Abstract
Data Monitoring Committees (DMCs) are responsible for safeguarding the interests of study participants and assuring the integrity and credibility of clinical trials. The independence of DMCs from sponsors and investigators is essential to achieving this mission. Creative approaches are needed to address ongoing and emerging challenges that potentially threaten DMCs’ independence and effectiveness. An expert panel of representatives from academia, industry and government sponsors, and regulatory agencies discussed these challenges and proposed best practices and operating principles for effective functioning of contemporary DMCs.
Prospective DMC members need better training. Options could include didactic instruction as well as apprenticeships to provide real-world experience. DMC members should be protected against legal liability arising from their service. While avoiding breaches in confidentiality of interim data remains a high priority, DMCs should have access to unblinded efficacy and safety data throughout the trial to enable informed judgments about risks and benefits. Because overly rigid procedures can compromise their independence, DMCs should have the flexibility necessary to best fulfill their responsibilities. DMC charters should articulate principles that guide the DMC process rather than list a rigid set of requirements. DMCs should develop their recommendations by consensus rather than through voting processes. The format for DMC meetings should maintain the independence of the DMC and clearly establish the leadership of the DMC chair. The independent statistical group at the Statistical Data Analysis Center should have sufficient depth of knowledge about the study at hand and experience with trials in general to ensure that the DMC has access to timely, reliable, and readily interpretable insights about emerging evidence in the clinical trial. Contracts engaging DMC members for industry-sponsored trials should have language customized to the unique responsibilities of DMC members rather than use language appropriate to consultants for product development. Regulatory scientists would benefit from experiencing DMC service that does not conflict with their regulatory responsibilities.
Keywords: Independence, training, experience, apprenticeship, indemnification, confidentiality, charter, regulatory, operating principles
Introduction
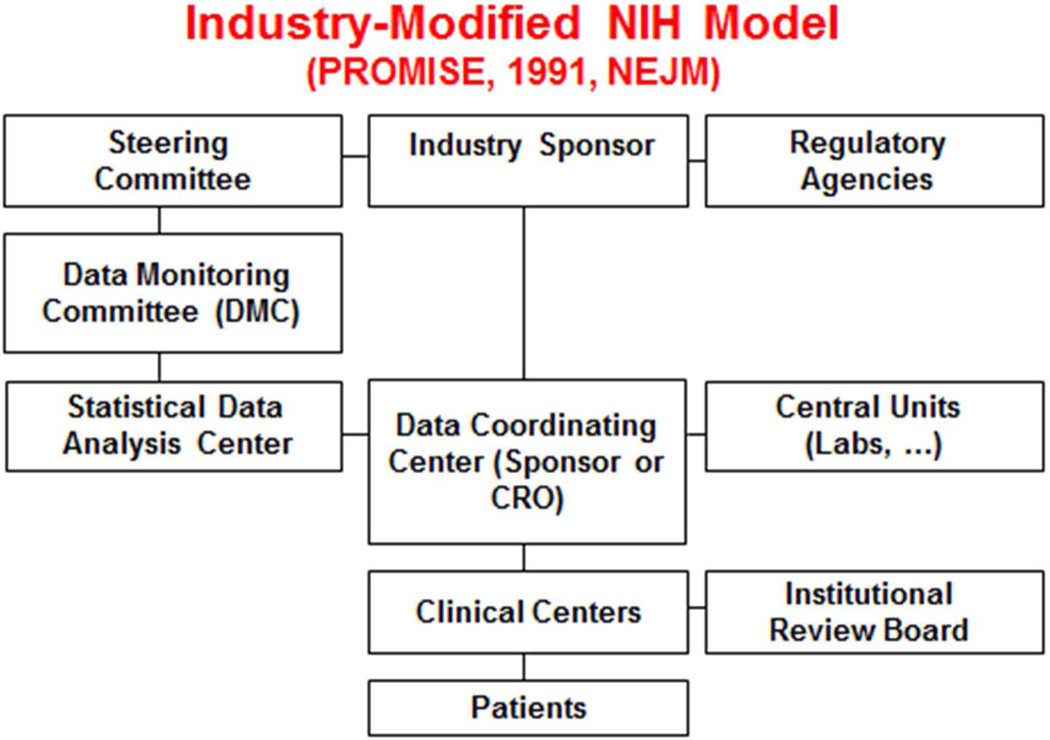
Properly designed and conducted randomized clinical trials are integral to achieving timely and reliable evidence about the benefits and risks of medical interventions. Oversight of these clinical trials requires coordination and ongoing review of safety, efficacy, quality, ethics, adjudication, operations, and logistics. Independent Data Monitoring Committees (DMCs) hold a unique place in clinical trial oversight (see Figure 1). DMCs periodically conduct unblinded reviews of accumulating safety and efficacy data and serve in an advisory role to the trial leadership and sponsor. Their primary mission is to safeguard the interests of study participants and to protect the integrity and credibility of the trial. A substantial body of literature describes the role, function, and experience of DMCs;1–21 see online Appendix 1 for the evolution of their role and scope.
Figure 1.
Organizational diagram of clinical trial stakeholders and participants. The Data Monitoring Committee occupies a central position and interacts directly with some of the groups that oversee, support, and manage the trial conduct.
The independence of DMCs from sponsors and investigators is essential to the ability of DMCs to achieve their mission; however, ongoing and emerging challenges threaten DMCs’ independence and effectiveness. An expert panel of representatives from academic medical centers, academic research organizations, the pharmaceutical and biotech industry, the National Institutes of Health, and the U.S. Food and Drug Administration discussed these challenges and proposed best practices and operating principles for the effective functioning of DMCs. This position paper summarizes these discussions and offers recommendations to improve the DMC process, highlighting insights from meeting participants who collectively have DMC experience in hundreds of clinical trials, and who widely represent areas of expertise in the DMC process.
Best practices and operating principles for DMCs
Achieving adequate training/experience in the DMC process
DMCs face complex challenges, such as balancing safety signals that arise from limited data against the value of continuing the trial to obtain more conclusive results. Most DMC members have not received formal training concerning their roles and responsibilities, in part because of a lack of infrastructure for providing such training.17–19 A recent unpublished survey reported that only 8% of DMC members indicated having been trained in DMC processes, while nearly all indicated their belief that such training would have been valuable prior to their initial DMC service. Few trial sponsors require formal training for DMC members.
