Abstract
Objective
Individuals with schizophrenia demonstrate cognitive, social cognitive and motivational deficits that contribute to impairment in real-world functioning. In the current study, we investigated the effects of supplementing computerized neurocognitive training with social cognitive exercises, as compared to neurocognitive training alone.
Methods
In this ongoing, double-blind, randomized controlled trial of 111 participants with psychosis, we compare the effects of supplementing intensive targeted cognitive training with social cognitive training exercises (TCT+SCT), to the effects of targeted cognitive training alone (TCT-only). Participants were assessed on cognition, symptoms, functional capacity and functional outcomes, as well as social cognition and measures related to reward processing.
Results
Both treatment groups showed significant improvement in multiple cognitive domains and improvement in functional capacity. However, as predicted, TCT+SCT group participants showed significant improvement in prosody identification and reward processing relative to TCT-only participants.
Conclusions and Implications for Practice
Our findings indicate that supplementing intensive computerized cognitive training with social cognitive exercises in people with psychosis confers greater benefits in prosody identification and reward processing relative to cognitive training alone, even though both approaches drive significant improvements in cognition and functional capacity. Impairments in both prosody identification and reward processing have been associated with greater negative symptoms and poorer functional outcomes in schizophrenia, raising the possibility that this form of treatment may lead to better long-term outcomes than traditional cognitive training approaches. Follow-up assessments will determine if results are durable and generalize over time to improvements in symptoms and functioning.
ClinicalTrials.gov Identifier
Keywords: Cognitive remediation, neuroplasticity, social cognition, emotion perception, motivation
Introduction
Schizophrenia is associated with significant deficits in neurocognitive functioning, including deficits in attention/vigilance, learning and memory, and executive functioning (Fioravanti et al., 2012). These deficits have been linked to poor social and occupational outcomes, and may account for 20–60% of the variance in real-world outcomes (Green et al., 2000). However, schizophrenia is also associated with significant deficits in social cognition -- the mental operations underlying social interactions (Brothers, L, 1990), including emotion recognition, social cue perception, and theory of mind (Penn et al., 1997; Green et al., 2008; Penn, Sanna, & Roberts, 2008; Pinkham et al., 2003). This can result in misinterpretations of the social intent of others, social withdrawal, and impaired daily social functioning (Fett et al., 2011; Green et al., 2012).
The unique contribution of social cognition deficits to psychosocial and clinical status in schizophrenia was first described by Penn and colleagues (Penn, et al., 1997). A seminal model developed shortly after by Green and Nuechterlein in 1999 proposed that basic neurocognitive deficits contribute to impaired social cognition in schizophrenia, which then impedes patients from developing successful social and occupational roles in real-world settings (Green & Nuechterlein, 1999). Indeed, a large body of research has since demonstrated that social cognitive abilities are unique and important determinants of functional outcome in schizophrenia (Bell et al., 2009; Couture et al., 2006; Vauth et al., 2004). Both meta-analytic work and statistical modeling studies demonstrate that social cognition is more strongly related to community functioning, and explains unique variance in outcomes, above and beyond neurocognition alone (de Jong, de Gelder, & Hodiamont, 2013; Fett et al., 2011; Schmidt, Mueller, & Roder, 2011). However, despite the robustness of these findings, a large proportion of the variance in functional outcome remains unaccounted for by cognitive or social cognitive measures (Couture et al., 2006; Fett et al., 2011), prompting research on additional contributors.
Motivation appears to be one such important contributing factor. In a mediation analysis of 1378 individuals with schizophrenia, Fervaha et al (2015) found that social cognition was related to functioning, and that this relationship was mediated by level of motivation. These findings suggest that: 1) Amotivation undermines social cognitive task performance, which leads to poor functioning, or, alternatively, 2) That poor social cognitive abilities impede motivation, which leads to poor functioning. While seemingly counterintuitive, several studies using path analysis, described below, support the latter hypothesis, and have very important treatment implications: If poor social cognitive abilities impact motivated behavior, will treatments targeting social cognitive deficits lead to enhancement of motivation, and ultimately, to improvement in functioning?
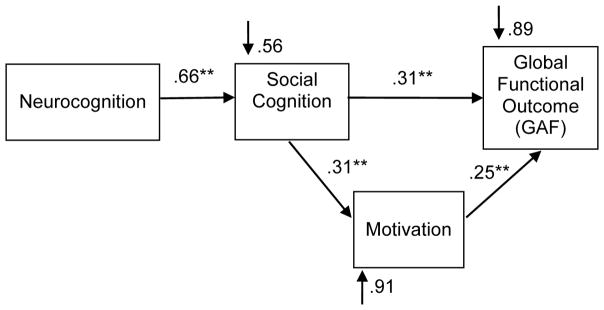
Green et al. (2012), using structural equation modeling, found a single path running through early visual perception, social cognition, and beliefs/motivation to functional outcome. We found a similar relationship using path analysis: social cognition had direct effects on both motivation and functional outcome, and motivation played a significant and mediating role between neurocognition, social cognition, and functional outcome (Gard et al., 2009) (Figure 1). Finally, a recent path analysis found significant direct and indirect paths between social and neurocognition, motivation and negative symptoms, and functional outcome (Bhagyavathi et al., 2015). Notably, 1) Motivation and other negative symptoms mediated the influence of social cognition on functional outcome, and 2) Social cognition was the strongest predictor of motivation and other negative symptoms. As the authors note, these results suggest that correcting social and neurocognitive deficits may improve functioning directly, as well as indirectly by improving motivation and other negative symptoms. The implications are clinically very important, as negative symptoms have so far been notoriously difficult to treat.
Figure 1.
A hypothesized model of the relationship between neurocognition, social cognition, motivation and functional outcome, adapted from Gard et al., 2009. Social cognition has significant direct effects on both motivation and functional outcome. Motivation is a significant mediating factor between social cognition and functional outcome.
The relationship linking social cognition to motivation and functioning in schizophrenia is not surprising, for we now know that the processing of socio-emotional stimuli is intimately integrated with neural systems related to reward, learning, and motivation (e.g. Adolphs, 2009; Millan & Bales, 2013). Social stimuli are highly rewarding and can serve as primary reinforcement, activating reward-related neural structures, such as the ventral striatum and ventromedial prefrontal cortex (Hooker et al., 2006; Kampe et al., 2001; O’Doherty et al., 2003). If people with schizophrenia have basic impairments in perceiving and accurately processing social stimuli, it is likely that this will affect operations in reward circuitry, and it suggests that improving these social cognitive operations might have a beneficial effect on reward processing pathways. A large body of work has linked impairments in reward processing with deficits in motivated behavior and with negative symptoms, suggesting that treatments that could improve reward processing in schizophrenia could also enhance motivated behavior (e.g., Segarra et al., 2015; Strauss, Waltz, & Gold, 2014; Young & Markou, 2015).
In the present study, we sought to capitalize upon this body of research on the relationship between cognition, social cognition, reward processing, and functional outcome and determine whether the effects of “cold” (i.e. non-social) cognitive training could be significantly enhanced by supplementation with computerized exercises that target social cognitive processes. In our previous studies, and consistent with a large body of work, we found that administering 40–50 hours of intensive computerized cognitive training of auditory processing yielded significant gains in multiple cognitive domains relative to a computer games control condition, but no gains in socio-emotional tasks or post-training assessments of functioning (Adcock et al., 2009; Fisher et al., 2009, 2010, and 2015). In contrast, in a pilot open-label study of 24 hours of the social cognitive training exercises used in the present study, and delivered as a stand-alone treatment in individuals with early psychosis, significant gains were shown on measures of social cognition, reward processing, and social functioning (Nahum et al., 2014).
Here, we delivered a large “dose” of targeted auditory and visual system training (TCT) supplemented with intensive social cognitive training (SCT) exercises, for a total of 70 hours of training (i.e. TCT+SCT); we compared the effects to the same number of hours of targeted cognitive training that did not contain any social cognitive exercises (TCT-only). We presented the auditory and visual training modules serially over the course of the study, to avoid potential interference between the two modules (e.g. Rass et al., 2012). We hypothesized that: 1) Both groups would show significant gains in cognition; 2) Supplementation with social cognitive exercises (TCT+SCT) would enhance social cognition relative to targeted cognitive training alone (TCT-only); and 3) The TCT+SCT group would show greater gains in measures of reward processing and functioning relative to the TCT-only group.
