Abstract
Air pollution is getting severe and concerns about its toxicity effects on airway and lung disease are also increasing. Particulate matter (PM) is major component of air pollutant. It causes respiratory diseases, such as asthma, chronic obstructive pulmonary disease, lung cancer, and so on. PM particles enter the airway and lung by inhalation, causing damages to them. Especially, PM2.5 can penetrate into the alveolus and pass to the systemic circulation. It can affect the cardiopulmonary system and cause cardiopulmonary disorders. In this review, we focused on PM-inducing toxicity mechanisms in the framework of oxidative stress, inflammation, and epigenetic changes. We also reviewed its correlation with respiratory diseases. In addition, we reviewed biomarkers related to PM-induced respiratory diseases. These biomarkers might be used for disease prediction and early diagnosis. With recent trend of using genomic analysis tools in the field of toxicogenomics, respiratory disease biomarkers associated with PM will be continuously investigated. Effective biomarkers derived from earlier studies and further studies might be utilized to reduce respiratory diseases.
Keywords: Particulate matter, Biomarker, Oxidative stress, Inflammation, Epigenetic change
INTRODUCTION
In modern society, development of industry causes environmental pollutions, including air, water, and soil pollutions. Among them, the air pollution has been revealed to be one of the harmful factors affecting human health by various studies.1–4 In accordance with World Health Organization (WHO) report, the exposure to air pollutant caused approximate 7 million of death in the world in 2012.5 Air pollutants consist of carbon monoxide, volatile organic compounds, persistent free radicals, particulate matter (PM), and so on.6 All these substances have some effects on human health. They especially induce lung and heart dysfunctions.
Among these major air pollutants, PM is the most harmful substance to human health by causing various diseases.7,8 It is classified as a Group 1 carcinogen (induces carcinogenesis in human body) by International Agency for Research on Cancer. PM induces the premature death in people with heart or lung disease. It also induces nonfatal heart attacks, irregular heartbeat, aggravated asthma, decreased lung function, and increased respiratory symptoms, such as irritation of the airways, coughing, and difficulty breathing.
PM is a complex mixture of extremely small particles and liquid droplet in the atmosphere.9 The complex mixture consists of organic carbon, ammonium, nitrates, sulphates, mineral dust, trace elements, and water. These substances exist as particles with diameters of less than 2.5 μm or less than 10 μm.10,11 Particles with diameters of less than 2.5 μm are called PM2.5 and those of less than 10 μm are called PM10.12 PM10 is composed of various dusts from sea salt, soil dust resuspension, construction/demolition, non-exhausted vehicle emissions, and industrial fugitives.13 Sources of PM2.5 are primarily emissions from natural event, such as forest fire, and industrial activities, such as mining and construction. Another sources of PM2.5 are secondary particles converted from chemical or gas generated by industrial activities.14 Heavy metals, such as Pb, Ni, Cd, Cr, V, Cu, and Mn, are well-known as hazardous substances. They are also sources of PM2.5.15
Depending on its diameter, PM has detrimental effects on human. It has different transport efficiency and penetration ratio to the respiratory system.16 PM10 leads to physical damage to the respiratory system, such as alveolus and larynx. It rarely induces chemical reaction to lung tissue.16 It can reach alveolus or bronchioles but cannot penetrate alveolus. In contrast, PM2.5 is able to penetrate into the alveolus and pass to the systemic circulation. It induces both physical and chemical damage to the respiratory system.16 Therefore, PM2.5 induces more serious damage to the lung than PM10.
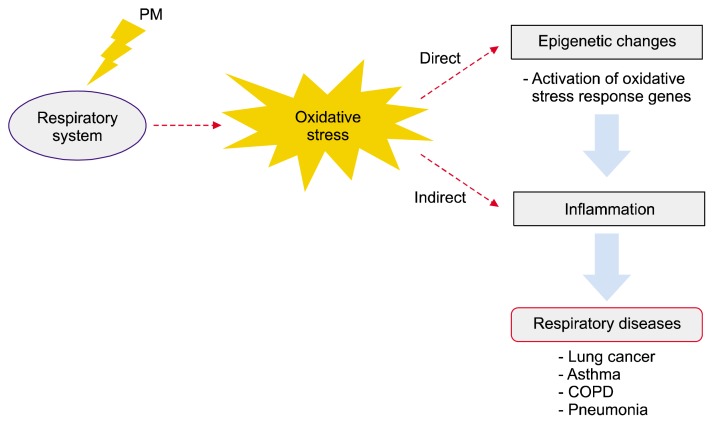
Respiratory system has physical contact with air pollutant by respiration. After being exposed to air pollutant such as PM, inhalation toxicity to the respiratory system might be occured. Numerous research studies have suggested that PM is associated with respiratory toxicity in in vitro, in vivo, and epidemiological studies.17–21 PM can induce oxidative stress and inflammation on respiratory organ tissue.22–25 It triggers the development and exacerbation of diverse diseases of the respiratory system, such as asthma,26,27 chronic obstructive pulmonary disease (COPD),28–30 and so on. Besides, PM can generate reactive oxygen species (ROS) and some oxidative metabolite, causing oxidative stress.31 It damages DNA and causes epigenetic changes.32,33 According to this reason, PM can eventually induce cancer (Fig. 1).34
Figure 1.
Schematic diagram of toxic effects of particulate matter (PM) on respiratory system. PM triggers oxidative stress in respiratory system, which induces epigenetic changes directly by activating oxidative stress response genes. Overproduced reactive oxygen species (ROS) and altered gene expressions can affect to inflammation indirectly as ROS stimulates signaling pathways. It contributes to development of respiratory diseases, such as asthma, chronic obstructive pulmonary disease (COPD), pneumonia, and lung cancer.
Once lung is damaged by chemical such as PM, regeneration of lung to normal state is almost impossible. Thus, prediction and early diagnosis of lung diseases are important and imperatively necessary. Biomarkers can be used for disease prediction.35 Various epigenetic biomarkers of lung diseases induced by PM exposure have been discovered in recent researches.36,37 Epigenetic changes do not alter the DNA sequences. However, they can modify methylation or acetylation of DNA and histone protein, and then induces changes in DNA structure and gene expression. In this review, we focused on PM-inducing toxicity mechanisms in the framework of oxidative stress, inflammation, and epigenetic changes. We also reviewed its correlation with respiratory diseases (Fig. 1). In addition, we reviewed biomarkers related to PM-induced respiratory diseases. These biomarkers might be used for disease prediction and early diagnosis.