Training options for DMC service could include a combination of didactic and experiential instruction. Training might include a formal curriculum with textbooks,5,6,8 articles,9–11 web-based lectures,12 or interactive courses. DMC members should understand basic clinical trial design and analysis13 as well as the fundamentals of clinical trial operations. More advanced training might include biomedical ethics, and methodologies of interim analyses. Formal DMC courses could be offered as part of comprehensive degree programs at schools of medicine or public health, or through training programs offered by Clinical Translational Science Award-funded institutions.22 Experienced DMC members could offer “short courses,” perhaps in conjunction with professional meetings. Such training could be supplemented by web-based modules designed to highlight past dilemmas DMCs have faced. Some universities offer similar types of instruction in other disciplines through open-source platforms such as Coursera, Open CourseWare, and iTunes U. These increasingly popular courses offer a low-cost mechanism to disseminate DMC training widely. Efforts also could target international settings, such as those by the Harvard Multiregional Clinical Trials Center in publishing insights about DMC responsibilities23 and in providing regional training opportunities to hundreds of clinical trialists from emerging regions by sending teams of clinical trial experts to developing countries to build expertise regarding the purpose, goals and responsibilities of DMCs.
Given the nature and complexity of DMC functions, a combination of training and experience may best prepare a new generation of qualified DMC members. An apprenticeship model for initial DMC service could complement didactic and web-based training. In one apprenticeship model, the DMC could include a ‘new member’ without prior DMC experience, yet with substantive expertise in areas of research or practice relevant to the deliberations of the DMC. This ‘new member’ could benefit from the mentorship provided by at least one experienced fellow member, ideally from a related area of specialization, enabling them to effectively engage in the DMC’s process of review and consensus development. While many expert panelists agreed with the potential value of such apprenticeships, such programs remain to be widely implemented.
Some apprenticeship-type opportunities have been provided through participation in clinical trial monitoring activities performed by committees that are ‘semi-independent’ because their membership includes researchers from the sponsoring organization who do not have leadership responsibilities in the trials. These could be standing internal committees formed by sponsors to monitor early-stage trials not having independent DMCs. Such a committee at Merck Research Laboratories involves both junior and senior members from clinical, quantitative, and regulatory organizations within Merck, and enables internal personnel to gain experience on safety and efficacy monitoring practices and techniques. In some settings, these semi-independent standing committees have been formed to be supportive to DMCs that are in place. For clinical trials conducted by HIV prevention cooperative groups sponsored by the National Institute of Allergy and Infectious Diseases, such committees monitor pooled data on quality of trial conduct measures, enhancing trial integrity while providing their members substantive insights into the monitoring process.24
While training and experience is important for all DMC members, this is especially true for the DMC Chair, whose leadership has considerable influence on the efficiency and effectiveness of the DMC process. The Chair should be prepared to serve in the lead role in all sessions of the DMC meeting, and in the development and dissemination of DMC recommendations and meeting minutes.
Training the next generation of DMC members will require commitment from academic institutions, government agencies, and professional organizations such as the Society of Clinical Trials, in order to develop curricula, options for “real-world” DMC experiences, and mentoring by experienced DMC members. A well-structured certification process could provide substantive experience for potential future DMC members. Defining the financial model needed to support such endeavors will be important in ensuring that DMC training options become widely available and sustainable.
Indemnification
DMC members face several sources of possible liability from clinical trial stakeholders,25 including trial participants or their advocates who might argue that participants experienced trial-related injuries that the DMC could have prevented, as well as investors and employees of companies who have a financial stake in the outcomes. Historically, few DMCs have been the subject of litigation; however, over the past decade, several DMC members have been involved as defendants or have been required to provide depositions for legal proceedings (personal communications). The possibility of litigation has many important ramifications for DMC members including significant personal financial costs, substantial lost time from normal work activities, personal stress, and damage to professional reputation.26
Indemnification for DMC members could influence their performance. Some participants on the expert panel suggested that comprehensive sponsor indemnification could encourage overly risky decision-making by the DMC; others observed that indemnification coverage has routinely been provided by academic institutions or industry to their employees, and automobile insurance policies typically provide coverage even in instances of negligence, presumably based on judgment that such practices do not lead to unacceptable increases in irresponsible behavior. Most of the expert panel and all authors supported the view that a robust indemnification process could favorably influence DMC decision-making by enabling members to engage in deliberations about how best to protect patient safety and trial integrity without having excessive concerns about potential legal and financial consequences of their recommendations.
Some points about indemnification deserve particular emphasis. A variety of indemnification options often seen in DMC contracts for industry-sponsored trials were widely considered to be unacceptable, including requiring DMC members to indemnify the sponsor, providing indemnification only if DMC members use the sponsor’s rather than their independent counsel if litigation is pursued against them, and providing an “opt out” of indemnification for negligence by the DMC member with no indication of how such judgments would be made. The indemnification process also is complicated for DMC members participating in National Institutes of Health-sponsored research, since the federal Antideficiency Act, Pub.L. 97–258, 96 Stat. 923, specifically prohibits indemnification of non-government employees.
Potential solutions to the indemnification problem have been proposed. First, DMC members as well as trial sponsors should be educated about issues such as personal liability and indemnification to ensure that they consider these when negotiating contracts for DMC membership. Second, standardized indemnification language could be developed for use in industry-sponsored DMC contracts to provide indemnification except in cases of a judicial finding of gross negligence, willful misconduct, or fraudulent acts.26 Third, in National Institutes of Health-sponsored trials, external sources could be pursued for liability coverage, such as the DMC member’s home institution or the trial’s data coordination center. Fourth, central sources of indemnification for individual DMC members could be pursued, such as creating a risk pool or other type of professional liability insurance group plan to mitigate individual risk. While the financial model needed to support such an endeavor is uncertain, key clinical trial stakeholders such as the Clinical Translational Science Award program and the National Patient-Centered Clinical Research Network could be engaged to develop viable strategies for collectively addressing this issue of such importance to DMC service.