Methods
Participants
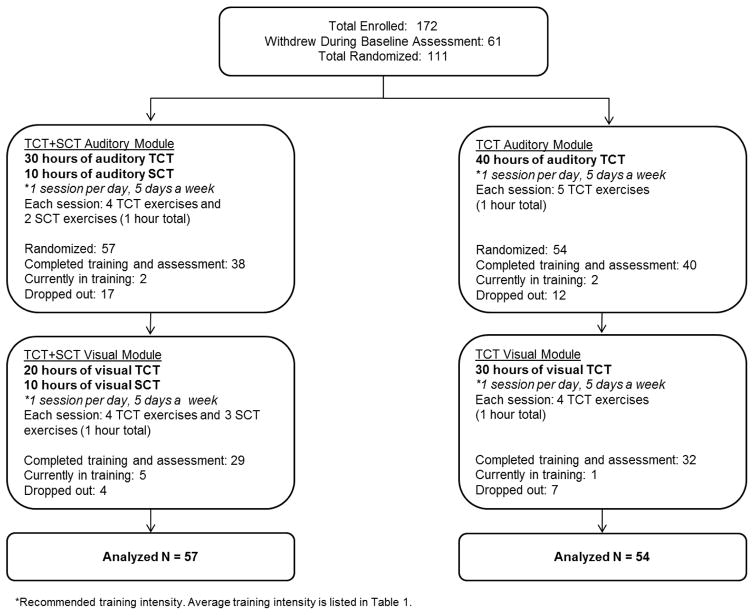
We describe below our sample of 111 randomized participants (ClinicalTrials.gov Identifier: NCT02105779). Clinically stable participants with psychosis were recruited from outpatient clinics and mental health treatment settings in the community. All participants in this study had a diagnosis of schizophrenia, schizoaffective disorder, or psychosis not otherwise specified (NOS). All participants gave written informed consent and underwent a series of baseline clinical and cognitive assessments. Participants were stratified by age, education, and gender and randomly assigned to either the neuroplasticity-based targeted cognitive training plus social cognition training (TCT+SCT) condition or to the targeted cognitive training only condition (TCT-only). Participants were receiving case management in the community but reported no prior cognitive remediation treatment. All participants received $5 at the end of each successful day of training, and an additional $20 bonus for every fifth day of training, which was contingent on attendance only. A CONSORT diagram of enrollment and allocation is shown in Figure 2. We note that a 6 month follow-up assessment is currently in progress and not included in this report. Demographic characteristics are presented in Table 1 and medication regimens are shown in Supplemental Table 1.
Figure 2.
Consort Diagram of Study Participants.
Table 1.
Demographics of Targeted Cognitive + Social Cognitive Training Participants (TCT+SCT) and Targeted Cognitive Training Only Participants (TCT).
| TCT+SCT (N=57) Mean (SD) |
TCT-only (N=54) Mean (SD) |
T-test (p-value) |
|
|---|---|---|---|
| Female(N) / Male(N)a | 13/44 | 19/35 | (0.21) |
|
| |||
| Age | 44.08 (13.05) | 42.37 (12.65) | 0.70 (0.48) |
|
| |||
| Years of Education | 13.77 (2.23) | 13.61 (2.26) | 0.38 (0.71) |
|
| |||
| Wechsler Test of Adult Reading-- Premorbid IQ Estimate | 102.04 (11.75) | 102.51 (11.06) | −0.22 (0.83) |
|
| |||
| Diagnosisb | |||
| Schizophrenia(N) | 39 | 31 | |
| Schizoaffective Disorder(N) | 17 | 22 | 1.48 (0.48) |
| Psychosis NOS (N) | 1 | 1 | |
|
| |||
| Positive and Negative Syndrome Scale Total | 64.90 (17.00) | 62.17 (14.31) | 0.90 (0.37) |
|
| |||
| Quality of Life Scale -- Average Item Rating | 3.10 (1.05) | 3.28 (1.11) | −0.84 (0.40) |
|
| |||
| Social Functioning Scale -- Average Subscale Total | 108.03 (8.22) | 107.92 (8.75) | 0.08 (0.94) |
|
| |||
| Module 1 Dose (hours of training) | 31.84 (15.39) | 33.40 (13.14) | −0.57 (0.57) |
|
| |||
| Module 1 Weeks of Training | 12.05 (6.72) | 13.62 (9.02) | 1.03 (0.30) |
|
| |||
| Module 1 Training Intensity (hours/week) | 2.85 (1.36) | 3.09 (1.77) | −0.80 (0.43) |
|
| |||
| Module 2 Dose (hours of training) | 27.21 (9.70) | 25.76 (10.36) | 0.62 (0.54) |
|
| |||
| Module 2 Weeks of Training | 11.53 (7.22) | 10.16 (8.49) | −0.74 (0.46) |
|
| |||
| Module 2 Training Intensity (hours/week) | 2.98 (1.61) | 3.46 (1.91) | −1.11 (0.27) |
Fisher’s Exact Test results.
Chi-Square Test results.
Interventions
Targeted Cognitive Training (TCT) Exercises
Targeted cognitive training (TCT) was provided by software developed by PositScience, Inc. and consisted of 40 hours of auditory processing training followed by 30 hours of visual processing training. The TCT+SCT group received 30 hours of the general auditory exercises supplemented with 10 hours of auditory social cognition exercises described further below, followed by 20 hours of general visual exercises supplemented with 10 hours of visual social cognition exercises. The TCT-only group received 40 hours of the general auditory exercises, followed by 30 hours of the general visual exercises (Figure 2). In the general auditory and visual exercises, participants were driven to make progressively more accurate distinctions about the spectro-temporal fine-structure of auditory and visual stimuli under conditions of increasing working memory load (i.e. increasing number of stimuli, and decreasing inter-stimulus intervals and duration of stimulus presentation). In the auditory exercises, stimuli across the exercises spanned the acoustic and organizational structure of speech, from very simple acoustic stimuli and tasks (e.g., time order judgments of rapidly successive frequency modulated sweeps) to the complex manipulations of continuous speech (e.g., narrative memory) (Fisher et al., 2009). In the visual exercises, stimuli ranged from simple visual sine-wave sweeps to more complex and ecologically-meaningful visual stimuli such as birds, or cars and road signs. The tasks in the visual exercises ranged from simple perceptual discrimination tasks (e.g. discriminate direction of visual sweeps), to more complex tasks requiring multiple object tracking, divided attention, and spatio-temporal working memory (Surti et al., 2011).
Both auditory and visual exercises were continuously adaptive in that they first established the precise parameters within each stimulus set required for an individual participant to maintain 80% correct performance; once that threshold was determined, task difficulty increased or decreased systematically and parametrically as performance improved or declined. In all exercises, correct performance was heavily rewarded in a game-like fashion: each correct response was followed by novel and amusing visual and auditory embellishments as well as the accumulation of points. After several correct responses, a longer and more elaborate animation was provided. These same principles were applied in the second training module, focused on the visual system. All TCT exercises are listed in Supplemental Table 2.
Social Cognition Training (SCT)
Social cognition training was provided by the SocialVille program, an online (browser / iPad-playable) program developed by Posit Science, and which is designed to treat social cognition deficits in schizophrenia (Nahum et al., 2014). SCT training in the current study comprised of 9 exercises, which collectively target perception, attention and memory in the social cognitive domains of vocal and visual affect perception and social cue perception (gazes and faces). The TCT+SCT group completed the auditory portion of SCT (10 hours) during the auditory module and the visual portion of SCT (10 hours) during the visual module, in order to avoid potential interference in training effects between visual and auditory modalities.
The SCT exercises have similar structure and progressions to those included in the TCT modules, but instead they employ socially-relevant stimuli and discriminations with the goal of improving speed and accuracy in brain systems dedicated to the processing of social information (e.g., Nahum, Lee, & Merzenich, 2013). Participants are driven to make progressively more accurate discriminations in socially-relevant features such as faces, facial expressions, prosodic fluctuations, and gaze directions. For example, in a facial expression exercise the users are required to find a matching emotional expression to that of a rapidly presented target face. Throughout training, the facial expressions become more subtle and more difficult to discriminate, and the pool of emotions becomes larger. As in the suite of auditory and visual exercises, some exercises emphasize processing speed and require speeded discriminations, while others focus on memory, working memory and attention load, which increase progressively in the course of training. All SocialVille exercises used in the study are listed in Supplemental Table 3.
