Abstract
The present study evaluated the effects of Androctonus amoreuxi scorpion venom, Cerastes cerastes snake venom and their mixture on prostate cancer cells (PC3). An MTT assay was used to determine the anti-proliferative effect of the venoms, while quantitative real time PCR was used to evaluate the expression of apoptosis-related genes (Bax and Bcl-2). Furthermore, colorimetric assays were used to measure the levels of malondialdehyde (MDA) and antioxidant enzymes. Our results show that the venoms significantly reduced PC3 cell viability in a dose-dependent manner. On the other hand, these venoms significantly decreased Bcl-2 gene expression. Additionally, C. cerastes venom significantly reduced Bax gene expression, while A. amoreuxi venom and a mixture of A. amoreuxi & C. cerastes venoms did not alter Bax expression. Consequently, these venoms significantly increased the Bax/Bcl-2 ratio and the oxidative stress biomarker MDA. Furthermore, these venoms also increased the activity levels of the antioxidant enzymes, catalase, superoxide dismutase, glutathione peroxidase, glutathione reductase, and glutathione-S-transferase. Overall, the venoms have cytotoxic and anti-proliferative effects on PC3 cells.
Keywords: Venoms, Apoptosis, Bcl-2 family proteins, Bax/Bcl-2 ratio, Oxidative stress
INTRODUCTION
Scorpion venom is a natural product that is a complex mixture of molecules and plays an important role in defense and prey capture. Some scorpion venoms show cytotoxic effects against a wide range of cancer cell lines. Indian black scorpion (Heterometrus bengalensis) venom has anti-proliferative, cytotoxic, and apoptogenic activities against human leukemic cell lines U937 and K562.1 Rhopalurus junceus scorpion venom induces apoptosis in HeLa cells and necrosis in A549 cells, which is mainly via modulation of apoptosis gene expression.2
Another class of natural product is snake venom toxin, which has been reported to possess cytotoxic effects against cancer cell lines. A heat stable protein toxin (drCT-I) from Indian Daboia russelli russelli venom has cytotoxic and apoptotic properties against human leukemic cells (U937 and K562), and it significantly decreases Ehrlich ascites carcinoma cell viability.3 Venom extracted from the Walterinnesia aegyptia snake in combination with silica nanoparticles down-regulates the expression of the Bcl-2 gene, enhances the activation of caspase-3, and induces apoptosis in human breast carcinoma cell lines (MDA-MB-231 and MCF-7).4
Apoptosis is regulated by various protein families, including the Bcl-2 family and the caspase family. Anti-apoptotic Bcl-2 family genes (e.g., Bcl-2, Bcl-XL, and Mcl-1) and pro-apoptotic (e.g., Bax and Bak) genes are demonstrated to be important proteins functioning in the apoptotic process or clinical cancer therapy.5 The Bcl-2 family plays an important role in the activation of caspases and dominates the intrinsic pathways of apoptosis. Additionally, agents that cause oxidative stress are considered as mediators of apoptosis. Some studies have reported that the exposure to toxins/venoms increases the levels of oxidative markers.6,7 Consequently, understanding the changes occurring in the expression of apoptosis-related genes and oxidative stress biomarkers and antioxidant enzyme activity levels is important for understanding the mechanisms of apoptosis in cancer cell lines.
The present study evaluated the effects of scorpion venom from Androctonus amoreuxi, snake venom from Cerastes cerastes, and a mix of the venoms from A. amoreuxi & C. cerastes on cell viability. In addition, we measured apoptosis-related genes (Bax and Bcl-2), the Bax/Bcl-2 ratio, malondialdehyde (MDA) levels, and the changes in antioxidant enzyme activities.
MATERIALS AND METHODS
1. Tumor cell line
The human prostate cancer cell line (PC3) was provided by VACSERA (Giza Governorate, Egypt). The cells were maintained at 37°C in a humidified incubator with 5% CO2 and RPMI 1640 media with L-glutamine (Lonza, Verviers, Belgium), which was supplemented with 10% heat inactivated FBS (Biowest, Nuaillé, France).
2. Venom source
Scorpion venom from A. amoreuxi and snake venom from C. cerastes were obtained from VACSERA (Egypt).
