studies were one-of-a-kind and anecdotal, we were unable to draw definitive conclusions about these virus' relationship with depression.
Based on above results, the directionality of the association between infection and depression should be concerned. We speculate that there might possibly be a causal relationship between BDV and the etiology of depression. The indirect evidence is that the overall response rate of the amantadine augmentation in the BDV-infected patients with regard to depressive symptoms was 68% after a mean of 2.9 weeks of treatment. In addition, the decrease of depression tended to correspond with the decrease in viral activity48. However, until now, there are no enough evidences that explain the directionality of the association between the other four microorganism (HSV-1, VZV, EBV, and C. trachomatis) and depression. Taking into account the selection bias, which come from the controls in the included studies were not completely enrolled the very same source population of cases (Supplement), thus, more researches correspond to the nested case/control (CC) study design with large sample size should be performed to cope with this problem in the future.
Methods
Literature search strategy
This study was composed of two components: a qualitative systematic review followed by a quantitative meta-analysis. A systematic review, by definition, implies an epidemiologic description of previously published articles that regards individual studies as “subjects”. A computerized literature search was conducted up to May 2013 using Medline (PubMed), ISI Web of Knowledge, PsycINFO, and the Cochrane Library. No race restriction was utilized. The following logical combinations of search terms were used: (“depression”, “depressive”, “depressed”, OR depression*) AND ([“virus” OR virus*] OR [“bacterium”, “bacteria”, OR bacteria*] OR [“fungus” OR fungi*] OR [“parasite” OR parasite*]). Reference lists of all discovered articles were examined for identification of additional eligible studies.
Inclusion and exclusion criteria
Included studies must have (i) enrolled patients with a current diagnosis of unipolar depression, consisting of either a primary depressive episode or recurrent depressive disorder, with the specific exclusion of patients with manic episodes, bipolar disorder, and other unclearly defined mood or affective disorders; (ii) enrolled controls who were defined as healthy individuals or those characterized by the absence of any history of psychiatric or neurological disease; and (iii) examined biochemical markers in both patients and controls. Excluded studies included non-controlled studies, animal studies, reviews, studies which did not present their results in an appropriate and/or explicit manner, and those that did not assess infectious agent(s).
Selection of studies, data extraction, and management
A customized data extraction form was used. Three authors (XL, XZ and YL) independently performed the database searches, reviewed each of the retrieved abstracts, selected those that appeared to meet the study's inclusion criteria, and reviewed each of the selected manuscripts in full. Where a study met the inclusion criteria, they independently extracted the data from each study. The following information was extracted: microorganism, first author name, publication year, diagnostic criteria for unipolar depression, sample of detection, technique, biomarker of determination, descriptive results in each group (Table 1). All authors reviewed and agreed on the study inclusion and exclusion criteria, and on the selected manuscripts. Any disagreement was resolved by consensus or third-party adjudication (XL). Study quality was assessed using the Newcastle-Ottawa Quality Assessment Scale (http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm; updated 10 February 2010).
Statistical analysis
The summary odds ratio (OR) was used as the effect parameter for this meta-analysis, and a 95% confidence interval (CI) was used to interpret the results. When the number of studies under analysis was greater than or equal to three (although five may be better), heterogeneity was assessed using the χ2 test and I2. A P-value of ≤0.10 was deemed statistically significant, and there were not substantial heterogeneity if a 95% CI for the I2 index contains the zero percentage. I2 values of 25%, 50%, and 75% represented low, moderate, and high degree of inconsistency, respectively. In cases of low heterogeneity for outcome data, a fixed-effect model was used for analysis; otherwise, a random-effect model was used. For I2 values greater than 75%, strong heterogeneity dictated the performance of an additional meta-regression using the Restricted Maximum Likelihood (REML) method. In such cases and if the number of studies to be included in each subgroup was big enough (greater than or equal to three), a more detailed sub-analysis of the different subgroups was performed. Finally, Begg's and Egger's tests were used in order to check for any particular publication biases and their magnitude. Data obtained from the included studies were analyzed using the R version 3.0.1 statistical package. All tests were two-sided, and a P < 0.05 was was deemed statistically significant unless otherwise specified.
Supplementary Material
Table S1
Acknowledgments
This work was supported by the National Basic Research Program of China (973 Program) (Grant No. 2009CB918300), the National Natural Science Foundation of China (Grant No. 30900456 and 21175099), and the Natural Science Foundation Project of Chongqing (CSTC, 2008BB5238 and 2010BB5393). The funders had no role in study design, data collection and analysis, publication decisions, or manuscript preparation. We sincerely thank Dr. N.D. Melgiri for his assistance in editing and proofreading the manuscript.
Footnotes
The authors declare no competing financial interests.