The sequence of training, hours of training, number of exercises completed per session and recommended training intensity are shown in Figure 2. Both groups received the same total number of hours of training, computer exposure, contact with research personnel, and monetary payments.
Average hours of training completed and training intensity during the auditory and visual modules are listed in Table 1. Seventy participants trained at the lab on laptops (39 TCT+SCT, 31 TCT-only) and 41 participants completed training off-site via iPad (18 TCT+SCT, 23 TCT-only). The difference in the number of participants in each group who trained via iPad was non-significant (Fisher’s Exact Test p=0.25). The iPad version of the exercises differed slightly from the laptop version in its user interface and exercise graphics, due to developments in Posit Science’s software packages over the years. However, the ‘active ingredients’ of each exercise were similar across platforms. These include the stimulus sets, stimulus progressions, adaptivity, and exercise logic.
Assessments
Diagnostic assessments were administered at baseline. All other assessments were administered at baseline, after 40 hours of training (the auditory module) and after 70 hours of training (both modules).
Diagnostic Assessment
At study entry, each participant received a standardized diagnostic evaluation performed by research personnel trained in research diagnostic techniques. Evaluations included the Structured Clinical Interview for DSM-IV Axis I Disorders (First et al., 2002), as well as review of clinical records and interview with patient informants (e.g., psychiatrists, therapists, social workers).
Neurocognitive Measures
The MATRICS Consensus Cognitive Battery (MCCB; Nuechterlein et al., 2008) was administered at baseline, and after 40 and 70 hours of training. All measures were distinct and independent from tasks practiced during training. In addition to the learning trials, the verbal and visual memory trials (i.e. delayed recall) of the Hopkins Verbal Learning Test-R (HVLT-R) and Brief Visuospatial Memory Test-R (BVMT-R) were administered. Alternate forms of the HVLT-R, BVMT-R, and Nab Mazes tests were administered and counterbalanced. All tests were scored and re-scored by a second staff member blind to the first scoring.
Social Cognition and Motivation Measures of Reward Processing
Social cognition was assessed with the Mayer-Salovey-Caruso Emotional Intelligence Test (MSCEIT) Perceiving Emotions and Managing Emotions branches (Mayer et al., 2003), the Prosody Identification Test (PROID; Juslin & Laukka, 2001), and the Faux Pas Recognition Test which assesses theory of mind (Gregory et al., 2002; Stone, Baron-Cohen, & Knight, 1998). Thirty-eight TCT+SCT and 40 TCT-only participants completed the PROID which was added to the battery after the study was initiated. Alternate forms of the Faux Pas Recognition Test were created (odd versus even numbered items) and counterbalanced.
The Temporal Experience of Pleasure Scale (TEPS; Gard, et al., 2006) was used as a proxy measure of reward processing related to motivated behavior. The TEPS assesses anticipatory pleasure and consummatory pleasure. Anticipatory pleasure is closely linked to motivation and goal-directed behavior while consummatory pleasure is associated with satiation (i.e. wanting versus liking). Research indicates that schizophrenia patients may have a deficit in anticipatory pleasure, but normal consummatory pleasure (Gard et al., 2007).
To assess engagement during training, participants rated their level of interest/enjoyment in the cognitive training exercises using the 7-item subscale of Interest/Enjoyment from the Intrinsic Motivation Inventory - a 1–7 Likert scale, with higher scores corresponding to greater interest/enjoyment (Deci et al., 1994).
Symptom and Functional Outcome Measures
Symptom severity was assessed using the Positive and Negative Syndrome Scale (PANSS, Kay, Fiszbein, & Opler, 1987). Functional capacity was assessed with the University of California, San Diego, Performance-Based Skills Assessment--Brief (UPSA-Brief; Mausbach et al., 2007), in which participants are asked to role-play tasks in 2 areas of functioning: Communication and Finances. Quality of life was assessed using an abbreviated version of the Quality of Life Scale (QLS, Bilker et al., 2003; Heinrichs, Hanlon, & Carpenter, 1984), and using the Social Functioning Scale (Birchwood et al., 1990).
Research staff who conducted clinical or cognitive testing first completed extensive training on testing/interviewing and scoring criteria of individual items (e.g., scoring videotaped sessions, observation of sessions conducted by experienced staff, and participating in mock sessions). In our laboratory, intra-class correlation coefficients (ICC) are greater than 0.85 for the PANSS and QLS Total and subscale scores. Participants and assessment personnel were blind to group assignment.
Statistical Analyses
The MCCB computerized scoring program was used to compute age and gender adjusted T-scores and the composite scores. All variables were screened and normally distributed after winsorising of outlying values. An intent-to-treat analysis of all randomized participants was conducted (N=111) using a linear mixed-effects model with group and time as fixed factors. Model parameters were estimated using restricted maximum likelihood. Participant groups were compared on the change in the MCCB T-scores (listed in Table 2), measures of social cognition and motivation, the PANSS, UPSA-Brief, QLS, SFS totals and subscale scores. Effect sizes (Cohen’s d) were calculated using mean change scores (baseline to post-training) and change score standard deviations. Independent Samples T-tests tested for group differences in demographic variables, hours of training, and training intensity. Fisher’s Exact Test or Chi-Sqaure Test tested for group differences in categorical variables (i.e. gender, diagnosis, medications, attrition).
Table 2.
Scores on Cognitive, Social Cognitive, and Reward Processing Outcome Measures, and Symptom Ratings and Functional Outcomes at Baseline, 40, and 70 Hours of Training in Participants who Received Targeted Cognitive Training plus Social Cognition Training (TCT+SCT) or Targeted Cognitive Training only (TCT-only).
| TCT+SCT | TCT-only | |||||||
|---|---|---|---|---|---|---|---|---|
| Outcome Measures | Baseline Mean (SE) N=57 |
40 Hours Mean (SE) N=38 |
70 Hours Mean (SE) N=29 |
Baseline Mean (SE) N=54 |
40 Hours Mean (SE) N=40 |
70 Hours Mean (SE) N=32 |
Main Effects of Time p Value |
Group x Time Interaction p Value |
| MATRICS Consensus Cognitive Batterya | ||||||||
| Global Cognition | 29.41 (1.89) | 31.69 (1.99) | 34.11 (2.22) | 31.84 (1.92) | 34.35 (1.99) | 35.09 (2.22) | <0.001 | 0.58 |
| Attention | 37.66 (1.73) | 37.46 (2.04) | 40.25 (2.08) | 39.36 (1.78) | 42.82 (2.04) | 43.16 (2.07) | 0.03 | 0.28 |