3. In vitro cell viability assay
The effect of the venoms studied (scorpion venom A. amoreuxi, snake venom C. cerastes, and a mix of A. amoreuxi & C. cerastes venoms [mixed 1:1]) on PC3 cell viability was determined by an MTT assay.8 PC3 cells were seeded in 96-well plates (Costar®, Corning, Switzerland) at a density of 1 × 104 cells/well and incubated for 24 hours at 37°C in a humidified incubator with 5% CO2. After incubation, the media was replaced with 100 μL/well of different concentrations of each venom or their mixture (0.39, 1.56, 6.25, 25, 100 μg/mL) dissolved in serum-free medium. After 24 hours of treatment, the medium was replaced with 100 μL/well of the MTT solution (0.5 mg/mL in PBS), followed by incubation for 3 to 4 hours at 37°C. The supernatants were carefully removed, and the formazan crystals were solubilized with dimethyl sulfoxide (150 μL/well). The plates were gently shaken for 15 minutes at 37°C. Absorbance was determined at 570 nm with a microplate reader (Bio Tek, Winooski, VT, USA). The percentage of viability was calculated using the following formula: Viability (%) = (A570 of treated cells/A570 of control cells) × 100.
4. RNA isolation and cDNA Synthesis
After treatment of the PC3 cells with the IC50 (median inhibitory concentration) values of the venoms for 24 hours at 37°C in a humidified incubator with 5% CO2, the cells were harvested. The total RNA was isolated from treated and untreated PC3 cells using an RNeasy mini Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s protocol. The concentration and purity of the isolated RNA were detected by NanoDrop 2000 (Thermo Fisher Scientific, Waltham, MA, USA).
cDNA synthesis was performed using High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) in 20 μL reaction mixture containing 10 μL RNA sample (1 μg), 2 μL 10× RT Buffer, 0.8 μL 25× dNTP Mix (100 mM), 2 μL 10× RT random primers, 1 μL MultiScribe Reverse Transcriptase, 1 μL RNase inhibitor, and 3.2 μL nuclease-free water. Reverse transcription was performed using thermal cycler (Applied Biosystems) with the following temperatures and times: Step 1, 10 minutes at 25°C; Step 2, 120 minutes at 37°C; Step 3, 5 minutes at 85°C; then Step 4, hold at 4°C.
5. Quantitative real-time-PCR
SYBR Green quantitative real-time-PCR (qPCR) was performed on cDNA extracted from treated and untreated PC3 cells. The expression of target genes was quantified with SYBR Green PCR master mix using StepOne real-time PCR system (Applied Biosystems). Each qPCR amplification reaction was performed in 20 μL reaction mixture containing 10 μL Power SYBR Green PCR master mix (2×), 2 μL cDNA sample (100 ng), 1 μL forward primer (10 μM), 1 μL reverse primer (10 μM), and 6 μL double-distilled water. The cycling conditions started with initial 10 minutes at 95°C, followed by 40 two-step cycles of 15 seconds at 95°C and 1 minute at 60°C. The amplification was checked by melting curve analysis. The relative gene expression was calculated using 2−ΔΔCT method,9 where ΔΔCT values of each sample were calculated from CT values; ΔΔCT = [CT target gene–CTGAPDH](treated sample) –[CT target gene–CT GAPDH](untreated sample). The primers used in the experiments were as follows:10,11 Bax: F, 5′-GCTGGACATTGGACTTCCTC-3′ and R, 5′-TCAGCCCATCTTCTTCCAGA-3′; Bcl-2: F, 5′-TGTGGATGACTGAGTACCTGAACC-3′ and R, 5′-CAGCCAGGAGAAATCAAACAGAG-3′; GAPDH (housekeeping gene): F, 5′-CGTCTGCCCTATCAACTTTCG-3′ and R, 5′-CGTTTCTCAGGCTCCCTCT-3′. The primers were synthesized by LGC Biosearch technologies (Novato, CA, USA).
6. Biochemical analysis of oxidative stress and anti-oxidant biomarkers
PC3 cells were treated with the IC50 values of the venoms for 24 hours in a humidified atmosphere of 5% CO2 at 37°C. After that treatment, the cells (floating and adherent cells) were harvested, washed twice with cold PBS (by centrifugation), and pelleted at 2,000 ×g for 5 minutes at 4°C. The cell lysate was prepared by resuspending the cell pellet in a cold PBS, followed by sonication on ice and then centrifugation at 4,000 ×g for 15 minutes at 4°C. The supernatant was used for assaying.