TOXIC EFFECTS OF PARTICULATE MATTER ON RESPIRATORY SYSTEM
1. Oxidative stress
Oxidative stress can be defined as damage resulting from imbalance of oxidation and reduction status of the body.38 As a result of oxidation, ROS can react with other molecules. Organism has antioxidant defense system for maintaining the stability of redox homeostasis.38 However, antioxidant system cannot overcome the effect of excessively produced ROS.38 Excessive ROS has various detrimental effects on the body, including cell function impairment and cell death.38 In human, oxidative stress causes various diseases, such as cancers, neurological diseases, heart diseases, atherosclerosis, and pulmonary disease.39,40
It is well-known that PM induces oxidative stress in the lung. Because various chemicals and compounds, including PMs, can enter the airway directly, lung has a unique protecting system itself.41 Higher glutathione (GSH) levels have been observed in alveolar epithelial surface (about 140 times higher than those of plasma).42–44 GSH plays important role in protecting lungs against oxidative stress45 by returning oxidized cell constituents to reduced form and by detoxifying lipid hyperoxides or other oxidants.46 Thus, depletion of GSH is associated with disease development. Numerous studies have investigated the relationship between GSH level and disease development.47–50 In addition, when there are high levels of oxidative stress in lung tissue, neutrophils will arrive and become activated, which can produce more ROS.38 These over-produced ROS may cause oxidative stress, consequently leading to inflammatory response in the airway.
2. Inflammation
Inflammatory response is a process that react on tissues receiving harmful stimuli. It protects human body involving immune cells. It is activated when immune cells express pattern recognition receptor which binds to pathogen-associated molecular patterns, such as lipopolysaccharide.51 Besides, other stimuli, such as cytokines, can bind to plasma membrane or stress signal and activate inflammatory response. A number of kinases are associated with inflammatory response.52 Many studies have shown that inflammatory response is related to PM exposure.53 It has been reported that bacteria-derived endotoxin bound to the surface of PM particle is one of the causative agent in PM-mediated lung injury.54–56 As the major factor of PM induces lung toxicity, transition metal content of PM10 cause oxidative stress.57,58 Fe and Cu are common chemicals inducing hydroxyl radical via Fenton reaction. When they are included in PM particles,59,60 they can induce oxidative stress and cause inflammatory diseases.61,62
In a recent animal study, PM exposure has been demonstrated to cause early immune suppression in severe allergic response of adult mouse.63 PM affects specific antigen tolerance when the level of immunoglobulin E is elevated. It also increases the risks of asthma. PM10 change can induce cell proliferation, leading to cancer development. In addition, chemokines and selectins induced by inflammatory response can be used by cancer cells and increase malignant effects. Although inflammatory response prevents cancer development in some case, inflammatory response by PM exposure can lead to lung cancer. Exposure to low concentration of PM induces inflammatory response and damage to lung tissues of healthy mouse.64 In addition, when human lung epithelial cells are exposed to PM10, pyruvate kinase related to carcinogenesis is up-regulated while annextin 1, an anti-inflammation response protein, is down-regulated.53 PM2.5 also induces inflammatory response as a result of oxidative stress caused by ROS. Oxidative stress changes the expression of proteins, such as NF-κB and interleukin (IL)-8 which are associated with inflammatory response.65
3. Epigenetic change
Epigenetic change means molecular change that regulates gene expression without changing DNA primary sequence.66 Epigenetic change is related to diverse biological mechanisms through alterations in gene expression or mRNA degradation. Epigenetic change occurs frequently by environmental factors, such as aging and diet.67,68 Recent studies have revealed that oxidative stress and redox status induced by PM regulates epigenetic changes, such as histone modification, methylation, acetylation, and chromatin remodeling.69–71
One of studies has shown that DNA methylation pattern in human is altered by exposure to traffic particles, such as PM and black carbon.72 Exposure to PM2.5 particles is associated with demethylation of long interspersed nucleotide element (LINE)-1.72 In addition, in in vitro and in vivo studies, black carbon, diesel exhausted particles (DEPs), and metal components of air pollutant particles, such as arsenic and cadmium, also induce DNA methylation.73,74 Several studies have suggested that histone modification is also induced by PM. For example, PM10 and DEP exposure have increased histone H4 acetylation at the IL-8 and COX-2 promoter in human lung and bronchial cells, respectively.75,76 Moreover, short-term exposure to PM can induces epigenetic changes, specifically in the promoter region of mitogen-activated protein kinase pathway genes such as LINE-1.71,72 These epigenetic changes might be associated with inflammation or increased oxidative stress.71 Thus, oxidative stress, inflammation, and epigenetic changes are intimately connected. They play important roles in the development and acceleration of respiratory diseases.
RESPIRATORY DISEASE INDUCED BY PARTICULATE MATTER EXPOSURE
Various lung diseases, such as COPD, asthma, and lung cancer, are related to PM exposure.77–79 COPD is a progressive disease causing abnormal inflammatory response by harmful particles or gas. It can lead symptoms, such as constantly cough, shortness of breath, wheezing, and chest tightness.80 When stable COPD patients are exposed to PM2.5, the number of patients who progress to acute exacerbation of COPD (AECOPD) is increased.7 It is caused by effects of PM2.5 exposure, such as increased phagocytosis, oxidant stress, and pro-inflammatory cytokines. One of epidemiologic investigations performed at Cleaveland, OH has revealed that risk of AECOPD was increased according to the concentration of PM2.5,81 supporting the relationship between COPD and PM2.5 exposure.
Asthma is a chronic illness that makes the airway sensitive and narrow. Symptoms of this disease are coughing, shortness of breath, chest tightness, and wheezing.82 One study has suggested that increased exposure to PM10 and biological endotoxins are important factors in asthma pathogenesis.83 PM10 induces pro-inflammatory response in lung tissue through toll-like receptor (TLR) pathway and affects NF-κB activation. It is probably one of the pathogenic factors of asthma. PM2.5 is also associated with the pathogenesis of asthma.84 Through meta-analysis using various database (PubMed, Ebsco, Ovid, and four Chinese), one study has shown that acute elevation of PM concentration in the air may increase hospital admission of Chinese children for asthma.84 When the concentration of PM2.5 is increased to 10 μg/m3, the increment of hospital admission for asthma is nearly twice than that when the concentration of PM10 is increased to 10 μg/m3.84
Lung cancer (also known as lung carcinoma) is one of the fatal cancers in worldwide. Lung cancer has two main types: small-cell lung carcinoma (SCLC) and non-small-cell lung carcinoma (NSCLC).1 WHO announced that cancer caused 8.2 million deaths in 2012 and lung cancer caused 1.59 million deaths.5 Among cancer deaths, lung cancer death had the highest number in 2012.5 A number of studies have determined the factors that lead to lung cancer. Through various studies, the causes of lung cancer include tobacco, radon gas, asbestos, genetic source, PM, and so on. Although major cause of lung cancer is known as smoking, recent studies have revealed that exposure to PM also leads to lung cancer.2,9 Lung cancer does not show initial symptom. Symptom of lung cancer is very similar to that of common cold. Thus, only a few patients are diagnosed of lung cancer at early stage. Most lung cancer patients are diagnosed after lung cancer has processed. As a result, untreated SCLC patients have a survival time of 6 to 17 weeks and treated SCLC patients have a survival periods of 40 to 70 weeks.85 Their 5-year survival rate is lower than 10%. The 5-years survival rate of all lung cancer patients (including male and female SCLC and NSCLS patients) during 2006 to 2010 year in South Korea was 19.7%.86 Therefore, it is important to prevent lung cancer to reduce mortality. Many clinical studies have reported the association between PM exposure and lung cancer occurrence. The concentrations of diverse compositions of PM have been measured by research staff at many cites. The long-term lung cancer occurrence rate and death rate are also recorded. Although detailed mechanisms of PM carcinogenic effect remain unclear, the relationship between PM and lung cancer is being studied by many institutions.
