Abstract
Background
The “first night effect” of polysomnography (PSG) has been studied, however the ability to quantify the level of sleep disruption has been confounded by using PSG on all nights. We used actigraphy to quantify disruption level, and examined characteristics associated with disruption.
Methods
778 older men (76.2 ± 5.4 years) from a population-based study at six US centers underwent one night of in-home PSG. Actigraphy was gathered on the PSG night and three subsequent nights. Actigraphically measured total sleep time (TST), sleep efficiency (SE), wake after sleep onset (WASO), and sleep onset latency (SOL) were compared from the PSG night and subsequent nights. Linear regression models were used to examine the association of characteristics and sleep disruption.
Results
On average, sleep on the PSG night was worse than the following night (p<0.05, TST 21 ± 85 min less, SE 2.3 ± 11.3% less, WASO 4.9 ± 51.8 min more, SOL 6.6 ± 56.2 min more). Compared to sleep two and three nights later, sleep on the PSG night was significantly worse. Characteristics associated with greater sleep disruption on the PSG night, included older age, higher apnea-hypopnea index, worse neuromuscular function, and more depressive symptoms. Minorities and men with excessive daytime sleepiness slept somewhat better on the PSG night.
Conclusions
Among older men, there was sleep disruption on the PSG night which may lead to an underestimation of sleep time. The increase of sleep on the night after the PSG suggests data from the second monitoring may overestimate sleep.
Keywords: polysomnography, first night effect, reverse first night effect, actigraphy
1. Introduction
Considerable effort has been directed at identifying whether there is a “first night effect” (FNE) of polysomnography (PSG) recordings, characterized by a disruption of sleep on the initial PSG recording when compared to measurements from PSG recordings from subsequent nights [1,2]. This disruption of sleep includes less total sleep time (TST), lower sleep efficiency (SE), longer sleep onset latency (SOL) and more wake after sleep onset (WASO). While there are many possible reasons for a FNE, in most cases it is likely due to discomfort caused by the PSG electrodes and cables, limitation of movement and/or anxiety caused by being under study [1]. If recordings were performed in a laboratory rather than an in-home setting, sleep disruption could also be due to discomfort associated with sleeping in an unfamiliar environment [3,4]. The FNE may be more pronounced among certain patient subgroups, such as in patients with depression [5], anxiety disorders [6,7], or insomnia [8–10], or in older patients [11,12], or minorities [13]. Concerns about FNE have led to conducting PSG on multiple nights, sometimes excluding data from the first night [9,10,14]. While this may improve the representativeness of sleep data, this practice is often impractical due to cost, limitation of resources, and patient burden. The increased use of in-home testing highlights the importance of examining the FNE of in-home PSG [15]. Therefore, quantifying the level of sleep disruption caused by in-home PSG recording and examining patient characteristics that are associated with this disruption is of value in understanding the limitations of single-night PSG.
Although prior research has examined how sleep quality the night of the first PSG recording compares to the following night, little research has examined the comparability of PSG-determined sleep from a single, first night study to sleep measured several nights immediately after the PSG study [13,16–18]. Perhaps the differences seen between the first PSG night and the night after are more extreme than differences seen between PSG and the patient’s typical sleep patterns due to the need for a night of “recovery” sleep.
All studies but one [5] have used PSG on all nights to measure sleep quality and estimate the FNE. While PSG is the “gold standard” for sleep assessment, using PSG to measure the sleep disruption caused by PSG may underestimate the true effect. If PSG is used on all nights to measure sleep, it is uncertain the level of sleep disruption caused by the device because the disruption may be present for all PSG recordings.
Wrist actigraphy has been shown to be a reasonable surrogate for measurement of sleep characteristics [20]. Actigraphy can be used concurrently the night of a PSG recording and on subsequent nights to quantify the amount of disruption caused by PSG. Actigraphs can record for numerous nights, which allows examination of the length of time of the PSG-related disruption. Another benefit is the absence of a FNE with actigraphy, so the level of sleep disruption caused by PSG can be directly quantified without possible confounding by continued use of PSG [21].
We used the data from 778 men in the Outcomes of Sleep Disorders in Older Men (MrOS Sleep) Study who had one night of in-home PSG with concurrent actigraphy recordings and two or more additional nights of actigraphy to examine our aims: 1) Is there PSG-related FNE among older community dwelling men, and if so what is the magnitude of the disruption to sleep. 2) How long did this disruption effect sleep patterns. 3) Was there “recovery” sleep the night after the PSG. 4) Are participant characteristics such as depression, anxiety, insomnia, race, and age related to the level of sleep disruption caused by PSG.
2. Material and methods
2.1. Participants
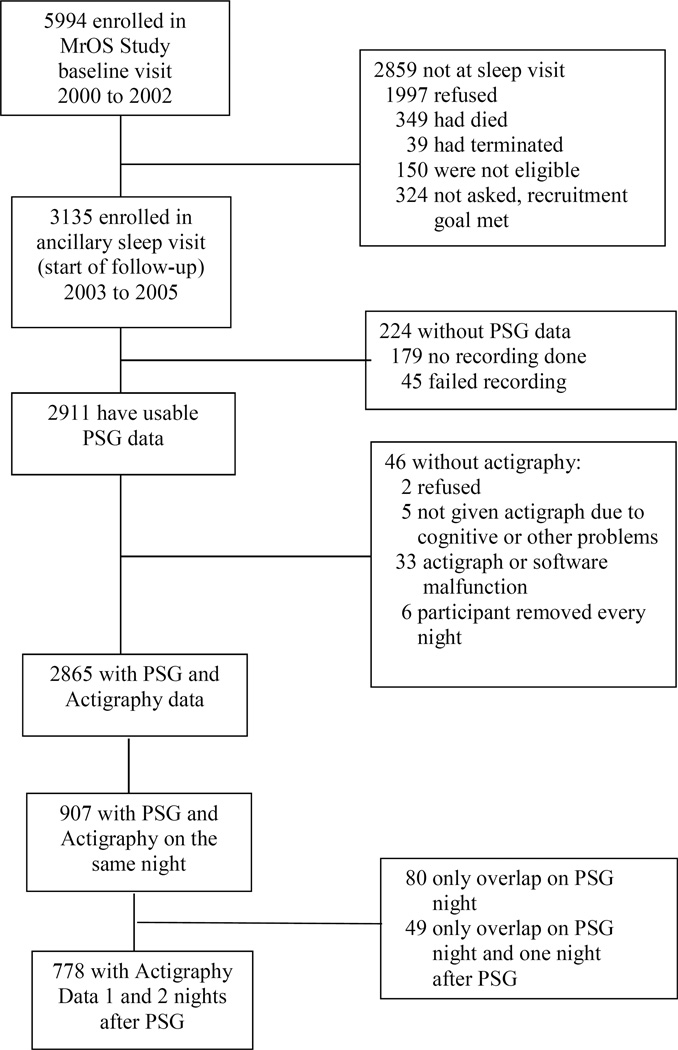
The MrOS Sleep Study recruited 3135 men between December 2003 and March 2005 from a larger study of 5994 men, the Osteoporotic Fractures in Men (MrOS) Study. Community-dwelling men aged 65 and older were recruited to participate in the MrOS study at six US clinical centers in Birmingham, Alabama; Minneapolis, Minnesota; Palo Alto, California; the Monongahela Valley near Pittsburgh, Pennsylvania; Portland, Oregon; and San Diego, California. At the time of the first visit, men were ineligible for the study if they had a history of a bilateral hip replacement or were unable to walk without the assistance of another person [22,23]. Of the 2859 men who did not participant in the ancillary MrOS Sleep Study, 349 died before the sleep visit, 40 had already terminated the study, 323 were not asked because recruitment goals had already been met, and 1997 refused. One hundred and fifty men were not eligible for the study due to an open tracheotomy or use of continuous positive airway pressure, bilevel positive airway pressure, sleeping with a mouthpiece for snoring or sleep apnea, or using oxygen therapy during sleep in the past three months with the inability to forgo use of these devices for the PSG recording. (Figure 1)
Figure 1.