| Speed of Processing | 34.75 (1.77) | 35.60 (2.12) | 37.51 (2.32) | 36.62 (1.80) | 38.55) (2.13) | 41.46 (2.31) | 0.001 | 0.64 |
| Working Memory | 39.61 (1.70) | 39.41 (1.74) | 41.01 (1.94) | 39.97 (1.75) | 41.23 (1.75) | 42.14 (1.90) | 0.21 | 0.84 |
| Verbal Learning | 36.45 (1.16) | 38.04 (1.32) | 38.90 (1.83) | 37.91 (1.20) | 40.23 (1.32) | 39.99 (1.75) | 0.02 | 0.70 |
| Verbal Memoryb | 29.87 (2.04) | 28.82 (2.45) | 29.44 (2.76) | 32.52 (2.10) | 33.83 (2.45) | 32.28 (2.65) | 0.96 | 0.55 |
| Visual Learning | 35.63 (1.80) | 37.48 (1.99) | 42.22 (2.25) | 39.44 (1.85) | 43.02 (1.98) | 41.92 (2.20) | 0.01 | 0.07 |
| Visual Memoryb | 33.86 (2.22) | 37.52 (2.38) | 40.24 (2.91) | 38.13 (2.26) | 39.35 (2.35) | 38.87 (2.85) | 0.12 | 0.29 |
| Problem Solving | 39.61 (1.41) | 38.88 (1.54) | 43.08 (1.70) | 41.38 (1.45) | 43.50 (1.55) | 44.41 (1.69) | 0.002 | 0.11 |
| Social Cognition and Reward Processing Measures | ||||||||
| MSCEIT Perceiving Emotions | 42.30 (1.45) | 43.47 (1.79) | 45.58 (2.03) | 44.18 (1.49) | 44.75 (1.79) | 43.53 (2.01) | 0.50 | 0.32 |
| MSCEIT Managing Emotions | 40.40 (1.29) | 41.57 (1.29) | 41.30 (1.32) | 38.99 (1.33) | 39.22 (1.29) | 38.97 (1.32) | 0.70 | 0.60 |
| Prosody Identification Correct Responsesc | 31.48 (1.21) | 35.61 (1.74) | 37.98 (1.98) | 34.68 (1.91) | 35.21 (1.68) | 37.07 (1.94) | 0.001 | 0.05 |
| Prosody Identification Reaction Time (ms) | 4883.10 (194.47) | 4413.47 (192.86) | 3954.28 (139.52) | 4280.49 (190.41) | 4249.95 (183.16) | 4138.21 (135.92) | 0.001 | 0.03 |
| Faux Pas Test Percent Correct | 74.19 (1.96) | 77.61 (2.38) | 80.03 (2.72) | 76.56 (2.02) | 77.86 (2.34) | 78.94 (2.64) | 0.03 | 0.64 |
| TEPS Total Mean Item Score | 4.14 (0.11) | 4.37 (0.13) | 4.52 (0.13) | 4.41 (0.12) | 4.33 (0.13) | 4.23 (0.13) | 0.53 | 0.02 |
| TEPS Anticipatory Pleasure | 4.05 (0.12) | 4.27 (0.14) | 4.43 (0.15) | 4.34 (0.12) | 4.25 (0.14) | 4.12 (0.14) | 0.72 | 0.06 |
| TEPS Consummatory Pleasure | 4.23 (0.14) | 4.47 (0.14) | 4.63 (0.15) | 4.47 (0.14) | 4.40 (0.14) | 4.35 (0.15) | 0.32 | 0.04 |
| Symptom Ratings | ||||||||
| PANSS Total | 64.90 (2.09) | 63.00 (2.65) | 64.16 (2.71) | 62.17 (2.20) | 59.97 (2.69) | 57.72 (2.63) | 0.33 | 0.40 |
| PANSS Positive Symptoms | 15.68 (0.71) | 14.49 (0.74) | 14.97 (0.85) | 14.97 (0.74) | 15.62 (0.75) | 13.96 (0.85) | 0.17 | 0.09 |
| PANSS Negative Symptoms | 16.53 (0.72) | 15.83 (0.75) | 15.83 (0.84) | 14.81 (0.75) | 13.35 (0.77) | 14.02 (0.81) | 0.09 | 0.14 |
| PANSS General Psychopathology | 32.68 (1.12) | 31.95 (1.54) | 32.08 (1.54) | 32.39 (1.17) | 31.17 (1.56) | 30.33 (1.48) | 0.42 | 0.97 |
| Functional Outcomes | ||||||||
| UPSA-Briefd Total | 69.60 (1.92) | 70.35 (2.29) | 71.05 (2.50) | 70.91 (1.97) | 72.95 (2.28) | 77.48 (2.43) | 0.05 | 0.30 |
| Quality of Life Scale Mean Item Score | 3.10 (0.14) | 3.22 (0.15) | 3.24 (0.17) | 3.28 (0.15) | 3.40 (0.16) | 3.32 (0.17) | 0.35 | 0.77 |
| Social Functioning Scale—Average Subscale Score | 108.03 (1.14) | 108.20 (1.27) | 108.46 (1.14) | 107.92 (1.17) | 108.91 (1.27) | 107.28 (1.13) | 0.55 | 0.53 |
MATRICS Consensus Cognitive Battery (MCCB) Measures: Global Cognition (composite T-score across all MCCB measures); Attention (Continuous Performance Task-Identical Pairs); Speed of Processing (Trail Making Test Part A; Category Fluency Animal Naming; BACS Symbol Coding); Working Memory (Letter-Number Span; WMS-III Spatial Span); Verbal Learning (HVLT-R Immediate Recall); Visual Learning (BVMT-R Immediate Recall); Problem Solving (NAB Mazes); MSCEIT Managing Emotions (listed in Table 3).
In addition to the MCCB, verbal and visual Delayed Recall from the HVLT-R and BVMT-R were administered;
78 of the 111 participants completed the Prosody Identification Test.
University of California, San Diego, Performance-Based Skills Assessment—Brief.
Results
Demographics, Baseline Performance, and Attrition
At baseline, there were no significant differences between the TCT+SCT and TCT-only groups in demographic characteristics, cognitive measures, symptom severity, functioning, or medication regimens (Table 1 and Supplemental Table 1). TCT+SCT participants rated the training as slightly more enjoyable (M=5.24, SD=1.25) relative to TCT-only (M=4.79, SD=1.44), a non-significant difference (t(47) = 1.18, p = 0.25).
Attrition rates were also similar between the two groups (see Figure 2). A total of 21 TCT+SCT (37%) and 19 TCT-only (35%) dropped out from the study, a non-significant difference (Fisher’s Exact Test p=1.00). Participants who dropped out from the study and those who remained in the study were compared on demographic variables, baseline cognitive performance, symptoms, functional outcomes, and hours of training and training intensity (Supplemental Table 4). All group differences were non-significant with the exception of hours of training and training intensity, where those who dropped out completed less total hours and at fewer hours per week. Participants who completed training via iPad versus desktop computer were compared on these same characteristics (Supplemental Table 5). Participants who completed iPad training were significantly younger, with higher premorbid IQ, lower symptom severity, and completed less hours of training per week (training intensity) compared to participants who completed training in the lab. All differences in baseline cognition and functioning were non-significant.
The Effects of Targeted Cognitive Training on Cognition
In the total sample, omnibus test results showed significant main effects of time in Global Cognition (d=.63), Attention (d=.30), Speed of Processing (d=.42), Verbal Learning (d=.28), Visual Learning (d=.50), and Problem Solving (d=.40) (Table 2). All group-by-time interactions were non-significant.
The Effects of Social Cognition Training on Social Cognition and Reward Processing
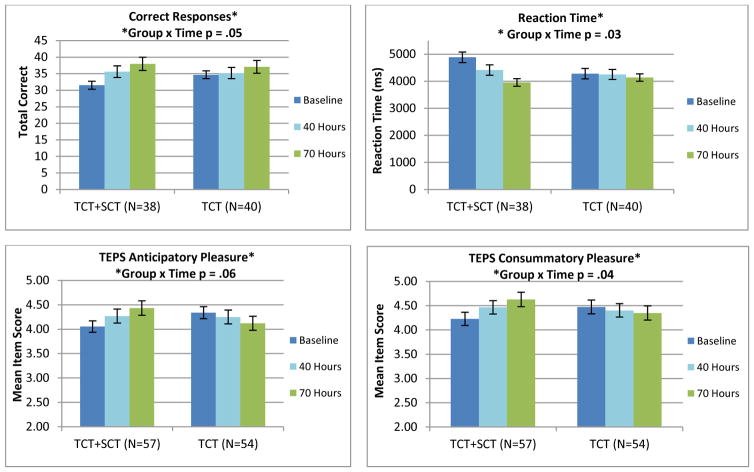
Omnibus test results showed significant group-by-time interactions with the TCT+SCT group showing greater improvements in the Prosody Identification Task Correct Responses (d=.53) and Reaction Time (d=-.74), the TEPS Total Mean Item Score (d=.81), the TEPS Consummatory Pleasure scale (d=.77), and group differences at trend level significance in the TEPS Anticipatory Pleasure (d=.68), relative to the TCT-only group. The Faux Pas Test showed a significant main effect of time, with both groups showing improvement (d=.31) (Table 2 and Figure 3)
Figure 3.
Performance in a) Prosody Identification, b) Prosody Identification Reaction Time, c) TEPS Anticipatory Pleasure, and d) TEPS Consummatory Pleasure at Baseline, 40, and 70 Hours of Training in the TCT+SCT and TCT-only Participant Groups.
The Effects of Targeted Cognitive Training on Symptoms and Functioning
The UPSA-Brief omnibus test showed a significant main effect of time (d=.37). All changes in symptom severity, the Quality of Life Scale, and Social Functioning Scale were non-significant (Table 2).