BIOMARKERS OF LUNG DISEASES CAUSED BY PARTICULATE MATTER
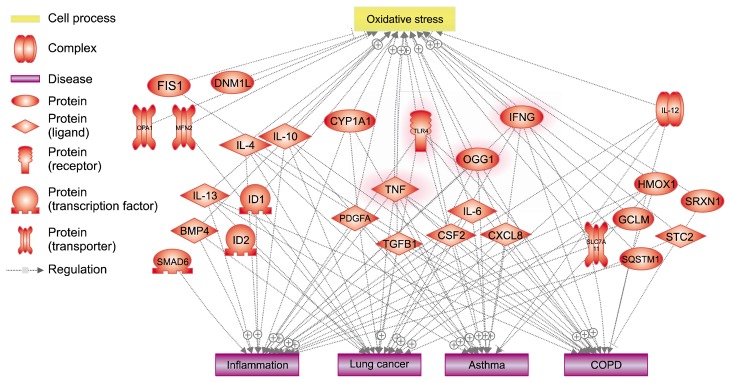
Genetic and epigenetic patterns associated with air pollutant, such as PM, might be useful biomarkers.87 Some studies have shown that epigenetic change caused by PM exposure can lead to increased susceptibility to lung diseases, including cancer. Epigenetic changes alter the expression of diverse genes and the regulation of mRNAs. Through screening of epigenetic patterns, it is possible to predict disease development of respiratory system. For this reason, biomarkers can be used to detect long-term effects of PM exposure. They are very useful for the prediction or early detection of respiratory diseases. Up to date, numerous studies have suggested that biomarkers are associated with PM exposure (Table 1, Fig. 2).88–93
Table 1.
Potential biomarkers of respiratory diseases induced by particulate matter
| Gene name | Description | Related process | Related disease | Reference |
|---|---|---|---|---|
| CYP1A1 | Cytochrome P450 family 1 subfamily | Oxidative stress | Lung cancer | 97 |
| A member 1 | Inflammation | Asthma | ||
| OGG1 | 8-oxoguanine DNA glycosylase | Oxidative stress | Lung cancer | 88 |
| Inflammation | Asthma COPD |
|||
| IFNG | Interferon gamma | Oxidative stress | Lung cancer | 97 |
| Inflammation | Asthma COPD |
|||
| TLR4 | Toll-like receptor 4 | Oxidative stress | Lung cancer | 92 |
| Inflammation | Asthma COPD |
|||
| IL-13 | Interleukin 13 | Oxidative stress | Lung cancer | 98 |
| Inflammation | Asthma COPD |
|||
| IL-10 | Interleukin 10 | Oxidative stress | Lung cancer | 98 |
| Inflammation | Asthma COPD |
|||
| IL-4 | Interleukin 4 | Oxidative stress | Lung cancer | 98 |
| Inflammation | Asthma COPD |
|||
| IL-13 | Interleukin 13 | Oxidative stress | Lung cancer | 98 |
| Inflammation | Asthma COPD |
|||
| IL-6 | Interleukin 6 | Oxidative stress | Lung cancer | 99 |
| Inflammation | Asthma COPD |
|||
| TNF | Tumor necrosis factor | Oxidative stress | Lung cancer | 99 |
| Inflammation | Asthma COPD |
|||
| PDGFA | Platelet-derived growth factor subunit A | Oxidative stress | Lung cancer | 99 |
| Inflammation | Asthma | |||
| TGFB1 | Transforming growth factor beta induced | Oxidative stress | Lung cancer | 99 |
| Inflammation | Asthma COPD |
|||
| CSF2 | Colony stimulating factor 2 | Oxidative stress | Lung cancer | 99 |
| Inflammation | Asthma COPD |
|||
| CXCL8 | C-X-C motif chemokine ligand 8 | Oxidative stress | Lung cancer | 99 |
| Inflammation | Asthma COPD |
|||
| BMP4 | Bone morphogenetic protein 4 | Oxidative stress Inflammation |
Lung cancer | 100 |
| SMAD6 | SMAD family member 6 | Inflammation | 100 | |
| ID1 | Inhibitor of DNA binding 1, HLH protein | Oxidative stress Inflammation |
Lung cancer | 100 |
| ID2 | Inhibitor of DNA binding 2, HLH protein | Lung cancer | 100 | |
| GCLM | Glutamate-cysteine ligase modifier subunit | Oxidative stress Inflammation |
100 | |
| HMOX1 | Heme oxygenase 1 | Oxidative stress | Lung cancer | 100 |
| Inflammation | Asthma COPD |
|||
| SLC7A11 | Solute carrier family 7 member 11 | Oxidative stress Inflammation |
Asthma | 100 |
| SQSTM1 | Sequestosome 1 | Oxidative stress Inflammation |
100 | |
| SRXN1 | Sulfiredoxin 1 | Oxidative stress | COPD | 100 |
| STC2 | Stanniocalcin 2 | Oxidative stress | Lung cancer | 100 |
| DNM1L | Dynamin 1 like | Oxidative stress | 101 | |
| FIS1 | Fission, mitochondrial 1 | Oxidative stress | COPD | 101 |
| MFN2 | Mitofusin 2 | Oxidative stress | Lung cancer | 101 |
| OPA1 | Mitochondrial dynamin like GTPase | Oxidative stress | 101 |
COPD, chronic obstructive pulmonary disease.
Figure 2.
