Abstract
Hand-Assisted Thoracoscopic Surgery for pulmonary metastasectomy through sternocostal triangle access allows manual palpation of both lungs, thus permitting effective treatment of lung metastases. In our research, 62 patients from November 2001 to January 2012 underwent our Hand-Assisted Thoracoscopic Surgery procedures for pulmonary metastasectomy. Clinical data, including the number of pulmonary metastases determined by Computed Tomography/Positron Emission Tomography-Computed Tomography, surgical findings and survival data of these patients were collected. We found that the median follow-up time was 23.7 months (range 2.4 to 85.6 months). 30 cases of them had post-operative recurrences and the median disease-free survival period was 27.4 months. For Computed Tomography scan, the overall sensitivity for proved metastases was 63% (115/182). 67 non-imaged malignant nodules were palpated and removed in 14 cases. For Positron Emission Tomography-Computed Tomography scan, the overall sensitivity was 66% (79/120). 41 non-imaged malignant nodules were palpated and removed in 12 cases. This study show that the Hand-Assisted Thoracoscopic Surgery provides an easier way for routine bilateral pleural exploration, and thus is critical and effective in detection of non-imaged malignant pulmonary metastases, which might contribute to long-term disease-free survival.
Complete resection of pulmonary metastases remains the standard treatment for selected patients with isolated pulmonary metastases from extra-thoracic primary malignancies, which is associated with a 5-year survival rate of 21% to 68%1.
When video-assisted thoracic surgery (VATS) was introduced as a minimally invasive surgery, it was quickly utilized in metastasectomy2. Some new approaches, such as the transxiphoid hand-assisted thoracoscopic surgery (HATS) developed by Mineo3 and Detterbeck4 as well as the trans-diaphragmatic HATS developed by Wright5, have been used in this field. We also developed a HATS procedure for bilateral lung metastasectomy through sternocostal triangle access, which has been reported previously6.
Some authors suggest that the sensitivity of a Computed Tomography (CT)scan is insufficient, and therefore complete manual exploration during thoracotomy should be considered for patients undergoing pulmonary metastasectomy7. In addition, without manual palpation, the high rate of missing occult metastases during VATS procedures is unacceptable8,9. This study assessed the reliability of our HATS approach in detecting occult metastases when comparing to preoperative helical CT and Positron Emission Tomography- Computed Tomography (PET-CT), and analyzed the survival of the patients who underwent this procedure.
Results
62 patients with pulmonary nodules and a history of solid organ malignancy underwent 65 HATS procedures for pulmonary metastasectomy by the same group of surgeons. Three patients underwent more than one pulmonary metastasectomy for recurrence. The characteristics of the procedures are shown in Table 1. The histological information of primary tumors was demonstrated in Table 2.
Table 1. The characteristics of the procedures.
| Characteristic | Procedures for Pulmonary Metastasectomy (n = 65) | |
|---|---|---|
| Sex (%) | ||
| Male | 42 | 65% |
| Female | 23 | 35% |
| CT/PET-CT unilateral/bilateral metastases (%) | ||
| Unilateral | 39 | 60% |
| Bilateral | 26 | 40% |
| Pathological unilateral/bilateral metastases (%) | ||
| Unilateral | 33 | 51% |
| Bilateral | 32 | 49% |
| Age (years) | ||
| Median | 49 | |
| Range | 12–88 | |
| Mean | 48 | |
| Follow-up(months) | ||
| Median | 19 | |
| Range | 1–86 | |
| Mean | 27 | |
| Length of stay (days) | ||
| Median | 6 | |
| Range | 2–24 | |
| Mean | 7 | |
| Resected lesion size (mm) | ||
| Mean | 12 | |
| Median | 8 | |
| Range | 1–80 | |
| No. of resected lesion (metastases/benign) | ||
| Metastases | 183 | 73% |
| Benign | 69 | 27% |
Table 2. Primary Tumor Types.
