Summary
Improvements in acute burn care have enabled patients to survive massive burns which would have once been fatal. Now up to 70% of patients develop hypertrophic scars following burns. The functional and psychosocial sequelae remain a major rehabilitative challenge, decreasing quality of life and delaying reintegration into society.
The current approach is to optimise the healing potential of the burn wound using targeted wound care and surgery in order to minimise the development of hypertrophic scarring. This approach often fails, and modulation of established scar is continued although the optimal indication, timing, and combination of therapies have yet to be established. The need for novel treatments is paramount, and future efforts to improve outcomes and quality of life should include optimisation of wound healing to attenuate or prevent hypertrophic scarring, well-designed trials to confirm treatment efficacy, and further elucidation of molecular mechanisms to allow development of new preventative and therapeutic strategies.
Introduction
Cutaneous scarring remains the pathognomonic feature following burns to the skin and characteristically underlies post-burn physical and psychosocial morbidity. The most common cicatrix formed following a burn is the hypertrophic scar, the prevalence of which has been reported as being as high as 70%.1 Over the past several decades, improvement in acute burn care has reduced mortality, enabling survival of burn injuries covering up to 100% of total body surface area (TBSA). Patients with these massive burns have extensive scarring and contractures, itch, and pain. They are dissatisfied with their appearance and experience restricted movement, itch, and loss of function for many years. The greatest unmet challenges in burn rehabilitation relate to decreased quality of life and delayed reintegration into society resulting from post-burn scar. In this, the third article in a series on burn injury in which metabolism and inhalation injury were examined, we discuss current strategies for burn wound and scar management, and identify areas where more research is needed to reduce post-burn scarring and improve burn survivors’ rehabilitation and reintegration into society.
Post-burn Scarring
Following cutaneous injury, the defect is healed through creation of a scar, with linear collagen deposition lacking the flexibility of uninjured skin. Although the desired result for any healing wound is scarless healing, the best result is usually a flat, pliable scar with slight discoloration. Deposition of excess collagen results in a pathologic scar that is thick, non-pliable, itchy, and painful.2 One of two types of pathologic scars arises from the burn wound – a hypertrophic scar or a keloid. The mechanisms underlying the development of either scar differ, and each scar type is managed differently.3–6 Although the differentiation between hypertrophic scar and keloid is not always clear, hypertrophic scar occurs within the confines of the original wound, matures within ~2 years, and does not return following excision (Figure 1a). Keloids grow beyond the edge of the initial wound with persistence of the proliferative phase for an extended time. A small number of burn patients, typically those with darkly pigmented skin, develop keloids (Figure 1b).3,7 Here we focus on the most common type of scar in the severely burned patient - the hypertrophic scar - and discuss aspects of wound healing and scar management that can modulate scarring.
Figure 1.
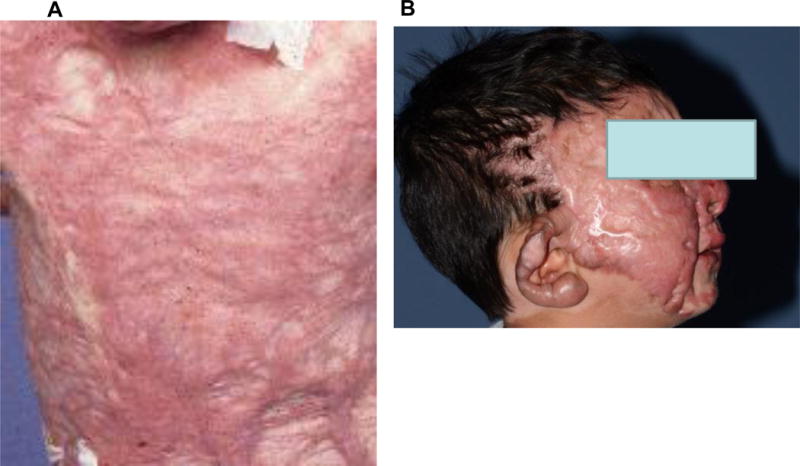
Hypertrophic scar (a) and Keloid (b) that developed following severe burn injuries.
The Pathophysiology of Wound Healing and Scarring
In post-natal tissue, wound healing occurs in three discrete phases that ultimately result in the formation of a scar: inflammation, proliferation, and remodeling.8 Modulation of the three phases can allow the wound to heal without scar or result in excessive fibrosis. Although a flat, less fibrotic scar is desired, when the acute inflammatory phase persists or wound healing is delayed, pathologic scars form (see inset panel). During the inflammatory phase, a fibrin clot forms, thereby creating a scaffold for the repair process.8 Release of cytokines and chemokines, including platelet-derived growth factor, transforming growth factor- β, epidermal growth factor, and insulin-like growth factor, recruits mast cells, fibroblasts, macrophages, and other cells to restore the skin barrier.3,8 Several days after the injury, the inflammatory healing response transitions into the proliferation phase, which persists for up to 6 weeks.9 Deep injuries, such as those created by burns and subsequent surgeries, activate the deep dermal fibroblasts - which are larger fibroblasts that proliferate slower, produce large quantities of collagen and inflammatory cytokines (including TGF-β), and synthesize less collagenase, thereby decreasing collagen degradation.2 These activated fibroblasts synthesize the extracellular matrix (ECM), comprised of hyaluronic acid, proteoglycans, elastin, and procollagen, to serve as a scaffold for cell movement and vascularization. From the bone marrow, fibrocytes migrate to the wound, differentiate into fibroblasts, and increase local TGF-β production, stimulating fibroblast differentiation into myofibroblasts. Subsequently the myofibroblasts contract to decrease the wound size9. The wound then enters the maturation phase that lasts for up to 24 months.
In optimal wound healing scenarios, the ECM is degraded and the early wound’s immature type III collagen is modified to form mature type I collagen, which strengthens the healing wound.9 A hallmark of the hypertrophic scar is perturbation of collagen production and/or degradation; this dysregulation results in disorganized bundles of collagen cross-linked tightly, while type I collagen expression is reduced and type III collagen is over-synthesized.10 Synthesis of fibronectin and hyaluronic acid is up-regulated while decorin production is down-regulated. Elastin, an elastic ECM protein that enables skin to return to its normal shape following stretching, is virtually absent for ~5 years following burns. These changes in the ECM affect the pliability and height of the scar.
T-helper cells (CD4+) influence the establishment of anti-fibrotic or pro-fibrotic wound phenotypes.2 An increase in collagenase activity occurs with the anti-fibrotic Type 1 helper T cell phenotype, where the CD4+ cells produce IL-2, IFN-gamma, and IL-12. An increase in the pro-fibrotic Types 2 and 3 helper cell phenotypes occurs with expression of IL-4/IL-5/IL-10 or TGF-β, respectively. Decreased collagenase activity also accompanies the Type 2 helper T cell phenotype.
Cutaneous wounds occurring in areas experiencing higher tension and greater stretching are more likely to form hypertrophic scars, due in part to tension inducing myofibroblast differentiation.4,11 Management of the wound and careful incision placement along the skin’s natural tension lines can reduce subsequent hypertrophic scarring, enabling the clinician to influence the healing environment through selection of wound covers, and surgical and non-surgical interventions.4,12
Acute Wound Care
Clinical evidence suggests that time to healing is related to burn wound depth. The majority of burn wounds – flash injuries and small scalds – are superficial second degree burns (or superficial partial-thickness burns) that usually result in an unobtrusive, non-hypertrophic scar.13,14 Delayed healing of these wounds may result from infection or a known permutation of wound healing such as diabetes or systemic corticosteroid use.15 Care for these wounds consists of washing with water and hand or chlorhexidine soap, and then covering with a product that can remain on for 5–7 days (e.g. a biosynthetic silicone wound dressing or foam dressings with or without silver). There are a wide array of topical agents and dressings available for use (Table 1: Commonly Used Products) that have been reviewed extensively in the burn literature.16–19 Product availability may be limited depending on location, cost, or facility purchasing policy.
Table 1.