Related cell process and diseases with potential disease biomarkers induced by particulate matter visualized by Pathway Studio. Most of all proteins known as potential biomarker have relationship with oxidative stress and inflammation. Respiratory diseases also relates to diverse biomarkers. Highlighted proteins represents entire connection with cell process (oxidative stress) and diseases (inflammation, lung cancer, asthma, and COPD). FIS1, mitochondrial fission 1; DNM1L, dynamin 1 like; OPA1, mitochondrial dynamin like GTPase; MFN2, mitofusin 2; IL, interleukin; ID1, inhibitor of DNA binding 1, HLH protein; ID2, inhibitor of DNA binding 2, HLH protein; CYP1A1, cytochrome P450 family 1 subfamily A member 1; TLR4, toll-like receptor 4; IFNG, IFN gamma; OGG1, 8-oxoguanine DNA glycosylase; PDGFA, platelet-derived growth factor subunit A; TGFB1, transforming growth factor beta induced; CSF2, colony stimulating factor 2; CXCL8, C-X-C motif chemokine ligand 8; HMOX1, heme oxygenase 1; SRXN1, sulfiredoxin 1; GCLM, glutamate-cysteine ligase modifier subunit; SLC7A11, solute carrier family 7 member 11; STC2, stanniocalcin 2; SQSTM1, sequestosome 1; COPD, chronic obstructive pulmonary disease.
It has been reported that 8-oxo-2′-deoxyguanosine (8-OHdG) is a biomarker of DNA damage.88,94 8-OHdG is one of several oxidative damage markers. It is induced by metal contents of PM, such as Fe, Cu, Ni, and Cd, in lung cells. Transition metals generates excessive ROS, which significantly increases oxidative stress.95,96 Formatted 8-OHdG causes DNA damage and mediate the cytotoxicity and carcinogenicity.88 LINE-1, one of oxidative damage-related markers, has been suggested as a gene expression and DNA methylation markers.71,72,97 Other markers, such as Arthrobacter luteus restriction endonuclease (Alu) and TLR-4, also alters their gene expression levels in response to ambient particles.97 In addition, 8-oxoguanine DNA glycosylase (OGG1) is regarded as a DNA methylation biomarker based on previous studies.88 Takano et al.98 have demonstrated that cytochrome P450 1A1 is induced by DEP exposure in the lung. Biomarkers are induced by acute inhalation exposure to DEP and may result in ROS generation, subsequently causing lung injury.98 In field of toxicogenomics, global gene expression profiles have been well studied in diverse researches.99,100 These studies provide important information on biomarkers which can be used to predict the development of lung diseases. Biomarkers might play important role in preventing disease occurrence.
CONCLUSION
PM air pollutant is well known to have harmful components in many studies. It has been demonstrated that respiratory diseases and cardiovascular diseases are associated with exposure to PM. PMs can trigger pulmonary pathogenic effect and cause pulmonary disease subsequently. Lung is a core organ of the respiratory system. Once lung is damaged, it is very hard to recover to normal pulmonary function.101 COPD, asthma, and lung cancer, referred in this review as major pulmonary diseases, are all caused by PM exposure. Although diverse mechanisms are involved in PM-induced respiratory disease, there are three major mechanisms: induction of oxidative stress, inflammation, and epigenetic changes. Oxidative stress is a well-known cause of various diseases, especially cancer. PM induces oxidative stress in the lung by producing ROS which damages DNA and leads to apoptosis and other symptoms. In addition, oxidative stress may induces inflammatory injuries and epigenetic changes. Therefore, these three mechanisms are connected to each other. Biomarkers of PM-induced diseases are based on these mechanisms. Recently, toxicogenomic studies are being performed very actively due to recent development of genomic analysis tools. With this highly advanced genomic tools such as NGS, the new studies for finding biomarkers of PM-induced diseases can be conducted. Through such studies, if the most effective bio-markers can be identified, they will be useful for the prediction of PM-induced diseases and early detection. Eventually, studies on biomarkers will contribute to the prevention of lung diseases.
ACKNOWLEDGMENTS
This subject was supported by Korea Ministry of Environment (MOE) as “The Environmental Health Action Program” (2016001360009).
Footnotes
CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
REFERENCES
- 1.Ekpenyong CE, Ettebong EO, Akpan EE, Samson TK, Daniel NE. Urban city transportation mode and respiratory health effect of air pollution: a cross-sectional study among transit and non-transit workers in Nigeria. BMJ Open. 2012;2:e001253. doi: 10.1136/bmjopen-2012-001253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu X, Yao T, Fung JC, Lin C. Estimation of health and economic costs of air pollution over the Pearl River Delta region in China. Sci Total Environ. 2016;566–567:134–43. doi: 10.1016/j.scitotenv.2016.05.060. [DOI] [PubMed] [Google Scholar]
- 3.Behndig AF, Mudway IS, Brown JL, Stenfors N, Helleday R, Duggan ST, et al. Airway antioxidant and inflammatory responses to diesel exhaust exposure in healthy humans. Eur Respir J. 2006;27:359–65. doi: 10.1183/09031936.06.00136904. [DOI] [PubMed] [Google Scholar]
- 4.Sydbom A, Blomberg A, Parnia S, Stenfors N, Sandström T, Dahlén SE. Health effects of diesel exhaust emissions. Eur Respir J. 2001;17:733–46. doi: 10.1183/09031936.01.17407330. [DOI] [PubMed] [Google Scholar]
- 5.WHO. [Accessed 2012];Burden of disease from Ambient Air Pollution for 2012. http://www.who.int/phe/health_topics/outdoorair/databases/AAP_BoD_results_March2014.pdf?ua=1.
- 6.Blanchard CL, Tanenbaum S, Lawson DR. Differences between weekday and weekend air pollutant levels in Atlanta; Baltimore; Chicago; Dallas-Fort Worth; Denver; Houston; New York; Phoenix; Washington, DC; and surrounding areas. J Air Waste Manag Assoc. 2008;58:1598–615. doi: 10.3155/1047-3289.58.12.1598. [DOI] [PubMed] [Google Scholar]
- 7.Ni L, Chuang CC, Zuo L. Fine particulate matter in acute exacerbation of COPD. Front Physiol. 2015;6:294. doi: 10.3389/fphys.2015.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cui Y, Sun Q, Liu Z. Ambient particulate matter exposure and cardiovascular diseases: a focus on progenitor and stem cells. J Cell Mol Med. 2016;20:782–93. doi: 10.1111/jcmm.12822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neri T, Pergoli L, Petrini S, Gravendonk L, Balia C, Scalise V, et al. Particulate matter induces prothrombotic microparticle shedding by human mononuclear and endothelial cells. Toxicol In Vitro. 2016;32:333–8. doi: 10.1016/j.tiv.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Barmpadimos I, Hueglin C, Keller J, Henne S, Prévôt ASH. Influence of meteorology on PM10 trends and variability in Switzerland from 1991 to 2008. Atmos Chem Phys. 2011;11:1813–35. doi: 10.5194/acp-11-1813-2011. [DOI] [Google Scholar]
- 11.McBride AC, Dale VH, Baskaran LM, Downing ME, Eaton LM, Efroymson RA, et al. Indicators to support environmental sustainability of bioenergy systems. Ecol Indic. 2011;11:1277–89. doi: 10.1016/j.ecolind.2011.01.010. [DOI] [Google Scholar]
- 12.EPA. [Accessed 2015];Air Quality: EPA’s 2013 Changes to the Particulate Matter (PM) Standard. https://www.fas.org/sgp/crs/misc/R42934.pdf.