| Types of Primary Cancer | Number of Procedures | Percent (%) |
|---|---|---|
| Colorectal Carcinoma | 18 | 27.7 |
| HCC | 10 | 15.4 |
| Renal Carcinoma | 5 | 7.7 |
| NPC | 4 | 6.2 |
| Cervical Carcinoma | 4 | 6.2 |
| Lung Cancer | 3 | 4.6 |
| Osteosarcoma | 3 | 4.6 |
| Melanoma | 2 | 3.1 |
| Hysteromyoma | 2 | 3.1 |
| Thyroid Cancer | 1 | 1.5 |
| Breast Cancer | 1 | 1.5 |
| Carcinoma of Urinary Bladder | 1 | 1.5 |
| Esophageal Carcinoma | 1 | 1.5 |
| Malignant Uteri Chorion-Epithelioma | 1 | 1.5 |
| Synovial Sarcoma | 1 | 1.5 |
| Nephroblastoma | 1 | 1.5 |
| Duodenal Carcinoma | 1 | 1.5 |
| Gastric Cancer | 1 | 1.5 |
| Adrenal Pheochromocytoma | 1 | 1.5 |
| Testicular Mixed Germ Cell Tumor | 1 | 1.5 |
| Liposarcoma | 1 | 1.5 |
| Tongue Cancer | 1 | 1.5 |
| Nasal Malignant Mixed Tumor | 1 | 1.5 |
| Total | 65 | 100 |
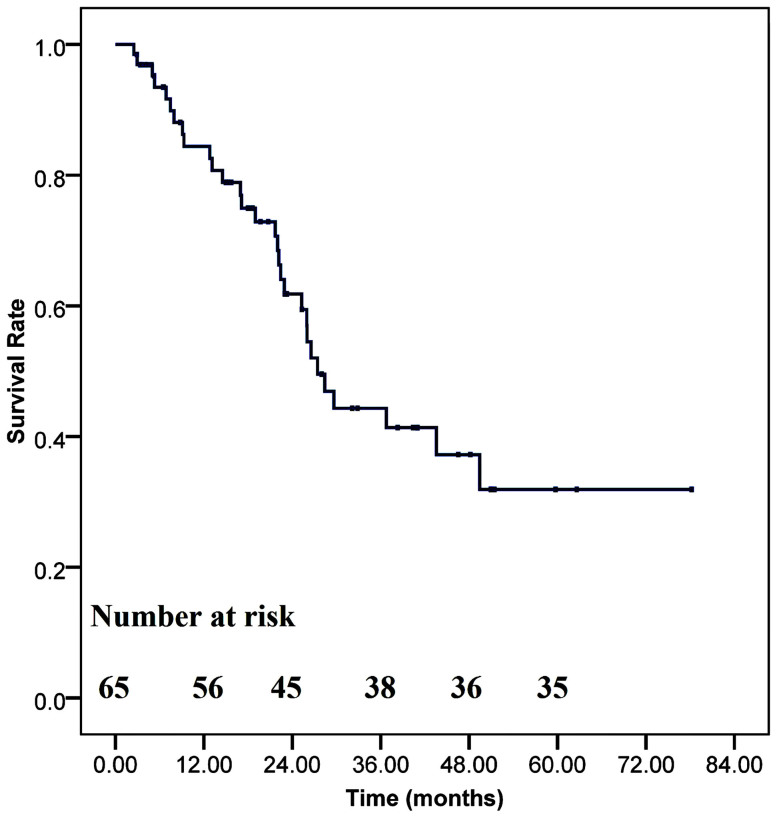
Median follow-up of HATS for bilateral lung metastasectomy through sternocostal triangle access patients was 23.7 months (range 2.4 to 85.6 months). No patient was lost of follow-up. Post-operative recurrences were found in 30 cases. The routine examinations in the follow-up included chest X-ray performed every three months with chest CT scan every six months for five years. Abdominal CT scan was done every six months to twelve months depending on the status of the patients and the classification of primary cancer. Five-year disease-free survival rate in our cohort was shown in Figure 1. One-year, Two-year, Three-year and five-year disease-free survival rates were 84.4%, 61.8%, 44.3% and 31.9%, respectively. Mean disease-free survival time was 40.5 months (log-rank, 95% confidence interval, 32.03 to 48.94). Median disease-free survival period was 27.4 months.