Common used partial-thickness or full-thickness burn wound dressings
| Dressing Agent | Active Substance | Presentation | Main Use | Advantages | Disadvantages |
|---|---|---|---|---|---|
| Bacitracin | Bacitracin | Ointment | Superficial burns, skin grafts | Gram (+) coverage | No G(−) or fungal coverage |
| Polymyxin | Polymyxin B | Ointment | Superficial burns, skin grafts | Gram (−) coverage | No G(+) or fungal coverage |
| Mycostatin | Nystatin | Ointment | Superficial burns, skin grafts | Good fungal coverage | No bacterial coverage |
| Silvadene | Silver sulfadiazine | Ointment | Deep burns | Good bacterial and fungal coverage, painless | Poor eschar penetration, sulfa moiety, leucopenia, pseudoeschar formation |
| Sulfamylon | Mafenide acetate | Ointment and liquid solution | Deep burns | Good bacterial coverage, good eschar penetration | Painful, poor fungal coverage, metabolic acidosis |
| Dakin’s | Sodium hypochlorite | Liquid solution | Superficial and deep burns | Good bacterial coverage, inexpensive and readily available | Very short half life |
| Silver | Silver nitrate, silver ion | Liquid solution, dressing sheets | Superficial burns | Good bacterial coverage, painless | Hyponatremia, dark staining of wounds and linens |
| Acetic Acid 2% | Acetic acid | Liquid solution, dressing | Superficial and deep burns | Good gram negative coverage | Painful, vinegary smell |
Deep dermal second degree and third degree wounds (deep partial- and full-thickness, respectively) can take longer to heal, and are therefore at greater risk for pathologic scarring.20 Recent technological advances have improved the objective determination of wound depth and healing potential with non-invasive monitoring techniques in comparison to subjective visual assessment.21 Diagnostic accuracy has improved with the use of Laser Doppler Imaging, Infrared Thermography, and Spectrophotometric Intracutaneous Analysis.22 The most frequently used technique for the prediction of the time to healing, to guide wound management, and to determine the need for surgical intervention is laser Doppler imaging.21 Despite the incorporation of improved techniques for determining burn wound depth into clinical practice, it is not always possible to distinguish between deep dermal tissue that should be excised versus that which will heal faster.
Deitch and colleagues found that burn wounds healing within 3 weeks had a low risk of hypertrophic scarring, while wounds taking longer than 3 weeks had a high risk for developing a pathologic, hypertrophic scar.13 A more recent study of more than 500 pediatric scald burn patients confirmed the time to healing as a strong predictor of hypertrophic scarring, with 21 to 25 days identified as the crucial time frame.14 The implication that strategies to reduce wound healing time could reduce scar formation resulted in surgeon-led efforts to modulate the wound environment. In the early 1970’s, early excision for deep partial and full-thickness burn wounds was introduced.23,24 Prior to this evolution in care, treatment was delayed until the eschar fell off and coverage was achieved with skin grafts. Early tangential excision and coverage with split-thickness skin autograft is the standard of care for burn patients with deep burn injuries that are not expected to heal within 3 weeks.23 The most effective prophylaxis and treatment of burn wound infection remains early excision, which directly impacts survival.25 Similarly, early excision of the wound is recommended as one of the most effective strategies to reduce severe scarring.14,22 However, donor site pain remains problematic, and may be associated with poor healing of the burn wounds due to systemic pain signaling.
The appearance, elasticity, and structure of the scar can be modulated through the choice of wound covering; dermal substitutes, consisting mainly of collagen and elastin, provide a scaffold to replace dermis in the burn wound that is later degraded and replaced by infiltrating cells.26,27 Significant resources have been invested in creating functional dermal and epidermal substitutes for the coverage of full-thickness burns,28,29 and a plethora of biologic and synthetic temporary wound coverings30–36 and permanent skin replacements37–41 are available (Table 2).
Table 2.
Products and solutions mentioned in this article
| Product | Description | Presentation | Main Use | Advantages | Disadvantages |
|---|---|---|---|---|---|
| Alloderm | Human cadaveric acellular matrix | Membrane | Full thickness deep burn in cosmetically important areas | Dermal substitute | Expensive |
| Amnion | Biological dressing | Membrane | Partial thickness facial burn due to ease of manipulation | Modable, Plaible, Monitoring of wound, contains growth factors, accelerated wound healing, reduced pain, decreased infections, | Allogenic, expensive |
| Biobrane | Biosynthetic collagen matrix | Membrane (large sheets, hands) | Superficial burns | No dressing change if adherent for 2 weeks; reduced pain; monitoring of wound; maintains moist wound environment | If not adherent risk of infection. |
| Cultured Epithelial Autograft (CEA) | Autologous keratinocytes and epithelial cells from single biospy | Thin sheet of skin | Large Burns | Large coverage with small donor, espicailly for burns ≥90% of TBSA; decreased mortality | Fragile, expensive, long-time to grow in-vitro, poor long term outcomes/scarring |
| Cultured Skin Substitute (CSS) | Autologous keratinocytes and fibroblasts from patient biopsy, combined into a bilayer with bovine collagen matrix | Sheet | Large burns ≥ 50% of the TBSA; full thickness burns | Combined epidermal and dermal substitute; reduced risk of epidermolysis and blistering; can include melanocytes or angiogenic cytokines | Currently not available, takes long time to grow |
| Integra | Bovin Collagen matrix with silicone layer on the surface | Membrane with silicone layer | Deep fullthickness burn, burn in cosmetically important areas, large burns | Good cosmetic outcome, dermal substitute | Infection risk, expensive |
| Suprathel | synthetic copolymer >70% DL-lactide polymerized with ε-caprolactone and methylenecarbonate | Membrane | Partial thickness burns and split thickness donor sites | Rapid re-epithelilization of wound, reduction in pain | Expensive Not available in the USA or Canada |
Newly developed autologous epidermal substitutes are available for increasing re-epithelialization of the deep second degree burn wound and as a last resort for catastrophic burns where donor sites may be lacking. Available technologies enable isolation of single cell subtypes or a mixture of fibroblasts, melanocytes, Langerhans cells, and keratinocytes from autologous split-thickness biopsies, followed by application of the freshly-isolated cell suspension onto the wound area. Improved wound healing, reduced compression garment use, and decreased dyschromia (hyper- or hypo-pigmentation) have been reported following application of the mixed cell population.42,43 Similarly, the application of non-cultured keratinocytes improved wound healing and reduced hypertrophic scarring.44 When sprayed onto meshed autograft, keratinocytes reduced wound healing time and wound contraction.45 At the present time, these treatments are largely experimental, and the long-term results have not been adequately studied.
Newer approaches have focused on developing biological trilaminate wound coverings with the potential to decrease scarring, and stem cells may enable development of these trilayer structures for wound closure. The discovery of adult mesenchymal stem cells in most tissues has fueled research into the regeneration of the dermis and acceleration of re-epithelialization with application of stem cells to the wound.46,47 Adipose-derived stem cells are of particular interest as they can be easily isolated from severely burned patients when the fat layer underlying the burn wound is excised.48,49 These cells can be used to create a multi-layer skin substitute.50
Full-thickness burns are either excised 24–48 hours post-injury and covered with autograft, or debrided with newly available non-surgical removal agents such as a bromelain-enriched enzymatic mixture which dissolves burn wound eschar. Debridement with a bromelain-containing agent reduced both the need for surgery and regrafting in patients with deep partial or full-thickness burns without affecting scarring or quality of life.51 If the burn wounds cover ≥30% of TBSA, then cadaver or pig skin can be used to temporarily cover the autograft and excised wounds.52 Recent work suggests that a biosynthetic silicone wound dressing can be used to temporarily cover the wound; standardized protocols for this application have yet to be established.53 Small burns, including deep partial and full-thickness burns, and those occurring on the face, hands, neck, and fingers where cosmesis is important should be covered with sheet split thickness skin graft. Larger burns can be covered with meshed split thickness grafts expanded to a ratio of 1:1, 2:1, 3:1, or 4:1. In patients with very limited donor sites, the Meek technique can be used to expand the skin to a maximum ratio of 9:1 for use in areas other than the face and hands. These grafts are then covered with cadaver allograft (“sandwich technique”), an overlay that increases the rate of wound healing and reduces wound infection rates.54 Although large areas can be covered by expanding the graft through meshing, complications introduced by the use of meshed grafts include creation of an uneven scar surface with heterogeneous pigmentation in the affected area (Figure 2). With larger expansion of split thickness skin graft, the time to complete re-epithelialization of the excised burn wound usually increases, along with the risk of infection and hypertrophic scarring. The utility of adding aersolized stem cells isolated from fat or skin harvested from the patient during operations when widely expanded grafting is utilized and applying them to the meshed area is of great potential.42,43
Figure 2.