- 13.Gugamsetty B, Wei H, Liu CN, Awasthi A, Hsu SC, Tsai CJ, et al. Source characterization and apportionment of PM10, PM2.5 and PM0.1 by using positive matrix factorization. Aerosol Air Qual Res. 2012;12:476–91. [Google Scholar]
- 14.Saffari A, Daher N, Shafer MM, Schauer JJ, Sioutas C. Seasonal and spatial variation in dithiothreitol (DTT) activity of quasi-ultrafine particles in the Los Angeles Basin and its association with chemical species. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2014;49:441–51. doi: 10.1080/10934529.2014.854677. [DOI] [PubMed] [Google Scholar]
- 15.Samara C, Voutsa D. Size distribution of airborne particulate matter and associated heavy metals in the roadside environment. Chemosphere. 2005;59:1197–206. doi: 10.1016/j.chemosphere.2004.11.061. [DOI] [PubMed] [Google Scholar]
- 16.Atkinson RW, Fuller GW, Anderson HR, Harrison RM, Armstrong B. Urban ambient particle metrics and health: a time-series analysis. Epidemiology. 2010;21:501–11. doi: 10.1097/EDE.0b013e3181debc88. [DOI] [PubMed] [Google Scholar]
- 17.Hirota JA, Marchant DJ, Singhera GK, Moheimani F, Dorscheid DR, Carlsten C, et al. Urban particulate matter increases human airway epithelial cell IL-1β secretion following scratch wounding and H1N1 influenza A exposure in vitro. Exp Lung Res. 2015;41:353–62. doi: 10.3109/01902148.2015.1040528. [DOI] [PubMed] [Google Scholar]
- 18.Gilmour PS, Schladweiler MC, Richards JH, Ledbetter AD, Kodavanti UP. Hypertensive rats are susceptible to TLR4-mediated signaling following exposure to combustion source particulate matter. Inhal Toxicol. 2004;16(Suppl 1):5–18. doi: 10.1080/08958370490442827. [DOI] [PubMed] [Google Scholar]
- 19.Clifford RL, Jones MJ, MacIsaac JL, McEwen LM, Goodman SJ, Mostafavi S, et al. Inhalation of diesel exhaust and allergen alters human bronchial epithelium DNA methylation. J Allergy Clin Immunol. 2017;139:112–21. doi: 10.1016/j.jaci.2016.03.046. [DOI] [PubMed] [Google Scholar]
- 20.Wang L, Zhao X, Xu W, Tang J, Jiang X. Correlation analysis of lung cancer and urban spatial factor: based on survey in Shanghai. J Thorac Dis. 2016;8:2626–37. doi: 10.21037/jtd.2016.09.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jansen KL, Larson TV, Koenig JQ, Mar TF, Fields C, Stewart J, et al. Associations between health effects and particulate matter and black carbon in subjects with respiratory disease. Environ Health Perspect. 2005;113:1741–6. doi: 10.1289/ehp.8153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Møller P, Jacobsen NR, Folkmann JK, Danielsen PH, Mikkelsen L, Hemmingsen JG, et al. Role of oxidative damage in toxicity of particulates. Free Radic Res. 2010;44:1–46. doi: 10.3109/10715760903300691. [DOI] [PubMed] [Google Scholar]
- 23.Lodovici M, Bigagli E. Oxidative stress and air pollution exposure. J Toxicol. 2011;2011:487074. doi: 10.1155/2011/487074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu Z, Xu X, Zhong M, Hotchkiss IP, Lewandowski RP, Wagner JG, et al. Ambient particulate air pollution induces oxidative stress and alterations of mitochondria and gene expression in brown and white adipose tissues. Part Fibre Toxicol. 2011;8:20. doi: 10.1186/1743-8977-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Eeden SF, Tan WC, Suwa T, Mukae H, Terashima T, Fujii T, et al. Cytokines involved in the systemic inflammatory response induced by exposure to particulate matter air pollutants (PM(10)) Am J Respir Crit Care Med. 2001;164:826–30. doi: 10.1164/ajrccm.164.5.2010160. [DOI] [PubMed] [Google Scholar]
- 26.Gavett SH, Koren HS. The role of particulate matter in exacerbation of atopic asthma. Int Arch Allergy Immunol. 2001;124:109–12. doi: 10.1159/000053685. [DOI] [PubMed] [Google Scholar]
- 27.Tecer LH, Alagha O, Karaca F, Tuncel G, Eldes N. Particulate matter (PM(2.5), PM(10–2.5), and PM(10)) and children’s hospital admissions for asthma and respiratory diseases: a bidirectional case-crossover study. J Toxicol Environ Health A. 2008;71:512–20. doi: 10.1080/15287390801907459. [DOI] [PubMed] [Google Scholar]
- 28.Ling SH, van Eeden SF. Particulate matter air pollution exposure: role in the development and exacerbation of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2009;4:233–43. doi: 10.2147/COPD.S5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Eeden SF, Yeung A, Quinlam K, Hogg JC. Systemic response to ambient particulate matter: relevance to chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2005;2:61–7. doi: 10.1513/pats.200406-035MS. [DOI] [PubMed] [Google Scholar]
- 30.de Hartog JJ, Ayres JG, Karakatsani A, Analitis A, Brink HT, Hameri K, et al. Lung function and indicators of exposure to indoor and outdoor particulate matter among asthma and COPD patients. Occup Environ Med. 2010;67:2–10. doi: 10.1136/oem.2008.040857. [DOI] [PubMed] [Google Scholar]
- 31.Donaldson K, Stone V, Borm PJ, Jimenez LA, Gilmour PS, Schins RP, et al. Oxidative stress and calcium signaling in the adverse effects of environmental particles (PM10) Free Radic Biol Med. 2003;34:1369–82. doi: 10.1016/S0891-5849(03)00150-3. [DOI] [PubMed] [Google Scholar]
- 32.Kawanishi S, Hiraku Y, Oikawa S. Mechanism of guanine-specific DNA damage by oxidative stress and its role in carcinogenesis and aging. Mutat Res. 2001;488:65–76. doi: 10.1016/S1383-5742(00)00059-4. [DOI] [PubMed] [Google Scholar]