The MDA level and antioxidant enzyme (catalase [CAT], superoxide dismutase [SOD], glutathione peroxidase [GPx], glutathione reductase [GR], and glutathione-S-transferase [GST]) activity levels were, according to the manufacturer’s instructions, assayed in treated and untreated PC3 cells using readymade kits provided by Biodiagnostic (Cairo, Egypt) and Milton Roy spectronic 21D UV-Visible spectrophotometer (USA). The total protein was determined in PC3 cells by the Bradford method.12 The MDA and the antioxidant enzyme activity levels were calculated per mg protein.
7. Statistical analysis
All experiments were independently performed at least three times. The IC50 values were determined using GraphPad Prism7 (GraphPad software, La Jolla, CA, USA). The P values were obtained by comparing the control group versus each treatment group using unpaired Student t-test. The differences were considered statistically significant at P < 0.05.
RESULTS AND DISCUSSION
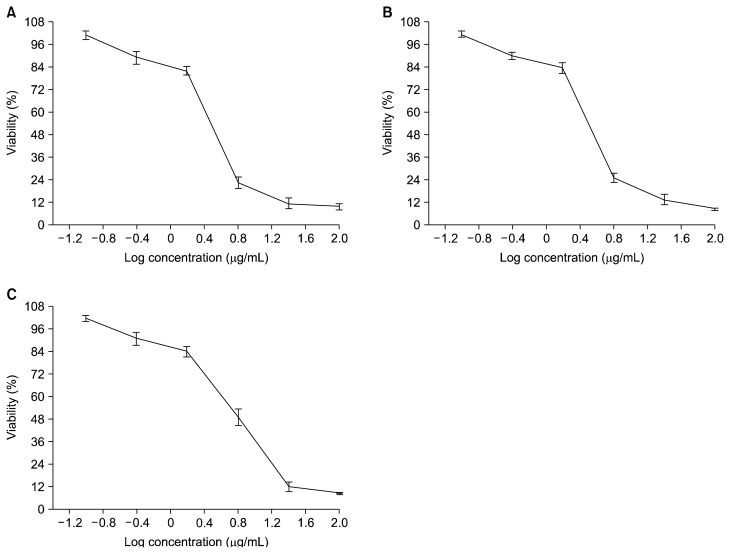
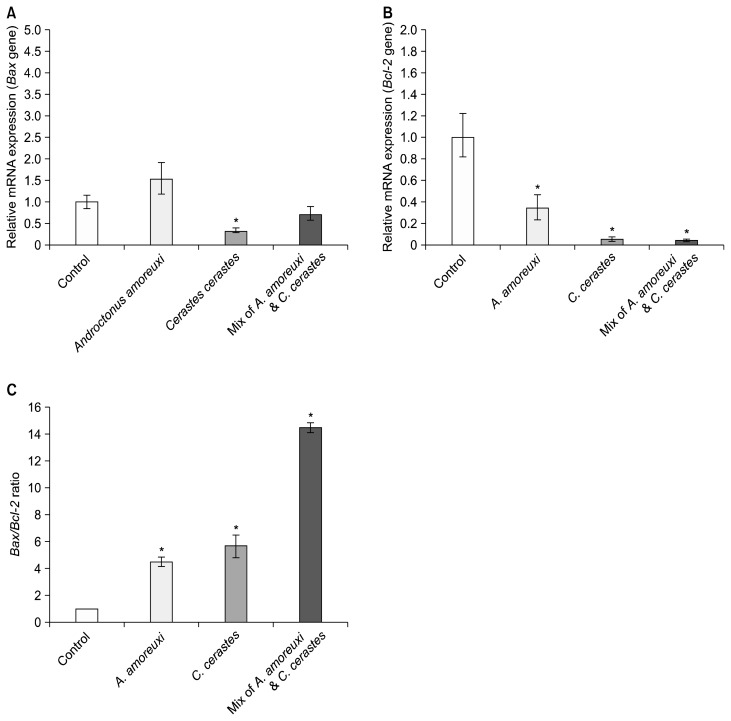
The present work evaluated the changes that occurred in a human PC3 cells treated with the venoms studied. Many reports have demonstrated the cytotoxic, anti-proliferative, and apoptotic effects of different snake and scorpion venoms against different cancer cell lines.1,4 This evidence, like our results, supports the potential of these venoms to act against an epithelial cancer cell line. The venom’s effect on the viability of PC3 cells was evaluated by an MTT assay. We observed that treatment with these venoms resulted in a dose-dependent decrease in the PC3 cell viability (Fig. 1). The IC50 values for A. amoreuxi venom, C. cerastes venom, and a mix of A. amoreuxi & C. cerastes venoms on PC3 cells were calculated as 3.04, 3.21, and 5.58 μg/mL, respectively, for a 24 hours treatment. The effect of the venom at its IC50 was studied on the expression of apoptosis-related genes (Bax and Bcl-2). Our results showed that a 24 hours treatment of PC3 cells with these venoms caused a significant down-regulation of Bcl-2 compared to control