Maintaining confidentiality
Preserving confidentiality of interim clinical trial data is essential to trial integrity as it reduces the risk for pre-judgments that could adversely impact future trial enrollment, trial participants’ adherence to the randomized therapies, and participants’ retention in the trial. Undermining these components of trial conduct threatens the ability of the trial to provide clear answers to the questions it was designed to address.3,5,21,27–31 In a recent well-publicized instance, a serious breach in confidentiality led to wide dissemination of misleading results and inability to complete the trial.31 While rare, breaches of confidentiality are extremely serious as they may irreversibly bias subsequent trial conduct, whether in fact or by perception.21
Trial sponsors with a financial or intellectual property interest in the outcome of a trial, as in industry trials, should not attend the closed DMC sessions when unblinded efficacy and safety data are reviewed. This restriction also holds for trial leadership, who might have a vested scientific interest in a specific trial outcome. Excluding non-members of the DMC protects the independence of the DMC from interference by entities with vested interests, and protects sponsors and investigators from any perception that they might have used interim results to interfere with the scientific process. Government-funded trials do not pose the same type of financial conflicts for the sponsors as do industry-funded trials. However, even if one accepts that a funding agency has no financial or intellectual property stake in the outcome of the trial and no institutional interest other than in assuring the trial be conducted in a scientifically rigorous and ethical manner, whether agency staff members should attend closed sessions continues to be debated.32 Many clinical trial experts caution against such attendance even in these settings since funding agencies (and in particular, agency staff who may have played an important role in obtaining funding for the study) have an intellectual stake in the trial that they designed and funded.3,5,28,30,32,33
An important aspect of trial conduct relating to confidentiality is the need, on occasion, to make changes to a protocol during the study. Data external to the trial, for example, may indicate the desirability of modifying the eligibility criteria or even changing the primary endpoint. The trial sponsor makes final decisions regarding such issues. A trial sponsor with access to the interim data, however, cannot make objective decisions about such changes, since it will usually be clear whether the change will increase or decrease the probability that the trial has the desired outcome. This issue is relevant to all trial sponsors, public or private.
While blinding other stakeholders is important to maintaining integrity of the trial, DMCs should have full access to unblinded accumulating data on safety and efficacy throughout the clinical trial. Some believe a DMC should receive only safety data or that a DMC that receives efficacy data only by blinded codes (e.g., Group A versus Group B) will be more objective in assessing interim data. The consensus of the expert panel was that such blinding was counterproductive, even potentially dangerous to the safety of the study participants. By having access to unblinded data on all relevant treatment outcomes, the DMC can develop timely insights about safety in the context of a benefit-to-risk assessment, as well as about irregularities in trial conduct or in the generation of the DMC reports.5,30,32,34,35 See online Appendix 2 for further discussion, including examples of experiences.
DMC members are expected to maintain confidentiality of emerging trial results. Typically, members sign confidentiality agreements that cover not only the period of trial conduct but also a substantial period of time following the conclusion of the trial. Given these typical agreements, how should the DMC proceed if the DMC believes the sponsor’s dissemination or lack of dissemination of information to the trial participants and the public has led to serious scientific or ethical concerns? In particular, what recourse do DMC members have to comment? While rare, such situations have occurred and have led to consideration about whether some type of mediation process for DMCs is needed in such situations.5
Creating an effective DMC charter: Avoiding rigid procedures
A DMC charter, and at times a DMC-specific statistical analysis plan or a performance standards document,5 should be developed to guide the DMC process. The DMC charter should specify the primary responsibilities of the DMC in relation to the sponsor and the trial leadership committees. The charter should address potential conflicts of interests DMC members may have, procedures for maintaining confidentiality, the format for DMC meeting sessions, reports and minutes, additional details regarding communication pathways, and the statistical guidelines for interim analyses.5
Over the years, the length of DMC charters has grown, and their tone has become increasingly legalistic rather than focusing upon a set of principles intended to serve as an operational guide. Some DMC charters have formulated restrictions that limit the number of meetings, or refer to “just reviewing safety data” rather than assessing safety in the context of benefit-to-risk, or have restricted the format and content of DMC reports. Some DMC charters require a rigid voting process rather than recognizing the importance of formulating DMC recommendations through consensus development.
The DMC should provide multidisciplinary scientific and ethical oversight by exploiting the synergy of members’ experience and expertise. The expert panel agreed that DMC recommendations are best formulated by in-depth discussion of the issues leading to consensus, rather than by voting. Extensive experience has shown broad success in achieving consensus when the DMC chair assumes responsibility for fostering communication and collaboration among the DMC members, maximizes the contributions from all members, ensures that relevant concerns are properly addressed, and guides the DMC toward developing final consensus recommendations.
Long, complex, and ritualistic DMC charters inappropriately constrain DMCs by eroding their independence and, perhaps, sometimes placing them in a legally and scientifically compromised position. Predefining exact operational procedures and contingencies for all potential scenarios is not possible. The DMC must have the flexibility to deal with unexpected challenges. The charter should articulate principles and processes that will provide guidance to the DMC in addressing its mission. To assure proper tone and content for the DMC charter, the DMC should share the lead responsibility for the charter’s development, usually in collaboration with trial leadership.
DMC meeting format
Traditionally, the format for DMC meetings includes both closed and open sessions.5,37 In the closed sessions attended only by DMC members and the unblinded independent statistician, the DMC reviews measures of efficacy, safety, and quality of trial conduct in an unblinded manner. In the open sessions which are attended by trial leadership and sponsors as well as the DMC members, the data reviewed are typically limited to measures of the quality of trial conduct, pooled across intervention groups. Closed sessions allow the DMC to maintain confidentiality of interim data, while the open sessions allow direct interaction between the DMC and the study team about trial conduct, evidence from external sources related to the study interventions, and relevant interactions between the sponsor and regulatory authorities.
The specific meeting format can impact the independence and integrity of the DMC process. Beginning the DMC meeting with a closed session emphasizes the autonomy of the DMC and enhances the likelihood that the DMC—not the trial leadership or sponsor—is in charge of the meeting and its agenda. This approach also offers an opportunity for DMC members to achieve a common understanding of the principal findings from interim data, and to organize and prioritize issues for discussion with the study investigators during the open session. This also provides an opportunity to remind DMC members about the confidential nature of the proceedings before they receive updates on the trial progress from the study investigators during the open session. For these reasons, we favor this format; however, alternative formats (e.g., beginning with an open session) can work if the DMC chair brings strong leadership and experience to the proceedings.