Discussion
Intensive Computerized Cognitive Training Drives a Range of Cognitive Improvements
Our interim results indicate that across both groups, a total of 70 hours of training -- 40 hours of auditory systems training followed by 30 hours of visual systems training -- drives significant gains in Global Cognition, Attention, Speed of Processing, Verbal Learning, Visual Learning, and Problem Solving. These results are consistent with our previous study (Fisher et al., 2010; Subramaniam et al., 2014; Subramaniam et al., 2012) where we found that a higher “dose” and a “broad spectrum” training of both auditory and visual systems applied serially drives improvements in a large number of cognitive domains. We found no differential effect in “cold” cognition gains in the TCT+SCT vs. TCT-only groups, despite the fact that the TCT+SCT group received fewer hours of pure cold cognition exercises than the TCT-only group (30 vs. 40 in the auditory module, and 20 vs. 30 in the visual module). This finding suggests that social cognitive training exercises can supplement a cognitive training regime without necessitating an increase in overall hours of training, and while still conserving the beneficial effects on general cognitive outcome measures. We note also that the magnitude of the effects on cognition in the present study ranged from small to medium, while in our prior studies, effect sizes ranged predominantly from medium to large. This difference is likely due to the computer games control condition used in our prior studies which drove small non-significant decreases in verbal cognitive measures in the control group, and which contributed to the overall magnitude of the effects.
Supplementation with Social Cognition Training Shows Positive Effects in Measures of Prosody Identification
As predicted, TCT+SCT participants showed significantly larger improvements in prosody identification, and in prosody identification reaction time, relative to TCT-only participants. In our pilot study (Nahum et al., 2014), we found that 24 hours of SocialVille training in recent-onset schizophrenia patients yielded similar improvements in prosody identification. Effect sizes in these social cognition measures were larger relative to effect sizes in the neurocognitive measures, and consistent with a recent meta-analysis (Grynszpan et al., 2011).
Using emotional prosody stimuli developed by the same authors as was used in the current study (Juslin & Laukka, 2001), Gold et al., 2012 found that patients with schizophrenia show a large deficit in the ability to recognize emotion based on tone of voice, and this was significantly associated with impairment in detecting the underlying acoustic features such as change in pitch. A second study (Kantrowitz et al., 2013) showed that this reduced sensitivity to acoustic features correlated not just with deficits in emotional prosody identification but also with negative symptoms—supporting the notion that sensory-based social cognition training strategies to improve emotional prosody identification might ultimately have a benefit on negative symptoms such as amotivation.
There were no significant group differences in the MSCEIT Perceiving Emotions subscale. The TCT+SCT group showed the expected pattern of gains from baseline to 70 hours of training (d =.25) and the TCT-only group showed a small decline (d = −.05). It is possible that the study was underpowered to detect these group differences; it is also possible that the MSCEIT Perceiving Emotions subscale does not detect the changes harnessed by the Socialville exercises used in this study: speed of processing and working memory for elemental prosodic and facial affect information. There were also no significant group differences in the MSCEIT Managing Emotions or Faux Pas test. These measures assess higher-order social cognitive operations: Managing Emotions assesses participants’ ability to judge the actions that are most effective in obtaining a specified emotional outcome for an individual in a story, while the Faux Pas test assesses theory of mind. Exercises that specifically target higher-order cognitive processes (e.g. social perception, theory of mind) are likely needed to drive improvements in these domains. A meta-analysis of controlled trials of social cognition training found large effect sizes in affect recognition, moderate effects on theory of mind, and non-significant effects on social perception and attributional style (Kurtz & Richardson, 2012). Recent reviews have echoed these findings (Fiszdon & Reddy, 2012; Kurtz et al., 2015).
Supplementation with Social Cognition Training Drives Improvements in Self-Report Measures of Reward Processing
A key result of the current study is that TCT+SCT participants showed significantly larger gains from baseline to 70 hours of training in self-ratings of TEPS Anticipatory and Consummatory Pleasure, relative to TCT-only participants. This result is consistent with similar changes in the TEPS Anticipatory Pleasure seen in our open-label pilot study (Nahum et al., 2014). Consummatory and anticipatory pleasure are basic behavioral manifestations of neural reward processing operations, and have been linked to motivated and/or goal-directed behavior (Cooper et al., 2015; Gard et al., 2007; Reddy et al., 2015; Strauss & Gold, 2012; Wang et al., 2015). Interestingly, in a recent baseline imaging study on a subset of our participants, we found an association between self-ratings of Consummatory Pleasure and abnormal patterns of activation in right inferior parietal lobe during anticipation of reward in the monetary incentive delay task (Subramaniam et al., 2015). Together, the emerging cognitive neuroscience data suggest that the subjective experience of anticipating and receiving reward are related to specific activation patterns in prefrontal-ventral striatal systems, that abnormal patterns are present in schizophrenia, and that impaired reward-processing is related to deficits in real-world motivation and negative symptoms (Segarra et al., 2015; Yan et al., 2015; Young & Markou, 2015; for reviews, see Kring & Barch, 2014; Simpson et al., 2012; and Strauss et al., 2014). To the best of our knowledge, ours is the first randomized controlled trial to demonstrate that intensive training of social cognitive operations in schizophrenia could serve as a critical leverage point into driving adaptive change in these systems (Gard et al., 2009; Green et al., 2012). If successful, this may represent an important new treatment paradigm for addressing negative symptoms, which are extremely difficult to treat and which are accompanied by a high degree of functional impairment. However, our results require confirmation with a larger sample size since the omnibus test of Anticipatory Pleasure was at trend level significance (p=.06).
Intensive Cognitive Training May Have a Beneficial Effect on Functional Capacity
Participants in the total sample showed significant gains in the UPSA-Brief after 70 hours of training -- a measure of functional capacity that involves role-playing of skills related to daily functioning (e.g., counting and making change, paying bills, making a doctor’s appointment). The UPSA-Brief is highly correlated with cognition, including the MCCB global cognition composite score (Buchanan et al., 2011). Thus, it is possible that the cognitive gains generalized to gains in functional capacity. However, we found no significant changes in the Quality of life Scale or Social Functioning Scale, consistent with our prior reports (Fisher et al., 2009 and 2014). It would be highly unlikely that any training-induced cognitive improvements would have an immediate direct effect on these measures. In the recent report by McGurk et al. (2015), better occupational functioning over an 18 month period was seen after a combination of supported employment and cognitive remediation in individuals with serious mental illness (vs. supported employment alone), suggesting that cognitive improvement can open a window of opportunity for consolidation of functional gains under the right psychosocial learning circumstances. Indeed, in our imaging studies we found a significant positive relationship between training-induced enhancements of prefrontal cortical activity and better ratings on social and occupational domains of the Quality of Life Scale six months after completion of training (Subramaniam et al., 2012 and 2014).
Comparison to Other Studies of Combined Cognitive and Social Cognitive Computerized Training
Our results show some consistency with three studies that have tested the effects of computerized cognitive training combined with computerized social cognition training. In a pilot, single-arm study of 19 individuals with schizophrenia, our group combined 50 hours of auditory training with 12 hours of computerized training in emotion identification, social perception, and theory of mind tasks (Sacks et al., 2013). Participants showed significant improvements on multiple measures of neurocognition, social cognition (including the MSCEIT Perceiving Emotions and Managing Emotions branches), self-referential source memory, and a significant decrease in positive symptoms.
Lindenmayer and colleagues (Lindenmayer et al., 2013) tested the effects of 36 hours of CogPack exercises versus 24 hours of CogPack exercises plus 12 hours of social cognition exercises in 59 individuals with schizophrenia. Both groups showed improvement in cognition, while participants who received the combined training showed greater improvement in emotion recognition and discrimination, in the MSCEIT Managing Emotions branch, and greater MCCB improvement in global cognition, attention/vigilance, speed of processing, and working memory. The combined training group also showed greater improvement in social functioning, but no change in the PANSS subscales.
A third study compared the effects of 30 hours of computerized cognitive and social cognitive exercises from NeuroPersonalTrainer to the effects of 30 hours of computer tasks (e.g. computer skills training, internet games, and documentary videos) in 53 patients early in the course of the illness (Fernandez-Gonzalo et al., 2015). The combined cognitive and social cognitive training group showed significantly greater gains in visual working memory, visual learning, and emotion recognition. Similar to our study, there were no gains in measures of higher-order social cognition (e.g. theory of mind), and both groups showed improvement in PANSS subscales.