Progression of participants through the Osteoporotic Fractures in Men (MrOS) and MrOS sleep studies
There were 2911 men with PSG data gathered, of which 2865 had actigraphy. The actigraph recording was typically started the day of the clinic exam (93%). The study protocol did not specifically require that the participants wear the actigraph while the PSG recording was performed, but rather that the PSG was performed within one month of the clinical examination. The PSG recording was often done later due to scheduling issues or availability of equipment. There were 907 men with concurrent PSG and actigraphy recordings. A minimum of 2 post-PSG nights are required to examine if the change in sleep parameters is nonlinear (u- or j-shaped). Of these 907 men, 778 also had actigraphic recordings the two nights after the PSG recording and comprise our analytic sample. There were 663 men who had a third post-PSG night, which is also summarized here. The institutional review boards at each clinic site approved the study, and written informed consent was obtained from all participants.
2.2. In-home polysomnography
In-home sleep studies were completed using unattended, portable polysomnography (Safiro, Compumedics, Inc.®, Melbourne, Australia). The 7 (0.9%) men who reported use of CPAP in the past 3 months had to forgo use during the PSG recording. Participants were not asked to modify any other activities for the PSG recording, specifically they were not asked to change sleep habits, medication use, or change alcohol or caffeine consumption.
The recording montage was as follows: C3/A2 and C4/A1 electroencephalograms (EEG), bilateral electrooculograms and a bipolar submental electromyogram to determine sleep status; thoracic and abdominal respiratory inductance plethysmography to determine respiratory effort; airflow (by nasal-oral thermocouple and nasal pressure cannula); finger pulse oximetry; lead I electrocardiogram (ECG); body position (mercury switch sensor); and bilateral tibialis leg movements (piezoelectric sensors). Centrally-trained and certified staff performed home visits to set up the unit, verify the values of the impedances for each channel, confirm calibration of position sensors and note any problems encountered during set-up, similar to the protocol used in the Sleep Heart Health Study [24]. Staff returned the next morning to collect the equipment and download the data to the Central Sleep Reading Center (Cleveland, OH) to be scored by certified research polysomnologists using standard criteria [25,26]. PSG data quality was excellent, with a failure rate of less than 4% and more than 70% of studies graded as being of excellent or outstanding quality.
Apneas were defined by the absence or near absence of airflow on the thermistor for >10 seconds, and hypopneas were scored if clear reductions in breathing amplitude (at least 30% below baseline breathing) occurred, and lasted >10 seconds, and were associated with desaturations of 3% or greater [27]. The apnea-hypopnea index (AHI) was defined as apneas plus hypopneas per hour of sleep time. Sleep disordered breathing was defined as AHI ≥15.
2.3. Wrist actigraphy
Objective characteristics of sleep-wake patterns were estimated using an actigraph (SleepWatch-O, Ambulatory Monitoring, Inc., Ardsley, NY) worn continuously. Participants were instructed to wear the actigraph securely fastened around their non-dominant wrist, to be removed only when bathing or during water sports. The actigraph is similar in size and weight to a standard wristwatch, and movement is detected via a piezoelectric bimorph-ceramic cantilever beam that generates a voltage each time the actigraph is moved. These voltages were gathered continuously and summarized over one-minute intervals. Data were collected in three modes but are reported here based on proportional integration mode [28]. ActionW-2 software (Ambulatory Monitoring, Inc., Ardsley, NY) was used to analyze the actigraphy data [29]. Details of the actigraphy scoring algorithms utilized in the study have been published elsewhere [30].
Participants completed sleep diaries for the time period they wore the actigraph. The diaries included time into and time out of bed and times the actigraph was removed. This information was used in editing the actigraphy data files to set intervals for when the participant was in bed trying to sleep (after “lights off”), and to delete time when the actigraph was removed. Inter-scorer reliability for editing the actigraphy data files has been found to be high in our group (intra-class coefficient = 0.95) [31]. Actigraphy has been shown to have good concordance with sleep parameters from PSG in a prior study in this cohort (intra-class correlation coefficients TST 0.57; SE 0.46; SOL 0.32; WASO 0.54) [30].
Variables estimated from actigraphy used in this analysis included: 1) total sleep time (TST): the hours per night spent sleeping while in bed after “lights off”; 2) sleep efficiency (SE): the percentage of time in bed after “lights off” spent sleeping; 3) wake after sleep onset (WASO): minutes of wake after sleep onset during the in bed interval, with sleep onset defined as the point when the participant achieved a 20-minute continuous block of sleep after “lights off”; 4) sleep onset latency (SOL): the minutes from “lights off” until sleep onset.
