Abstract
Background
Evidence suggests that patients are generally accepting of their enrollment in trials for emergency care conducted under Exception From Informed Consent (EFIC). It is unknown whether individuals with more severe initial injuries or worse clinical outcomes have different perspectives. Determining whether these differences exist may help to structure post-enrollment interactions.
Methods
Primary clinical data from the Progesterone for the Treatment of Traumatic Brain Injury trial (ProTECT III) were matched to interview data from the Patients' Experiences in Emergency Research-Progesterone for the Treatment of Traumatic Brain Injury (PEER-ProTECT) study. Answers to 3 key questions from PEER-ProTECT were analyzed in the context of enrolled patients' initial injury severity (initial Glasgow Coma Scale and Injury Severity Score) and principal clinical outcomes (Extended Glasgow Outcome Scale and Extended Glasgow Outcome Scale relative to initial injury severity). The 3 key questions from PEER-ProTECT addressed: participants' general attitude toward inclusion in the ProTECT III trial (general trial inclusion), their specific attitude toward being included in ProTECT III under the exception from informed consent (personal EFIC enrollment), and their attitude toward the use of EFIC in the ProTECT III trial in general (general EFIC enrollment). Qualitative analysis of interview transcripts was performed to provide contextualization and to determine the extent to which respondents framed their attitudes in terms of clinical experience.
Results
Clinical data from ProTECT III were available for all 74 patients represented in the PEER-ProTECT study (including 46 patients for whom the surrogate was interviewed due to the patient's cognitive status or death). No significant difference was observed regarding acceptance of general trial inclusion or acceptance of general EFIC enrollment between participants with favorable neurological outcomes and those with unfavorable outcomes relative to initial injury. Agreement with personal enrollment in ProTECT III under EFIC, however, was significantly higher among participants with favorable outcomes compared to those with unfavorable outcomes (89% vs. 59%, p= 0.003). There was also a statistically significant relationship between more severe initial injury and increased acceptance of personal EFIC enrollment (p= 0.040) or general EFIC use (p= 0.034) in ProTECT III. Many individuals referenced personal experience as a basis for their attitudes, but these references were not used to support negative views.
Conclusions
Patients and surrogates of patients with unfavorable clinical outcomes were somewhat less accepting of their own inclusion in the ProTECT III trial under EFIC than were patients or surrogates of patients with favorable clinical outcomes. These findings suggest a need to identify optimal strategies for communicating with patients and their surrogates regarding EFIC enrollment when clinical outcomes are poor.
Keywords: Research ethics, bioethics, informed consent, acute care research, emergency research, traumatic brain injury
Introduction
Regulations allowing an Exception From Informed Consent (EFIC) for research in emergency settings have facilitated studies in which critically ill patients are not able to provide prospective consent but therapy must be initiated before a surrogate can be identified.1, 2 Experience with EFIC research has grown since passage of these regulations, 3-6 as has understanding of how patients and the public view these studies.7-12
Because enrolled patients and their family members are directly affected by EFIC enrollment, understanding their views and experiences is important. Prior interview studies with patients enrolled in EFIC studies and their surrogates, as well as reports of community consultation efforts conducted in preparation for EFIC research, have reported generally high levels of acceptance of EFIC enrollment.5, 13, 14 In fact, enrolled patients and surrogates appear to be slightly more accepting on the whole than the general public.9, 11, 15
One obvious question is whether individuals with more severe initial injuries or less favorable outcomes and their family members have more negative attitudes toward EFIC enrollment than those with more favorable outcomes or less severe injury. One recent pediatric study suggests that outcomes may influence the experience of EFIC enrollment and that research teams may be uncomfortable discussing enrollment with patients or family members with undesirable outcomes.16 Understanding these relationships may help researchers to optimize communication strategies after EFIC enrollment has occurred.