Figure 1. Kaplan-Meier plot of disease free survival for patients who underwent our HATS approach.
CT scans were performed before 62 HATS procedures. The median interval between metastasectomy and the examination was 13 days (range, 2–52 days). Based on the information of CT scans, 111 pulmonary nodules that were not identified in the images preoperatively were found in 26 cases, and 67 non-imaged malignant nodules were palpated and removed in 14 cases. 29 (29/42) cases who had predicted unilateral metastases at CT scan were confirmed to be unilateral.
PET-CT scans were performed before 43 HATS procedures. The median interval between metastasectomy and the examination was 9 days (range, 2–74 days). If the pulmonary nodules detected by PET-CT were defined as visualization, 76 pulmonary nodules that were not identified in the images preoperatively were found in 20 cases, and 41 non-imaged malignant nodules were palpated and removed in 12 cases. 25 (25/30) cases who had been predicted to have unilateral metastases by PET-CT scan were finally confirmed to be unilateral. While if we change the definition into the high metabolic nodules, 76 pulmonary nodules that were not imaged preoperatively were found in 19 cases, and 43 non-imaged malignant nodules were palpated and removed in 12 cases. 18 (18/25) of the cases who had been predicted to have unilateral metastases were confirmed to be unilateral. Sensitivity and positive predictive value for CT/PET-CT are shown in Tables 3.
Table 3. Nodules Revealed at Surgery and on Computed Tomography/Positron Emission Tomography- Computed Tomography.
| Procedures enrolled to the analysis(N = 65) | ||||||
|---|---|---|---|---|---|---|
| Surgery | TRUE Positive | FALSE Negative | Sensitivity | FALSE Positive | PPV | |
| Nodules | 252 | 139 | 113 | 55% | 21 | 87% |
| Metastases | 183 | 126 | 57 | 69% | 33 | 79% |
PPV: Positive Predictive Value.
Discussion
Accurate thoracic imaging brings reliable prediction of the metastatic disease. It is very important to select those patients who would benefit from resection, and palliate those patients who would not10. Technological progress has resulted in the widespread availability of helical CT scanners11, which has demonstrated superior detection rates compared to conventional CT12. Overlapping image reconstruction may enhance the number of visible nodules and reduce the number of false-positive nodules attributable to cross sections of pulmonary vessels11. Therefore, some researchers suggested that helical CT should be the better choice than the palpation of lungs11. Unfortunately, recent studies have compared helical CT prediction to surgical exploration and reported a high risk of missing lesions in CT scan13.
VATS has been widely used in thoracic surgery in the last decade. Although it is a minimally invasive surgery, VATS makes the palpation of the lungs difficult and incomprehensive, and therefore might be not suitable for pulmonary metastasectomy. The previous studies of VATS showed unsatisfactory results in detection of pulmonary metastatic lesions when compared to our HATS procedure (Table 4)8,9,14. Indeed, VATS is suitable for diagnosis of most undefined peripheral pulmonary nodules including pulmonary metastasis15. However, the role of therapeutic VATS metastasectomy remains largely unknown12.
Table 4. Sensitivity of Helical CT in Detecting Pulmonary Metastases.