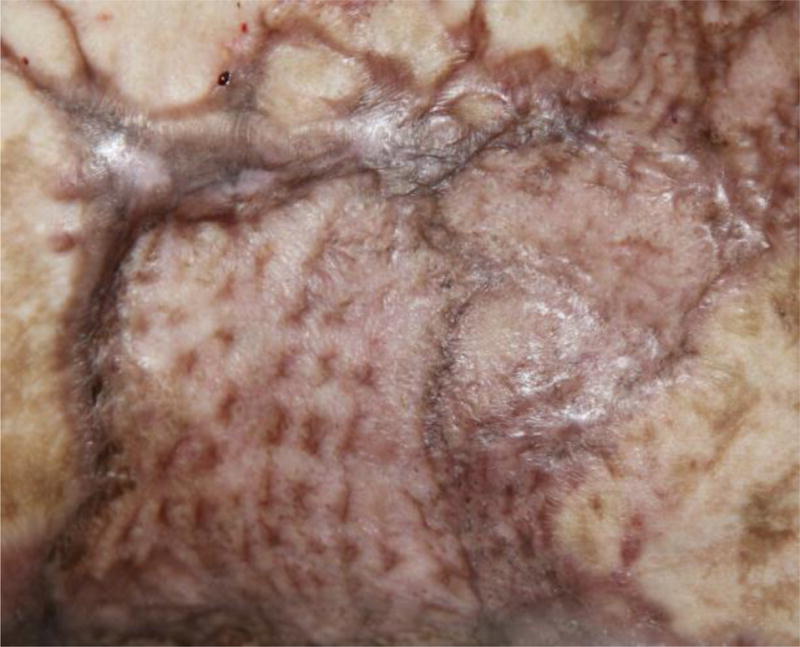
Typical scar pattern resulting from using a meshed graft
Modulation of the Burn scar
To determine whether interventions are successful and effective, objective assessment techniques and experimental models are utilized. Even in these areas, we currently lack depth.
Assessing the Impact of Scar
Attempts at scar manipulation or modulation should be quantified by improvements in scar quality or quality of life related to post-burn scarring. Subjective and objective quantification of scars are therefore essential in both clinical and research practice. These evaluations depend on photographs, clinical measurements, or patient input to assess the quality of the scar, the efficacy of scar-reducing therapies, or the impact of the scar on life quality. A variety of devices such as colorimeters or spectrophotometers, laser Doppler imaging, pneumatonometers, cutometers, or ultrasound, are employed to assess parameters such as colour, perfusion, pliability, elasticity, or thickness, respectively.55 Blood flow and angiogenesis can be measured by detecting the fluctuation in laser light reflected that occurs with blood cell movement through the vasculature using a laser speckle imaging system.56 These devices are non-invasive, accurate, and easy-to-use, and allow objective assessment of the wound or scar in a reliable and reproducible manner. There is a lack of consensus as to the most appropriate tools to use, and studies have shown that combinations of technologies may improve and refine scar assessments.57,58
Several scar scales have been developed that are mainly subjective evaluations of colour, vascularity, extent, thickness, pliability, texture, pigmentation, pain, and itching.55 Such scales are susceptible to inter-assessor and inter-patient variations55. Photographic evaluation of scar severity can be achieved through one of several methods utilizing standard or 3-dimensional photography59. The Seattle Scar Scale is one such method that employs photographs to compare the scar height, surface, color, and thickness to the bordering normal skin,60 and has been validated in the paediatric burn population.61 The Vancouver Scar Scale (VSS)62 and the Patient and Observer Scar Assessment Scale (POSAS)63 are the most commonly used scales for the physical evaluation of burn scars57. The VSS is conducted by a trained observer who evaluates the vascularity, pigmentation, and pliability of the scar. Vascularity, pigmentation, thickness, and pliability are evaluated with the POSAS, which combines the clinician’s and patient’s evaluation of the scar. The novel aspect of the POSAS is the inclusion of the patient’s perception of pain and itch, which occurs in more than 90% of burn patients and persists in 40% of burn survivors.64 The development of hypertrophic scar is one of the most striking predictors for pruritus.64 The Visual Analog Scale and the 5-D Itch Scale allow the patient to describe and quantify itch, while the Itch Man Scale was developed for use in paediatric burn patients.65
As post-burn scar reduces the quality of life experienced by burn survivors, quantifying this effect is important. The Boston Outcomes Questionnaire,66 the Visual Analog Scale,64 the 5-D itch scale,64 the Scars Problems Questionnaire,67 and the Brisbane Burn Scar Impact Profile68 are used to determine the long-term impact of post-burn scarring on life quality and to elucidate the relationships between variables underlying scarring.64 Although parameters such as pain, itch, limited range of motion, and sleep disturbances decrease between the time of burn and two years post-injury, persistence in wanting to hide scarred areas, dissatisfaction with the ability to accurately portray facial expressions, and unhappiness with skin discoloration endure.67
Models for Studying Post-burn Scar
Elucidation of the molecular mechanisms underlying hypertrophic scar, and the effects of therapeutic interventions on the scarring process, has been limited largely to biopsies from human patients used for histology or derivation of skin- or cell-cultures; the lack of animal models which healed and scar similarly to people hindered research, until the recent development of several animal models enabled studies of hypertrophic scar. The female (or castrated male) red Duroc pig develops hypertrophic scar following excisional or burn wounding.69–74 This model is used to study the ontogeny of hypertrophic scar and intervention efficacy.74 Another exciting development has been the grafting of human scar tissue onto the backs of immune-compromised mice; this model has facilitated studies of the scarring process itself, including the role of immune cells in hypertrophic scar by utilizing animals with various immune cells knocked out.75,76 With these models becoming more widely available, it will be possible to conduct tightly controlled studies to evaluate therapy efficacy and to perform mechanistic studies.
The development of a standardized wound and scarring model in patient volunteers has provided a reproducible, controlled model for studying scarring that can be applied to other scarring pathologies beyond post-burn scarring.2 A wound with varying depth is created, thus enabling the comparison of normal scarring to pathologic scarring in the same patient. Finally, the effects of therapies on the whole skin or scar can be studied by obtaining a biopsy for ex vivo organ culture.77–80 The major advantage of this type of culture is that all of the cell types that participate in wound healing and scarring are present, allowing better elucidation of the mechanics of healing when compared to in vitro studies utilizing a single cell type, such as fibroblasts. These models can be used to study how to modulate the healing process to mimic that seen in tissues which do not scar.81,82
Interventions
Compression garments, massage, laser therapy, intense pulsed light, steroids, exercise, and injection of fat into the scar have been used to reduce hypertrophic scar83. As these therapies are not 100% effective on their own, patients may benefit from combining several approaches. If the scar remains problematic following manipulation, surgical revision is used to correct deficiencies and deformities. More cost-effective therapies can be used, including splints constructed of leather and wood, or ace wraps for pressure.
Non-Surgical Approaches to Scar Modification
Physical Approaches
Since the 1970s, compression therapy has been used to reduce the post-burn scar by decreasing blood flow and modulating collagen remodeling.84–86 Additional studies demonstrated significantly increased pliability and decreased thickness of the scar with compression therapy,84,87,88 while a few studies reported no efficacy with the use of pressure garments89,90. A meta-analysis of compression therapy revealed a small decrease in scar height with pressure, although the clinical significance is unknown.90 More recently, Engrav et al compared the effects of <5 mm Hg (low compression) to 17–24 mm Hg (“normal” compression) in 67 burn patients with a forearm burn at least 4 cm in diameter that after 3 weeks required grafting and/or did not heal.88 Scars treated with normal compression became significantly thinner and softer, and had a better clinical appearance than scars treated with low compression.88 Current clinical practice dictates that patients with wounds taking longer than 3 weeks to heal wear pressure garments for at least 23 hours a day until the hypertrophic scar has matured, typically ~2 years post injury. Thus, compliance with this protocol can be challenging. Studies to determine the optimal pressure and duration of therapy are needed to refine current treatment strategies.