2.4. Other measurements
At the time of the Sleep Visit all participants completed questionnaire data, which included questions about demographics, marital status, medical history, self-reported health, alcohol use, and smoking status. Caffeine consumption was estimated based on self-report of the average daily number of cups of caffeinated coffee, tea, or soda consumed [32]. Physical activity was assessed using the Physical Activity Scale for the Elderly (PASE) [33]. The Geriatric Depression Scale (GDS) was used to assess depressive symptoms, with the standard cutoff of ≥6 symptoms used to define depression [34]. Information to compute the Goldberg anxiety scale was also collected, and the cutpoint of five or more was used to define clinically significant anxiety [35]. Participants with insomnia symptoms were defined as those who reported inability getting to sleep within 30 minutes or waking up in the middle of the night or early morning three or more times per week. The Epworth Sleepiness Scale (ESS) was completed with the cutpoint of ESS>10 used to define excessive daytime sleepiness [36,37] The Modified Mini-Mental State examination (3MS) was administered to assess cognitive function, with higher scores on a scale of 0 to 100 representing better cognition [38]. Functional status was assessed by collecting information on impairments of five instrumental activities of daily living (IADL), which included walking two to three blocks on level ground, climbing up to ten steps, preparing meals, doing heavy housework, and shopping for groceries or clothing [39,40]. Neuromuscular function was measured with walking speed (time in seconds to walk six meters at usual pace expressed as meters/sec). Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters. Obesity was defined as a BMI of 30 kg/m2 or more. All prescription and nonprescription medications taken within the past 30 days were collected by the clinics and stored in an electronic medications inventory database (San Francisco Coordinating Center, San Francisco, CA). Each medication was matched to its ingredient(s) based on the Iowa Drug Information Service (IDIS) Drug Vocabulary (College of Pharmacy, University of Iowa, Iowa City, IA) [41]. The morning after the PSG recording men were asked how the night of sleep compared to their usual sleep quality, and if the sleeping arrangements during the PSG recording were similar to their usual arrangement.
2.5. Statistical analysis
Characteristics of this subset of 778 men and the remaining 2357 in the MrOS Sleep Study cohort were summarized by means and standard deviations (SD) for continuous variables, and counts and percentages for categorical variables. Characteristics between the two groups were compared using t-tests for continuous normally distributed variables, Wilcoxon rank-sum tests for continuous data with skewed distributions, and chi-square tests for categorical characteristics.
All objectively measured sleep parameters were assessed with actigraphy. TST, SE, WASO and SOL on the night of the PSG recording and one, two, and three nights after the PSG recording were summarized as mean ± SD, and mean difference from values from the night of the PSG recording ± SD. Distributions were examined for potential outliers. Comparisons between the nights were examined using paired t-tests for normally distributed variables (TST, SE), paired Wilcoxon signed rank tests for skewed variables (WASO, SOL). Although these analyses were pre-specified, a Bonferroni correction was applied to all significant p-values to examine if the associations observed held after correction for multiple comparisons.
Similar analyses were performed on the time the participant got into and out of bed and the duration of the time in bed. These analyses were performed to examine if the participant changed their usual sleep routine on the night of the PSG recording.
All characteristics were screened for an association to the change of sleep parameters from the PSG night to the following night. TST was also screened as a covariate, because those with more sleep have a potential for a larger amount of disruption. The TST covariate used was the average of actigraphic TST on the second and third night after the PSG recording, because potentially those nights were less effected by PSG disruption. Linear regression models were performed to examine these potential associations. These models were adjusted for clinic site. The dependent variable in these models was the difference in the parameter from the PSG night and the subsequent night, which was normally distributed for all sleep parameters. Those covariates with significant associations were included in a multivariable model to examine if associations were independent. Results are presented as beta coefficients with 95% confidence intervals (CI).
The primary analysis presents means of the sleep parameters on each night, comparing the sleep parameters from each night to that of the night of the PSG recording. Similar analytical methods were used in the majority of prior studies, and were selected here to allow for comparability. A sensitivity analysis using random-effects models was preformed to allow for inclusion of all 907 men who had overlapping PSG and actigraphy data, regardless of how many post-PSG nights with actigraphy data were available. This allows for more robust estimates and limits possible bias caused by excluding men with less data. These models account for between-participant variation and within-participant correlation of repeated outcomes. The random effect terms included the intercept and the slope of the parameters over time, allowing for individual time trends for each participant. Variances and covariances were estimated using the restricted maximum likelihood method. Time was modeled as a continuous covariate, measured as nights from the PSG recording. A quadratic term for time was considered to test for a nonlinear time trend. The values for WASO and SOL were log-transformed to meet model requirements. Beta coefficients from these models were used to calculate estimates for the parameters at each timepoint.
All significance levels reported were two-sided and all statistical analyses were performed using SAS software, version 9.4 (SAS Institute, Inc, Cary, NC).
3. Results
Of the 2865 men with both PSG and actigraphy data, 778 (27%) had concurrent data from both methods plus two or more subsequent nights of actigraphic data. These 778 men were 76.2 years old on average, and 95% Caucasian (Table 1). Compared to the remaining 2357 participants in the MrOS Sleep Study cohort, this subset of 778 men were more likely to be Caucasian, and on average had less depressive symptoms, slightly higher levels of cognitive functioning, and lower rates of excessive daytime sleepiness (p<0.05).
Table 1.