Defining the role of the statistical data analysis center (SDAC)
To perform its functions effectively, a DMC relies on reports containing timely and accurate data on efficacy, safety, and quality of trial conduct.5 Independent biostatisticians at the SDAC generate these reports.38,39 These biostatisticians typically serve as a liaison between the DMC and the database (see Figure 1), attending the open and closed sessions of the DMC, and providing additional information requested by the DMC between and during the meetings.
The SDAC may be embedded within an academic research organization or function as an independent entity. The same entity may serve not only as the SDAC but also as the data analysis center for data management and site management; in this arrangement, which is common for federally-funded trials, there are blinded and unblinded personnel in the same organization. In such settings, it is important to ensure that the unblinded statisticians can carry out their responsibilities with the DMC confidentially and independently of others in the organization. Some have expressed a concern that an organization with dual roles might create a conflict of interest for the independent statistician because producing a report likely to result in a DMC recommendation for terminating the trial would adversely affect the organization. DMCs should be cognizant of this potential conflict of interest. The independent statistician, whether as part of the organization that performs data and site management or as part of a separate SDAC, should have the knowledge of the protocol and data collection plan and experience to provide the DMC with timely, reliable, and readily interpretable closed reports. This capability should be the primary consideration in the selection of an SDAC.
In current practice, the length and content of DMC reports vary tremendously. Some reports are as brief as a dozen tables with perhaps some accompanying text while others resemble a lengthy clinical study report with thousands of pages of figures, tables, and listings. The DMC needs streamlined concise documents that display important data in optimally informative ways to foster efficient and intelligent decision-making. As such, at the pre-trial DMC organizational meeting, the DMC should help to define the format and content of critical tables and graphs. Thoughtful development of figures and table is important; graphical presentations of data with backup tables in an organized appendix for reference as needed, are encouraged.40 DMC reports should begin with a brief protocol synopsis, a listing of new amendments, a reminder of previous recommendations of the DMC, a concise summary of interim data, and an explanation of complex medical or statistical issues that may influence the interpretation of the data.
The contract for the SDAC should ensure that the independent statistician is experienced with clinical trials and with DMC processes, and is provided adequate time and resources to evaluate the interim data and generate an informative but concise report (see Table 1). The SDAC contract should also recognize the need for flexibility to respond to DMC needs and requests to modify the report during the course of the trial. Reports should not be limited to analyses planned at study onset, as the DMC may need additional explanatory and exploratory analyses to help interpret emerging findings. This capability will require that SDACs routinely have access to all trial data, not only the subset of data required to produce the initially specified reports.41 The SDAC should not have to obtain permission or additional data from the sponsor to add or change the number of analyses, tables, or graphs, as such requests endanger the confidentiality of the emerging data. The initial SDAC budget should build in some funding for unplanned or additional analyses the DMC may request. An experienced SDAC will generally be able to estimate quite accurately these needs.
Table 1.
Core responsibilities of the Statistical Data Analysis Center & key attributes of the DMC report
| Core Responsibilities | Best Practices for Report Content |
|---|---|
|
Interim data summary report should be in one document with page numbers. The report should be:
|
DMC contracting process
Discussion regarding DMC contracting for industry-sponsored trials focused on the difference between the type of services offered by someone to assist the sponsor in product development, and the type of service a DMC member offers a company. In the former, intellectual property issues are very important. In the latter, avoidance of conflicts of interest and even the reduction of the perception of such risks is of considerable importance. While some industry sponsors have developed “independent scientist” agreements for DMC members, many continue to use the same contracts assigned to product development consultants. Such generic contracts are often complex and written in technical and legally binding language that is not consistent with the mission of DMC members. Specialized contracting procedures and language are needed to enlist DMC members; the language should recognize the DMC members as independent scientists whose primary focus is to protect patient safety and trial integrity.
The DMC’s independence, either real or perceived, also could be influenced by who engages the DMC members, manages the contracting process and provides payment for their services. While it is appropriate for the trial sponsor to assume these roles, DMC independence could be enhanced by other approaches. For example, in industry sponsored trials, academic leadership of a study steering committee could engage the DMC members and they (or the trial’s data coordinating center or Statistical Data Analysis Center) could provide the payments to DMC members for their services.42 See online Appendix 3 for further discussion of addressing conflicts of interest.
More effectively integrating regulatory authorities into the DMC process
The work of DMCs is relevant to regulatory agencies, especially when a trial has been terminated early for benefit or harm. As a result, some regulatory agencies have developed guidelines that provide an important set of principles for the DMC process, including the 2006 Food and Drug Administration Guidance Document,3 the 2007 European Medicines Agency Guideline on DMCs,43 and the 2013 Guideline on DMCs from Japan’s Pharmaceuticals and Medical Devices Agency.44
Given the importance of the DMC’s role in ensuring appropriate and safe conduct of trials, additional guidance from global regulatory authorities would be useful, in part to help address challenges we have discussed that potentially threaten the DMC’s independence and effectiveness. Regulatory authorities may benefit from having direct experience in the DMC process. Clinicians and statisticians working in regulatory agencies should have the opportunity to observe the DMC process or even serve on DMCs, ideally in specialty areas unrelated to their regulatory review functions. Such experience would enhance regulators’ understanding of DMC operations and practices and thus their ability to periodically advise sponsors about best practices for DMC procedures in light of the evolving landscape of clinical trials and trial oversight.
Conclusions: DMC best practices and operating principles
DMCs play an important role in safeguarding the interests of study participants and enhancing the integrity and credibility of clinical trials. The independence of DMCs from sponsors and investigators is essential to achieving this mission; however, ongoing and emerging challenges in the current clinical research environment provide multiple threats to the effectiveness and independence of DMCs. To address these issues, creative approaches are needed in many areas. Training options for prospective DMC members should be more widely developed and used. These could include didactic instruction along with apprenticeships to provide real-world experience. Indemnification for DMC members is needed, with an “opt-out” only in cases of a judicial finding of gross negligence, willful misconduct, or fraudulent acts, and with the ability of DMC members to select and retain their own independent counsel when faced with litigation. Adequately flexible procedures are needed to protect the independence of the DMC process. In that regard, the preferred approach is for DMC charters to articulate principles guiding the DMC process rather than providing a rigid set of requirements, and for DMC recommendations to be developed by consensus rather than through rigid voting processes. The format of DMC meetings, such as the order of closed and open sessions, should maintain independence of the DMC from the sponsor and investigators. The leadership role of the DMC chair should be clear. DMC members and the Statistical Data Analysis Center independent statisticians should have access to unblinded efficacy and safety data throughout the trial to enable informed judgments. The independent statisticians at the Statistical Data Analysis Center should have the depth of knowledge and experience to ensure that the DMC has access to timely, reliable, and readily interpretable insights about emerging evidence in the clinical trial. Real and perceived conflicts of interest should be identified and procedures should be followed to avoid the creation of such conflicts. DMC members in industry-sponsored trials should be engaged using approaches that recognize members’ role as independent scientists focused on protecting patient safety and trial integrity rather than as consultants for product development, in part through the development of a customized independent scientist agreement for DMC members. Finally, regulatory authorities should have the opportunity to observe or even to serve on DMCs in trial settings that would not create conflicts with their regulatory responsibilities, enhancing their ability to provide informed guidance regarding the DMC process.