Author Contributions Conceived and designed the experiments: P.X., X.W., L.Z. and Y.L. Performed the experiments: X.L., X.Z. and Y.L. Analyzed the data: X.W., M.W. and L.Y. Contributed reagents/materials/analysis tools: L.Z. and S.F. Wrote the paper: X.W., L.Z., Y.L. and X.L.
References
- Ustun T. B. et al. Multiple-informant ranking of the disabling effects of different health conditions in 14 countries. WHO/NIH Joint Project CAR Study Group. Lancet 354, 111–115 (1999). [DOI] [PubMed] [Google Scholar]
- Murray C. J. & Lopez A. D. Evidence-based health policy--lessons from the Global Burden of Disease Study. Science (New York, N.Y.) 274, 740–743 (1996). [DOI] [PubMed] [Google Scholar]
- Nestler E. J. et al. Neurobiology of depression. Neuron 34, 13–25 (2002). [DOI] [PubMed] [Google Scholar]
- Lohoff F. W. Overview of the genetics of major depressive disorder. Curr Psychiatry Rep 12, 539–546, 10.1007/s11920-010-0150-6 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks-Gunn J. & Duncan G. J. The effects of poverty on children. The future of children, 55–71 (1997). [PubMed] [Google Scholar]
- Mason L. H., Mathews M. J. & Han D. Y. Neuropsychiatric symptom assessments in toxic exposure. Psychiatr Clin North Am 36, 201–208, 10.1016/j.psc.2013.02.001 (2013). [DOI] [PubMed] [Google Scholar]
- Schlossberg K., Massler A. & Zalsman G. Environmental risk factors for psychopathology. Isr J Psychiatry Relat Sci 47, 139 (2010). [PubMed] [Google Scholar]
- Sayers I. Low spirits after virus infections. BMJ 2, 440 (1976). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buka S. L., Cannon T. D., Torrey E. F. & Yolken R. H. Maternal exposure to herpes simplex virus and risk of psychosis among adult offspring. Biol Psychiatry 63, 809–815, 10.1016/j.biopsych.2007.09.022 (2008). [DOI] [PubMed] [Google Scholar]
- Halonen P. E., Rimon R., Arohonka K. & Jantti V. Antibody levels to herpes simplex type I, measles and rubella viruses in psychiatric patients. Br J Psychiatry 125, 461–465 (1974). [DOI] [PubMed] [Google Scholar]
- Allen A. D. & Tilkian S. M. Depression correlated with cellular immunity in systemic immunodeficient Epstein-Barr virus syndrome (SIDES). J Clin Psychiatry 47, 133–135 (1986). [PubMed] [Google Scholar]
- Pokorny A. D., Rawls W. E., Adam E. & Mefferd R. B. Jr Depression, psychopathy, and herpesvirus type I antibodies. Lack of relationship. Arch Gen Psychiatry 29, 820–822 (1973). [DOI] [PubMed] [Google Scholar]
- Sinanan K. & Hillary I. Post-influenzal depression. Br J Psychiatry 138, 131–133 (1981). [DOI] [PubMed] [Google Scholar]
- King D. J. et al. A survey of serum antibodies to eight common viruses in psychiatric patients. Br J Psychiatry 147, 137–144 (1985). [DOI] [PubMed] [Google Scholar]
- Fu Z. F. et al. Detection of Borna disease virus-reactive antibodies from patients with affective disorders by western immunoblot technique. J Affect Disord 27, 61–68 (1993). [DOI] [PubMed] [Google Scholar]
- Hornig M. et al. Absence of evidence for bornavirus infection in schizophrenia, bipolar disorder and major depressive disorder. Mol Psychiatry 17, 486–493, 10.1038/mp.2011.179 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode L., Riegel S., Lange W. & Ludwig H. Human infections with Borna disease virus: seroprevalence in patients with chronic diseases and healthy individuals. J Med Virol 36, 309–315 (1992). [DOI] [PubMed] [Google Scholar]
- Lycke E., Norrby R. & Roos B. E. A serological study on mentally ill patients with particular reference to the prevalence of herpes virus infections. Br J Psychiatry 124, 273–279 (1974). [DOI] [PubMed] [Google Scholar]
- Haeri S. et al. Maternal depression and Epstein-Barr virus reactivation in early pregnancy. Obstet Gynecol 117, 862–866, 10.1097/AOG.0b013e31820f3a30 (2011). [DOI] [PubMed] [Google Scholar]
- Johnson N. et al. Depression in pregnancy: is there an association with Epstein-Barr virus (EBV) reactivation? Am J Obstet Gynecol 204, S322–S322, 10.1016/j.ajog.2010.10.848 (2011). [DOI] [Google Scholar]
- Fellerhoff B., Laumbacher B. & Wank R. High risk of schizophrenia and other mental disorders associated with chlamydial infections: hypothesis to combine drug treatment and adoptive immunotherapy. Med Hypotheses 65, 243–252, 10.1016/j.mehy.2005.03.013 (2005). [DOI] [PubMed] [Google Scholar]