Study Limitations and Future Directions
One limitation of this study is the interim analysis conducted. While the findings suggest improvement in cognition and functional capacity in the total sample, and greater gains in prosody identification and reward processing in the TCT+SCT group relative to the TCT-only group, we did not report on the effects at 6 months post-training since these follow-up assessments are still in progress.
The attrition rate is another limitation. By 70 hours of training, 37% of the TCT+SCT participants and 35% of the TCT-only participants dropped out from the study. This attrition rate is greater relative to other studies of combined cognitive and social cognitive computerized training exercises for individuals with schizophrenia. Lindenmayer et al. (2013) showed a low attrition rate of 7% when training was provided for inpatients in the context of a 20-hour per week psychiatric rehabilitation program. Fernandez-Gonzalo et al. (2015) showed an attrition rate of 25% in outpatients who were in the early phases of the illness. Neuropsychologists in this study played an active role during all training sessions and provided individual coaching. The goal of the present study was to test the effects of the training when delivered as a stand-alone treatment. While both groups rated the training as “somewhat enjoyable,” the high attrition rate has implications for the delivery of treatment in real-world settings, and additional research is needed to determine how to increase adherence and retention.
Third, participants in this study were well-educated, with an average age of 43, which may limit the generalizability of our results to other samples. Finally, while we found significant improvement in functional capacity in the total sample, neither group showed significant improvement in symptom severity, quality of life, or social functioning immediately after training. In contrast, an open-label study of the Socialville exercises in young adults early in the course of schizophrenia showed significant gains in social functioning immediately after training (Nahum et al., 2014). It may be that gains in functioning in individuals with a longer course of schizophrenia are not evident immediately after training and will be evident only after a longer follow-up period. Or the difference between these studies might be the result of differences in the measures of functional outcome used.
Our findings indicate that an intensive cognitive training approach drives improvements in cognition and functional capacity in people with persistent psychotic illness, and that supplementation with social cognition training enhances prosody identification and self-report measures of reward processing. Follow-up studies must determine whether gains in prosody identification and measures of reward processing translate into reduced negative symptoms and enhanced functioning—the holy grail of treatment research in schizophrenia (Keefe & Kraus, 2012).
Supplementary Material
References
- Adcock RA, Dale C, Fisher M, Aldebot S, Genevsky A, Simpson GV, … Vinogradov S. When top-down meets bottom-up: auditory training enhances verbal memory in schizophrenia. Schizophrenia Bulletin. 2009;35(6):1132–1141. doi: 10.1093/schbul/sbp068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R. The Social Brain: Neural Basis of Social Knowledge. Annual Review of Psychology. 2009;60:693–716. doi: 10.1146/annurev.psych.60.110707.163514. http://doi.org/10.1146/annurev.psych.60.110707.163514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell M, Tsang HWH, Greig TC, Bryson GJ. Neurocognition, social cognition, perceived social discomfort, and vocational outcomes in schizophrenia. Schizophrenia Bulletin. 2009;35(4):738–747. doi: 10.1093/schbul/sbm169. http://doi.org/10.1093/schbul/sbm169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagyavathi HD, Mehta UM, Thirthalli J, Kumar CN, Kumar JK, Subbakrishna DK, Gangadhar BN. Cascading and combined effects of cognitive deficits and residual symptoms on functional outcome in schizophrenia–A path-analytical approach. Psychiatry Research. 2015;229(1–2):264–271. doi: 10.1016/j.psychres.2015.07.022. http://doi.org/10.1016/j.psychres.2015.07.022. [DOI] [PubMed] [Google Scholar]
- Bilker WB, Brensinger C, Kurtz MM, Kohler C, Gur RC, Siegel SJ, Gur RE. Development of an abbreviated schizophrenia quality of life scale using a new method. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology. 2003;28(4):773–7. doi: 10.1038/sj.npp.1300093. http://doi.org/Research Support, U.S. Gov’t, P.H.S. [DOI] [PubMed] [Google Scholar]
- Birchwood M, Smith J, Cochrane R, Wetton S, Copestake S. The Social Functioning Scale. The development and validation of a new scale of social adjustment for use in family intervention programmes with schizophrenic patients. The British Journal of Psychiatry: The Journal of Mental Science. 1990;157:853–859. doi: 10.1192/bjp.157.6.853. [DOI] [PubMed] [Google Scholar]
- Brothers L. The Social Brain: A project for integrating primate behavior and neurophysiology in a new domain. Concepts in Neuroscience. 1990;1:27–61. [Google Scholar]
- Buchanan RW, Keefe RSE, Umbricht D, Green MF, Laughren T, Marder SR. The FDA-NIMH-MATRICS guidelines for clinical trial design of cognitive-enhancing drugs: what do we know 5 years later? Schizophrenia Bulletin. 2011;37(6):1209–1217. doi: 10.1093/schbul/sbq038. http://doi.org/10.1093/schbul/sbq038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S, Lavaysse LM, Gard DE. Assessing motivation orientations in schizophrenia: Scale development and validation. Psychiatry Research. 2015;225(1–2):70–78. doi: 10.1016/j.psychres.2014.10.013. http://doi.org/10.1016/j.psychres.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couture SM, Penn DL, Roberts DL. The functional significance of social cognition in schizophrenia: a review. Schizophrenia Bulletin. 2006;32(Suppl 1):S44–63. doi: 10.1093/schbul/sbl029. http://doi.org/10.1093/schbul/sbl029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deci EL, Eghrari H, Patrick BC, Leone DR. Facilitating internalization: the self-determination theory perspective. Journal of Personality. 1994;62(1):119–142. doi: 10.1111/j.1467-6494.1994.tb00797.x. [DOI] [PubMed] [Google Scholar]
- de Jong JJ, de Gelder B, Hodiamont PPPG. Sensory processing, neurocognition, and social cognition in schizophrenia: towards a cohesive cognitive model. Schizophr Res. 2013;146(1–3):209–16. doi: 10.1016/j.schres.2013.02.034. http://doi.org/10.1016/j.schres.2013.02.034. [DOI] [PubMed] [Google Scholar]
- Fernandez-Gonzalo S, Turon M, Jodar M, Pousa E, Hernandez Rambla C, García R, Palao D. A new computerized cognitive and social cognition training specifically designed for patients with schizophrenia/schizoaffective disorder in early stages of illness: A pilot study. Psychiatry Research. 2015;228(3):501–509. doi: 10.1016/j.psychres.2015.06.007. http://doi.org/10.1016/j.psychres.2015.06.007. [DOI] [PubMed] [Google Scholar]
- Fervaha G, Siddiqui I, Foussias G, Agid O, Remington G. Motivation and Social Cognition in Patients with Schizophrenia. Journal of the International Neuropsychological Society. 2015;21(06):436–443. doi: 10.1017/S1355617715000375. http://doi.org/10.1017/S1355617715000375. [DOI] [PubMed] [Google Scholar]