Many factors, including evolving circumstances, have led to these ongoing and emerging challenges. Some issues, such as those related to the need for better training and experience, have intensified with the rapid increase in clinical trials having DMCs, while others, such as the need for protection against legal liability, have grown as DMCs have become more visible. By adopting consensus best practices and operating principles for effective functioning of contemporary DMCs, the role of the DMC can continue to evolve to safeguard patients’ interests effectively and to enhance trial integrity, in support of obtaining ethical, timely, and reliable evaluations of new interventions for the prevention and treatment of diseases.
Acknowledgments
Administrative and funding support for the expert panel meeting
The expert panel meeting, held on June 4–5, 2015, was organized by the lead authors of the manuscript, together with colleagues at the Duke Clinical Research Institute. Marelle Molbert from the Duke Clinical Research Institute made significant contributions to the management of the expert panel. Funding support for the meeting was provided through registration fees from Amgen, Inc., AstraZeneca, Bristol-Myers Squibb Co., Johnson & Johnson/Janssen Pharmaceutical, Merck & Co., Inc., Roche/Genentech, Rhythm Pharmaceutical and St. Jude Medical. No government funds were used for this meeting. All meeting participants were invited to contribute to the development of this position paper, with those making substantive contributions being recognized as authors. The authors determined the content and direction of the position paper and it presents their views.
The authors appreciate the thoughtful comments provided by the reviewers and editorial board of the journal.
Appendix 1. The Evolution of the Role and Scope of the DMC’s Mission in Clinical Trials
An NIH advisory committee, chaired by Dr. Bernard Greenberg, was convened in 1967 to offer guidance on the organization and conduct of large-scale clinical trials. Among the recommendations of the Greenberg Report was establishment of an external board to review the study protocol, monitor the conduct of the trial, and develop a mechanism for early termination of the trial if necessary to protect patient safety.1 These recommendations were first implemented in the Coronary Drug Project2; variations on the Coronary Drug Project model were adopted by many National Institutes of Health institutes and other federal agencies. The Food and Drug Administration issued initial guidance in 2001, minimally revised and issued as a final guidance in 2006,3 that addressed the role of a DMC in clinical trials involving Agency-regulated products.4 A substantial body of literature on the role, function and experience of DMCs has been accumulated.5–13
The DMC is an important component of a trial’s organization (Figure 1).5,7 It receives data and analyses from a Statistical Data Analysis Center, an organization that is crucial to supporting the DMC’s mission. The DMC typically reports its recommendations concerning continuation, modification or termination of a trial to the trial leadership and sponsors. Proper interactions among the various trial stakeholders and the DMC is important to achieving optimal DMC conduct.
Although all trials should have careful monitoring of safety and data quality, an independent DMC often is not needed or required.3 Significant heterogeneity exists in the use of DMCs in clinical trials, with evidence of increasing involvement of DMCs, especially in earlier phase trials.14 An analysis of DMC use in randomized clinical trials published in high-impact journals revealed that only 10% of trials published in these medical journals explicitly reported DMC use in 1990, compared with 25% in 2000.15 Although information regarding DMCs is not a mandatory element in registering a clinical trial in the United States ClinicalTrials.gov database, an analysis in 2010 of 33,615 interventional trials across the domains of cardiovascular disease, oncology, and mental health, showed that 41% reported the use of a DMC.16 Notably, this analysis demonstrated that trials sponsored by the National Institutes of Health were nine times more likely to report having a DMC than were trials sponsored by industry; late-stage, phase III trials were most likely to incorporate a DMC, yet even earlier stage phase I-II trials that typically focused upon non-clinical endpoints made frequent use of DMCs.16 Growing evidence suggests that DMCs are implemented broadly across all phases of clinical trials in contemporary practice.17–21
The 2006 Food and Drug Administration guidance on DMCs provides insight into when DMCs may be useful or warranted. The guidance is not prescriptive but emphasizes the utility of DMCs in large phase III trials, especially those with mortality and other clinical endpoints that reflect serious morbidity.3 The Food and Drug Administration requires a DMC for trials in emergency situations when obtaining the patient’s informed consent or consent from a legally authorized representative is not feasible or practical; this is the only situation in which United States regulation requires independent DMCs. The Food and Drug Administration also recommended that sponsors consider using a DMC for trials which entail an expected high risk to study participants or where the presence of a DMC may be particularly important to ensure scientific validity; however, the Food and Drug Administration did not generally expect DMC involvement for trials of products at an early stage of development, or for trials with less serious outcomes. The broader use of DMCs draws upon a limited pool of experienced clinicians and biostatisticians with the necessary qualifications to serve on a DMC. On the other hand, in many clinical settings where DMCs have been used they have made significant contributions to enhancing the quality of trial conduct in addition to safeguarding the interests of study participants. The perspectives of all stakeholders for clinical trials are important when considering the need for independent trial oversight by a DMC.
Appendix 2. Importance of Unblinded Review by DMC Members
The DMC should have access to all the data practically available in order to carry out its primary responsibilities. These data include the actual treatment assignments, not simply codes distinguishing the treatment arms. The DMC is uniquely positioned to be enlightened by interim data without an undue risk for prejudgment. Ideally its members are knowledgeable about the interpretation of data when analyses are conducted repeatedly over calendar time, are as free of conflicts of interest as possible, and are well informed about the importance of maintaining confidentiality of interim data from ongoing trials.
Some have advocated that DMC members review data only by treatment codes (e.g. A vs B), without access to the actual treatments. This practice, presumably intended to increase even further the objectivity of the DMC, is misguided, as it can hamper rapid recognition of potential emerging harm.35 In addition, it is impractical in many studies in which the comparison of rates of specific adverse events would clearly reveal the actual treatments.