cells (P value < 0.05; Fig. 2B). These latter results agree with studies that have reported the down-regulating effect of some snake and scorpion venoms on Bcl-2 gene expression.2,13 We also found that Bax expression was not altered (P = 0.10) in the PC3 cells treated with A. amoreuxi venom, which agrees with reports that showed a non-significant effect of some scorpion venoms on Bax. Díaz-García et al.2 reported that R. junceus scorpion venom does not alter Bax expression and it down-regulates the Bcl-2 gene in A549 lung carcinoma cells. Bax expression was significantly decreased (P = 0.01) after C. cerastes venom treatment, which is consistent with data that showed the down-regulating effect of some venoms on pro-apoptotic and anti-apoptotic genes. For example, Pang et al.14 reported the decreases in both Bax and Bcl-2 genes in HCT-8 cancer cells after treatment with Agkistrodon acutus snake venom. Díaz-García et al.2 reported that R. junceus scorpion venom down-regulates both p53 and Bcl-2 genes in A549 cell line. On the other hand, Bax was not altered (P = 0.10) after a mixture of A. amoreuxi and C. cerastes venoms was applied. Some studies have reported the up-regulation of Bax expression after treatment with snake or scorpion venom,15,16 which is in contrast with our results. The effect of the venoms studied on Bax expression is presented in Figure 2A.
Figure 1.
Anti-proliferative effects of (A) Scorpion venom from Androctonus amoreuxi. (B) Snake venom from Cerastes cerastes. (C) Mix of A. amoreuxi & C. cerastes venoms on PC3 cells. The cells were exposed to various concentrations (0.39, 1.56, 6.25, 25, 100 μg/mL) of the venoms for 24 hours. The data are expressed as the mean ± SD.
Figure 2.
The effects of the venoms studied at the IC50 values on (A) Bax gene expression, (B) Bcl-2 gene expression, and (C) Bax/Bcl-2 ratio in PC3 cells after 24 hours of treatment. Fig. 2A and 2B represent RQ (relative quantification) values – Error bars are ± RQmax, RQmin – while values of Fig. 2C represent the mean ± SD. *This mark indicates significant difference.