- Fellerhoff B., Laumbacher B., Mueller N., Gu S. & Wank R. Associations between Chlamydophila infections, schizophrenia and risk of HLA-A10. Mol Psychiatry 12, 264–272, 10.1038/sj.mp.4001925 (2007). [DOI] [PubMed] [Google Scholar]
- Conejero-Goldberg C., Torrey E. F. & Yolken R. H. Herpesviruses and Toxoplasma gondii in orbital frontal cortex of psychiatric patients. Schizophr Res 60, 65–69 (2003). [DOI] [PubMed] [Google Scholar]
- de la Torre J. C. [Family Bornaviridae]. Virus taxonomy: classification and nomenclature of viruses: seventh report of the International Committee on Taxonomy of Viruses [Regenmortel, M. (ed.)] [531–538] (Academic Press, London, 2000). [Google Scholar]
- Stitz L., Krey H. & Ludwig H. Borna disease in rhesus monkeys as a models for uveo-cerebral symptoms. J Med Virol 6, 333–340 (1981). [DOI] [PubMed] [Google Scholar]
- Sprankel H., Richarz K., Ludwig H. & Rott R. Behavior alterations in tree shrews (Tupaia glis, Diard 1820) induced by Borna disease virus. Med Microbiol Immun 165, 1–18 (1978). [DOI] [PubMed] [Google Scholar]
- Wagner K., Ludwig H. & Paulsen J. Fluorescence serological demonstration of Borna virus antigen. Berl Munch Tierarztl 81, 395–396 (1968). [PubMed] [Google Scholar]
- Na K. S., Tae S. H., Song J. W. & Kim Y. K. Failure to detect borna disease virus antibody and RNA from peripheral blood mononuclear cells of psychiatric patients. Psychiatry Investig 6, 306–312, 10.4306/pi.2009.6.4.306 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode L. et al. Borna disease virus-specific circulating immune complexes, antigenemia, and free antibodies--the key marker triplet determining infection and prevailing in severe mood disorders. Mol Psychiatr 6, 481–491, 10.1038/sj.mp.4000909 (2001). [DOI] [PubMed] [Google Scholar]
- Bode L., Zimmermann W., Ferszt R., Steinbach F. & Ludwig H. Borna disease virus genome transcribed and expressed in psychiatric patients. Nat Med 1, 232–236 (1995). [DOI] [PubMed] [Google Scholar]
- Gotlieb-Stematsky T. et al. Antibodies to Epstein-Barr virus, herpes simplex type 1, cytomegalovirus and measles virus in psychiatric patients. Arch Virol 67, 333–339 (1981). [DOI] [PubMed] [Google Scholar]
- Amsterdam J. D. & Hernz W. J. Serum antibodies to herpes simplex virus types I and II in depressed patients. Biol Psychiat 34, 417–420 (1993). [DOI] [PubMed] [Google Scholar]
- Riaza G. M., de la Torre E. M., Mencía B. S., Molina C. J. & Tamariz-Martel M. A. Complications of varicella in children. An Esp Pediatr 50, 259 (1999). [PubMed] [Google Scholar]
- Irwin M. et al. Cellular immunity to varicella-zoster virus in patients with major depression. J Infect Dis 178 Suppl 1, S104–108 (1998). [DOI] [PubMed] [Google Scholar]
- Carter G. I., Taylor G. R. & Crow T. J. Search for viral nucleic acid sequences in the post mortem brains of patients with schizophrenia and individuals who have committed suicide. J Neurol Neurosurg Psychiatry 50, 247–251 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg D. B. Depression, anxiety, and Epstein-Barr virus infection. Ann Intern Med 104, 449 (1986). [DOI] [PubMed] [Google Scholar]
- Cooke R. G., Warsh J. J., Hasey G. M., McLaughlin B. J. & Jorna T. Epstein-Barr virus antibodies and severity of depression. Biol Psychiat 29, 621–623 (1991). [DOI] [PubMed] [Google Scholar]
- Amsterdam J. D. et al. Serum antibodies to Epstein-Barr virus in patients with major depressive disorder. Am J Psychiatry 143, 1593–1596 (1986). [DOI] [PubMed] [Google Scholar]
- Buka S. L. et al. Maternal infections and subsequent psychosis among offspring. Arch Gen Psychiatry 58, 1032–1037 (2001). [DOI] [PubMed] [Google Scholar]
- Phillips A. C., Carroll D., Khan N. & Moss P. Cytomegalovirus is associated with depression and anxiety in older adults. Brain Behav Immun 22, 52–55, 10.1016/j.bbi.2007.06.012 (2008). [DOI] [PubMed] [Google Scholar]
- Deborah D. [Lessons and Challenges Arising from the “First Wave of Chlamydia Genome Sequencing”]. Chlamydia: genomics and pathogenesis [Patrik, M. (ed.)] [14] (Horizon Scientific Press, 2006). [Google Scholar]