| Approaches | Study | N | No. of metastases | Sensitivity (%) |
|---|---|---|---|---|
| Thoracotomy | Kayton et al 2006[16] | 54 | 209 | 87.6 |
| Thoracotomy | Nakajima et al 2007[14] | 43 | 130 | 85.4 |
| Thoracotomy | Kang et al 2008[17] | 27 | 198 | 77 |
| Thoracotomy | Pfannschmidt et al 2008[18] | 125 | 331 | 83.7 |
| Thoracotomy | Ellis et al 2011[9] | — | 73 | 88.3 |
| Thoracotomy | Chung et al 2011[19] | 120 | 368 | 26.7% (32/120) have occult metastases |
| VATS | McCormack et al 1996[8] | 18 | 19 | * |
| VATS | Nakajima et al 2007[14] | 79 | 120 | 90 |
| VATS | Ellis et al 2011[9] | — | 35 | * |
| Transxiphoid HATS | Mineo et al 1999[3] | 13 | 21 | 76.2 |
| Transxiphoid HATS | Ambrogi et al 2000[10] | 22 | 49 | 84 |
| Transxiphoid HATS | Mineo et al 2001[24] | 29 | 60 | 81.7 |
| Substernal HATS | Detterbeck et al 2004[4] | 16 | — | 18.8% (3/16) have occult metastases |
| Transxiphoid HATS | Mineo et al 2007[25] | 71 | 178 | 87.1 |
| Mix 1 | Parsons et al 2004[11] | 41 | 88 | 78 |
| Mix 2 | Parsons et al 2007[7] | 60 | 135 | 60 |
| Our HATS | Current study | 62 | 182 | 6322.6% (14/62) have occult metastases |
N: Number of cases.
—: No data obtained.
*: The number of pulmonary nodules palpated and removed is less than the number of pulmonary nodules detected on CT scan.
Mix1: The approaches consists of sternotomy (n = 19), unilateral thoracotomy (n = 11), VATS with a substernal handport (n = 11).
Mix2: The approaches consists of sternotomy (n = 30), unilateral thoracotomy (n = 19), VATS with a substernal handport (n = 11).
The standard approach of metastasectomy is to palpate the lungs at the time of resection in order to completely remove all metastatic deposits1. Some researchers recommended sternotomy or thoracotomy1. Studies of these conventional procedures have found that the sensitivity of helical CT in detection of pulmonary metastatic lesions varies from 77% to 88.3% (Table 4)9,14,16,17,18,19. The corresponding figure in our study was 63%. These studies also demonstrated that the sensitivity of PET-CT varies from 64.3% to 67.5% (Table 5)13,20,21, while our figure was 66%. The above results demonstrated that our HATS procedure, which may be more minimally invasive compared with sternotomy or thoracotomy6, had similar ability in detection of occult pulmonary metastases.
Table 5. Sensitivity of PET-CT in Detecting Pulmonary Metastases.
| Approaches | Study | N | No. of metastases | Sensitivity(%) |
|---|---|---|---|---|
| Thoracotomy | Fortes et al 2008[20] | 104 | 154 | 67.5 |
| Thoracotomy | Cerfolio et al 2009[13] | 57 | 67 | 64.3 |
| Thoracotomy | Cerfolio et al 2011[21] | 152 | — | 21.1% (32/152) have occult metastases |
| Our HATS | Current study | 43 | 116 | 66%27.9% (12/43) have occult metastases |
N: Number of cases.
—: No data obtained.
Although the mortality of conventional thoracotomy is low1, increasing effort has been devoted to new approaches that may reduce the morbidity. Under this circumstance, the HATS procedure, which allows bilateral pleural exploration with a hand, would be an appropriate technique for patients undergoing metastasectomy with curative intent3,4,5,6. Mineo, Wright and Detterbeck have reported their HATS approaches that allow bilateral manual palpation of the lung in 1999, 2003 and 2004, respectively3,4,5. The above three HATS approaches demonstrated analogous results in detection of occult pulmonary metastatic lesions (Table 4). As an improvement for HATS, we have some variation in this surgical procedure to overcome some limitations of previous ones. For example, the change of resection from xiphoid appendix to sternocostal triangle access reduced the morbidity of intraoperative rhythm disturbance. Furthermore, keeping the integrity of diaphragm may reduce the risk of post-operative diaphragmatic hernia6.