Access to unblinded efficacy and safety data not only allows timely insights about benefits and risks, but also increases the DMC’s ability to identify irregularities in trial conduct or in the generation of the DMC reports.5 In many cases, a fully unblinded DMC has been able to identify substantive deficiencies in DMC reports. In a clinical trial conducted in an acute care setting, there had been a sufficiently large excess of irreversible morbidity/mortality events on the experimental arm that it appeared to the DMC that early termination of the trial was justified due to excess risks, (see example 2.5 in reference 5); however, unexpected patterns for treatment effect on some important laboratory and toxicity measures led the DMC to delay their recommendation regarding termination until the independent statistician could verify that the treatment groups had been correctly coded in the DMC Closed Report. During the following week, the DMC’s suspicion was confirmed that the treatment codes in that report had been reversed. A second somewhat similar experience occurred in an oncology trial where the DMC was able to identify a reversal of treatment codes in the DMC report relatively early in the trial based on their recognition of unexpected patterns in the relationship of the intervention with some measures of safety and treatment discontinuation. A third experience arose in a clinical trial with an array of neurological endpoints, (see section 5.3 in reference 5). The DMC discovered that the data in the DMC Closed Report not only were coded A/B for the principal outcome measures, but that a different permutation had been performed for each measure. The DMC was granted access to fully unblinded data that enabled more timely identification of clinically important unfavorable effects of the experimental intervention.
Sometimes sponsors try to limit the role of the DMC to reviews of “safety data” which limits its scope in a potentially dangerous way. For example, in a trial of antiplatelet therapy, bleeding is a key safety outcome. However, increase in the risk of bleeding examined without any reference to the decreased risk of ischemic events, would create a one-sided and misleading view of safety.36 It is essential that the DMC has access to all data that is necessary to make an informed decision about safety in the context of a benefit-to-risk assessment.
Appendix 3. Conflicts of Interest
Because real or perceived conflicts of interest can be detrimental to the integrity of the DMC process, they should be properly disclosed and carefully addressed prior to and during the clinical trial. Typically, financial conflicts are straightforward and generally more readily identified than intellectual or academic conflicts. Perceived conflicts can be challenging to address, as DMC members with deep expertise in the science of the study intervention typically have research arrangements and relationships with many different investigators, as well as with industry and government trial sponsors.
The following conflict of interests should preclude membership on a DMC: employment by sponsors for products that are being evaluated or that are competitive with those being evaluated; ownership of stock in affected companies; having a leadership role in the scientific development of the products being evaluated by the clinical trial; potential involvement in the clinical care of trial participants; or potential to have regulatory or other oversight responsibilities for the trial products being investigated.21 Certain other activities, such as service on multiple DMCs of competing products or even time-limited consulting agreements on issues not related to the ongoing trial, either with the trial’s sponsor or with competitors having related products, typically would not constitute unacceptable conflicts of interest, but members should report such arrangements annually, or whenever a new potential conflict arises, to the DMC Chair.
The DMC meeting venues should be arranged by the sponsor in collaboration with the DMC and the Statistical Data Analysis Center. Locations for in-person meetings should be chosen for ease of access rather than attractiveness of venue. Socializing between the DMC and the sponsor or trial leadership at dinners and functions held in advance or after the DMC meetings is discouraged as being too collegial a relationship between the DMC and sponsor.
Appendix 4. Conference Participant List
John Adler, PhD, Tomas Andersson, MD, PhD, Patrick Archdeacon, MD, PhD, Charles Asare, MD, MPH, Raymond Bain, PhD, Norman Bohidar, PhD, Vicki Brunn, Karim Calis, PharmD, MPH, Gregory Campbell, PhD, David Chonzi, MD, David DeMets, PhD, Mehul Desai, MD, Pete DiBattiste, MD, Susan Ellenberg, PhD, Owen Faris, PhD, Thomas Fleming, PhD, Lawrence Friedman, MD, Diptee Gajjar, B. Pharm, PhD, David Gordon, MD, Charles Hennekens, MD, DrPh, Narimon Honarpour, MD, H.M. James Hung, PhD, Jonathan Jarow, MD, John Jenkins, MD, Marvin Konstam, MD, Scott Korn, MD, Mary Elizabeth Kozar, JD, Chris Kurtz, MD, John Lachin, ScD, Lisa LaVange, PhD, Jack Lawrence, MD, Kerry Lee, PhD, Serio Ley, MD, Thomas Liu, PhD, Kenneth Mahaffey, MD, Alan Meisel, JD, Marelle Molbert, James Neaton, PhD, Gail Pearson, MD, ScD, Michael Pencina, PhD, Marc Pfeffer, MD, PhD, Matthew Roe, MD, MHS, Estelle Russek-Cohen, PhD, Tamara Shipman, BSN, Mat Soukup, PhD, Nusrath Sultana, MD, Robert Temple, MD, Amit Vora, MD, MPH, Janet Wittes, PhD, Bram Zuckerman, MD.
Footnotes
The views expressed in this article are those of the authors and do not necessarily represent the views and policies of the United States Food and Drug Administration; National Heart, Lung, and Blood Institute; National Institutes of Health; or the United States Department of Health and Human Services.
Disclosures
Fleming: This research was partially supported by funding provided by a National Institutes of Health (NIH)/National Institute of Allergy and Infectious Diseases (NIAID) grant titled “Statistical Issues in AIDS Research” (R37 AI 29168). Thomas Fleming serves as a chair or member of many DMCs for both government and industry.
DeMets: David DeMets serves as a member of many DMCs for both government and industry.
Roe: Financial disclosures available at https://www.dcri.org/about-us/conflict-ofinterest/COI%20-Roe_2015.pdf.
Wittes: Dr. Wittes sits on many DMCs for both government and industry. Her company, Statistics Collaborative, serves as the SDAC for many trials sponsored by industry.
Calis: None to report.
Vora: Amit Vora is funded by NIH T-32 training grant T32 HL069749 and L30 HL124592; no other relevant disclosures.
Meisel: None to report.
Bain: None to report.
Konstam: Service on several DMCs and consulting fees from several companies, but nothing representing a conflict of interest for this manuscript.