Characteristics of the analytic cohort compared to the remaining MrOS Sleep cohort
| Characteristic | Remaining Cohort (N = 2357) |
Analytic Subset (N = 778) |
p-value |
|---|---|---|---|
| Age, y | 76.49 ± 5.60 | 76.23 ± 5.44 | 0.27 |
| Caucasian | 2081 (88.29) | 735 (94.47) | <0.0001 |
| Self-reported health status good or excellent | 2035 (86.45) | 679 (87.28) | 0.56 |
| ≥ IADL impairment | 511 (21.69) | 156 (20.05) | 0.33 |
| Alcohol intake, drinks/week | |||
| 0–2 | 1402 (59.89) | 449 (57.79) | 0.24 |
| 3–13 | 803 (34.30) | 290 (37.32) | |
| 14+ | 136 (5.81) | 38 (4.89) | |
| Average caffeine intake, mg/day | 236.18 ± 242.99 | 232.75 ± 257.17 | 0.17 |
| Current smoker | 52 (2.21) | 12 (1.54) | 0.26 |
| Married or in married-like relationship | 1942 (82.64) | 652 (83.80) | 0.45 |
| Current antidepressant use | 193 (8.19) | 55 (7.07) | 0.31 |
| Current benzodiazepine use | 103 (4.37) | 36 (4.63) | 0.76 |
| Current prescription sleep medication use | 52 (2.21) | 10 (1.29) | 0.11 |
| ≥1 selected medical conditionsa | 979 (41.57) | 313 (40.23) | 0.51 |
| Geriatric Depression Scale score (range 0–15) | 1.83 ± 2.17 | 1.68 ± 2.19 | <0.01 |
| Depression (Geriatric Depression Scale ≥6) | 161 (6.85) | 50 (6.43) | 0.69 |
| Goldberg anxiety score (0–9) | 0.98 ± 1.89 | 0.96 ± 1.90 | 0.24 |
| Anxiety (Goldberg anxiety score ≥5) | 205 (8.73) | 72 (9.25) | 0.66 |
| 3MS score (range 0–100) | 92.35 ± 6.69 | 93.42 ± 5.36 | <0.0001 |
| Walking speed, m/s | 1.14 ± 0.23 | 1.14 ± 0.23 | 0.64 |
| PASE score (physical activity) | 146.35 ± 72.20 | 143.33 ± 71.12 | 0.31 |
| Body mass index, kg/m2 | 27.15 ± 3.87 | 27.31 ± 3.78 | 0.32 |
| Body mass index ≥30 kg/m2 | 468 (19.88) | 172 (22.11) | 0.18 |
| Apnea-hypopnea index | 16.95 ± 14.97 | 17.39 ± 15.50 | 0.63 |
| Apnea-hypopnea index ≥15 | 924 (43.32) | 340 (43.70) | 0.85 |
| Insomnia symptoms | 1444 (61.34) | 495 (63.62) | 0.26 |
| Epworth sleepiness scale (range 0–24) | 6.25 ± 3.77 | 5.91 ± 3.44 | 0.02 |
| Excessive daytime sleepiness (ESS >10) | 328 (13.92) | 78 (10.03) | <0.01 |
| Not usual sleeping arrangement during PSG | 278 (13.06) | 92 (11.84) | 0.38 |
Data shown as mean ± standard deviation or n (%). p-values for continuous variables from a t-test for normally distributed variables, a Wilcoxon rank sum test for skewed variables. p-values for categorical variables from a chi-square test.
Medical conditions include stroke, diabetes, Parkinson’s disease, chronic obstructive pulmonary disease/emphysema, myocardial infarction, angina, and congestive heart failure.
IADL = instrumental activities of daily living; 3MS = Modified Mini-Mental State examination; PASE = Physical Activity Scale for the Elderly; ESS = Epworth Sleepiness Scale.
When asked to compare the sleep quality from the PSG night to their usual sleep pattern, 56% of men in the analysis subset reported sleeping worse than usual, 36% reported no difference, and 8% reported sleeping better than usual on the PSG night. The majority of men (88%) reported that their sleeping arrangement on the night of PSG was the same as their usual arrangement. Of those 92 men who did not have their usual sleeping arrangement on the night of PSG, 88 reported sleeping alone when they usually slept in the same bed (n=80) or the same room (n=8) as someone else. There was no significant difference between the analysis cohort and the remaining MrOS men in the proportion reporting not having their usual sleeping arrangement during PSG. (p=0.38) On average, men did alter their time into and out of bed on the night of the PSG recording compared to the 3 subsequent nights. On the night of the PSG recording, men got into bed earlier compared to subsequent nights (PSG night: 10:22PM ± 69 min; 1 night later: 10:37PM ± 82 min; 2 nights later: 10:46PM ± 80 min; 3 nights later: 10:46PM ± 82 min, p<0.001 for all). Participants also got out of bed earlier on the morning after PSG compared to subsequent mornings (morning after PSG: 6:35AM ± 64 min; 1 morning later: 7:01AM ± 79 min; 2 mornings later: 7:02AM ± 77 min; 3 mornings later: 7:03AM ± 77 min, p<0.001 for all). The duration of time in bed on the PSG night was on average 13 minutes shorter than on the night after the PSG (8.21 ± 1.2 hrs vs. 8.42 ± 1.4 hrs, p<0.0001) but did not differ from the duration on 2 and 3 nights after the recording (8.3 ± 1.4 hrs and 8.3 ± 1.4 hrs, respectively; p≥0.12).
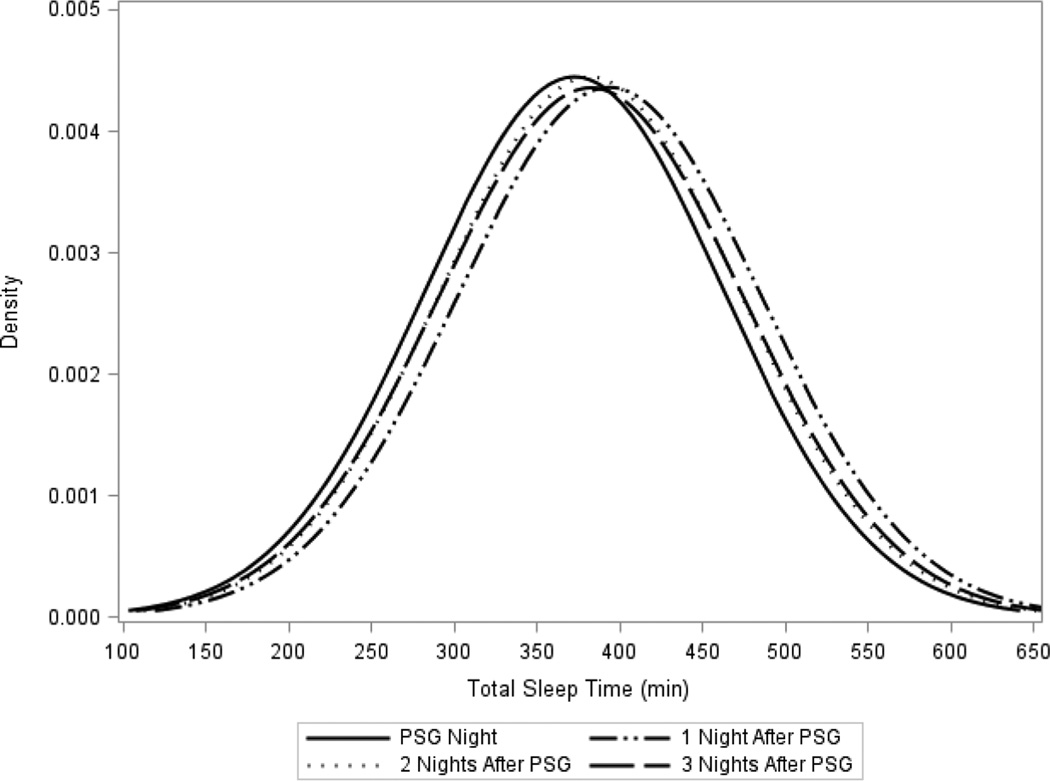
There was a statistically significant difference in all actigraphically measured sleep parameters from the night of the PSG recording and the next night (p<0.01, Table 2). There were also significant differences in TST, SE, and SOL between the PSG recording night and the second and third night after PSG (p ≤0.03). On average, on the night of PSG men slept 21 min less compared to the first night after the recording, and about 9 to 10 min less than two and three nights later. A similar pattern was seen with SE, on average, with SE on the night of PSG 2.3% lower compared to the first night after the recording, and about 1.2 to 1.3% lower than two and three nights later. For all sleep parameters there was a larger mean difference between the PSG recording night and first night after PSG than to the second and third night after PSG. The second and third night after the PSG recording were similar to each other (p>0.05), but were statistically different from the first night after the PSG recording for TST and SE (Figure 2, TST). In Table 2 we performed 12 tests of significance. Adjusting the significance level of 0.05 with a Bonferroni correction would lead to a significance cutpoint of P < 0.004 (0.05/12). After applying this more stringent cutpoint for significance to these results the significant associations comparing the PSG night to one night after the PSG recording remained significant, while tests comparing 2 nights after the recording to the PSG night remained significant for TST and SE, and tests comparing 3 nights after the recording to the PSG night was significant for TST.