Scar massage by either manual or mechanical techniques (compressed air, vacuotherapy, showers) is commonly used in the management of post-burn hypertrophic scars. Reported benefits include reduction in pain and itching, increased range of motion, and decreased anxiety. However, the evidence base is weak, with a recent meta-analysis showing only anecdotal evidence for effectiveness.91
Silicone is commonly applied topically to hypertrophic scar in the form of sheets, strips, gels, creams, sprays, or foams. Silicone is thought to influence collagen remodelling via multiple mechanisms including hydration, increasing local temperature, polarization of the scar tissue, local chemical effects, elevated local oxygen tension, and an increased local mast cell population. But evidence have yielded contradictory conclusions regarding the whether silicone gel sheeting is efficacious in preventing post-burn hypertrophic scar.5
Injections of corticosteroids into the hypertrophic scar may reduce the height and volume of the scar, decrease pain and pruritus, and make the scars more pliable.4,92 Intralesional corticosteroids are thought to modulate many aspects of hypertrophic scar development, including: decreasing collagen synthesis; attenuating proliferation of fibroblasts and keratinocytes; reducing oxygen and nutrient delivery via vasoconstriction; enhancing collagen degradation by activating collagenases within the wound; suppressing TGF-β; and inhibiting migration and phagocytosis by immunoregulatory cells.3,93 Triamcinolone acetonide (10–40 mg/ml), a long-acting corticosteroid, is commonly used with or without lignocaine, and can be combined with other therapies including silicone gel sheeting, pulsed dye laser treatment, cryotherapy, and irradiation.4 The optimal dose and area that can be treated have yet to be determined.4,5
Microneedling, or percutaneous collagen induction (PCI), has been applied to atrophic acne scars and burn scars. Piercing the stratum corneum with the microneedles is thought to produce micro-channels or tiny wounds in the epidermis and papillary dermis that result in neocollagenesis and neovascularisation, but evidence is limited.5
Laser and Light Therapy94
The incorporation of laser and light therapy into scar management has increased the clinician’s ability to reduce hypertrophic scar by decreasing erythema, reducing height, and increasing pliability of scar.95 Additional benefits included reduced pain, pruritus, colour, and abnormal texture.95 Non-ablative or ablative fractional lasers have become a standard therapy in many burn centers to reduce stiffness in post-burn hypertrophic scar by inducing collagen remodeling.96–101 The pulsed dye laser, the ablative fractional CO2 laser, and intense pulse light reduce particular aspects of the hypertrophic scar. Pulsed dye laser decreases vascularity by inducing necrosis in the targeted capillaries, thereby reducing scar volume, erythema, pliability, hyperemia, itch, and pruritus.94,95
Hultman et al have developed a treatment paradigm that includes pulsed dye laser, ablative fractional laser, and intense pulsed light in a sequential manner to manipulate specific aspects of the maturing hypertrophic scar.102 Inflammation in the immature scar is reduced by coagulating the microvessels with pulsed dye laser. The ablative fractional laser (CO2) is employed to ablate the collagen, allowing formation of more normal collagen to occur along with fibroblast apoptosis. Intense pulsed light is then used to correct dyschromia and reduce remaining inflammation. The initial therapeutic intervention significantly reduces scar quality, as measured by the VSS. The improvements resulting from the laser and light therapy were long-lasting; the scar remained stable throughout the 2-year study. Although laser therapy is a novel and exciting intervention for improving scar quality after burns, it requires more fundamental research and clinical trials.
Autologous fat transfer
A significant advance has been structural fat grafting to achieve better wound healing and cosmesis.103 Wound closure can be enhanced by injecting fat, processed to remove the lipids and blood cells, around the wound. Fibrosis and scar height were reduced and scar pliability increased.104 To reduce post-burn scar, fat can be injected into the scar every 8–12 weeks and repeated up to four times, significantly reducing skin hardness as measured with a Durometer and decreasing scarring assessed with the POSAS.105 Adipose-derived stem cells are believed to underlie the positive effects on the grafted fat,106 with preliminary work suggesting that fibroblast ECM production is altered by interactions with adipose-derived stem cells.107
Surgical Approaches
Hypertrophic scar that is refractory to management via non-surgical methods and functionally or aesthetically disabling can be considered for surgical intervention. Scars can be incised to release contracture, excised, and/or repositioned.2 Excision and direct closure can be considered for small scars and is often combined with repositioning the scar parallel to the lines of minimum skin tension.11 If primary closure is not possible or excessive force is required to close the wound then the use of local, adjacent, or distant tissue must be considered in the form of local, regional, or distant (free) flaps. Challenging reconstructive problems can be addressed using tissue expansion, skin grafts, or dermal substitutes82.
Composite tissue allotransplantation108 is a newer reconstructive plastic surgery discipline that utilizes allotransplantation of organs and tissues from a donor to restore deformed or amputated tissue in the recipient. The long-term survival of transplanted limbs was demonstrated in 1998, with partial and total face transplants successfully performed in 2005 and 2010. These transplants can include nerves, skin, blood vessels, mucosa, soft tissues, muscle, salivary glands, cartilage, lymph nodes, and bones. Due to the nature of the tissue for transplant across the major histocompatibility complex, the long-term acceptance of the face or limbs depends on immune tolerance, making regulation of the immune response following composite tissue allotransplantation critical. Modulation of the immune response beginning immediately post-operatively is necessary for tissue survival and positive functional outcomes. The immunological microenvironment within the skin, consisting of resident cellular elements and transitory inflammatory and immunological cells – including antigen-presenting cells, is referred to as the skin-associated lymphoid tissue; this lymphoid tissue drains into the regional lymph nodes, where the immunological response and tolerance can be monitored. Immunosuppression is a critical component of the successful composite tissue allotransplantation transplant, and complications are minimized by reducing the dose: effect ratio. Despite these measures, up to 85% of composite tissue allotransplantation cases result in acute rejection although chronic rejection is a rare occurrence. Current research investigations focus on utilizing stem cell therapy or infusion of the bone marrow from the donor to increase long-term tolerance. Although the burn patient may become highly sensitized after the utilization of skin allografts and large volumes of blood transfused,109 burn patients should be considered candidates for composite allotransplantation, as they can undergo these procedures with high success rates.110 Clinical experience with more than 14 burn patients having received VCA - hands and faces has shown good success rates with only one patient showing active rejection with alloantibodies.
Genetics of Burn Scar
Although the elucidation of the genetic component to scarring is in its infancy, DNA genotyping has been used to identify the variant genes associated with severity of hypertrophic scar. In a study of predominantly white males, a particular variant of the CUB and Sushi multiple domains 1 (CSMD1) gene was associated with less severe post-burn hypertrophic scarring. The role of the resultant protein in hypertrophic scar is yet unknown; the protein produced by the CSMD1 gene is a putative tumor suppressor, the expression of which is elevated in cells isolated from head and neck cancers. The reduction in post-burn hypertrophic scar in the presence of the re11136645 variant is postulated to be related to inflammation via complement activation, regeneration of neurons, or signaling via the TGF-β/SMAD1 pathway – a key pathway known to modulate post-burn hypertrophic scarring.2 As this discovery is quite recent, there is little information to support how or why this gene variant affects hypertrophic scarring. Further genetic studies including more diverse patient populations may lead to identification of additional genes that influence post-burn fibrosis.
Conclusion
Despite the short-comings of many of the studies, long-term objective studies have altered clinical care such that the methods used to heal burn wounds and reduce post-burn hypertrophic scar are improving. When treating the severely burned patient, prevention of hypertrophic scar is preferred over treating a resultant scar. The current approach of reducing hypertrophic scar formation with early acute surgical intervention and various non-surgical modalities, in combination with scar revision or removal, has resulted in greater patient satisfaction, but are not enough. Going forward, questions that are critical to improving patient outcomes and quality of life include how can the wound be modulated to heal faster, what can be used to best predict who will scar badly, and how can hypertrophic scar development be attenuated or prevented? Improvement in how we accurately and objectively determine whether a wound has healed well or the severity of scar will enable the accurate assessment of treatment modalities. Once standardized, these outcome measures can then be used to inform well designed randomized controlled trials to confirm the effects of existing or new treatments that are proposed to improve post-burn scarring. Further elucidation of the molecular mechanisms involved in scarring will allow novel preventative and therapeutic strategies to be developed that will improve the lives of survivors of severe burns.