To address this question, we linked clinical data from the Progesterone for the Treatment of Traumatic Brain Injury (ProTECT III) trial17 with interview data from the Patients' Experiences in Emergency Research-Progesterone for the Treatment of Traumatic Brain Injury (PEER-ProTECT) interview study.14
Methods
Objective and population
The principal objective of this study was to assess the impact of clinical factors on attitudes of patients and family members toward EFIC enrollment. To examine this relationship, we correlated measures of initial injury severity and 6-month neurologic outcomes with interview data on patients' and surrogates' attitudes toward the ProTECT III trial, a Phase III, multicenter, randomized, placebo-controlled trial of progesterone in moderate to severe traumatic brain injury.17
PEER-ProTECT was an interview study assessing views of EFIC enrollment with 74 patients or surrogates enrolled in the ProTECT III trial at 12 sites.14 Surrogates were interviewed when the patient was unable due to impairment or death. Interviews were conducted at least 3 months post-enrollment (average 192 days) in order to maximize opportunities to interview patients. Clinical data for patients represented in PEER-ProTECT were extracted from the ProTECT III database using the unique study identifier. This analysis was planned at the outset of PEER-ProTECT but could not be performed until completion of ProTECT III and publication of results.
Informed consent for ProTECT III (after initial EFIC enrollment) and PEER-ProTECT were previously obtained. Participating sites' Institutional Review Boards approved both studies.
Data collection and management
Clinical data included two measures of initial injury severity: Glasgow Coma Scale and Injury Severity Score. The primary clinical outcome measure was the Extended Glasgow Outcome Scale score at 6 months. As in the primary ProTECT III analysis, the Extended Glasgow Outcome Scale was evaluated independently as an ordinal variable and also as a dichotomized variable (favorable or unfavorable) relative to initial injury severity.17
We analyzed participants' responses to 3 key questions from PEER-ProTECT.14 Participants were asked to respond to each question on a 5-point Likert scale, and interviewers asked follow up “probe” questions to assess participants' reasons for their views. The first question focused on general attitudes toward inclusion in ProTECT III without reference to EFIC (general trial inclusion): “I am glad that I was included in this research study.” The second focused on acceptance of personal enrollment in ProTECT III under EFIC (personal EFIC): “I think that it was ok for researchers to include me in the PROTECT III research study without asking me for permission first.” The third focused on acceptance of the use of EFIC in general for ProTECT III (general EFIC): “I think that it was ok for researchers to include people in the PROTECT research study without asking them for permission first.” Questions were asked in this order to minimize the impact of views specific to consent on general attitudes toward trial inclusion.
Data analysis
As in the primary PEER-ProTECT report,14 5-point Likert-scale responses were collapsed for analysis. Agreement or acceptance was defined as a response of 1 or 2; other responses were classified as “not agree.” Cutoff values for initial injury severity by the Glasgow Coma Scale score and six-month outcome by the Extended Glasgow Outcome Scale score were identical to those employed in the primary ProTECT III analysis.17 Index Glasgow Coma Scale scores of 4-5 were considered severe; scores 6-8 were considered moderate to severe; scores 9-12 were considered moderate (Table 1). An Extended Glasgow Outcome Scale score of 3-8 was considered a favorable outcome in patients with severe injury; 5 to 8 was considered favorable in patients with moderate-severe injury; and 7 or 8 was considered favorable in patients with moderate injury.
Table 1. Patients' Experiences in Emergency Research-Progesterone for the Treatment of Traumatic Brain Injury Participant Clinical Baseline Characteristics and Outcomes compared to those of Progesterone for the Treatment of Traumatic Brain Injury III study.