The analysis of 5,206 patients with pulmonary metastasis confirmed that complete removal of all metastatic deposits was a potentially curative treatment, which is associated with long-term survival1. The survival data of VATS, obtained from patients with fewer metastases, did not illustrate a frustrating result compared with conventional thoracotomy or HATS procedure (Table 6)2,12,15,22. The DFS of metastatic tumors treated with conventional thoracotomy or other HATS procedures, both of which allowed palpation of the lungs, were reported from less than 10 months to 29 months in the literatures (Table 6), while the figure in our cohort was similar (27.4 months). There is only one study that reported a better DFS than ours. In this study only the patients with lung metastasis of colorectal cancer were enrolled. In addition, the mean number of pulmonary lesions was 1.73, which was much less than ours (3.88). Therefore, the primary tumors with better prognosis and fewer lung metastases would contribute to the better survival23.
Table 6. Disease-free survival of metastasis tumor.
| Approaches | Study | N | DFS (months) | Survival Rate | |||
|---|---|---|---|---|---|---|---|
| 1-year | 2-year | 3-year | 5-year | ||||
| Thoracotomy | Mutsaerts et al 2002[22] | 19 | <10 | — | 42 | — | — |
| Thoracotomy | Nakajima et al 2008[2] | 71 | 27 | 57.6 | — | 26.2 | 21.1 |
| Thoracotomy | Carballo et al 2009[15] | 135 | 24.8 | 87.4 | — | 67.9 | 58.8 |
| Thoracotomy | Nakas et al 2009[12] | 27 | 29 | — | — | — | — |
| VATS | Mutsaerts et al 2002[22] | 16 | <10 | — | 50 | — | — |
| VATS | Nakajima et al 2008[2] | 72 | 23.8 | 64.6 | — | 42.6 | 34.4 |
| VATS | Carballo et al 2009[15] | 36 | 25.6 | 91.7 | — | 69.6 | 69.6 |
| VATS | Nakas et al 2009[12] | 25 | 20 | — | — | — | — |
| Transxiphoid HATS | Mineo et al 2001[24] | 29 | 19 | — | — | — | — |
| Transxiphoid HATS | Mineo et al 2007[25] | 71 | 12 | — | — | — | — |
| Our HATS | Current study | 65 | 27.4 | 84.4 | 61.8 | 44.3 | 31.9 |
N: Number of cases.
—: No data obtained.
There are several limitations in this study. First, all the data were retrospectively collected. Second, although the radiographic images were reviewed by the same thoracic surgeon and consultant radiologist, the operative findings were recorded according to the operation notes and pathologic reports. Third, the involvement of different surgeons throughout the time of the study may bring in some bias, even though they were all trained in the same group. For example, results of finger palpation or the findings of additional metastatic lesions may not be consistent in all cases. A further well-controlled prospective study is needed to verify the value of our HATS approach in detection of occult pulmonary metastases and the survival data of patients with pulmonary metastases.
In conclusion, our HATS approach provides an easier way for routine bilateral pleural exploration similar to conventional sternotomy or thoracotomy regardless of unilateral or bilateral nodules on preoperative radiographic imaging6. Using this HATS approach, we detected and removed occult pulmonary metastases, which might contribute to long-term DFS.
Methods
This study was approved by the institutional review board of Sun Yat-sen University Cancer Center (SYSUCC) and informed consent was obtained from each participant. Chart review was performed on 62 consecutive patients who suffered from pulmonary metastases and underwent 65 HATS procedures through sternocostal triangle access between November 2001 and January 2012. Patients clinical and pathological data were obtained. Patients undergoing our HATS procedures were selected according to two mandatory prerequisites: (a) complete control of the primary tumor; (b) absent or resectable extrapulmonary metastases. Exclusion criteria were a history of pleuritis or pleurodesis, or the presence of cardiomegaly or arrhythmia.
CT examination were performed in 62 cases before metastasectomy, while PET-CT examination in 43 cases. The Philips twin flash CT was used from 1995 to 2005 with 10-mm slice collimation and 10-mm reconstruction. The Philips Brilliance 16 CT (Philips Healthcare, Andover, MA) with 5-mm slice collimation and 5-mm reconstruction was introduced in 2005. A Toshiba Aquilion 64 CT scanner (Toshiba American Medical Systems Inc, Tustin, CA) with 2-mm slice collimation and 5-mm reconstruction was introduced in 2009 and a Discovery ST16 PET-CT made by General Electric (GE Healthcare, Piscataway, NJ) was introduced in 2005.