Literature Search.
A key word search was performed in PubMed (http://www.ncbi.nlm.nih.gov/pubmed) for manuscripts with combinations of the words ‘burn’, ‘thermal’, ‘cicatrix’, ‘hypertrophic’, and ‘keloid’ in the abstract and/or title that had been published between January 2006 to April 2016. From this search result, manuscripts where large cohorts of patients with appropriate control groups had been studied were preferentially selected for inclusion. Due to the limitations on space, recent review articles – as opposed to primary publications - have been cited to direct the reader to more extensive presentation of data or concepts. Finally, several non-PubMed indexed review articles were included due to the scope of information covered.
Inset panel: Characteristics of normal and hypertrophic scars.
Normal Scar
Increased collagenase activity
Lower TGF-βexpression
Macrophages have Th1 phenotype
Hypertrophic Scar
Decreased collagenase activity and collagen breakdown
Increased collagen synthesis
Higher TGF-β expression
Macrophages have Th2 phenotype
Increased presence of myofibroblasts
Increased PDGF
Fibrocytes present
More likely to have been grafted
Acknowledgments
This project was supported by grants from: the National Institutes of Health R01-GM56687 and P50-GM-60338 to D.N.H., R01-GM-112936 to C.C.F.; from the Shriners Hospitals for Children to D.N.H. (84080, 79141) and to C.C.F. (84202); from the Anderson Foundation and the Gillson Longenbaugh Foundation to C.C.F. and D.N.H.; and to M.G.J. from CIHR Funds (123336), and CFI Leader’s Opportunity Fund (Project #25407). Additional support was provided by UTMB’s Institute for Translational Sciences, supported in part by a Clinical and Translational Science Award (UL1TR000071) from the National Center for Advancing Translational Sciences (NIH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest and Contributor Statement
The authors have no relevant conflict of interest to disclose. C.C.F., M.G.J., L.K.B., J.P.B., P.D., and D.N.H. drafted the manuscript and produced the Figures. All authors participated in the critical revisions. All authors approved the final version of the manuscript.
References
- 1.Bombaro KM, Engrav LH, Carrougher GJ, et al. What is the prevalence of hypertrophic scarring following burns? Burns. 2003;29:299–302. doi: 10.1016/s0305-4179(03)00067-6. [DOI] [PubMed] [Google Scholar]
- 2.Tredget EE, Levi B, Donelan MB. Biology and principles of scar management and burn reconstruction. Surg Clin North Am. 2014;94:793–815. doi: 10.1016/j.suc.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Niessen FB, Spauwen PH, Schalkwijk J, Kon M. On the nature of hypertrophic scars and keloids: a review. Plastic and reconstructive surgery. 1999;104:1435–58. doi: 10.1097/00006534-199910000-00031. [DOI] [PubMed] [Google Scholar]
- 4.Ogawa R. The most current algorithms for the treatment and prevention of hypertrophic scars and keloids. Plastic and reconstructive surgery. 2010;125:557–68. doi: 10.1097/PRS.0b013e3181c82dd5. [DOI] [PubMed] [Google Scholar]
- 5.Arno AI, Gauglitz GG, Barret JP, Jeschke MG. Up-to-date approach to manage keloids and hypertrophic scars: a useful guide. Burns. 2014;40:1255–66. doi: 10.1016/j.burns.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel PA, Bailey JK, Yakuboff KP. Treatment outcomes for keloid scar management in the pediatric burn population. Burns: journal of the International Society for Burn Injuries. 2012;38:767–71. doi: 10.1016/j.burns.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Gauglitz GG, Korting HC, Pavicic T, Ruzicka T, Jeschke MG. Hypertrophic scarring and keloids: pathomechanisms and current and emerging treatment strategies. Mol Med. 2011;17:113–25. doi: 10.2119/molmed.2009.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tredget EE, Nedelec B, Scott PG, Ghahary A. Hypertrophic scars, keloids, and contractures. The cellular and molecular basis for therapy. The Surgical clinics of North America. 1997;77:701–30. doi: 10.1016/s0039-6109(05)70576-4. [DOI] [PubMed] [Google Scholar]
- 9.Slemp AE, Kirschner RE. Keloids and scars: a review of keloids and scars, their pathogenesis, risk factors, and management. Current opinion in pediatrics. 2006;18:396–402. doi: 10.1097/01.mop.0000236389.41462.ef. [DOI] [PubMed] [Google Scholar]
- 10.Oliveira GV, Hawkins HK, Chinkes D, et al. Hypertrophic versus non hypertrophic scars compared by immunohistochemistry and laser confocal microscopy: type I and III collagens. Int Wound J. 2009;6:445–52. doi: 10.1111/j.1742-481X.2009.00638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Son D, Harijan A. Overview of surgical scar prevention and management. Journal of Korean medical science. 2014;29:751–7. doi: 10.3346/jkms.2014.29.6.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kerwin LY, El Tal AK, Stiff MA, Fakhouri TM. Scar prevention and remodeling: a review of the medical, surgical, topical and light treatment approaches. International journal of dermatology. 2014;53:922–36. doi: 10.1111/ijd.12436. [DOI] [PubMed] [Google Scholar]
- 13.Deitch EA, Wheelahan TM, Rose MP, Clothier J, Cotter J. Hypertrophic burn scars: analysis of variables. J Trauma. 1983;23:895–8. [PubMed] [Google Scholar]
- 14.Cubison TC, Pape SA, Parkhouse N. Evidence for the link between healing time and the development of hypertrophic scars (HTS) in paediatric burns due to scald injury. Burns: journal of the International Society for Burn Injuries. 2006;32:992–9. doi: 10.1016/j.burns.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 15.Guo S, Dipietro LA. Factors affecting wound healing. Journal of dental research. 2010;89:219–29. doi: 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rashaan ZM, Krijnen P, Klamer RR, Schipper IB, Dekkers OM, Breederveld RS. Nonsilver treatment vs. silver sulfadiazine in treatment of partial-thickness burn wounds in children: a systematic review and meta-analysis. Wound repair and regeneration: official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2014;22:473–82. doi: 10.1111/wrr.12196. [DOI] [PubMed] [Google Scholar]
- 17.Vloemans AF, Hermans MH, van der Wal MB, Liebregts J, Middelkoop E. Optimal treatment of partial thickness burns in children: a systematic review. Burns: journal of the International Society for Burn Injuries. 2014;40:177–90. doi: 10.1016/j.burns.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 18.Wasiak J, Cleland H, Campbell F, Spinks A. Dressings for superficial and partial thickness burns. The Cochrane database of systematic reviews. 2013:CD002106. doi: 10.1002/14651858.CD002106.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aziz Z, Abu SF, Chong NJ. A systematic review of silver-containing dressings and topical silver agents (used with dressings) for burn wounds. Burns: journal of the International Society for Burn Injuries. 2012;38:307–18. doi: 10.1016/j.burns.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 20.Dunkin CS, Pleat JM, Gillespie PH, Tyler MP, Roberts AH, McGrouther DA. Scarring occurs at a critical depth of skin injury: precise measurement in a graduated dermal scratch in human volunteers. Plast Reconstr Surg. 2007;119:1722–32. doi: 10.1097/01.prs.0000258829.07399.f0. discussion 33–4. [DOI] [PubMed] [Google Scholar]
- 21.Jaskille AD, Ramella-Roman JC, Shupp JW, Jordan MH, Jeng JC. Critical review of burn depth assessment techniques: part II. Review of laser doppler technology. Journal of burn care & research: official publication of the American Burn Association. 2010;31:151–7. doi: 10.1097/BCR.0b013e3181c7ed60. [DOI] [PubMed] [Google Scholar]