Table 2.
Comparing sleep parameters measured by actigraphy from the night of polysomnography to subsequent nights
| Sleep Parameter /Statistic |
PSG Night | 1 Night After PSG |
2 Nights After PSG |
3 Nights After PSG |
|---|---|---|---|---|
|
Total Sleep Time, min |
||||
| N | 778 | 778 | 778 | 663 |
| Mean ± SD | 372.96 ± 89.81 | 394.10 ± 91.59 | 382.35 ± 89.83 | 382.90 ± 91.67 |
| Mean difference from PSG Night ± SD* |
21.14 ± 85.12 | 9.39 ± 80.37 | 9.88 ± 81.15 | |
| p-value | <0.001 | <0.01 | <0.01 | |
| Sleep Efficiency, % | ||||
| N | 778 | 778 | 778 | 663 |
| Mean ± SD | 75.82 ± 15.27 | 78.12 ± 13.35 | 77.10 ± 13.62 | 76.99 ± 14.08 |
| Mean difference from PSG Night ± SD* |
2.29 ± 11.33 | 1.28 ± 11.02 | 1.22 ± 11.22 | |
| p-value | <0.001 | <0.01 | <0.01 | |
| WASO, min | ||||
| N | 773 | 777 | 774 | 661 |
| Mean ± SD | 83.33 ± 54.73 | 78.60 ± 56.24 | 80.90 ± 54.65 | 80.82 ± 53.95 |
| Mean difference from PSG Night ± SD* |
−4.86 ± 51.78 | −2.45 ± 52.74 | −3.05 ± 49.86 | |
| p-value | <0.001 | 0.07 | 0.06 | |
|
Sleep Onset Latency, min |
||||
| N | 773 | 777 | 774 | 661 |
| Mean ± SD | 38.46 ± 53.9 | 33.40 ± 50.21 | 33.34 ± 44.99 | 34.68 ± 48.81 |
| Mean difference from PSG Night ± SD* |
−6.59 ± 56.19 | −5.48 ± 59.16 | −3.97 ± 59.26 | |
| p-value | <0.01 | <0.01 | 0.03 |
Subsequent night – PSG night.
p-values for total sleep time and sleep efficiency from a paired t-test. P-values for wake after sleep onset and sleep latency from a paired Wilcoxon signed rank test.
PSG = polysomnography; SD = standard deviation; WASO = wake after sleep onset.
Figure 2.
Distribution of actigraphic total sleep time the night of polysomnography and three subsequent nights
In sensitivity analyses, inclusion of all 907 men in random effects models had little effect on the estimates of the parameters across nights. The estimates of TST (min) across nights using this method was: PSG night=373.76; 1 night after PSG=386.30; 2 nights after PSG=388.13; 3 nights after PSG=373.76. The estimates of SE (%) across nights was: PSG night=76.03; 1 night after PSG=77.56; 2 nights after PSG=77.81; 3 nights after PSG=76.78. The estimates of WASO (min) across nights was: PSG night=83.36; 1 night after PSG=80.03; 2 nights after PSG=79.91; 3 nights after PSG=81.51. The estimates of SOL (min) across nights was: PSG night=38.02; 1 night after PSG=33.69; 2 nights after PSG=32.71; 3 nights after PSG=35.05. Quadratic terms for all models were <0.04, implying a u- or j-shaped association.
Those characteristics with a significant association between change in sleep parameters from the PSG night to the following night are shown in Table 3. Age, race, and AHI had the most consistent associations with PSG-related sleep disruption. As age and AHI increased the level of disruption of TST, SE and WASO increased. Compared to Caucasians, minority men had an improvement of sleep the night PSG was recorded. Slower walking speed, a measure of neuromuscular function, was related to more sleep disruption in TST and SE. Those men with excessive daytime sleepiness had an improvement in sleep on the night of the PSG compared to the next night (TST, SE). Other characteristics had less consistent relationships with sleep disruption. Presence of an IADL impairment, more depressive symptoms, more TST and lower cognitive function were associated to higher levels of disruption of TST. Lower levels of physical activity and presence of insomnia symptoms were related to more disruption of SE.
Table 3.