No patients turned out to have non-small cell lung cancer among the ones whose primary tumors were not. All the radiographic images were reviewed by the same thoracic surgeon and consultant radiologist, and the number, type of the pulmonary nodules that were palpated and removed or detected by CT/PET-CT were recorded. The radiologist would record each nodule which was suspicious regardless of its size.
Sensitivity was determined by the number of true-positive metastases identified by the radiologist divided by the number of total metastases identified by pathology (true positives + false negatives). Positive predictive value was determined by the number of true-positive metastases identified by the radiologist divided by the total number of nodules called by the radiologist (true positives + false positives). Because it was not possible to quantify true negative findings for pulmonary nodules in patients with multiple lesions, the specificity and negative predictive value could not be calculated.
Statistics analyses were performed on all operations regardless of the fact that how many operations each patient had. Disease-free survival (DFS) curves were estimated using the Kaplan-Meier method. Time to event was calculated from the date of resection of the pulmonary metastases to the time of diagnosis of new metastases, was reported in this article. Data analysis was performed using SPSS 18.0 (PASW Statistics 18) for Windows (SPSS Inc, Chicago, IL).
Acknowledgments
This work was supported by a grant from Sun Yat-sen University Clinical Research 5010 plan ChiCTR-TRC-00000142. We wish to thank Prof. Yan from Department of English, School of Foreign Languages (SYSU) for language editing.
Footnotes
The authors declare no competing financial interests.
Author Contributions H.L. and L.J. designed and performed most experiments. L.J. and Y.B.L. wrote the main manuscript text. D.R.S.T. and Y.Z. collected the clinical data. L.J., Y.G.Z. and G.W.M. performed data analysis. H.L. designed and directed the overall project.
References
- Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. The International Registry of Lung Metastases. J Thorac Cardiovasc Surg 113, 37 (1997). [DOI] [PubMed] [Google Scholar]
- Nakajima J., Murakawa T., Fukami T. & Takamoto S. Is thoracoscopic surgery justified to treat pulmonary metastasis from colorectal cancer? Interact Cardiovasc Thorac Surg 7, 212–216 (2008). [DOI] [PubMed] [Google Scholar]
- Mineo T. C., Pompeo E., Ambrogi V. & Pistolese C. Video-assisted approach for transxiphoid bilateral lung metastasectomy. Ann Thorac Surg 67, 1808 (1999). [DOI] [PubMed] [Google Scholar]
- Detterbeck F. C. & Egan T. M. Thoracoscopy using a substernal handport for palpation. Ann Thorac Surg 78, 1031 (2004). [DOI] [PubMed] [Google Scholar]
- Wright G. M., Clarke C. P. & Paiva J. M. Hand-assisted thoracoscopic surgery. Ann Thorac Surg 75, 1665 (2003). [DOI] [PubMed] [Google Scholar]
- Long H. et al. Hand-assisted thoracoscopic surgery for bilateral lung metastasectomy through sternocostal triangle access. Ann Thorac Surg 91, 852 (2011). [DOI] [PubMed] [Google Scholar]
- Parsons A. M. et al. Helical computed tomography inaccuracy in the detection of pulmonary metastases: can it be improved? Ann Thorac Surg 84, 1830 (2007). [DOI] [PubMed] [Google Scholar]
- McCormack P. M. et al. Role of video-assisted thoracic surgery in the treatment of pulmonary metastases: results of a prospective trial. Ann Thorac Surg 62, 213–216 (1996). [DOI] [PubMed] [Google Scholar]