Androgen-independent prostate cancer cells, such as PC3 cells, show an increase in Bcl-2.17,18 The down-regulating effect caused by the venoms on Bcl-2 is noteworthy since Bcl-2 is a highly conserved member of the Bcl-2 family, and it constitutes an important regulator of apoptosis. Bcl-2 inhibits and protects cells from apoptosis by blocking cytochrome c release from mitochondria and preventing the activation of the caspase 3 dependent pathway.19 In contrast, the attenuation of Bcl-2 may also prove favorable in certain clinical settings to enhance alternative modes of cell death. This is because Bcl-2 is not only a mediator of apoptosis but is also involved in programmed necrosis.20 Bax and Bcl-2 genes express membrane-bound pore-forming proteins that interact through heterodimerization, and Bax binds to Bcl-2 to counteract its function. The Bax/Bcl-2 ratio appears more important than the individual Bax or Bcl-2 level in determining a cell’s vulnerability to apoptosis. High Bax/Bcl-2 ratios lead to greater apoptotic activity.21,22 Our results showed that the Bax/Bcl-2 ratio was significantly increased (P value range from 0.0004 to 0.02; Fig. 2C) after the treatment with the venoms at their IC50 concentrations. This was because of the significant down-regulation of Bcl-2 gene expression, which agrees with studies showing an increase in the Bax/Bcl-2 ratio after snake or scorpion venom application.2,23 The increase in the Bax/Bcl-2 ratio indicates the involvement of mitochondria-mediated apoptosis after venom treatment since the Bax/Bcl-2 ratio is recognized as a key factor for apoptosis in cell by regulating cytochrome c release from the mitochondria to the cytosol.24
The changes that occurred in oxidative stress were tested in PC3 cells after venom treatment. Generally, oxidative stress is due to the imbalance between the antioxidants and pro-oxidants in favor of the oxidants.25 Venom/toxin-induced oxidative stress results in increased levels of oxidative markers,6,7 and it sometimes causes malfunctioning of vital organs through membrane destruction, enzyme release, and protein loss.26 Lipid peroxidation was measured by the MDA level, which is a biomarker of oxidative stress and cellular damage, that is evoked by stressors. A 24 hours treatment of PC3 cells with the IC50 of the venoms caused a significant increase in MDA in the cell lysate compared to the control cells (Table 1). This agrees with reports that showed an elevation of lipid peroxidation levels after treatment with various types of venoms,27,28 and increased lipoperoxidation may result in membrane damage. In the defense against oxidative stress, the cellular antioxidant enzyme system plays an important role. This system includes CAT, SOD, GPx, GR, and GST (the antioxidant enzymes studied). CAT converts H2O2 to H2O, SOD catalyzes the dismutation of the superoxide radical anion, GPx catalyzes GSH (the reduced form of glutathione) oxidation to oxidized glutathione (GSSG) at the expense of hydrogen peroxide or other organic peroxides, GR recycles GSSG back to GSH using NADPH, and GST catalyzes the conjugation of GSH to xenobiotic substrates for detoxification of nucleic acids. The induction of antioxidant enzymes is necessary for the host defense to protect the cell against oxidative stress. Antioxidant enzymes are working simultaneously to prevent the formation of highly cytotoxic hydroxyl radicals. The antioxidant defense system in PC3 cells was tested in response to venom. Our results show that a 24 hours treatment of PC3 cells with the IC50 of these venoms caused a significant increase in the activity of the antioxidant enzymes in the cell lysate compared to the control cells (Table 1). This agrees with reports that showed an increase in lipid peroxidation level and antioxidant enzymes when using venom as a treatment.28,29 In addition, da Silva et al.7 reported increased activities of CAT and GST in venom-injected experimental animals, which also agrees with our results. However, in contrast to our results, other studies showed decreases in some antioxidant enzymes in case of treatment with venoms.30
Table 1.
Effect of the venoms at the IC50 value on the activity of the antioxidant enzymes and MDA level in PC3 cells
| Variable/mg protein | Control cell | PC3 cells treated with | ||
|---|---|---|---|---|
|
| ||||
| Androctonus amoreuxi | Cerastes cerastes | Mix of A. amoreuxi & C. cerastes venoms | ||
| CAT (mU) | 0.84 ± 0.10 | 1.49 ± 0.12 (0.02) | 2.90 ± 0.33 (0.04) | 4.00 ± 0.27 (0.003) |
| SOD (U) | 168.75 ± 0.81 | 232.20 ± 9.01 (0.03) | 385.75 ± 32.78 (0.03) | 638.24 ± 41.81 (0.01) |
| GPx (mU) | 4.60 ± 0.31 | 7.64 ± 0.48 (0.02) | 14.05 ± 0.49 (< 0.001) | 13.03 ± 0.38 (0.0002) |
| GR (mU) | 17.82 ± 0.43 | 27.73 ± 0.79 (0.003) | 47.29 ± 4.01 (0.03) | 86.27 ± 7.34 (0.02) |
| GST (mU) | 3.09 ± 0.10 | 5.22 ± 0.26 (< 0.01) | 8.97 ± 0.29 (0.004) | 9.68 ± 0.24 (0.0002) |
| MDA level (nmol) | 4.43 ± 0.20 | 6.77 ± 0.49 (0.04) | 14.17 ± 0.90 (0.01) | 16.17 ± 1.13 (0.01) |
Values are present as mean ± SE (P value). PC3 cells were treated with the venoms for 24 hours. MDA, malondialdehyde; PC3, prostate cancer cell line; CAT, catalase; SOD, superoxide dismutase; GPx, glutathione peroxidase; GR, glutathione reductase, GST, glutathione-S-transferase.