| Patient Outcomes | PEER-ProTECT population | ProTECT population |
|---|---|---|
| Total participants | 74 | 882 |
| Index GCS at randomization—no. (%) a | ||
| Severe | 9 (12.2) | 156 (17.7) |
| Moderate-to-severe | 42 (56.8) | 472 (53.5) |
| Moderate | 23 (31.1) | 254 (28.8) |
| Injury Severity Score (ISS) b | 25.2 ± 9.7 | 24.4 ± 11.4 |
| 6-month outcome based on GOS-E score—no.(%) c | ||
| 1-2: Death/Vegetative state | 4 (5.4) | 164 (18.6) |
| 3-4: Severe disability | 20 (27.0) | 184 (20.9) |
| 5-6: Moderate disability | 27 (36.5) | 253(28.7) |
| 7-8: Good recovery | 23 (31.1) | 229 (26.0) |
| 6-month outcome relative to initial injury severity—no. (%) | ||
| Favorable | 44 (59.5) | 445 (50.5) |
| Unfavorable | 29 (39.2) | 385 (43.6) |
| Missing data | 1 (1.4) | 52 (5.9) |
PEER-ProTECT= Patients' Experiences in Emergency Research-Progesterone for the Treatment of Traumatic Brain Injury ProTECT= Progesterone for the Treatment of Traumatic Brain Injury
Index Glasgow Coma Scale (GCS), defined as the highest reliable GCS score documented prior to randomization, ranges from 3 to 15. Lower scores indicate lower level of consciousness. The overall GCS score is the sum of scores given for the motor (range 1-6), verbal, and eye-opening components.
The Injury Severity Score ranges from 0 to 75. Higher scores suggest greater injury severity. Plus–minus values are means and SD.
An Extended Glasgow Outcome Scale (GOS-E) score of 1 indicates death, and 2, vegetative state; 3 or 4 severe disability; 5 or 6 moderate disability; and 7 or 8 good recovery.
Quantitative data were analyzed using SAS 9.3 (SAS institute, Cary, NC). Descriptive statistics and bivariate analyses (chi-squared and Fisher exact tests) were calculated, consistent with the study's hypothesis-generating goals. Simple logistic regression analysis was used to examine the relationship between ordinal categorical Glasgow Coma Scale and Extended Glasgow Outcome Scale scores and attitudes. Responses to the 3 key questions in PEER-ProTECT were previously coded using MAXQDA 10 (VERBI GMbH, Berlin, Germany). Open-ended answers to follow-up questions were analyzed using template analytic method to facilitate qualitative description of the ways in which patients or surrogates described clinical experiences impacting their views on ProTECT III. These codes were developed inductively and the final codebook was used to code all interviews. Coded segments were reviewed by two authors to ensure they reflected coherent themes.
Results
Study population
Clinical data were obtained for all 74 patients enrolled in PEER-ProTECT. Of the 74 participants, 28 were patients (37.8 %) and 46 were surrogates (62.2%). Four patients died before the 6-month follow-up assessment for ProTECT and were assigned an Extended Glasgow Outcome Scale score of 1. One patient's 6-month outcome evaluation was completed outside of the study window; those data were excluded from analysis by outcome.
Clinically, patients included in PEER-ProTECT differed from the overall ProTECT III trial population in several ways (Table 1). There was a higher rate of death or vegetative state within the ProTECT III trial overall (5.4% vs. 18.6%). There was a marginally lower rate of favorable clinical outcome (50.5% vs. 59.5%) in ProTECT III. The two groups were similar in other respects.
Impact of clinical characteristics on attitudes toward EFIC and enrollment in ProTECT III
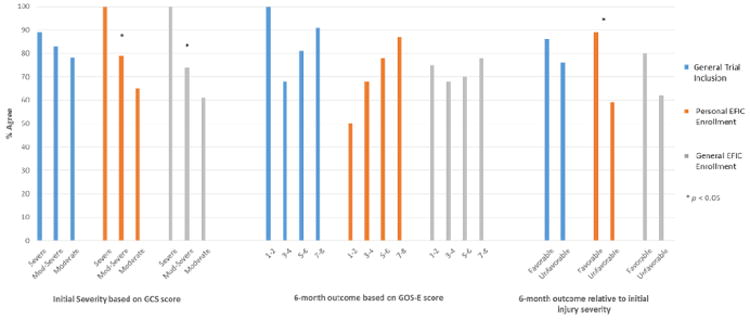
Among patients with more severe initial injury by the Glasgow Coma Scale, there was a significantly higher level of acceptance of personal EFIC (p= 0.040) and general EFIC enrollment (p= 0.034; Table 2). That relationship did not exist for acceptance of general trial inclusion. Initial injury severity by the Injury Severity Score was not associated with rates of acceptance of any of the key questions.