Pencina: DSMB member for: Theracos, Corvia Medical, Cardiovascular Sciences Foundation.
Gordon: De minimus (<$15,000) shares of stock in GE, Johnson & Johnson, and Pfizer in a generation-skipping trust for my children, which I inherited from my mother in March 2015.
Mahaffey: Financial disclosures prior to August 1, 2013, can be viewed at https://www.dcri.org/about-us/conflict-of-interest/Mahaffey-COI_2011-2013.pdf; disclosures after August 1, 2013, can be viewed at http://med.stanford.edu/profiles/kenneth-mahaffey.
Hennekens: Funded by the Charles E. Schmidt College of Medicine at Florida Atlantic University; serves as an independent scientist in an advisory role to investigators and sponsors as Chair or Member of Data and Safety Monitoring Boards for Amgen, AstraZeneca, Bayer, Bristol Myers-Squibb, British Heart Foundation, Cadila, Canadian Institutes of Health Research, DalCor, Esperion, Genzyme, Lilly, Regeneron, Sanofi, and the Wellcome Foundation; to the United States (U.S.) Food and Drug Administration and UpToDate; legal counsel for Pfizer and Takeda; receives royalties for authorship or editorship of 3 textbooks and as co-inventor on patents for inflammatory markers and cardiovascular disease that are held by Brigham and Women’s Hospital; has an investment management relationship with the West-Bacon Group within SunTrust Investment Services, which has discretionary investment authority and does not own any common or preferred stock in any pharmaceutical or medical device company.
Neaton: None to report.
Pearson: None to report.
Andersson: Full-time employee of AstraZeneca.
Pfeffer: Research Grant Support: Amgen, Celladon, Novartis, Sanofi; Consultant: AstraZeneca, Bayer, Boehringer Ingelheim, DalCor, Genzyme, GlaxoSmithKline, Lilly, Medicines Company, Merck, Novartis, Relypsa, Salix, Sanderling, Sanofi, Takeda, Teva, Thrasos and Vericel; Other: The Brigham and Women’s Hospital has patents for the use of inhibitors of the renin-angiotensin system in selected survivors of MI with Novartis. Dr. Pfeffer is a co-inventor. His share of the licensing agreement is irrevocably and unconditionally assigned to Rockford College.
Ellenberg: DMC: Bristol-Myers Squibb; one-time consulting fees from Acadia Pharmaceuticals, Sarepta Pharmaceuticals, Amgen, Takeda, Optimal Strategix.
References
- 1.Organization, review, and administration of cooperative studies (Greenberg Report): a report from the Heart Special Project Committee to the National Advisory Heart Council, May 1967. Contemp Clin Trials. 1988;9:137–148. doi: 10.1016/0197-2456(88)90034-7. [DOI] [PubMed] [Google Scholar]
- 2.Practical aspects of decision making in clinical trials-the Coronary Drug Project as a case study. Control Clin Trials. 1981;1:363–376. doi: 10.1016/0197-2456(81)90041-6. [DOI] [PubMed] [Google Scholar]
- 3.U.S. Food and Drug Administration. The establishment and operation of clinical trial data monitoring committees for clinical trial sponsors. [2016, accessed 8 February 2016]; http://www.fda.gov/RegulatoryInformation/Guidances/ucm127069.htm.
- 4.DeMets D, Califf R, Dixon D, et al. Issues in regulatory guidelines for data monitoring committees. Clin Trials. 2004;1:162–169. doi: 10.1191/1740774504cn019xx. [DOI] [PubMed] [Google Scholar]
- 5.Ellenberg SS, Fleming TR, DeMets DL. Data monitoring committees in clinical trials: a practical perspective. New York, NY: Wiley; 2003. [Google Scholar]
- 6.Herson J. Data and safety monitoring committees in clinical trials. Boca Raton, FL: CRC Press; 2009. [Google Scholar]
- 7.Fisher MR, Roecker EB, DeMets DL. The role of an independent statistical analysis center in the industry-modified National Institutes of Health model. Drug Inf J. 2001;35:115–129. [Google Scholar]
- 8.DeMets DL, Furberg C, Friedman LM. Data monitoring in clinical trials: a case studies approach. New York, NY: Springer; 2006. [Google Scholar]
- 9.Wittes J, Barrett-Connor E, Braunwald E, et al. Monitoring the randomized trials of the Women’s Health Initiative: the experience of the Data and Safety Monitoring Board. Clin Trials. 2007;4:218–234. doi: 10.1177/1740774507079439. [DOI] [PubMed] [Google Scholar]
- 10.Cairns JA, Wittes J, Wyse DG, et al. Monitoring the ACTIVE-W trial: some issues in monitoring a noninferiority trial. Am Heart J. 2008;155:33–41. doi: 10.1016/j.ahj.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 11.Pocock S, Wilhelmsen L, Dickstein K, et al. The data monitoring experience in the MOXCON trial. Eur Heart J. 2004;25:1974–1978. doi: 10.1016/j.ehj.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 12.DeMets D, Neaton J. Online video for training DMC members. [Accessed 8 February 2016];UW Institute for Clinical and Translational Research Web site. https://ictr.wisc.edu/DMCvideotrg.
- 13.Friedman L, Furberg C, DeMets D, et al. Fundamentals of clinical trials. 5th. New York, NY: Springer; 2015. [Google Scholar]
- 14.Inrig JK, Califf RM, Tasneem A, et al. The landscape of clinical trials in nephrology: a systematic review of Clinicaltrials.gov. Am J Kidney Dis. 2014;63:771–780. doi: 10.1053/j.ajkd.2013.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sydes MR, Altman DG, Babiker AB, et al. Reported use of data monitoring committees in the main published reports of randomized controlled trials: a cross-sectional study. Clin Trials. 2004;1:48–59. doi: 10.1191/1740774504cn003oa. [DOI] [PubMed] [Google Scholar]
- 16.Califf RM, Zarin DA, Kramer JM, et al. Characteristics of clinical trials registered in ClinicalTrials.gov, 2007–2010. JAMA. 2012;307:1838–1847. doi: 10.1001/jama.2012.3424. [DOI] [PubMed] [Google Scholar]
- 17.CTTI Data Monitoring Committees Project Expert Meeting: July 28–29, 2015. [Accessed 8 February 2016]; http://www.ctti-clinicaltrials.org/files/DMC/DMC-ExpMtgPresentation-July2015.pdf.