Characteristics related to level of disruption of sleep from polysomnography (one night after polysomnography night-polysomnography night)
| Covariate | TST, min Beta Coefficient (95% CI) |
SE, % Beta Coefficient (95% CI) |
WASO, min Beta Coefficient (95% CI) |
SOL, min Beta Coefficient (95% CI) |
|---|---|---|---|---|
| Age, per 5 year ↑ | 8.0 (2.5, 13.4) | 1.1 (0.4, 1.8) | −4.5 (−7.8, −1.1) | NS |
| Minority race | −31.4 (−57.6, −5.3) | −4.8 (−8.3, −1.4) | 19.5 (3.5, 35.5) | NS |
| Self-reported health status good or excellent |
NS | NS | −18.7 (−29.6, −7.8) | NS |
| ≥ IADL impairment |
15.3 (0.4, 30.1) | NS | NS | NS |
| Current smoker | NS | NS | 34.9 (5.5, 64.3) | NS |
| Benzodiazepine use |
NS | NS | NS | −27.7 (−46.5, −8.9) |
| GDS score, per 1 unit ↑ |
3.3 (0.5, 6.0) | NS | NS | NS |
| 3MS score, per 1 SD ↓ (5.4) |
7.4 (1.5, 13.3) | NS | NS | NS |
| Walking speed, per 1 SD ↓ (0.23 m/s) |
10.2 (4.1, 16.3) | 1.1 (0.3, 1.9) | NS | NS |
| PASE score, Per 1 SD ↓ (71.1) |
NS | 0.9 (0.1, 1.7) | NS | NS |
| BMI, per 1 SD ↑ (3.8 kg/m2) |
NS | NS | 4.7 (0.9, 8.4) | −5.2 (−9.2, −1.1) |
| ESS, per 1 point ↑ | −3.1 (−4.8, −1.4) | −0.3 (−0.5, −0.1) | NS | NS |
| Excessive daytime sleepiness |
−28.3 (−48.1, −8.6) | −3.4 (−6.1, −0.8) | NS | NS |
| AHI, per 5 unit ↑ |
2.1 (0.1, 4.0) | 0.5 (0.2, 0.7) | −1.4 (−2.6, −0.2) | NS |
| Insomnia symptoms |
NS | −1.7 (−3.4, −0.1) | NS | NS |
| TST*, per 30 min ↑ |
3.0 (0.8, 5.2) | NS | NS | NS |
| Not usual sleeping arrangement during PSG |
NS | NS | NS | NS |
All values shown have p<0.05. Models adjusted for clinic site. NS=not significant.
The average TST from 2 and 3 nights after the polysomnography recording.
TST = total sleep time; SE = sleep efficiency; WASO = wake after sleep onset; SOL = sleep onset latency; CI= confidence interval; SD = standard deviation; IADL = instrumental activities of daily living; GDS = Geriatric Depression Scale; 3MS = Modified Mini-Mental State examination; PASE = Physical Activity Scale for the Elderly; BMI = body mass index; ESS = Epworth Sleepiness Scale; AHI = apneahypopnea index.
For the outcome of TST disruption, after multivariate adjustment the characteristics of race, AHI, TST, and excessive daytime sleepiness remained significant. For the outcome of SE disruption, age, race and AHI remained significant after multivariate adjustment. For the outcome of WASO disruption, race, self-reported heath, BMI and AHI remained significant after adjustment.
4. Discussion
This analysis comparing actigraphic data from the night of in-home PSG to actigraphic data from subsequent nights provides evidence of PSG-related sleep disruption in older men. However, the differences in sleep characteristics were greatest when comparing the PSG night to the subsequent night, with attenuation of differences two and three nights later. This suggests that the immediate post-PSG night may have reflected a “recovery” period. While sleep duration and quality (measured by SE and WASO) may be modestly underestimated on the PSG night, these sleep parameters may be overestimated when using data from the night after the initial PSG recording. Larger amounts of sleep disruption were associated with older age, greater AHI, and lower levels of neuromuscular function. Other characteristics such as depression and presence of insomnia symptoms had a less consistent relationship to level of disruption.
There is a wide range of estimates of the FNE from other studies using in-home PSG, which may be due to the diverse study populations. Our study found an average FNE for TST of 21 minutes among community-dwelling older men. Most studies that measured FNE using in-home PSG on control groups, healthy subjects, or community-dwelling participants observed similar average FNE effect sizes for TST (min): 5.7 (12 normal subjects)[18]; 8.5 (32 normal sleepers)[8]; 24.9 (12 good sleepers)[42]; 26.2 (285 middle-aged women)[13]; 26.6 (26 young healthy subjects)[16]. Studies using in-home PSG to measure FNE for TST on those with sleep complaints had an average FNE of: −7.4 (32 insomniacs)[8]; 27.8 (20 patients with difficulty initiating and maintaining sleep (DIMS))[47]; 46 (12 sleep maintenance insomniacs)[42]; 64 (21 patients with DIMS)[7].
Unlike many other studies examining the FNE [5–7,9,14,43,44], participants in the MrOS Sleep Study were not selected based on disease status. This allowed for examination of characteristics that may be associated to the FNE. The results from the MrOS Sleep cohort confirmed findings from other studies that there was an association of magnitude of FNE with depression, age and insomnia [5,7,9,12,14]. Interestingly, men with excessive daytime sleepiness did not have a FNE. This is similar to the results from Ma and colleagues who saw no FNE among Chinese snorers with an average Epworth Sleepiness Scale of 10.5 [45]. Pittsley and colleagues found no FNE among older participants with complaints of snoring or excessive daytime sleepiness [46].
It is possible that the estimate of the FNE was exaggerated when comparing the PSG night to the next night. The first night after PSG may have been a “recovery” night. The true level of PSG disruption may be better measured by comparing sleep on the PSG night to sleep two nights later. In this cohort, on average sleep parameters on the first night after PSG improved compared to the PSG night, but the second and third night after PSG had less improvement over the PSG night. This pattern was seen in the other studies that had data for two or more nights after the first PSG recording [13,16–18,47]. In the current study, TST on the first night after PSG was on average 21 minutes longer, the second night 9 minutes longer, implying a “recovery” time of 12 minutes. Other studies using in-home PSG had an average “recovery” time of (min): 5.7 (12 good sleepers)[42]; 5.8 (12 normal subjects)[18]; 8.6 (26 young healthy subjects)[16]; 11 (285 middle-aged women)[13]; 21.7 (20 patients with DIMS)[47]; 29.2 (12 sleep maintenance insomniacs)[42]. The duration of time in bed the night after the PSG recording is on average longer than all other nights examined and tests for a possible nonlinear u- or j-shaped association of all parameters with time were significant, lending further support of the idea of a “recovery” night. For all sleep parameters examined, the average values on the second and third night after the PSG recordings are very similar. This consistency in values implies robustness of the measurement.
This study had several limitations. The findings may not be generalizable to populations other than community-dwelling older men, or to PSG recordings performed in the laboratory. The study protocol did not require concurrent PSG and actigraphy recording, making it impossible to include all men with data from both measures in this analysis. Those men not included did differ from our analysis subset on some characteristics, most notably levels of excessive daytime sleepiness, race and depressive symptoms. Actigraphy is a surrogate measure for sleep assessment, and while it compares reasonably well to PSG measurement of these variables examined in our cohort [30], there is measurement error. Based on cutpoints for ICCs, the agreement between PSG and actigraphy in this study is considered moderate for TST, SE and WASO, fair for SOL [48].
In conclusion, in-home PSG disrupts sleep when compared to sleep measured on subsequent nights after the PSG. Some levels of disruption were modest in magnitude and of uncertain clinical relevance. However, a bias in sleep measurement may differentially affect certain groups. Our data indicate the need to cautiously interpret data from a second night sleep assessment, which may modestly overestimate sleep duration due to “recovery” effects.