In conclusion, the venoms studied have cytotoxic and anti-proliferative activities against PC3 cells.
ACKNOWLEDGMENTS
The authors gratefully thank Dr. Aly Fahmy Mohamed (VACSERA, Egypt) for his valuable technical advises.
Footnotes
CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
REFERENCES
- 1.Das Gupta S, Debnath A, Saha A, Giri B, Tripathi G, Vedasiromoni JR, et al. Indian black scorpion (Heterometrus bengalensis Koch) venom induced antiproliferative and apoptogenic activity against human leukemic cell lines U937 and K562. Leuk Res. 2007;31:817–25. doi: 10.1016/j.leukres.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Díaz-García A, Morier-Díaz L, Frión-Herrera Y, Rodríguez-Sánchez H, Caballero-Lorenzo Y, Mendoza-Llanes D, et al. In vitro anti-cancer effect of venom from Cuban scorpion Rhopalurus junceus against a panel of human cancer cell lines. J Venom Res. 2013;4:5–12. [PMC free article] [PubMed] [Google Scholar]
- 3.Gomes A, Choudhury SR, Saha A, Mishra R, Giri B, Biswas AK, et al. A heat stable protein toxin (drCT-I) from the Indian Viper (Daboia russelli russelli) venom having antiproliferative, cytotoxic and apoptotic activities. Toxicon. 2007;49:46–56. doi: 10.1016/j.toxicon.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 4.Al-Sadoon MK, Abdel-Maksoud MA, Rabah DM, Badr G. Induction of apoptosis and growth arrest in human breast carcinoma cells by a snake (Walterinnesia aegyptia) venom combined with silica nanoparticles: crosstalk between Bcl2 and caspase 3. Cell Physiol Biochem. 2012;30:653–65. doi: 10.1159/000341446. [DOI] [PubMed] [Google Scholar]
- 5.Kim R, Emi M, Tanabe K. Role of mitochondria as the gardens of cell death. Cancer Chemother Pharmacol. 2006;57:545–53. doi: 10.1007/s00280-005-0111-7. [DOI] [PubMed] [Google Scholar]
- 6.Al Asmari A, Al Moutaery K, Manthari RA, Khan HA. Time-course of lipid peroxidation in different organs of mice treated with Echis pyramidum snake venom. J Biochem Mol Toxicol. 2006;20:93–5. doi: 10.1002/jbt.20121. [DOI] [PubMed] [Google Scholar]
- 7.da Silva JG, da Silva Soley B, Gris V, do Rocio Andrade Pires A, Caderia SM, Eler GJ, et al. Effects of the Crotalus durissus terrificus snake venom on hepatic metabolism and oxidative stress. J Biochem Mol Toxicol. 2011;25:195–203. doi: 10.1002/jbt.20376. [DOI] [PubMed] [Google Scholar]
- 8.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 9.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 10.Mahoney S, Arfuso F, Millward M, Dharmarajan A. The effects of phenoxodiol on the cell cycle of prostate cancer cell lines. Cancer Cell Int. 2014;14:110. doi: 10.1186/s12935-014-0110-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shandiz SAS, Ardestani MS, Irani S, Shahbazzadeh D. Imatinib induces down-regulation of Bcl-2 an anti-apoptotic protein in prostate cancer PC-3 cell line. Adv Stud Biol. 2015;7:17–27. doi: 10.12988/asb.2015.41052. [DOI] [Google Scholar]
- 12.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 13.Lee HL, Park MH, Son DJ, Song HS, Kim JH, Ko SC, et al. Anti-cancer effect of snake venom toxin through down regulation of AP-1 mediated PRDX6 expression. Oncotarget. 2015;6:22139–51. doi: 10.18632/oncotarget.4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pang Z, Lu W, Zhai R. The agkistrodon acutus venom componets of X in vitro anti-tumor effect and mechanism. Life Sci J. 2010;7:41–5. [Google Scholar]