- Fett AKJK, Viechtbauer W, Dominguez MGD, Penn DL, van Os J, Krabbendam L. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neurosci Biobehav Rev. 2011;35(3):573–88. doi: 10.1016/j.neubiorev.2010.07.001. http://doi.org/10.1016/j.neubiorev.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Fioravanti M, Bianchi V, Cinti ME. Cognitive deficits in schizophrenia: an updated metanalysis of the scientific evidence. BMC Psychiatry. 2012;12:64. doi: 10.1186/1471-244X-12-64. http://doi.org/10.1186/1471-244X-12-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis 1 Disorders, Research Version, Patient Edition. (SCID-I/P) New York, NY: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Fisher M, Holland C, Merzenich MM, Vinogradov S. Using neuroplasticity-based auditory training to improve verbal memory in schizophrenia. The American Journal of Psychiatry. 2009;166(7):805–811. doi: 10.1176/appi.ajp.2009.08050757. http://doi.org/10.1176/appi.ajp.2009.08050757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Holland C, Subramaniam K, Vinogradov S. Neuroplasticity-based cognitive training in schizophrenia: an interim report on the effects 6 months later. Schizophrenia Bulletin. 2010;36(4):869–879. doi: 10.1093/schbul/sbn170. http://doi.org/10.1093/schbul/sbn170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Loewy R, Carter C, Lee A, Ragland JD, Niendam T, … Vinogradov S. Neuroplasticity-Based Auditory Training Via Laptop Computer Improves Cognition in Young Individuals With Recent Onset Schizophrenia. Schizophrenia Bulletin. 2015;41(1):250–258. doi: 10.1093/schbul/sbt232. http://doi.org/10.1093/schbul/sbt232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiszdon JM, Reddy LF. Review of social cognitive treatments for psychosis. Clinical Psychology Review. 2012;32(8):724–740. doi: 10.1016/j.cpr.2012.09.003. http://doi.org/10.1016/j.cpr.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Gard DE, Fisher M, Garrett C, Genevsky A, Vinogradov S. Motivation and its relationship to neurocognition, social cognition, and functional outcome in schizophrenia. Schizophr Res. 2009;115(1):74–81. doi: 10.1016/j.schres.2009.08.015. http://doi.org/10.1016/j.schres.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard DE, Gard MG, Kring AM, John OP. Anticipatory and consummatory components of the experience of pleasure: A scale development study. Journal of Research in Personality. 2006;40(6):1086–1102. http://doi.org/10.1016/j.jrp.2005.11.001. [Google Scholar]
- Gard DE, Kring AM, Gard MG, Horan WP, Green MF. Anhedonia in schizophrenia: distinctions between anticipatory and consummatory pleasure. Schizophrenia Research. 2007;93(1–3):253–260. doi: 10.1016/j.schres.2007.03.008. http://doi.org/10.1016/j.schres.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold R, Butler P, Revheim N, Leitman DI, Hansen JA, Gur RC, … Javitt DC. Auditory Emotion Recognition Impairments in Schizophrenia: Relationship to Acoustic Features and Cognition. American Journal of Psychiatry. 2012;169(4):424–432. doi: 10.1176/appi.ajp.2011.11081230. http://doi.org/10.1176/appi.ajp.2011.11081230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Hellemann G, Horan WP, Lee J, Wynn JK. From perception to functional outcome in schizophrenia: modeling the role of ability and motivation. Arch Gen Psychiatry. 2012;69(12):1216–24. doi: 10.1001/archgenpsychiatry.2012.652. http://doi.org/10.1001/archgenpsychiatry.2012.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophrenia Bulletin. 2000;26(1):119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH. Should schizophrenia be treated as a neurocognitive disorder? Schizophrenia Bulletin. 1999;25(2):309–319. doi: 10.1093/oxfordjournals.schbul.a033380. [DOI] [PubMed] [Google Scholar]
- Green MF, Penn DL, Bentall R, Carpenter WT, Gaebel W, Gur RC, … Heinssen R. Social cognition in schizophrenia: an NIMH workshop on definitions, assessment, and research opportunities. Schizophrenia Bulletin. 2008;34(6):1211–1220. doi: 10.1093/schbul/sbm145. http://doi.org/10.1093/schbul/sbm145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory C, Lough S, Stone V, Erzinclioglu S, Martin L, Baron-Cohen S, Hodges JR. Theory of mind in patients with frontal variant frontotemporal dementia and Alzheimer’s disease: theoretical and practical implications. Brain: A Journal of Neurology. 2002;125(Pt 4):752–764. doi: 10.1093/brain/awf079. [DOI] [PubMed] [Google Scholar]
- Grynszpan O, Perbal S, Pelissolo A, Fossati P, Jouvent R, Dubal S, Perez-Diaz F. Efficacy and specificity of computer-assisted cognitive remediation in schizophrenia: a meta-analytical study. Psychological Medicine. 2011;41(01):163–173. doi: 10.1017/S0033291710000607. [DOI] [PubMed] [Google Scholar]
- Heinrichs DW, Hanlon TE, Carpenter WT. The Quality of Life Scale: an instrument for rating the schizophrenic deficit syndrome. Schizophrenia Bulletin. 1984;10(3):388–98. doi: 10.1093/schbul/10.3.388. http://doi.org/Research Support, U.S. Gov’t, P.H.S. [DOI] [PubMed] [Google Scholar]
- Hooker CI, Germine LT, Knight RT, D’Esposito M. Amygdala Response to Facial Expressions Reflects Emotional Learning. The Journal of Neuroscience. 2006;26(35):8915–8922. doi: 10.1523/JNEUROSCI.3048-05.2006. http://doi.org/10.1523/JNEUROSCI.3048-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juslin PN, Laukka P. Impact of intended emotion intensity on cue utilization and decoding accuracy in vocal expression of emotion. Emotion (Washington, DC) 2001;1(4):381–412. doi: 10.1037/1528-3542.1.4.381. [DOI] [PubMed] [Google Scholar]
- Kampe KKW, Frith CD, Dolan RJ, Frith U. Psychology: Reward value of attractiveness and gaze. Nature. 2001;413(6856):589–589. doi: 10.1038/35098149. http://doi.org/10.1038/35098149. [DOI] [PubMed] [Google Scholar]
- Kantrowitz JT, Leitman DI, Lehrfeld JM, Laukka P, Juslin PN, Butler PD, … Javitt DC. Reduction in tonal discriminations predicts receptive emotion processing deficits in schizophrenia and schizoaffective disorder. Schizophrenia Bulletin. 2013;39(1):86–93. doi: 10.1093/schbul/sbr060. http://doi.org/10.1093/schbul/sbr060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bulletin. 1987;13(2):261–76. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Keefe RSE, Kraus MS. Clues to the cognitive and perceptual origins of social isolation and psychosis in schizophrenia. The American Journal of Psychiatry. 2012;169(4):354–357. doi: 10.1176/appi.ajp.2012.12010042. http://doi.org/10.1176/appi.ajp.2012.12010042. [DOI] [PubMed] [Google Scholar]
- Kring AM, Barch DM. The motivation and pleasure dimension of negative symptoms: neural substrates and behavioral outputs. European Neuropsychopharmacology: The Journal of the European College of Neuropsychopharmacology. 2014;24(5):725–736. doi: 10.1016/j.euroneuro.2013.06.007. http://doi.org/10.1016/j.euroneuro.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz MM, Gagen E, Rocha NBF, Machado S, Penn DL. Comprehensive treatments for social cognitive deficits in schizophrenia: A critical review and effect-size analysis of controlled studies. Clinical Psychology Review. n.d doi: 10.1016/j.cpr.2015.09.003. http://doi.org/10.1016/j.cpr.2015.09.003. [DOI] [PubMed]
- Kurtz MM, Richardson CL. Social cognitive training for schizophrenia: a meta-analytic investigation of controlled research. Schizophr Bull. 2012;38(5):1092–104. doi: 10.1093/schbul/sbr036. http://doi.org/10.1093/schbul/sbr036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz MM, Rose J, Wexler BE. Predictors of participation in community outpatient psychosocial rehabilitation in schizophrenia [published online ahead of print July 30, 2010] Community Ment Health J. 2010;47(6):622–7. doi: 10.1007/s10597-010-9343-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenmayer JPP, McGurk SR, Khan A, Kaushik S, Thanju A, Hoffman L, … Herrmann E. Improving social cognition in schizophrenia: a pilot intervention combining computerized social cognition training with cognitive remediation. Schizophr Bull. 2013;39(3):507–17. doi: 10.1093/schbul/sbs120. http://doi.org/10.1093/schbul/sbs120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mausbach BT, Harvey PD, Goldman SR, Jeste DV, Patterson TL. Development of a brief scale of everyday functioning in persons with serious mental illness. Schizophrenia Bulletin. 2007;33(6):1364–1372. doi: 10.1093/schbul/sbm014. http://doi.org/10.1093/schbul/sbm014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer JD, Salovey P, Caruso DR, Sitarenios G. Measuring emotional intelligence with the MSCEIT V2.0. Emotion (Washington, DC) 2003;3(1):97–105. doi: 10.1037/1528-3542.3.1.97. [DOI] [PubMed] [Google Scholar]