Table 2. Exception From Informed Consent Acceptance by Participant Clinical Baseline Characteristics and Outcomes (NTotal = 74).
| Patient Outcomes | General trial inclusion | Personal EFIC enrollment | General EFIC enrollment | |||
|---|---|---|---|---|---|---|
| NAgree (% Agree) | p | NAgree (% Agree) | p | NAgree (% Agree) | p | |
| Overall | 61 (82) | 57 (77) | 54 (73) | |||
|
| ||||||
| Initial injury severity based on GCS score x | ||||||
| Severe | 8 (89) | 0.457a | 9 (100) | 0.040a | 9 (100) | 0.034a |
| Moderate-Severe | 35 (83) | 33 (79) | 31 (74) | |||
| Moderate | 18 (78) | 15 (65) | 14 (61) | |||
| Initial injury severity based on ISS score y | ||||||
| 1-15: Less severe | 10 (83) | 1.000b,c | 11 (92) | 0.274b | 9 (75) | 1.000b,c |
| 16-75: More severe | 51 (82) | 46 (74) | 45 (73) | |||
| 6-month outcome based on GOS-E score* z | ||||||
| 1-2: Death/Vegetative state | 4 (100) | 0.263a | 2 (50) | 0.066a | 3 (75) | 0.575a |
| 3-4: Severe disability | 13 (68) | 13 (68) | 13 (68) | |||
| 5-6: Moderate disability | 22 (81) | 21 (78) | 19 (70) | |||
| 7-8: Good recovery | 21 (91) | 20 (87) | 18 (78) | |||
| 6-month outcome relative to initial injury severity* | ||||||
| Favorable | 38 (86) | 0.251d | 39 (89) | 0.003d | 35 (80) | 0.101d |
| Unfavorable | 22 (76) | 17 (59) | 18 (62) | |||
EFIC= exception from informed consent.
Simple logistic regression
Fisher's exact test
Two-sided p value for Fisher exact test is 1.000 due to rounding and corresponds to the probability of observing the given difference or any more extreme differences with the same fixed marginal
Chi-Square test
6-month GOS-E score not available for 1 participant, n=73
Index Glasgow Coma Scale (GCS), defined as the highest reliable GCS score documented prior to randomization, ranges from 3 to 15. Lower scores indicate lower level of consciousness. The overall GCS score is the sum of scores given for motor (range 1-6), verbal, and eye-opening components.
The Injury Severity Score ranges from 0 to 75. Higher scores suggest greater injury severity.
An Extended Glasgow Outcome Scale (GOS-E) score of 1 indicates death, and 2, vegetative state; 3 or 4 severe disability; 5 or 6 moderate disability; and 7 or 8 good recovery.
Agreement with personal EFIC enrollment was significantly higher among participants with favorable outcomes compared to those with unfavorable outcomes (89% vs. 59%, p= 0.003). A similar trend approaching significance (p =0.066) was observed between personal EFIC acceptance and the Extended Glasgow Outcome Scale outcome strata, independent of initial injury severity. There was also numerically greater, though not statistically significant, acceptance of general EFIC enrollment among those with favorable outcomes.
Reference to clinical experience in responses to EFIC and enrollment in ProTECT III
Among PEER-ProTECT participants, 92% referenced personal clinical experiences in at least one response to the three key questions. Though some participants stated that clinical experience played a role in how they viewed trial enrollment, no participant specifically referenced a negative outcome as a basis for a negative attitude toward general trial inclusion, personal EFIC enrollment, or general EFIC enrollment.
Interviewees used clinical experiences to support their attitudes toward EFIC or trial inclusion in four different ways. One group of participants perceived direct clinical benefit from trial inclusion. Another group stated uncertainty about inclusion because it was unclear whether they received clinical benefit. A third group referenced their own experience as a reason for wanting to contribute to research. The fourth group specifically discussed that the severity of injury or poor prognosis led them to embrace any chance for benefit.
Discussion
This study is the first to link clinical characteristics and outcomes of adult patients involved in an EFIC trial with attitudes toward trial inclusion and the use of EFIC. It provides novel information regarding both the impact of clinical factors on attitudes and ways patients and surrogates use personal clinical experiences to frame opinions on the trial and EFIC.