- 18.Califf RM, Morse MA, Wittes J, et al. Toward protecting the safety of participants in clinical trials. Control Clin Trials. 2003;24:256–271. doi: 10.1016/s0197-2456(03)00005-9. [DOI] [PubMed] [Google Scholar]
- 19.Hess CN, Roe MT, Gibson CM, et al. Independent data monitoring committees: preparing a path for the future. Am Heart J. 2014;168:135–141. doi: 10.1016/j.ahj.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dixon DO, Freedman RS, Herson J, et al. Guidelines for data and safety monitoring for clinical trials not requiring traditional data monitoring committees. Clin Trials. 2006;3:314–319. doi: 10.1191/1740774506cn149oa. [DOI] [PubMed] [Google Scholar]
- 21.Fleming TR, Hennekens CH, Pfeffer MA, et al. Enhancing trial integrity by protecting the independence of data monitoring committees in clinical trials. J Biopharm Stat. 2014;24:968–975. doi: 10.1080/10543406.2014.925719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clinical & Translational Science Awards. [Accessed 8 February 2016];About the CTSA Consortium. https://ctsacentral.org/about-us/ctsa/
- 23.Bierer BE, Li R, Seltzer J, et al. Responsibilities of data monitoring committees: consensus recommendations. Ther Innov Regul Sci. doi: 10.1177/2168479016646812. Epub ahead of print 13 May 2016. [DOI] [PubMed] [Google Scholar]
- 24.Fleming TR. Addressing missing data in clinical trials. Ann Intern Med. 2011;54:113–117. doi: 10.1059/0003-4819-154-2-201101180-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tereskerz PM. Data and safety monitoring boards: legal and ethical considerations for research accountability. Account Res. 2010;17:30–50. doi: 10.1080/08989620903520313. [DOI] [PubMed] [Google Scholar]
- 26.DeMets DL, Fleming TR, Rockhold F, et al. Liability issues for data monitoring committee members. Clin Trials. 2004;1:525–531. doi: 10.1191/1740774504cn54oa. [DOI] [PubMed] [Google Scholar]
- 27.Fleming TR. Protecting the confidentiality of interim data: addressing current challenges. Clin Trials. 2015;12:5–11. doi: 10.1177/1740774514561243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fleming TR, Sharples K, McCall J, et al. Maintaining confidentiality of interim data to enhance trial integrity and credibility. Clin Trials. 2008;5:157–167. doi: 10.1177/1740774508089459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wittes J, Schactman M. On independent data monitoring committees in oncology clinical trials. Chin Clin Oncol. 2014;3:40. doi: 10.3978/j.issn.2304-3865.2014.06.01. [DOI] [PubMed] [Google Scholar]
- 30.Anand SS, Wittes J, Yusuf S. What information should a sponsor of a randomized trial receive during its conduct? Clin Trials. 2011;8:716–719. doi: 10.1177/1740774511424421. [DOI] [PubMed] [Google Scholar]
- 31.Nissen SE, Wolski KE, Prcela L, et al. Effect of Naltrexone-Bupropion on major adverse cardiovascular events in overweight and obese patients with cardiovascular risk factors: A randomized clinical trial. JAMA. 2016;315:990–1004. doi: 10.1001/jama.2016.1558. [DOI] [PubMed] [Google Scholar]
- 32.Office of Inspector General. [Accessed 8 February 2016];Data and safety monitoring boards in NIH clinical trials: Meeting guidance, but facing some issues. http://oig.hhs.gov/oei/reports/oei-12-11-00070.asp.
- 33.Yusuf S, Whitley DR, Ascenzo R, et al. Panel discussion: the operation of data monitoring committees. Stat Med. 1993;12:527–542. [Google Scholar]
- 34.Meinert CL. Masked monitoring in clinical trials--blind stupidity? N Engl J Med. 1998;338:1381–1382. doi: 10.1056/NEJM199805073381911. [DOI] [PubMed] [Google Scholar]
- 35.Friedman LM, Bristow JD, Hallstrom A, et al. Data monitoring in the Cardiac Arrhythmia Suppression Trial. Online J Curr Clin Trials. 1993 Doc;(No 79) doi: 10.1007/0-387-30107-0_18. [DOI] [PubMed] [Google Scholar]
- 36.Kereiakes DJ, Yeh RW, Driscoll-Shempp P, et al. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med. 2014;371:2155–2166. doi: 10.1056/NEJMoa1409312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DeMets DL, Ellenberg S, Fleming TR, et al. The data and safety monitoring board and acquired immune deficiency syndrome (AIDS) clinical trials. Control Clin Trials. 1995;16:408–412. doi: 10.1016/s0197-2456(95)00073-9. [DOI] [PubMed] [Google Scholar]
- 38.Chow SC, Corey R, Lin M. On the independence of data monitoring committee in adaptive design clinical trials. J Biopharm Stat. 2012;22:853–867. doi: 10.1080/10543406.2012.676536. [DOI] [PubMed] [Google Scholar]
- 39.DeMets DL, Fleming TR. The independent statistician for data monitoring committees. Stat Med. 2004;23:1513–1517. doi: 10.1002/sim.1786. [DOI] [PubMed] [Google Scholar]
- 40.University of Wisconsin-Madison Stastical Data Analysis Center. [Accessed 8 February 2016];Sample closed session DMC Report. 2014 Oct 15; https://www.biostat.wisc.edu/sites/default/files/sample_report_closed_2.pdf.
- 41.Ellenberg SS. Protecting clinical trial integrity and protecting data integrity: are we meeting the challenges? PLoS Med. 2012;9:e1001234. doi: 10.1371/journal.pmed.1001234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Drazen JM, Wood AJ. Don’t mess with the DSMB. N Engl J Med. 2010;363:477–478. doi: 10.1056/NEJMe1007445. [DOI] [PubMed] [Google Scholar]
- 43.European Medicines Agency. Committee for medicinal products for human use. [Accessed 16 June 2016];Guideline on Data Monitoring Committees. http://osp.od.nih.gov/sites/default/files/resources/WC500003635.pdf.
- 44.Pharmaceuticals and Medical Devices Agency. [Accessed 16 June 2016];Guideline on Data Monitoring Committees (PFSB/ELD notification No. 0404-1) Issued in Japanese on April 4, 2013. https://www.pmda.go.jp/files/000204620.pdf.