- 33.Kryston TB, Georgiev AB, Pissis P, Georgakilas AG. Role of oxidative stress and DNA damage in human carcinogenesis. Mutat Res. 2011;711:193–201. doi: 10.1016/j.mrfmmm.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 34.Au WW. Usefulness of biomarkers in population studies: from exposure to susceptibility and to prediction of cancer. Int J Hyg Environ Health. 2007;210:239–46. doi: 10.1016/j.ijheh.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 35.van der Velde AR, Meijers WC, de Boer RA. Biomarkers for risk prediction in acute decompensated heart failure. Curr Heart Fail Rep. 2014;11:246–59. doi: 10.1007/s11897-014-0207-7. [DOI] [PubMed] [Google Scholar]
- 36.Hou L, Zhang X, Tarantini L, Nordio F, Bonzini M, Angelici L, et al. Ambient PM exposure and DNA methylation in tumor suppressor genes: a cross-sectional study. Part Fibre Toxicol. 2011;8:25. doi: 10.1186/1743-8977-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu C, Xu J, Chen Y, Guo X, Zheng Y, Wang Q, et al. Characterization of genome-wide H3K27ac profiles reveals a distinct PM2.5-associated histone modification signature. Environ Health. 2015;14:65. doi: 10.1186/s12940-015-0052-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gomes EC, Florida-James G. Lung Inflammation. Rijeka: InTech; 2014. Lung inflammation, oxidative stress and air pollution. [Google Scholar]
- 39.Powers SK, DeRuisseau KC, Quindry J, Hamilton KL. Dietary antioxidants and exercise. J Sports Sci. 2004;22:81–94. doi: 10.1080/0264041031000140563. [DOI] [PubMed] [Google Scholar]
- 40.Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. 3rd ed. New York: Oxford University Press; 2007. [Google Scholar]
- 41.Boyd MR, Stiko A, Statham CN, Jones RB. Protective role of endogenous pulmonary glutathione and other sulfhydryl compounds against lung damage by alkylating agents. Investigations with 4-ipomeanol in the rat. Biochem Pharmacol. 1982;31:1579–83. doi: 10.1016/0006-2952(82)90383-5. [DOI] [PubMed] [Google Scholar]
- 42.Griffith OW, Bridges RJ, Meister A. Transport of gamma-glutamyl amino acids: role of glutathione and gamma-glutamyl transpeptidase. Proc Natl Acad Sci U S A. 1979;76:6319–22. doi: 10.1073/pnas.76.12.6319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cantin AM, Bégin R. Glutathione and inflammatory disorders of the lung. Lung. 1991;169:123–38. doi: 10.1007/BF02714149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meister A. New aspects of glutathione biochemistry and transport: selective alteration of glutathione metabolism. Fed Proc. 1984;43:3031–42. [PubMed] [Google Scholar]
- 45.Ray LE, Prescott JM. Isolation and some characteristics of glutathione reductase from rabbit erythrocytes (38548) Proc Soc Exp Biol Med. 1975;148:402–9. doi: 10.3181/00379727-148-38548. [DOI] [PubMed] [Google Scholar]
- 46.Rahman Q, Abidi P, Afaq F, Schiffmann D, Mossman BT, Kamp DW, et al. Glutathione redox system in oxidative lung injury. Crit Rev Toxicol. 1999;29:543–68. doi: 10.1080/10408449991349276. [DOI] [PubMed] [Google Scholar]
- 47.Rahman I, MacNee W. Lung glutathione and oxidative stress: implications in cigarette smoke-induced airway disease. Am J Physiol. 1999;277:L1067–88. doi: 10.1152/ajplung.1999.277.6.L1067. [DOI] [PubMed] [Google Scholar]
- 48.Moss M, Guidot DM, Wong-Lambertina M, Ten Hoor T, Perez RL, Brown LA. The effects of chronic alcohol abuse on pulmonary glutathione homeostasis. Am J Respir Crit Care Med. 2000;161:414–9. doi: 10.1164/ajrccm.161.2.9905002. [DOI] [PubMed] [Google Scholar]
- 49.Smith CV, Jones DP, Guenthner TM, Lash LH, Lauterburg BH. Compartmentation of glutathione: implications for the study of toxicity and disease. Toxicol Appl Pharmacol. 1996;140:1–12. doi: 10.1006/taap.1996.0191. [DOI] [PubMed] [Google Scholar]
- 50.Dalton TP, Chen Y, Schneider SN, Nebert DW, Shertzer HG. Genetically altered mice to evaluate glutathione homeostasis in health and disease. Free Radic Biol Med. 2004;37:1511–26. doi: 10.1016/j.freeradbiomed.2004.06.040. [DOI] [PubMed] [Google Scholar]
- 51.Lee JY, Zhao L, Youn HS, Weatherill AR, Tapping R, Feng L, et al. Saturated fatty acid activates but polyunsaturated fatty acid inhibits Toll-like receptor 2 dimerized with Toll-like receptor 6 or 1. J Biol Chem. 2004;279:16971–9. doi: 10.1074/jbc.M312990200. [DOI] [PubMed] [Google Scholar]
- 52.Sopko R, Andrews BJ. Linking the kinome and phosphorylome--a comprehensive review of approaches to find kinase targets. Mol Biosyst. 2008;4:920–33. doi: 10.1039/b801724g. [DOI] [PubMed] [Google Scholar]
- 53.Jeon YM, Son BS, Lee MY. Proteomic identification of the differentially expressed proteins in human lung epithelial cells by airborne particulate matter. J Appl Toxicol. 2011;31:45–52. doi: 10.1002/jat.1566. [DOI] [PubMed] [Google Scholar]
- 54.Becker S, Soukup JM, Gilmour MI, Devlin RB. Stimulation of human and rat alveolar macrophages by urban air particulates: effects on oxidant radical generation and cytokine production. Toxicol Appl Pharmacol. 1996;141:637–48. doi: 10.1006/taap.1996.0330. [DOI] [PubMed] [Google Scholar]
- 55.Dong W, Lewtas J, Luster MI. Role of endotoxin in tumor necrosis factor alpha expression from alveolar macrophages treated with urban air particles. Exp Lung Res. 1996;22:577–92. doi: 10.3109/01902149609046043. [DOI] [PubMed] [Google Scholar]
- 56.Long CM, Suh HH, Kobzik L, Catalano PJ, Ning YY, Koutrakis P. A pilot investigation of the relative toxicity of indoor and outdoor fine particles: in vitro effects of endotoxin and other particulate properties. Environ Health Perspect. 2001;109:1019–26. doi: 10.1289/ehp.011091019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Frampton MW, Ghio AJ, Samet JM, Carson JL, Carter JD, Devlin RB. Effects of aqueous extracts of PM(10) filters from the Utah valley on human airway epithelial cells. Am J Physiol. 1999;277:L960–7. doi: 10.1152/ajplung.1999.277.5.L960. [DOI] [PubMed] [Google Scholar]