- 22.Monstrey S, Hoeksema H, Verbelen J, Pirayesh A, Blondeel P. Assessment of burn depth and burn wound healing potential. Burns. 2008;34:761–9. doi: 10.1016/j.burns.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 23.Janzekovic Z. A new concept in the early excision and immediate grafting of burns. J Trauma. 1970;10:1103–8. [PubMed] [Google Scholar]
- 24.Monafo WW. Tangential excision. Clin Plast Surg. 1974;1:591–601. [PubMed] [Google Scholar]
- 25.Herndon DN, Barrow RE, Rutan RL, Rutan TC, Desai MH, Abston S. A comparison of conservative versus early excision. Therapies in severely burned patients. Annals of surgery. 1989;209:547–52. doi: 10.1097/00000658-198905000-00006. discussion 52–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pirayesh A, Hoeksema H, Richters C, Verbelen J, Monstrey S. Glyaderm((R)) dermal substitute: clinical application and long-term results in 55 patients. Burns. 2015;41:132–44. doi: 10.1016/j.burns.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 27.Li X, Meng X, Wang X, et al. Human acellular dermal matrix allograft: A randomized, controlled human trial for the long-term evaluation of patients with extensive burns. Burns. 2015;41:689–99. doi: 10.1016/j.burns.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 28.Branski LK, Herndon DN, Pereira C, et al. Longitudinal assessment of Integra in primary burn management: a randomized pediatric clinical trial. Critical care medicine. 2007;35:2615–23. doi: 10.1097/01.CCM.0000285991.36698.E2. [DOI] [PubMed] [Google Scholar]
- 29.Heimbach DM, Warden GD, Luterman A, et al. Multicenter postapproval clinical trial of Integra dermal regeneration template for burn treatment. J Burn Care Rehabil. 2003;24:42–8. doi: 10.1097/00004630-200301000-00009. [DOI] [PubMed] [Google Scholar]
- 30.Haberal M, Oner Z, Bayraktar U, Bilgin N. The use of silver nitrate-incorporated amniotic membrane as a temporary dressing. Burns, including thermal injury. 1987;13:159–63. doi: 10.1016/0305-4179(87)90108-2. [DOI] [PubMed] [Google Scholar]
- 31.Salisbury RE, Carnes RW, Enterline D. Biological dressings and evaporative water loss from burn wounds. Annals of plastic surgery. 1980;5:270–2. doi: 10.1097/00000637-198010000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Salisbury RE, Carnes R, McCarthy LR. Comparison of the bacterial clearing effects of different biologic dressings on granulating wounds following thermal injury. Plast Reconstr Surg. 1980;66:596–8. doi: 10.1097/00006534-198010000-00017. [DOI] [PubMed] [Google Scholar]
- 33.Quinby WC, Jr, Hoover HC, Scheflan M, Walters PT, Slavin SA, Bondoc CC. Clinical trials of amniotic membranes in burn wound care. Plast Reconstr Surg. 1982;70:711–17. doi: 10.1097/00006534-198212000-00009. [DOI] [PubMed] [Google Scholar]
- 34.Uhlig C, Rapp M, Hartmann B, Hierlemann H, Planck H, Dittel KK. Suprathel-an innovative, resorbable skin substitute for the treatment of burn victims. Burns. 2007;33:221–9. doi: 10.1016/j.burns.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 35.Schwarze H, Kuntscher M, Uhlig C, et al. Suprathel, a new skin substitute, in the management of donor sites of split-thickness skin grafts: results of a clinical study. Burns. 2007;33:850–4. doi: 10.1016/j.burns.2006.10.393. [DOI] [PubMed] [Google Scholar]
- 36.Schwarze H, Kuntscher M, Uhlig C, et al. Suprathel, a new skin substitute, in the management of partial-thickness burn wounds: results of a clinical study. Annals of plastic surgery. 2008;60:181–5. doi: 10.1097/SAP.0b013e318056bbf6. [DOI] [PubMed] [Google Scholar]
- 37.Munster AM. Cultured skin for massive burns. A prospective, controlled trial. Ann Surg. 1996;224:372–5. doi: 10.1097/00000658-199609000-00013. discussion 5–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sood R, Roggy D, Zieger M, et al. Cultured epithelial autografts for coverage of large burn wounds in eighty-eight patients: the Indiana University experience. J Burn Care Res. 2010;31:559–68. doi: 10.1097/BCR.0b013e3181e4ca29. [DOI] [PubMed] [Google Scholar]
- 39.van Zuijlen PPM, Gardien KLM, Jaspers MEH, et al. Tissue engineering in burn scar reconstruction. Burns & Trauma. 2015;3 doi: 10.1186/s41038-015-0017-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boyce ST, Rice RK, Lynch KA, et al. Assessment of replication rates of human keratinocytes in engineered skin substitutes grafted to athymic mice. Wound Repair Regen. 2012;20:544–51. doi: 10.1111/j.1524-475X.2012.00807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boyce ST, Kagan RJ, Yakuboff KP, et al. Cultured skin substitutes reduce donor skin harvesting for closure of excised, full-thickness burns. Ann Surg. 2002;235:269–79. doi: 10.1097/00000658-200202000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wood F, Martin L, Lewis D, et al. A prospective randomised clinical pilot study to compare the effectiveness of Biobrane(R) synthetic wound dressing, with or without autologous cell suspension, to the local standard treatment regimen in paediatric scald injuries. Burns: journal of the International Society for Burn Injuries. 2012;38:830–9. doi: 10.1016/j.burns.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 43.Gravante G, Di Fede MC, Araco A, et al. A randomized trial comparing ReCell system of epidermal cells delivery versus classic skin grafts for the treatment of deep partial thickness burns. Burns: journal of the International Society for Burn Injuries. 2007;33:966–72. doi: 10.1016/j.burns.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 44.Zweifel CJ, Contaldo C, Kohler C, Jandali A, Kunzi W, Giovanoli P. Initial experiences using non-cultured autologous keratinocyte suspension for burn wound closure. Journal of plastic, reconstructive & aesthetic surgery: JPRAS. 2008;61:e1–4. doi: 10.1016/j.bjps.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 45.Reid MJ, Currie LJ, James SE, Sharpe JR. Effect of artificial dermal substitute, cultured keratinocytes and split thickness skin graft on wound contraction. Wound Repair Regen. 2007;15:889–96. doi: 10.1111/j.1524-475X.2007.00313.x. [DOI] [PubMed] [Google Scholar]
- 46.Butler KL, Goverman J, Ma H, et al. Stem cells and burns: review and therapeutic implications. J Burn Care Res. 2010;31:874–81. doi: 10.1097/BCR.0b013e3181f9353a. [DOI] [PubMed] [Google Scholar]
- 47.Wu Y, Zhao RC, Tredget EE. Concise review: bone marrow-derived stem/progenitor cells in cutaneous repair and regeneration. Stem Cells. 2010;28:905–15. doi: 10.1002/stem.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Loder S, Peterson JR, Agarwal S, et al. Wound healing after thermal injury is improved by fat and adipose-derived stem cell isografts. Journal of burn care & research: official publication of the American Burn Association. 2015;36:70–6. doi: 10.1097/BCR.0000000000000160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wurzer P, Branski LK, Kamolz LP, Herndon DN, Finnerty CC. Fat and Adipose-Derived Stem Cell Grafts in Acute Burns. J Burn Care Res. 2016;37:e302. doi: 10.1097/BCR.0000000000000234. [DOI] [PubMed] [Google Scholar]
- 50.Chan R, Z DO, Wrice NL, Baer DG, Renz EM, Christy RJ, Natesan S. Development of a vascularized skin construct using adipose-derived stem cells from debrided burned skin. Stem Cells International. 2012;2012:841203. doi: 10.1155/2012/841203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosenberg L, Krieger Y, Bogdanov-Berezovski A, Silberstein E, Shoham Y, Singer AJ. A novel rapid and selective enzymatic debridement agent for burn wound management: a multi-center RCT. Burns: journal of the International Society for Burn Injuries. 2014;40:466–74. doi: 10.1016/j.burns.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 52.Leon-Villapalos J, Eldardiri M, Dziewulski P. The use of human deceased donor skin allograft in burn care. Cell and tissue banking. 2010;11:99–104. doi: 10.1007/s10561-009-9152-1. [DOI] [PubMed] [Google Scholar]