Most notably, these data demonstrate a potentially meaningful, though modest, relationship between worse clinical outcomes and more negative views toward personal EFIC enrollment. Despite this relationship, participants did not cite clinical outcomes as a basis for less favorable attitudes. Moreover, outcome-based differences were most pronounced in questions that specifically asked about EFIC rather than attitude toward trial inclusion. These findings suggest that, even when present, connections between clinical outcome and attitudes toward EFIC enrollment may not be overt. Our results support the findings of one recent study of parents' attitudes toward pediatric EFIC enrollment, which reported that negative experiences were often not obvious to research teams.16 Together, these data suggest that researchers should be attuned to these connections with outcome when discussing EFIC trial enrollment.
We observed that more severe initial injury was associated with more positive attitudes toward EFIC. The degree of initial injury was not, however, associated with different attitudes toward trial enrollment. Though it warrants further exploration, this finding suggests that individuals with more severe initial injuries may be more aware of the necessity of EFIC and less likely to question its use in the emergency setting. They may also perceive a more favorable risk-benefit ratio from trial participation.
These findings have two important implications for EFIC research. First, they raise the possibility that individuals with negative clinical outcomes may harbor more concerns about EFIC specifically than has been recognized. If so, it may be helpful for research teams to carefully consider specific needs that these patients or their families may have. Second, these data highlight the post-enrollment period as one that warrants attention. Most scholarship on ethical aspects of EFIC research has centered on initial enrollment and the community consultation process.15, 18, 19 Developing best practices for discussing EFIC enrollment that has already occurred, answering questions, and addressing patients' and families' needs, however, is essential to enhance their experiences with emergency research and EFIC. Attention to these issues is particularly relevant in the context of public sensitivity to the idea of enrollment in clinical trials without prospective consent and the importance of public trust for research in general.
Several limitations are important to mention. First, this study was a secondary analysis and is primarily hypothesis-generating. Second, PEER-ProTECT was a descriptive study, and small sample size prevents us from accounting for potential variation in attitudes based on surrogate status, racial or other demographic factors. Third, it is possible that views toward the trial evolved during the time from enrollment to PEER-ProTECT interview in ways that we were unable to capture. As reported previously, there were small decreases in acceptance with a longer interval between enrollment and interview.14 However, our small sample size limits the ability to adjust for this and other factors. Of note, the ProTECT III trial was prematurely stopped, but neither interviewers nor participants knew the study outcome at the time of the PEER-ProTECT interview.
Conclusions
Enrollment without prospective consent is necessary to advance research in emergency settings. EFIC research is widely accepted, but little attention has been devoted to the post-enrollment process. This study suggests that patients with unfavorable clinical outcomes may be somewhat less accepting of personal EFIC enrollment, though they have generally positive attitudes toward being in the trial. These findings suggest the importance of post-enrollment communication in enhancing experiences of EFIC enrollment. Future studies focusing on patient-centered approaches to the post-enrollment period may help to maximize the extent to which patients and families feel respected in the context of EFIC enrollment.
Figure 1. Acceptance of Key Questions by Participant Initial Injury Severity and Outcomes.
Acknowledgments
We would like to thank Michael Lunney for his assistance in managing the clinical data from the ProTECT III data set for our project and Candace Speight for her assistance with data analysis.
Funding: This work was supported by the Greenwall Foundation. The parent trial was supported by the National Institute for Neurological Disorders and Stroke (5U01NS062778).