- Ellis M. C. et al. Comparison of pulmonary nodule detection rates between preoperative CT imaging and intraoperative lung palpation. Am J Surg 201, 619 (2011). [DOI] [PubMed] [Google Scholar]
- Ambrogi V., Paci M., Pompeo E. & Mineo T. C. Transxiphoid video-assisted pulmonary metastasectomy: relevance of helical computed tomography occult lesions. Ann Thorac Surg 70, 1847 (2000). [DOI] [PubMed] [Google Scholar]
- Parsons A. M., Detterbeck F. C. & Parker L. A. Accuracy of helical CT in the detection of pulmonary metastases: is intraoperative palpation still necessary? Ann Thorac Surg 78, 1910–1916 (2004). [DOI] [PubMed] [Google Scholar]
- Nakas A. et al. Video-assisted versus open pulmonary metastasectomy: the surgeon's finger or the radiologist's eye? Eur J Cardiothorac Surg 36, 469 (2009). [DOI] [PubMed] [Google Scholar]
- Cerfolio R. J., McCarty T. & Bryant A. S. Non-imaged pulmonary nodules discovered during thoracotomy for metastasectomy by lung palpation. Eur J Cardiothorac Surg 35, 786–791 (2009). [DOI] [PubMed] [Google Scholar]
- Nakajima J. et al. Is finger palpation at operation indispensable for pulmonary metastasectomy in colorectal cancer? Ann Thorac Surg 84, 1680 (2007). [DOI] [PubMed] [Google Scholar]
- Carballo M., Maish M. S., Jaroszewski D. E. & Holmes C. E. Video-assisted thoracic surgery (VATS) as a safe alternative for the resection of pulmonary metastases: a retrospective cohort study. J Cardiothorac Surg 4, 13 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayton M. L. et al. Computed tomographic scan of the chest underestimates the number of metastatic lesions in osteosarcoma. J Pediatr Surg 41, 200–201 (2006). [DOI] [PubMed] [Google Scholar]
- Kang M. C. et al. Accuracy of 16-channel multi-detector row chest computed tomography with thin sections in the detection of metastatic pulmonary nodules. Eur J Cardiothorac Surg 33, 473 (2008). [DOI] [PubMed] [Google Scholar]
- Pfannschmidt J. et al. Diagnosis of pulmonary metastases with helical CT: the effect of imaging techniques. Thorac Cardiovasc Surg 56, 471 (2008). [DOI] [PubMed] [Google Scholar]
- Chung C. C. et al. Accuracy of helical computed tomography in the detection of pulmonary colorectal metastases. J Thorac Cardiovasc Surg 141, 1207 (2011). [DOI] [PubMed] [Google Scholar]
- Fortes D. L. et al. The sensitivity of 18F-fluorodeoxyglucose positron emission tomography in the evaluation of metastatic pulmonary nodules. Eur J Cardiothorac Surg 34, 1223 (2008). [DOI] [PubMed] [Google Scholar]
- Cerfolio R. J., Bryant A. S., McCarty T. P. & Minnich D. J. A prospective study to determine the incidence of non-imaged malignant pulmonary nodules in patients who undergo metastasectomy by thoracotomy with lung palpation. Ann Thorac Surg 91, 1696–1700 (2011). [DOI] [PubMed] [Google Scholar]
- Mutsaerts E. L. et al. Long term survival of thoracoscopic metastasectomy vs metastasectomy by thoracotomy in patients with a solitary pulmonary lesion. Eur J Surg Oncol 28, 864 (2002). [DOI] [PubMed] [Google Scholar]
- Siegel R., Naishadham D. & Jemal A. Cancer statistics, 2012. CA Cancer J Clin 62, 10 (2012). [DOI] [PubMed] [Google Scholar]
- Mineo T. C. et al. Transxiphoid bilateral palpation in video-assisted thoracoscopic lung metastasectomy. Arch Surg 136, 783 (2001). [DOI] [PubMed] [Google Scholar]
- Mineo T. C., Ambrogi V., Mineo D. & Pompeo E. Transxiphoid hand-assisted videothoracoscopic surgery. Ann Thorac Surg 83, 1978 (2007). [DOI] [PubMed] [Google Scholar]