- Shrier L. A. et al. Depressive symptoms and sexual risk behavior in young, chlamydia-infected, heterosexual dyads. J Adolesc Health 45, 63–69, 10.1016/j.jadohealth.2008.11.016 (2009). [DOI] [PubMed] [Google Scholar]
- Prats F., Porta Serra M., Yazbeck H., Herrera R. & Gasso J. M. The prevalence of serological markers for the human immunodeficiency virus and the hepatitis B virus in a psychiatric hospital. Gac Sanit 4, 179–183 (1990). [DOI] [PubMed] [Google Scholar]
- Franson T. R., Ksobiech L. J. & Simonsen H. W. Prevalence of hepatitis B carriers in a mental health in-patient facility: implications for employee screening and vaccination. Psychiatr Hosp 17, 81–83 (1986). [PubMed] [Google Scholar]
- Arling T. A. et al. Toxoplasma gondii antibody titers and history of suicide attempts in patients with recurrent mood disorders. J Nerv Ment Dis 197, 905–908, 10.1097/NMD.0b013e3181c29a23 (2009). [DOI] [PubMed] [Google Scholar]
- Groer M. W. et al. Prenatal depression and anxiety in Toxoplasma gondii-positive women. Am J Obstet Gynecol 204(433), e431–437, 10.1016/j.ajog.2011.01.004 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar N. & Misra B. Toxoplasma seropositivity and depression: a case report. BMC psychiatry 4, 1, 10.1186/1471-244x-4-1 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich D. E. et al. Amantadine in depressive patients with Borna disease virus (BDV) infection: an open trial. Bipolar Disord 2, 65–70 (2000). [DOI] [PubMed] [Google Scholar]
- Amsterdam J. D. et al. Borna disease virus. A possible etiologic factor in human affective disorders? Arch Gen Psychiatry 42, 1093–1096 (1985). [DOI] [PubMed] [Google Scholar]
- Igata-Yi R. et al. Borna disease virus and the consumption of raw horse meat. Nat Med 2, 948–949 (1996). [DOI] [PubMed] [Google Scholar]
- Salvatore M., Morzunov S., Schwemmle M. & Lipkin W. I. Borna disease virus in brains of North American and European people with schizophrenia and bipolar disorder. Bornavirus Study Group. Lancet 349, 1813–1814 (1997). [DOI] [PubMed] [Google Scholar]
- Fukuda K. et al. Immunological and PCR analyses for Borna disease virus in psychiatric patients and blood donors in Japan. J Clin Microbiol 39, 419–429, 10.1128/jcm.39.2.419-429.2001 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebain P. et al. Borna disease virus and psychiatric disorders. Schizophr Res 57, 303–305 (2002). [DOI] [PubMed] [Google Scholar]
- Zou D. Z. et al. Detection of Borna disease virus P24 RNA from peripheral blood mononuclear cells in psychiatric patients. Chinese journal of psychiatry 36, 228–230 (2003) (in Chinese). [Google Scholar]
- Wang Z. H., Xie P., Han Y. X. & Zhan J. Study on molecular epidemiology of Borna disease virus in Ningxia and vicinal regions. Chinese journal of epidemiology 27, 479–482 (2006) (in Chinese). [PubMed] [Google Scholar]
- Zhao L. B. et al. Molecular Biological Research on Borna Disease Virus Infection in Depressive Patients of Chongqing. Chinese journal of nervous and mental diseases 33, 18–22 (2007). [Google Scholar]
- Flower R. L. et al. Human Borna disease virus infection in Australia: serological markers of infection in multi-transfused patients. APMIS. Supplementum, 89–93 (2008). [DOI] [PubMed] [Google Scholar]
- Shah F. & Dar S. I. Prevalence of Hepatitis C in depressed population. Pak J Med Res 43, 200–202 (2004). [Google Scholar]
- Alvarado Esquivel C., Arreola Valenzuela M. A., Mercado Suarez M. F. & Espinoza Andrade F. Hepatitis B virus infection among inpatients of a psychiatric hospital of Mexico. Clin Pract Epidemiol Ment Health 1, 10, 10.1186/1745-0179-1-10 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado-Esquivel C. et al. Seroepidemiology of Toxoplasma gondii infection in psychiatric inpatients in a northern Mexican city. BMC Infect Dis 6, 178, 10.1186/1471-2334-6-178 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetinkaya Z., Yazar S., Gecici O. & Namli M. N. Anti-Toxoplasma gondii antibodies in patients with schizophrenia--preliminary findings in a Turkish sample. Schizophr Bull 33, 789–791, 10.1093/schbul/sbm021 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1