- 58.Ghio AJ, Devlin RB. Inflammatory lung injury after bronchial instillation of air pollution particles. Am J Respir Crit Care Med. 2001;164:704–8. doi: 10.1164/ajrccm.164.4.2011089. [DOI] [PubMed] [Google Scholar]
- 59.Halliwell B, Gutteridge JM. Free radicals in biology and medicine. 2nd ed. Oxford: Clarendon Press; 1989. [Google Scholar]
- 60.Lloyd RV, Hanna PM, Mason RP. The origin of the hydroxyl radical oxygen in the Fenton reaction. Free Radic Biol Med. 1997;22:885–8. doi: 10.1016/S0891-5849(96)00432-7. [DOI] [PubMed] [Google Scholar]
- 61.Harrison RM, Yin J. Particulate matter in the atmosphere: which particle properties are important for its effects on health? Sci Total Environ. 2000;249:85–101. doi: 10.1016/S0048-9697(99)00513-6. [DOI] [PubMed] [Google Scholar]
- 62.Lloyd DR, Carmichael PL, Phillips DH. Comparison of the formation of 8-hydroxy-2′-deoxyguanosine and single- and double-strand breaks in DNA mediated by fenton reactions. Chem Res Toxicol. 1998;11:420–7. doi: 10.1021/tx970156l. [DOI] [PubMed] [Google Scholar]
- 63.Saravia J, You D, Thevenot P, Lee GI, Shrestha B, Lomnicki S, et al. Early-life exposure to combustion-derived particulate matter causes pulmonary immunosuppression. Mucosal Immunol. 2014;7:694–704. doi: 10.1038/mi.2013.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Riva DR, Magalhães CB, Lopes AA, Lanças T, Mauad T, Malm O, et al. Low dose of fine particulate matter (PM2.5) can induce acute oxidative stress, inflammation and pulmonary impairment in healthy mice. Inhal Toxicol. 2011;23:257–67. doi: 10.3109/08958378.2011.566290. [DOI] [PubMed] [Google Scholar]
- 65.Ma J, Xu H, Wu J, Qu C, Sun F, Xu S. Linalool inhibits cigarette smoke-induced lung inflammation by inhibiting NF-κB activation. Int Immunopharmacol. 2015;29:708–13. doi: 10.1016/j.intimp.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 66.Loke YJ, Hannan AJ, Craig JM. The role of epigenetic change in autism spectrum disorders. Front Neurol. 2015;6:107. doi: 10.3389/fneur.2015.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fraga MF, Ballestar E, Paz MF, Ropero S, Setien F, Ballestar ML, et al. Epigenetic differences arise during the lifetime of monozygotic twins. Proc Natl Acad Sci U S A. 2005;102:10604–9. doi: 10.1073/pnas.0500398102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bjornsson HT, Sigurdsson MI, Fallin MD, Irizarry RA, Aspelund T, Cui H, et al. Intra-individual change over time in DNA methylation with familial clustering. JAMA. 2008;299:2877–83. doi: 10.1001/jama.299.24.2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rahman I, MacNee W. Role of transcription factors in inflammatory lung diseases. Thorax. 1998;53:601–12. doi: 10.1136/thx.53.7.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–61. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 71.Carmona JJ, Sofer T, Hutchinson J, Cantone L, Coull B, Maity A, et al. Short-term airborne particulate matter exposure alters the epigenetic landscape of human genes associated with the mitogen-activated protein kinase network: a cross-sectional study. Environ Health. 2014;13:94. doi: 10.1186/1476-069X-13-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baccarelli A, Wright RO, Bollati V, Tarantini L, Litonjua AA, Suh HH, et al. Rapid DNA methylation changes after exposure to traffic particles. Am J Respir Crit Care Med. 2009;179:572–8. doi: 10.1164/rccm.200807-1097OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Belinsky SA, Snow SS, Nikula KJ, Finch GL, Tellez CS, Palmisano WA. Aberrant CpG island methylation of the p16(INK4a) and estrogen receptor genes in rat lung tumors induced by particulate carcinogens. Carcinogenesis. 2002;23:335–9. doi: 10.1093/carcin/23.2.335. [DOI] [PubMed] [Google Scholar]
- 74.Wright RO, Baccarelli A. Metals and neurotoxicology. J Nutr. 2007;137:2809–13. doi: 10.1093/jn/137.12.2809. [DOI] [PubMed] [Google Scholar]
- 75.Gilmour PS, Rahman I, Donaldson K, MacNee W. Histone acetylation regulates epithelial IL-8 release mediated by oxidative stress from environmental particles. Am J Physiol Lung Cell Mol Physiol. 2003;284:L533–40. doi: 10.1152/ajplung.00277.2002. [DOI] [PubMed] [Google Scholar]
- 76.Cao D, Bromberg PA, Samet JM. COX-2 expression induced by diesel particles involves chromatin modification and degradation of HDAC1. Am J Respir Cell Mol Biol. 2007;37:232–9. doi: 10.1165/rcmb.2006-0449OC. [DOI] [PubMed] [Google Scholar]
- 77.Stevanović I, Jovasević-Stojanović M, Stosić JJ. Association between ambient air pollution, meteorological conditions and exacerbations of asthma and chronic obstructive pulmonary disease in adult citizens of the town of Smederevo. Vojnosanit Pregl. 2016;73:152–8. doi: 10.2298/VSP141111026S. [DOI] [PubMed] [Google Scholar]
- 78.Schwartz J. Particulate air pollution and chronic respiratory disease. Environ Res. 1993;62:7–13. doi: 10.1006/enrs.1993.1083. [DOI] [PubMed] [Google Scholar]
- 79.Raaschou-Nielsen O, Beelen R, Wang M, Hoek G, Andersen ZJ, Hoffmann B, et al. Particulate matter air pollution components and risk for lung cancer. Environ Int. 2016;87:66–73. doi: 10.1016/j.envint.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 80.Higenbottam T. Chronic cough and the cough reflex in common lung diseases. Pulm Pharmacol Ther. 2002;15:241–7. doi: 10.1006/pupt.2002.0341. [DOI] [PubMed] [Google Scholar]