Supplementary Material
Highlights.
The “first night effect” of polysomnography (PSG) recordings is characterized by sleep disruption.
There is a PSG-related first night effect among community-dwelling older men.
The magnitude of the PSG-related first night effect was small in this cohort.
There appeared to be a “recovery” night of sleep after PSG.
Certain characteristics effect the level of the PSG-related first night effect.
Acknowledgments
Funding Sources
The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute on Aging (NIA), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Center for Advancing Translational Sciences (NCATS), and NIH Roadmap for Medical Research under the following grant numbers: U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160, and UL1 TR000128.
The National Heart, Lung, and Blood Institute (NHLBI) provides funding for the MrOS Sleep ancillary study "Outcomes of Sleep Disorders in Older Men" under the following grant numbers: R01 HL071194, R01 HL070848, R01 HL070847, R01 HL070842, R01 HL070841, R01 HL070837, R01 HL070838, and R01 HL070839.
The funding agencies had no direct role in the conduct of the study; the collection, management, analyses and interpretation of the data; or preparation or approval of the manuscript.
Dr. Redline has received grant funding from the NIH via a subcontract from CPMC Research Institute.
Abbreviations
- FNE
first night effect
- PSG
polysomnography
- TST
total sleep time
- SE
sleep efficiency
- SOL
sleep onset latency
- WASO
wake after sleep onset
- MrOS Sleep
Outcomes of Sleep Disorders in Older Men Study
- MrOS Study
Osteoporotic Fractures in Men Study
- EEG
electroencephalogram
- ECG
electrocardiogram
- AHI
apnea-hypopnea index
- PASE
Physical Activity Scale for the Elderly
- GDS
Geriatric Depression Scale
- ESS
Epworth Sleepiness Scale
- 3MS
Modified Mini-Mental State examination
- IADL
instrumental activities of daily living
- BMI
Body mass index
- IDIS
Iowa Drug Information Service
- SD
standard deviation
- CI
confidence interval
- NS
not significant
- DIMS
difficulty initiating and maintaining sleep
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
Terri Blackwell, Dr. Paudel and Dr. Stone have no conflicts of interest to report. Dr. Ancoli-Israel is a consultant or on the advisory board for Merck, Pfizer, Purdue Pharma LP, and Jansen.
References
- 1.Agnew HW, Jr, Webb WB, Williams RL. The first night effect: an EEG study of sleeph. Psychophysiology. 1966;2:263–266. doi: 10.1111/j.1469-8986.1966.tb02650.x. [DOI] [PubMed] [Google Scholar]
- 2.Mendels J, Hawkins DR. Sleep laboratory adaptation in normal subjects and depressed patients ("first night effect") Electroencephalogr Clin Neurophysiol. 1967;22:556–558. doi: 10.1016/0013-4694(67)90063-6. [DOI] [PubMed] [Google Scholar]
- 3.Coble P, McPartland RJ, Silva WJ, Kupfer DJ. Is there a first night effect? (a revisit) Biol Psychiatry. 1974;9:215–219. [PubMed] [Google Scholar]
- 4.Browman CP, Cartwright RD. The first-night effect on sleep and dreams. Biol Psychiatry. 1980;15:809–812. [PubMed] [Google Scholar]
- 5.McCall C, McCall WV, Objective vs. subjective measurements of sleep in depressed insomniacs: first night effect or reverse first night effect? J Clin Sleep Med. 2012;8:59–65. doi: 10.5664/jcsm.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le Bon O, Minner P, Van Moorsel C, et al. First-night effect in the chronic fatigue syndrome. Psychiatry Res. 2003;120:191–199. doi: 10.1016/s0165-1781(03)00185-9. [DOI] [PubMed] [Google Scholar]
- 7.Saletu B, Klösch G, Gruber G, Anderer P, Udomratn P, Frey R. First-night-effects on generalized anxiety disorder (GAD)-based insomnia: laboratory versus home sleep recordings. Sleep. 1996;19:691–697. [PubMed] [Google Scholar]
- 8.Edinger JD, Fins AI, Sullivan RJ, Jr, et al. Sleep in the laboratory and sleep at home: comparisons of older insomniacs and normal sleepers. Sleep. 1997;20:1119–1126. doi: 10.1093/sleep/20.12.1119. [DOI] [PubMed] [Google Scholar]
- 9.Hauri PJ, Olmstead EM. Reverse first night effect in insomnia. Sleep. 1989;12:97–105. doi: 10.1093/sleep/12.2.97. [DOI] [PubMed] [Google Scholar]
- 10.Newell J, Mairesse O, Verbanck P, Neu D. Is a one-night stay in the lab really enough to conclude? First-night effect and night-to-night variability in polysomnographic recordings among different clinical population samples. Psychiatry Res. 2012;200:795–801. doi: 10.1016/j.psychres.2012.07.045. [DOI] [PubMed] [Google Scholar]
- 11.Moser D, Kloesch G, Fischmeister FP, Bauer H, Zeitlhofer J. Cyclic alternating pattern and sleep quality in healthy subjects--is there a first-night effect on different approaches of sleep quality? Biol Psychol. 2010;83:20–26. doi: 10.1016/j.biopsycho.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 12.Webb WB, Campbell SS. The first night effect revisited with age as a variable. Waking Sleeping. 1979;3:319–324. [PubMed] [Google Scholar]
- 13.Zheng H, Sowers M, Buysse DJ, et al. Sources of variability in epidemiological studies of sleep using repeated nights of in-home polysomnography: SWAN Sleep Study. J Clin Sleep Med. 2012;8:87–96. doi: 10.5664/jcsm.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scholle S, Scholle HC, Kemper A, et al. First night effect in children and adolescents undergoing polysomnography for sleep-disordered breathing. Clin Neurophysiol. 2003;114:2138–2145. doi: 10.1016/s1388-2457(03)00209-8. [DOI] [PubMed] [Google Scholar]
- 15.Kim RD, Kapur VK, Redline-Bruch J, et al. An Economic Evaluation of Home Versus Laboratory-Based Diagnosis of Obstructive Sleep Apnea. Sleep. 2015;38:1027–1037. doi: 10.5665/sleep.4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le Bon O, Staner L, Hoffmann G, et al. The first-night effect may last more than one night. J Psychiatr Res. 2001;35:165–172. doi: 10.1016/s0022-3956(01)00019-x. [DOI] [PubMed] [Google Scholar]