- 53.Tan H, Wasiak J, Paul E, Cleland H. Effective use of Biobrane as a temporary wound dressing prior to definitive split-skin graft in the treatment of severe burn: A retrospective analysis. Burns: journal of the International Society for Burn Injuries. 2015;41:969–76. doi: 10.1016/j.burns.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 54.Alexander JW, MacMillan BG, Law E, Kittur DS. Treatment of severe burns with widely meshed skin autograft and meshed skin allograft overlay. The Journal of trauma. 1981;21:433–8. [PubMed] [Google Scholar]
- 55.Fearmonti R, Bond J, Erdmann D, Levinson H. A review of scar scales and scar measuring devices. Eplasty. 2010;10:e43. [PMC free article] [PubMed] [Google Scholar]
- 56.Rege A, Thakor NV, Rhie K, Pathak AP. In vivo laser speckle imaging reveals microvascular remodeling and hemodynamic changes during wound healing angiogenesis. Angiogenesis. 2012;15:87–98. doi: 10.1007/s10456-011-9245-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brusselaers N, Pirayesh A, Hoeksema H, Verbelen J, Blot S, Monstrey S. Burn scar assessment: A systematic review of objective scar assessment tools. Burns: journal of the International Society for Burn Injuries. 2010;36:1157–64. doi: 10.1016/j.burns.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 58.Oliveira GV, Chinkes D, Mitchell C, Oliveras G, Hawkins HK, Herndon DN. Objective assessment of burn scar vascularity, erythema, pliability, thickness, and planimetry. Dermatologic surgery: official publication for American Society for Dermatologic Surgery [et al] 2005;31:48–58. doi: 10.1111/j.1524-4725.2005.31004. [DOI] [PubMed] [Google Scholar]
- 59.Lee KC, Dretzke J, Grover L, Logan A, Moiemen N. A systematic review of objective burn scar measurements. Burns & Trauma. 2016;4 doi: 10.1186/s41038-016-0036-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yeong EK, Mann R, Engrav LH, et al. Improved burn scar assessment with use of a new scar-rating scale. The Journal of burn care & rehabilitation. 1997;18:353–5. doi: 10.1097/00004630-199707000-00014. discussion 2. [DOI] [PubMed] [Google Scholar]
- 61.Mecott GA, Finnerty CC, Herndon DN, et al. Reliable scar scoring system to assess photographs of burn patients. The Journal of surgical research. 2015;199:688–97. doi: 10.1016/j.jss.2014.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nedelec B, Shankowsky HA, Tredget EE. Rating the resolving hypertrophic scar: comparison of the Vancouver Scar Scale and scar volume. J Burn Care Rehabil. 2000;21:205–12. doi: 10.1067/mbc.2000.104750. [DOI] [PubMed] [Google Scholar]
- 63.van der Wal MB, Tuinebreijer WE, Bloemen MC, Verhaegen PD, Middelkoop E, van Zuijlen PP. Rasch analysis of the Patient and Observer Scar Assessment Scale (POSAS) in burn scars. Quality of life research: an international journal of quality of life aspects of treatment, care and rehabilitation. 2012;21:13–23. doi: 10.1007/s11136-011-9924-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carrougher GJ, Martinez EM, McMullen KS, et al. Pruritus in adult burn survivors: postburn prevalence and risk factors associated with increased intensity. Journal of burn care & research: official publication of the American Burn Association. 2013;34:94–101. doi: 10.1097/BCR.0b013e3182644c25. [DOI] [PubMed] [Google Scholar]
- 65.Morris V, Murphy LM, Rosenberg M, Rosenberg L, Holzer CE, 3rd, Meyer WJ., 3rd Itch assessment scale for the pediatric burn survivor. Journal of burn care & research: official publication of the American Burn Association. 2012;33:419–24. doi: 10.1097/BCR.0b013e3182372bfa. [DOI] [PubMed] [Google Scholar]
- 66.Kazis LE, Liang MH, Lee A, et al. The development, validation, and testing of a health outcomes burn questionnaire for infants and children 5 years of age and younger: American Burn Association/Shriners Hospitals for Children. J Burn Care Rehabil. 2002;23:196–207. doi: 10.1097/00004630-200205000-00009. [DOI] [PubMed] [Google Scholar]
- 67.Wurzer P, Forbes AA, Hundeshagen G, et al. Long-term outcome of scarring and distress in children with severe burns. Disability and rehabilitation. 2016 doi: 10.1080/09638288.2016.1209579. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tyack Z, Ziviani J, Kimble R, et al. Measuring the impact of burn scarring on health-related quality of life: Development and preliminary content validation of the Brisbane Burn Scar Impact Profile (BBSIP) for children and adults. Burns. 2015;41:1405–19. doi: 10.1016/j.burns.2015.05.021. [DOI] [PubMed] [Google Scholar]
- 69.Sood RF, Muffley LA, Seaton ME, et al. Dermal Fibroblasts from the Red Duroc Pig Have an Inherently Fibrogenic Phenotype: An In Vitro Model of Fibroproliferative Scarring. Plast Reconstr Surg. 2015;136:990–1000. doi: 10.1097/PRS.0000000000001704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhu KQ, Carrougher GJ, Gibran NS, Isik FF, Engrav LH. Review of the female Duroc/Yorkshire pig model of human fibroproliferative scarring. Wound Repair Regen. 2007;15(Suppl 1):S32–9. doi: 10.1111/j.1524-475X.2007.00223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mauskar NA, Sood S, Travis TE, et al. Donor site healing dynamics: molecular, histological, and noninvasive imaging assessment in a porcine model. J Burn Care Res. 2013;34:549–62. doi: 10.1097/BCR.0b013e3182839aca. [DOI] [PubMed] [Google Scholar]
- 72.Travis TE, Mino MJ, Moffatt LT, et al. Biphasic presence of fibrocytes in a porcine hypertrophic scar model. J Burn Care Res. 2015;36:e125–35. doi: 10.1097/BCR.0000000000000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim JY, Dunham DM, Supp DM, Sen CK, Powell HM. Novel burn device for rapid, reproducible burn wound generation. Burns. 2016;42:384–91. doi: 10.1016/j.burns.2015.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim JY, Willard JJ, Supp DM, et al. Burn Scar Biomechanics after Pressure Garment Therapy. Plast Reconstr Surg. 2015;136:572–81. doi: 10.1097/PRS.0000000000001507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Momtazi M, Kwan P, Ding J, et al. A nude mouse model of hypertrophic scar shows morphologic and histologic characteristics of human hypertrophic scar. Wound Repair Regen. 2013;21:77–87. doi: 10.1111/j.1524-475X.2012.00856.x. [DOI] [PubMed] [Google Scholar]
- 76.Wang J, Ding J, Jiao H, et al. Human hypertrophic scar-like nude mouse model: characterization of the molecular and cellular biology of the scar process. Wound Repair Regen. 2011;19:274–85. doi: 10.1111/j.1524-475X.2011.00672.x. [DOI] [PubMed] [Google Scholar]
- 77.Hodgkinson T, Bayat A. Ex vivo evaluation of acellular and cellular collagen-glycosaminoglycan flowable matrices. Biomed Mater. 2015;10:041001. doi: 10.1088/1748-6041/10/4/041001. [DOI] [PubMed] [Google Scholar]
- 78.Mendoza-Garcia J, Sebastian A, Alonso-Rasgado T, Bayat A. Optimization of an ex vivo wound healing model in the adult human skin: Functional evaluation using photodynamic therapy. Wound Repair Regen. 2015;23:685–702. doi: 10.1111/wrr.12325. [DOI] [PubMed] [Google Scholar]
- 79.Mendoza-Garcia J, Sebastian A, Alonso-Rasgado T, Bayat A. Ex vivo evaluation of the effect of photodynamic therapy on skin scars and striae distensae. Photodermatology, photoimmunology & photomedicine. 2015;31:239–51. doi: 10.1111/phpp.12180. [DOI] [PubMed] [Google Scholar]
- 80.Syed F, Bagabir RA, Paus R, Bayat A. Ex vivo evaluation of antifibrotic compounds in skin scarring: EGCG and silencing of PAI-1 independently inhibit growth and induce keloid shrinkage. Laboratory investigation; a journal of technical methods and pathology. 2013;93:946–60. doi: 10.1038/labinvest.2013.82. [DOI] [PubMed] [Google Scholar]