- 81.Kumar N, Liang D, Comellas A, Chu AD, Abrams T. Satellite-based PM concentrations and their application to COPD in Cleveland, OH. J Expo Sci Environ Epidemiol. 2013;23:637–46. doi: 10.1038/jes.2013.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Globe G, Martin M, Schatz M, Wiklund I, Lin J, von Maltzahn R, et al. Symptoms and markers of symptom severity in asthma--content validity of the asthma symptom diary. Health Qual Life Outcomes. 2015;13:21. doi: 10.1186/s12955-015-0217-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ortiz-Martínez MG, Rodríguez-Cotto RI, Ortiz-Rivera MA, Pluguez-Turull CW, Jiménez-Vélez BD. Linking endotoxins, African Dust PM10 and Asthma in an urban and rural environment of Puerto Rico. Mediators Inflamm. 2015;2015:784212. doi: 10.1155/2015/784212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ding L, Zhu D, Peng D. Meta-analysis of the relationship between particulate matter (PM(10) and PM(2.5)) and asthma hospital admissions in children. Zhonghua Er Ke Za Zhi. 2015;53:129–35. [PubMed] [Google Scholar]
- 85.Lee JE, Park HS, Jung SS, Kim JO, Kim SY. Phase II study of a 3-week schedule of irinote can combined with cisplatin in previously untreated extensive-stage small-cell lung cancer. Oncology. 2007;73:76–80. doi: 10.1159/000120632. [DOI] [PubMed] [Google Scholar]
- 86.Jung KW, Won YJ, Kong HJ, Oh CM, Shin A, Lee JS. Survival of Korean adult cancer patients by stage at diagnosis, 2006–2010: national cancer registry study. Cancer Res Treat. 2013;45:162–71. doi: 10.4143/crt.2013.45.3.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ho SM. Environmental epigenetics of asthma: an update. J Allergy Clin Immunol. 2010;126:453–65. doi: 10.1016/j.jaci.2010.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hemmingsen JG, Jantzen K, Møller P, Loft S. No oxidative stress or DNA damage in peripheral blood mononuclear cells after exposure to particles from urban street air in overweight elderly. Mutagenesis. 2015;30:635–42. doi: 10.1093/mutage/gev027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Totlandsdal AI, Cassee FR, Schwarze P, Refsnes M, Låg M. Diesel exhaust particles induce CYP1A1 and pro-inflammatory responses via differential pathways in human bronchial epithelial cells. Part Fibre Toxicol. 2010;7:41. doi: 10.1186/1743-8977-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.He M, Ichinose T, Yoshida S, Nishikawa M, Mori I, Yanagisawa R, et al. Urban particulate matter in Beijing, China, enhances allergen-induced murine lung eosinophilia. Inhal Toxicol. 2010;22:709–18. doi: 10.3109/08958371003631608. [DOI] [PubMed] [Google Scholar]
- 91.Fujii T, Hayashi S, Hogg JC, Vincent R, Van Eeden SF. Particulate matter induces cytokine expression in human bronchial epithelial cells. Am J Respir Cell Mol Biol. 2001;25:265–71. doi: 10.1165/ajrcmb.25.3.4445. [DOI] [PubMed] [Google Scholar]
- 92.Sun H, Shamy M, Kluz T, Muñoz AB, Zhong M, Laulicht F, et al. Gene expression profiling and pathway analysis of human bronchial epithelial cells exposed to airborne particulate matter collected from Saudi Arabia. Toxicol Appl Pharmacol. 2012;265:147–57. doi: 10.1016/j.taap.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li R, Kou X, Geng H, Xie J, Yang Z, Zhang Y, et al. Effect of ambient PM(2.5) on lung mitochondrial damage and fusion/fission gene expression in rats. Chem Res Toxicol. 2015;28:408–18. doi: 10.1021/tx5003723. [DOI] [PubMed] [Google Scholar]
- 94.Valavanidis A, Vlahoyianni T, Fiotakis K. Comparative study of the formation of oxidative damage marker 8-hydroxy-2′-deoxyguanosine (8-OHdG) adduct from the nucleoside 2′-deoxyguanosine by transition metals and suspensions of particulate matter in relation to metal content and redox reactivity. Free Radic Res. 2005;39:1071–81. doi: 10.1080/10715760500188671. [DOI] [PubMed] [Google Scholar]
- 95.Tsou TC, Chen CL, Liu TY, Yang JL. Induction of 8-hydroxydeoxyguanosine in DNA by chromium(III) plus hydrogen peroxide and its prevention by scavengers. Carcinogenesis. 1996;17:103–8. doi: 10.1093/carcin/17.1.103. [DOI] [PubMed] [Google Scholar]
- 96.Prahalad AK, Inmon J, Dailey LA, Madden MC, Ghio AJ, Gallagher JE. Air pollution particles mediated oxidative DNA base damage in a cell free system and in human airway epithelial cells in relation to particulate metal content and bio-reactivity. Chem Res Toxicol. 2001;14:879–87. doi: 10.1021/tx010022e. [DOI] [PubMed] [Google Scholar]
- 97.Bellavia A, Urch B, Speck M, Brook RD, Scott JA, Albetti B, et al. DNA hypomethylation, ambient particulate matter, and increased blood pressure: findings from controlled human exposure experiments. J Am Heart Assoc. 2013;2:e000212. doi: 10.1161/JAHA.113.000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Takano H, Yanagisawa R, Ichinose T, Sadakane K, Inoue K, Yoshida S, et al. Lung expression of cytochrome P450 1A1 as a possible biomarker of exposure to diesel exhaust particles. Arch Toxicol. 2002;76:146–51. doi: 10.1007/s00204-002-0323-0. [DOI] [PubMed] [Google Scholar]
- 99.Chu JH, Hart JE, Chhabra D, Garshick E, Raby BA, Laden F. Gene expression network analyses in response to air pollution exposures in the trucking industry. Environ Health. 2016;15:101. doi: 10.1186/s12940-016-0187-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang T, Garcia JG, Zhang W. Epigenetic regulation in particulate matter-mediated cardiopulmonary toxicities: a systems biology perspective. Curr Pharmacogenomics Person Med. 2012;10:314–21. doi: 10.2174/187569212803901792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Crosbie WA, Snowden S, Parsons V. Changes in lung capillary permeability in renal failure. Br Med J. 1972;4:388–90. doi: 10.1136/bmj.4.5837.388. [DOI] [PMC free article] [PubMed] [Google Scholar]