References
- 1.Emanuel EJ, Wendler D, Grady C. What makes clinical research ethical? JAMA. 2000;283:2701–2711. doi: 10.1001/jama.283.20.2701. [DOI] [PubMed] [Google Scholar]
- 2.Largent EA, Wendler D, Emanuel E, et al. Is emergency research without initial consent justified?: the consent substitute model. Arch Intern Med. 2010;170:668–674. doi: 10.1001/archinternmed.2010.80. [DOI] [PubMed] [Google Scholar]
- 3.United States Department of Health and Human Services. Protection of Human Subjects. Washington, DC: 2009. Title 45 (Code of Federal Regulations), Part 46. [Google Scholar]
- 4.United States Food and Drug Administration. Protection of Human Subjects. Silver Spring, MD: 2004. Title 21 (Code of Federal Regulations), Part 50.24. [Google Scholar]
- 5.Potter JE, McKinley S, Delaney A. Research participants' opinions of delayed consent for a randomised controlled trial of glucose control in intensive care. Intensive Care Med. 2013;39:472–480. doi: 10.1007/s00134-012-2732-8. [DOI] [PubMed] [Google Scholar]
- 6.van Belle G, Mentzelopoulos SD, Aufderheide T, et al. International variation in policies and practices related to informed consent in acute cardiovascular research: Results from a 44 country survey. Resuscitation. 2015;91:76–83. doi: 10.1016/j.resuscitation.2014.11.029. [DOI] [PubMed] [Google Scholar]
- 7.Kasner SE, Baren JM, Le Roux PD, et al. Community views on neurologic emergency treatment trials. Ann Emerg Med. 2011;57:346–354.e6. doi: 10.1016/j.annemergmed.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 8.Dickert NW, Kass NE. Patients' perceptions of research in emergency settings: a study of survivors of sudden cardiac death. Soc Sci Med. 2009;68:183–191. doi: 10.1016/j.socscimed.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kamarainen A, Silfvast T, Saarinen S, et al. Conduct of emergency research in patients unable to give consent--experiences and perceptions of patients, their consent providing next of kin, and treating physicians following a prehospital resuscitation trial. Resuscitation. 2012;83:81–85. doi: 10.1016/j.resuscitation.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 10.Biros MH, Sargent C, Miller K. Community attitudes towards emergency research and exception from informed consent. Resuscitation. 2009;80:1382–1387. doi: 10.1016/j.resuscitation.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lecouturier J, Rodgers H, Ford GA, et al. Clinical research without consent in adults in the emergency setting: a review of patient and public views. BMC Med Ethics. 2008;9:9. doi: 10.1186/1472-6939-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abboud PA, Heard K, Al-Marshad AA, et al. What determines whether patients are willing to participate in resuscitation studies requiring exception from informed consent? J Med Ethics. 2006;32:468–472. doi: 10.1136/jme.2005.012633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dickert NW, Mah VA, Baren JM, et al. Enrollment in research under exception from informed consent: the Patients' Experiences in Emergency Research (PEER) study. Resuscitation. 2013;84:1416–1421. doi: 10.1016/j.resuscitation.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dickert NW, Scicluna VM, Baren JM, et al. Patients' perspectives of enrollment in research without consent: the patients' experiences in emergency research-progesterone for the treatment of traumatic brain injury study. Crit Care Med. 2015;43:603–612. doi: 10.1097/CCM.0000000000000747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lo B. Strengthening community consultation in critical care and emergency research. Crit Care Med. 2006;34:2236–2238. doi: 10.1097/01.CCM.0000229632.85246.3A. [DOI] [PubMed] [Google Scholar]
- 16.Woolfall K, Frith L, Gamble C, et al. How parents and practitioners experience research without prior consent (deferred consent) for emergency research involving children with life threatening conditions: a mixed method study. BMJ Open. 2015;5:e008522. doi: 10.1136/bmjopen-2015-008522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wright DW, Yeatts SD, Silbergleit R, et al. Very early administration of progesterone for acute traumatic brain injury. N Engl J Med. 2014;371:2457–2466. doi: 10.1056/NEJMoa1404304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dickert NW, Mah VA, Biros MH, et al. Consulting communities when patients cannot consent: a multicenter study of community consultation for research in emergency settings. Crit Care Med. 2014;42:272–280. doi: 10.1097/CCM.0b013e3182a27759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lynch CA, Houry DE, Dai D, et al. Evidence-based community consultation for traumatic brain injury. Acad Emerg Med. 2011;18:972–976. doi: 10.1111/j.1553-2712.2011.01153.x. [DOI] [PubMed] [Google Scholar]