- 17.Lorenzo JL, Barbanoj MJ. Variability of sleep parameters across multiple laboratory sessions in healthy young subjects: the "very first night effect". Psychophysiology. 2002;39:409–413. doi: 10.1017.S0048577202394010. [DOI] [PubMed] [Google Scholar]
- 18.Sharpley AL, Solomon RA, Cowen PJ. Evaluation of first night effect using ambulatory monitoring and automatic sleep stage analysis. Sleep. 1988;11:273–276. doi: 10.1093/sleep/11.3.273. [DOI] [PubMed] [Google Scholar]
- 19.Lord S, Sawyer B, O'Connell D, et al. Night-to-night variability of disturbed breathing during sleep in an elderly community sample. Sleep. 1991;14:252–258. [PubMed] [Google Scholar]
- 20.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 21.van Hilten JJ, Braat EA, van der Velde EA, Middelkoop HA, Kerkhof GA, Kamphuisen HA. Ambulatory activity monitoring during sleep: an evaluation of internight and intrasubject variability in healthy persons aged 50–98 years. Sleep. 1993;16:146–150. doi: 10.1093/sleep/16.2.146. [DOI] [PubMed] [Google Scholar]
- 22.Blank JB, Cawthon PM, Carrion-Petersen ML, et al. Overview of recruitment for the osteoporotic fractures in men study (MrOS) Contemp Clin Trials. 2005;26:557–568. doi: 10.1016/j.cct.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men(MrOS) study--a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26:569–585. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 24.Redline S, Sanders MH, Lind BK, et al. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep Heart Health Research Group. Sleep. 1998;21:759–767. [PubMed] [Google Scholar]
- 25.Rechtschaffen A, Kales A, editors. A manual of standardized terminology, techniques, and scoring system for sleep stages of human subjects. Washington DC: National Institutes of Health; 1968. NIH publication 204. [Google Scholar]
- 26.American Sleep Disorders Association. EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–184. [PubMed] [Google Scholar]
- 27.Quan SF, Howard BV, Iber C, et al. The Sleep Heart Health Study: design, rationale, and methods. Sleep. 1997;20:1077–1085. [PubMed] [Google Scholar]
- 28.Motionlogger® User’s Guide: Act Millenium. Ardsley NY: Ambulatory Monitoring, Inc.; [Google Scholar]
- 29.Action-W User’s Guide, Version 2.0. Ardsley NY: Ambulatory Monitoring, Inc.; [Google Scholar]
- 30.Blackwell T, Ancoli-Israel S, Redline S, Stone KL for the Osteoporotic Fractures in Men (MrOS) Study Group. Factors that May Influence the Classification of Sleep-Wake by Wrist Actigraphy: The MrOS Sleep Study. J Clin Sleep Med. 2011;7:357–367. doi: 10.5664/JCSM.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blackwell T, Ancoli-Israel S, Gehrman PR, Schneider JL, Pedula KL, Stone KL. Actigraphy scoring reliability in the study of osteoporotic fractures. Sleep. 2005;28:1599–1605. doi: 10.1093/sleep/28.12.1599. [DOI] [PubMed] [Google Scholar]
- 32.Barone JJ, Roberts HR. Caffeine consumption. Food Chem Toxicol. 1996;34:119–129. doi: 10.1016/0278-6915(95)00093-3. [DOI] [PubMed] [Google Scholar]
- 33.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): Development and Evaluation. J Clin Epidemiol. 1993;46:153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 34.Sheikh J, Yesavage J. Geriatric Depression Scale: recent evidence and development of a shorter version. Clin Gerontol. 1986;5:165–173. [Google Scholar]
- 35.Goldberg D, Bridges K, Duncan-Jones P, Grayson D. Detecting anxiety and depression in general medical settings. Bmj. 1988;297:897–899. doi: 10.1136/bmj.297.6653.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 37.Johns MW. Sensitivity and specificity of the multiple sleep latency test (MSLT), the maintenance of wakefulness test and the epworth sleepiness scale: failure of the MSLT as a gold standard. J Sleep Res. 2000;9:5–11. doi: 10.1046/j.1365-2869.2000.00177.x. [DOI] [PubMed] [Google Scholar]
- 38.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 39.Fitti JE, Kovar MG. The supplement on aging to the 1984 National Health Interview Survey. Vital & Health Statistics-series 1: Programs & collection procedures. 1987;21:1–115. [PubMed] [Google Scholar]
- 40.Pincus T, Summey JA, Soraci SA, Jr, Wallston KA, Hummon NP. Assessment of patient satisfaction in activities of daily living using a modified Stanford Health Assessment Questionnaire. Arthritis Rheum. 1983;26:1346–1353. doi: 10.1002/art.1780261107. [DOI] [PubMed] [Google Scholar]
- 41.Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994;10:405–411. doi: 10.1007/BF01719664. [DOI] [PubMed] [Google Scholar]
- 42.Coates TJ, George JM, Killen JD, Marchini E, Hamilton S, Thorensen CE. First night effects in good sleepers and sleep-maintenance insomniacs when recorded at home. Sleep. 1981;4:293–298. doi: 10.1093/sleep/4.3.293. [DOI] [PubMed] [Google Scholar]
- 43.Marzec ML, Selwa LM, Malow BA. Analysis of the first night effect and sleep parameters in medically refractory epilepsy patients. Sleep Med. 2005;6:277–280. doi: 10.1016/j.sleep.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 44.Milross MA, Piper AJ, Norman M, et al. Night-to-night variability in sleep in cystic fibrosis. Sleep Med. 2002;3:213–219. doi: 10.1016/s1389-9457(02)00030-8. [DOI] [PubMed] [Google Scholar]
- 45.Ma J, Zhang C, Zhang J, et al. Prospective study of first night effect on 2-night polysomnographic parameters in adult Chinese snorers with suspected obstructive sleep apnea hypopnea syndrome. Chin Med J (Engl) 2011;124:4127–4131. [PubMed] [Google Scholar]
- 46.Pittsley M, Gehrman P, Cohen-Zion M, Stepnowsky C, Marler M, Ancoli-Israel S. Comparing night-to-night variability of sleep measures in elderly African Americans and Whites. Behav Sleep Med. 2005;3:63–72. doi: 10.1207/s15402010bsm0302_1. [DOI] [PubMed] [Google Scholar]
- 47.Edinger JD, Marsh GR, McCall WV, Erwin CW, Lininger AW. Sleep variability across consecutive nights of home monitoring in older mixed DIMS patients. Sleep. 1991;14:13–17. [PubMed] [Google Scholar]
- 48.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.