- 15.Zhang YY, Wu LC, Wang ZP, Wang ZX, Jia Q, Jiang GS, et al. Anti-proliferation effect of polypeptide extracted from scorpion venom on human prostate cancer cells in vitro. J Clin Med Res. 2009;1:24–31. doi: 10.4021/jocmr2009.01.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park MH, Son DJ, Kwak DH, Song HS, Oh KW, Yoo HS, et al. Snake venom toxin inhibits cell growth through induction of apoptosis in neuroblastoma cells. Arch Pharm Res. 2009;32:1545–54. doi: 10.1007/s12272-009-2106-0. [DOI] [PubMed] [Google Scholar]
- 17.Lu S, Tsai SY, Tsai MJ. Molecular mechanisms of androgen-independent growth of human prostate cancer LNCaP-AI cells. Endocrinology. 1999;140:5054–9. doi: 10.1210/endo.140.11.7086. [DOI] [PubMed] [Google Scholar]
- 18.Lian J, Wu X, He F, Karnak D, Tang W, Meng Y, et al. A natural BH3 mimetic induces autophagy in apoptosis-resistant prostate cancer via modulating Bcl-2-Beclin1 interaction at endoplasmic reticulum. Cell Death Differ. 2011;18:60–71. doi: 10.1038/cdd.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, et al. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–32. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 20.Sasi N, Hwang M, Jaboin J, Csiki I, Lu B. Regulated cell death pathways: new twists in modulation of BCL2 family function. Mol Cancer Ther. 2009;8:1421–9. doi: 10.1158/1535-7163.MCT-08-0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–19. doi: 10.1016/0092-8674(93)90509-O. [DOI] [PubMed] [Google Scholar]
- 22.Yang E, Korsmeyer SJ. Molecular thanatopsis: a discourse on the BCL2 family and cell death. Blood. 1996;88:386–401. [PubMed] [Google Scholar]
- 23.Hammouda MB, Montenegro MF, Sánchez-Del-Campo L, Zakraoui O, Aloui Z, Riahi-Chebbi I, et al. Lebein, a snake venom dis-integrin, induces apoptosis in human melanoma cells. Toxins (Basel) 2016;8:E206. doi: 10.3390/toxins8070206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adams JM, Cory S. Bcl-2-regulated apoptosis: mechanism and therapeutic potential. Curr Opin Immunol. 2007;19:488–96. doi: 10.1016/j.coi.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sies H, Jones DP. Oxidative stress. In: Fink G, editor. Encyclopedia of Stress. Amsterdam: Elsevier; 2007. pp. 45–8. [Google Scholar]
- 26.Yamasaki SC, Villarroel JS, Barone JM, Zambotti-Villela L, Silveira PF. Aminopeptidase activities, oxidative stress and renal function in Crotalus durissus terrificus envenomation in mice. Toxicon. 2008;52:445–54. doi: 10.1016/j.toxicon.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 27.AL-Johany AM, Al-Sadoon MK, Abdel Moneim AE, Bauomy AA, Diab MSM. Effects of pyramid viper, echis pyramidum crude venom on hepatic redox status and BAX expression in rats. J Pure Appl Microbio. 2014;8:429–36. [Google Scholar]
- 28.Sebastin Santhosh M, Hemshekhar M, Thushara RM, Devaraja S, Kemparaju K, Girish KS. Vipera russelli venom-induced oxidative stress and hematological alterations: amelioration by crocin a dietary colorant. Cell Biochem Funct. 2013;31:41–50. doi: 10.1002/cbf.2858. [DOI] [PubMed] [Google Scholar]
- 29.Guo J, Zuo L, Liu J, Hu X, Li C, Zhao Y, et al. Effect of P38 MAPK on the apoptosis of human lung adenocarcinoma cell induced by the spider venom. Thorac Cancer. 2010;1:77–82. doi: 10.1111/j.1759-7714.2010.00009.x. [DOI] [PubMed] [Google Scholar]
- 30.Al Asmari AK, Khan HA, Manthiri RA, Al Yahya KM, Al Otaibi KE. Effects of Echis pyramidum snake venom on hepatic and renal antioxidant enzymes and lipid peroxidation in rats. J Biochem Mol Toxicol. 2014;28:407–12. doi: 10.1002/jbt.21578. [DOI] [PubMed] [Google Scholar]