- 81.Bellayr IH, Walters TJ, Li Y. Scarless wound healing. The journal of the American College of Certified Wound Specialists. 2010;2:40–3. doi: 10.1016/j.jcws.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Leavitt T, Hu MS, Marshall CD, Barnes LA, Lorenz HP, Longaker MT. Scarless wound healing: finding the right cells and signals. Cell and tissue research. 2016 doi: 10.1007/s00441-016-2424-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Friedstat JS, Hultman CS. Hypertrophic burn scar management: what does the evidence show? A systematic review of randomized controlled trials. Annals of plastic surgery. 2014;72:S198–201. doi: 10.1097/SAP.0000000000000103. [DOI] [PubMed] [Google Scholar]
- 84.Larson DL, Abston S, Evans EB, Dobrkovsky M, Linares HA. Techniques for decreasing scar formation and contractures in the burned patient. J Trauma. 1971;11:807–23. doi: 10.1097/00005373-197110000-00001. [DOI] [PubMed] [Google Scholar]
- 85.Linares HA, Larson DL, Willis-Galstaun BA. Historical notes on the use of pressure in the treatment of hypertrophic scars or keloids. Burns. 1993;19:17–21. doi: 10.1016/0305-4179(93)90095-p. [DOI] [PubMed] [Google Scholar]
- 86.MacMillan BG. The effect of pressure on the healing burn wound. In: Longacre JJ, editor. The ultrastructure of collagen; its relation to the healing of wounds and to the management of hypertrophic scar. Springfield, IL: Thomas; 1976. pp. 67–92. [Google Scholar]
- 87.Candy LH, Cecilia LT, Ping ZY. Effect of different pressure magnitudes on hypertrophic scar in a Chinese population. Burns. 2010;36:1234–41. doi: 10.1016/j.burns.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 88.Engrav LH, Heimbach DM, Rivara FP, et al. 12-Year within-wound study of the effectiveness of custom pressure garment therapy. Burns. 2010;36:975–83. doi: 10.1016/j.burns.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 89.Mustoe TA, Cooter RD, Gold MH, et al. International clinical recommendations on scar management. Plast Reconstr Surg. 2002;110:560–71. doi: 10.1097/00006534-200208000-00031. [DOI] [PubMed] [Google Scholar]
- 90.Anzarut A, Olson J, Singh P, Rowe BH, Tredget EE. The effectiveness of pressure garment therapy for the prevention of abnormal scarring after burn injury: a meta-analysis. Journal of plastic, reconstructive & aesthetic surgery: JPRAS. 2009;62:77–84. doi: 10.1016/j.bjps.2007.10.052. [DOI] [PubMed] [Google Scholar]
- 91.Shin TM, Bordeaux JS. The role of massage in scar management: a literature review. Dermatologic surgery: official publication for American Society for Dermatologic Surgery [et al] 2012;38:414–23. doi: 10.1111/j.1524-4725.2011.02201.x. [DOI] [PubMed] [Google Scholar]
- 92.Monstrey S, Middelkoop E, Vranckx JJ, et al. Updated scar management practical guidelines: non-invasive and invasive measures. Journal of plastic, reconstructive & aesthetic surgery: JPRAS. 2014;67:1017–25. doi: 10.1016/j.bjps.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 93.Al-Attar A, Mess S, Thomassen JM, Kauffman CL, Davison SP. Keloid pathogenesis and treatment. Plastic and reconstructive surgery. 2006;117:286–300. doi: 10.1097/01.prs.0000195073.73580.46. [DOI] [PubMed] [Google Scholar]
- 94.Donelan MB, Parrett BM, Sheridan RL. Pulsed dye laser therapy and z-plasty for facial burn scars: the alternative to excision. Annals of plastic surgery. 2008;60:480–6. doi: 10.1097/SAP.0b013e31816fcad5. [DOI] [PubMed] [Google Scholar]
- 95.Hultman CS, Edkins RE, Lee CN, Calvert CT, Cairns BA. Shine on: Review of Laser- and Light-Based Therapies for the Treatment of Burn Scars. Dermatology research and practice. 2012;2012:243651. doi: 10.1155/2012/243651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Haedersdal M, Moreau KE, Beyer DM, Nymann P, Alsbjorn B. Fractional nonablative 1540 nm laser resurfacing for thermal burn scars: a randomized controlled trial. Lasers in surgery and medicine. 2009;41:189–95. doi: 10.1002/lsm.20756. [DOI] [PubMed] [Google Scholar]
- 97.Haedersdal M. Fractional ablative CO(2) laser resurfacing improves a thermal burn scar. Journal of the European Academy of Dermatology and Venereology: JEADV. 2009;23:1340–1. doi: 10.1111/j.1468-3083.2009.03215.x. [DOI] [PubMed] [Google Scholar]
- 98.Waibel J, Wulkan AJ, Lupo M, Beer K, Anderson RR. Treatment of burn scars with the 1,550 nm nonablative fractional Erbium Laser. Lasers in surgery and medicine. 2012;44:441–6. doi: 10.1002/lsm.22038. [DOI] [PubMed] [Google Scholar]
- 99.Taudorf EH, Danielsen PL, Paulsen IF, et al. Non-ablative fractional laser provides long-term improvement of mature burn scars–a randomized controlled trial with histological assessment. Lasers in surgery and medicine. 2015;47:141–7. doi: 10.1002/lsm.22289. [DOI] [PubMed] [Google Scholar]
- 100.Shumaker PR, Kwan JM, Badiavas EV, Waibel J, Davis S, Uebelhoer NS. Rapid healing of scar-associated chronic wounds after ablative fractional resurfacing. Archives of dermatology. 2012;148:1289–93. doi: 10.1001/2013.jamadermatol.256. [DOI] [PubMed] [Google Scholar]
- 101.Ozog DM, Liu A, Chaffins ML, et al. Evaluation of clinical results, histological architecture, and collagen expression following treatment of mature burn scars with a fractional carbon dioxide laser. JAMA dermatology. 2013;149:50–7. doi: 10.1001/2013.jamadermatol.668. [DOI] [PubMed] [Google Scholar]
- 102.Hultman CS, Friedstat JS, Edkins RE, Cairns BA, Meyer AA. Laser resurfacing and remodeling of hypertrophic burn scars: the results of a large, prospective, before-after cohort study, with long-term follow-up. Ann Surg. 2014;260:519–29. doi: 10.1097/SLA.0000000000000893. discussion 29–32. [DOI] [PubMed] [Google Scholar]
- 103.Coleman SR. Structural Fat Grafting. In: Thorne CH, editor. Grabb and Smith’s Plastic Surgery. 6. Lippincott Williams & Wilkins; 2007. pp. 480–5. [Google Scholar]
- 104.Piccolo NS, Piccolo MS, Piccolo MT. Fat grafting for treatment of burns, burn scars, and other difficult wounds. Clinics in plastic surgery. 2015;42:263–83. doi: 10.1016/j.cps.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 105.Klinger M, Caviggioli F, Klinger FM, et al. Autologous fat graft in scar treatment. The Journal of craniofacial surgery. 2013;24:1610–5. doi: 10.1097/SCS.0b013e3182a24548. [DOI] [PubMed] [Google Scholar]
- 106.Embaby A, El-Shaer W, Abdl-Hasib A, Korany M. Histological study of the role of fat injection in scar remodeling following burn injury. The Egyptian Journal of Histology. 2012;35:437. [Google Scholar]
- 107.Alharbi S, Prasai A, El Ayadi A, Herndon D, Finnerty C. Adipose Derived Stem Cells Alter Fibroblast Extra Cellular Matrix Production. TISSUE ENGINEERING PART A. 2015;21:S94–S5. [Google Scholar]
- 108.Barret JPt, V. Immunological Aspects and Immunomodulation. Face Transplantation. 2015 [Google Scholar]
- 109.Duhamel P, Suberbielle C, Grimbert P, et al. Anti-HLA sensitization in extensively burned patients: extent, associated factors, and reduction in potential access to vascularized composite allotransplantation. Transplant international: official journal of the European Society for Organ Transplantation. 2015;28:582–93. doi: 10.1111/tri.12540. [DOI] [PubMed] [Google Scholar]
- 110.Win TS, Frew Q, Taylor CJ, Peacock S, Pettigrew G, Dziewulski P. Allosensitization following skin allografts in acute burn management: Are burns patients suitable face transplant candidates? Journal of plastic, reconstructive & aesthetic surgery: JPRAS. 2015;68:1155–7. doi: 10.1016/j.bjps.2015.04.018. [DOI] [PubMed] [Google Scholar]