- McGurk SR, Mueser KT, Xie H, Welsh J, Kaiser S, Drake RE, … Wolfe R. Cognitive Enhancement Treatment for People With Mental Illness Who Do Not Respond to Supported Employment: A Randomized Controlled Trial. American Journal of Psychiatry. 2015 doi: 10.1176/appi.ajp.2015.14030374. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Bales KL. Towards improved animal models for evaluating social cognition and its disruption in schizophrenia: The CNTRICS initiative. Neuroscience & Biobehavioral Reviews. 2013;37(9 Part B):2166–2180. doi: 10.1016/j.neubiorev.2013.09.012. http://doi.org/10.1016/j.neubiorev.2013.09.012. [DOI] [PubMed] [Google Scholar]
- Nahum M, Fisher M, Loewy R, Poelke G, Ventura J, Nuechterlein KH, … Vinogradov S. A novel, online social cognitive training program for young adults with schizophrenia: A pilot study. Schizophr Res Cogn. 2014;1(1):e11–e19. doi: 10.1016/j.scog.2014.01.003. http://doi.org/10.1016/j.scog.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahum M, Lee H, Merzenich MM. Principles of neuroplasticity-based rehabilitation. Prog Brain Res. 2013;207:141–71. doi: 10.1016/B978-0-444-63327-9.00009-6. http://doi.org/10.1016/B978-0-444-63327-9.00009-6. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, … Marder SR. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. The American Journal of Psychiatry. 2008;165(2):203–213. doi: 10.1176/appi.ajp.2007.07010042. http://doi.org/10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Winston J, Critchley H, Perrett D, Burt DM, Dolan RJ. Beauty in a smile: the role of medial orbitofrontal cortex in facial attractiveness. Neuropsychologia. 2003;41(2):147–155. doi: 10.1016/s0028-3932(02)00145-8. http://doi.org/10.1016/S0028-3932(02)00145-8. [DOI] [PubMed] [Google Scholar]
- Penn DL, Corrigan PW, Bentall RP, Meg J, Newman L. Social cognition in schizophrenia. Psychological Bulletin. 1997;121(1):114–132. doi: 10.1037/0033-2909.121.1.114. http://doi.org/10.1037/0033-2909.121.1.114. [DOI] [PubMed] [Google Scholar]
- Penn DL, Sanna LJ, Roberts DL. Social cognition in schizophrenia: an overview. Schizophrenia Bulletin. 2008;34(3):408–411. doi: 10.1093/schbul/sbn014. http://doi.org/10.1093/schbul/sbn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkham AE, Penn DL, Perkins DO, Lieberman J. Implications for the neural basis of social cognition for the study of schizophrenia. The American Journal of Psychiatry. 2003;160(5):815–824. doi: 10.1176/appi.ajp.160.5.815. [DOI] [PubMed] [Google Scholar]
- Rass O, Forsyth JK, Bolbecker AR, Hetrick WP, Breier A, Lysaker PH, O’Donnell BF. Computer-assisted cognitive remediation for schizophrenia: a randomized single-blind pilot study. Schizophrenia Research. 2012;139(1–3):92–98. doi: 10.1016/j.schres.2012.05.016. http://doi.org/10.1016/j.schres.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy LF, Horan WP, Green MF. Motivational Deficits and Negative Symptoms in Schizophrenia: Concepts and Assessments. Current Topics in Behavioral Neurosciences. 2015 doi: 10.1007/7854_2015_379. http://doi.org/10.1007/7854_2015_379. [DOI] [PubMed]
- Sacks S, Fisher M, Garrett C, Alexander P, Holland C, Rose D, … Vinogradov S. Combining computerized social cognitive training with neuroplasticity-based auditory training in schizophrenia. Clinical Schizophrenia & Related Psychoses. 2013;7(2):78–86A. doi: 10.3371/CSRP.SAFI.012513. http://doi.org/10.3371/CSRP.SAFI.012513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt SJ, Mueller DR, Roder V. Social cognition as a mediator variable between neurocognition and functional outcome in schizophrenia: empirical review and new results by structural equation modeling. Schizophr Bull. 2011;37(suppl 2):S41–54. doi: 10.1093/schbul/sbr079. http://doi.org/10.1093/schbul/sbr079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segarra N, Metastasio A, Ziauddeen H, Spencer J, Reinders NR, Dudas RB, … Murray GK. Abnormal Frontostriatal Activity During Unexpected Reward Receipt in Depression and Schizophrenia: Relationship to Anhedonia. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.370. http://doi.org/10.1038/npp.2015.370. [DOI] [PMC free article] [PubMed]
- Simpson EH, Waltz JA, Kellendonk C, Balsam PD. Schizophrenia in translation: dissecting motivation in schizophrenia and rodents. Schizophrenia Bulletin. 2012;38(6):1111–1117. doi: 10.1093/schbul/sbs114. http://doi.org/10.1093/schbul/sbs114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone VE, Baron-Cohen S, Knight RT. Frontal lobe contributions to theory of mind. Journal of Cognitive Neuroscience. 1998;10(5):640–656. doi: 10.1162/089892998562942. [DOI] [PubMed] [Google Scholar]
- Strauss GP, Gold JM. A new perspective on anhedonia in schizophrenia. The American Journal of Psychiatry. 2012;169(4):364–373. doi: 10.1176/appi.ajp.2011.11030447. http://doi.org/10.1176/appi.ajp.2011.11030447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Waltz JA, Gold JM. A review of reward processing and motivational impairment in schizophrenia. Schizophrenia Bulletin. 2014;40(Suppl 2):S107–116. doi: 10.1093/schbul/sbt197. http://doi.org/10.1093/schbul/sbt197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam K, Hooker CI, Biagianti B, Fisher M, Nagarajan S, Vinogradov S. Neural signal during immediate reward anticipation in schizophrenia: Relationship to real-world motivation and function. NeuroImage: Clinical. 2015;9:153–163. doi: 10.1016/j.nicl.2015.08.001. http://doi.org/10.1016/j.nicl.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam K, Luks TL, Fisher M, Simpson GV, Nagarajan S, Vinogradov S. Computerized cognitive training restores neural activity within the reality monitoring network in schizophrenia. Neuron. 2012;73(4):842–853. doi: 10.1016/j.neuron.2011.12.024. http://doi.org/10.1016/j.neuron.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam K, Luks TL, Garrett C, Fisher M, Nagarajan S, Vinogradov S. Intensive cognitive training in schizophrenia enhances working memory and associated prefrontal cortical efficiency in a manner that drives long-term functional gains. NeuroImage. 2014 doi: 10.1016/j.neuroimage.2014.05.057. http://doi.org/doi:10.1016/j.neuroimage.2014.05.057. [DOI] [PMC free article] [PubMed]
- Surti TS, Corbera S, Bell MD, Wexler BE. Successful computer-based visual training specifically predicts visual memory enhancement over verbal memory improvement in schizophrenia. Schizophrenia Research. 2011;132(2–3):131–134. doi: 10.1016/j.schres.2011.06.031. http://doi.org/10.1016/j.schres.2011.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twamley EW, Burton CZ, Vella L. Compensatory Cognitive Training for Psychosis: Who Benefits? Who Stays in Treatment? Schizophrenia Bulletin. 2011;37(suppl 2):S55–S62. doi: 10.1093/schbul/sbr059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vauth R, Rüsch N, Wirtz M, Corrigan PW. Does social cognition influence the relation between neurocognitive deficits and vocational functioning in schizophrenia? Psychiatry Res. 2004;128(2):155–65. doi: 10.1016/j.psychres.2004.05.018. http://doi.org/10.1016/j.psychres.2004.05.018. [DOI] [PubMed] [Google Scholar]
- Wang J, Huang J, Yang XH, Lui SSY, Cheung EFC, Chan RCK. Anhedonia in schizophrenia: Deficits in both motivation and hedonic capacity. Schizophrenia Research. 2015;168(1–2):465–474. doi: 10.1016/j.schres.2015.06.019. http://doi.org/10.1016/j.schres.2015.06.019. [DOI] [PubMed] [Google Scholar]
- Yan C, Yang T, Yu QJ, Jin Z, Cheung EFC, Liu X, Chan RCK. Rostral medial prefrontal dysfunctions and consummatory pleasure in schizophrenia: a meta-analysis of functional imaging studies. Psychiatry Research. 2015;231(3):187–196. doi: 10.1016/j.pscychresns.2015.01.001. http://doi.org/10.1016/j.pscychresns.2015.01.001. [DOI] [PubMed] [Google Scholar]
- Young JW, Markou A. Translational Rodent Paradigms to Investigate Neuromechanisms Underlying Behaviors Relevant to Amotivation and Altered Reward Processing in Schizophrenia. Schizophrenia Bulletin. 2015;41(5):1024–1034. doi: 10.1093/schbul/sbv093. http://doi.org/10.1093/schbul/sbv093. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.