Abstract
Background
B lymphocyte hyperactivity is a main characteristic of systemic lupus erythematosus (SLE), and B lymphocytes play a prominent pathogenic role in the development and progression of SLE. The aim of this study was to investigate the role of Sirtuin 1 (Sirt1) in B lymphocytes.
Material/Methods
Mouse B lymphocytes BaF3 was transfected with Sirt1 vector or shRNA against Sirt1. Then the transfected cells viability and apoptosis were respectively determined by MTT assay and flow cytometry. In addition, the mRNA levels of three pro-inflammatory cytokines and p53 were detected by RT-PCR. Furthermore, the expression levels of nuclear factor-kappa B (NF-κB) pathway proteins were measured by Western blot.
Results
Overexpression of Sirt1 significantly increased cell proliferation (p<0.05 or p<0.01) and significantly suppressed apoptosis (p<0.05). The mRNA level expressions of interleukin 1 (IL-1), IL-6, and tumor necrosis factor-α (TNF-α) were significantly upregulated (p<0.05 or p<0.01), whereas p53 was significantly downregulated (p<0.05) by Sirt1 overexpression. In addition, the inhibitory subunit of NF-κB (IκBα) and p65 were significantly activated and phosphorylated (p<0.01 or p<0.001), and B-Cell CLL/Lymphoma 3 (Bcl-3) was significantly upregulated (p<0.05) by Sirt1 overexpression.
Conclusions
These results suggested that Sirt1 overexpression could promote BaF3 cell proliferation, inhibit apoptosis, and upregulate pro-inflammatory cytokines. The NF-κB pathway might be involved in these effects of Sirt1 on BaF3 cells, and Sirt1 might be a potential risk factor of SLE.
MeSH Keywords: Antigens, Differentiation, B-Lymphocyte; Apoptosis; Cell Proliferation
Background
Systemic lupus erythematosus (SLE), also known as lupus, is an autoimmune disease in which the body’s immune system mistakenly attacks healthy tissue in various organs, such as brain, blood, and kidney [1–3]. The exact cause of SLE is still unclear, while hormonal abnormalities, living environment, and genetics identified as main risk factors [4]. Although glucocorticoids, antimalarial drugs, and immunosuppressive agents can control its activity, the complications of SLE and the severe side effects of these drugs include secondary infection [5,6]. Thus, a better understanding of the molecular mechanisms of SLE will be helpful for finding novel therapeutic strategy.
Sirtuin 1 (Sirt1) is an NAD-dependent deacetylase that plays an important role in regulating a variety of cellular processes, such as energy metabolism, cell-cycle progression, apoptosis, aging, and migration [7–9]. Additionally, Sirt1 is becoming an important target for new therapies in the treatment of several diseases, including inflammation and autoimmune diseases [10,11]. Hu et al. observed Sirt1 overexpression was implicated in the pathogenesis of SLE, and knockdown of Sirt1 mitigated the damage of SLE in vivo [12]. Consiglio et al. found that Sirt1 could modify the morbidity of SLE in a Southern Brazilian population [7]. However, the mechanism of its effect on the occurrence and development of SLE remains elusive.
B lymphocyte hyperactivity is a main characteristic of SLE [13], and B lymphocytes play a prominent pathogenic role in the development and progression of SLE [14]. Thus, mouse B lymphocyte BaF3 was used in the present study to investigate the role of Sirt1 in SLE, and to explore its possible underling mechanisms. To be more specific, BaF3 cells were transfected with Sirt1 vector or shRNA against Sirt1 to establish Sirt1 overexpressed or suppressed cells. After transfection, the effects of Sirt1 expression on cell viability and apoptosis were detected. Moreover, the mRNA level expressions of three pro-inflammatory cytokines shown to be closely related to the pathophysiology of SLE, as well as the apoptosis-related factor p53, were measured. Furthermore, the protein expressions of nuclear factor-kappa B (NF-κB) pathway proteins were determined. This study might provide evidence that Sirt1 plays a critical role in the development of SLE, and the NF-κB pathway might be relevant to the aggravation of SLE severity.
Material and Methods
Cell culture and transfection
Mouse B lymphocyte BaF3 was obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA). Cells were cultured in RPMI-1640 (Gibco, Grand Island, NY, USA) medium supplemented with 10% fetal bovine serum (FBS; Gibco, USA) and 2 ng/mL murine IL-3 (R&D Systems, Inc., Minneapolis, MN, USA) at 37°C in a humidified atmosphere of 5% CO2.
A Sirt1 expression vector pc-Sirt1 was constructed by subcloning the full-length wild-type Sirt1 coding sequence into pcDNA3.1 (Sangon Biotech, Shanghai, China). The empty construct pcDNA3.1 plasma was transfected into cells as a control group. Moreover, Sirt1-shRNA vector was constructed by GenePharma (Shanghai, China). Scrambled shRNA (GenePharma, Shanghai, China) acted as the blank control. Cell transfections were conducted with Lipofectamine 2000 reagent (Invitrogen) following the manufacturer’s protocol. G418 (Gibco, Paisley, UK) was used for selection of stable Sirt1 transfectants.
Cell viability assay
Cell viability was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay [15]. Briefly, after 48 hours of transfection, cells were seeded in 96-well plates at a density of 2×103 cells/well and cultured for 1–4 days. Then 20 μL MTT (Sigma, St. Louis, MO, USA) was added to each well and incubated for another 4 hours at 37°C. Afterwards, 150 μL dimethylsulfoxide (DMSO) was added and the plates were shaken for 10 minutes. The absorbance at 450 nm was measured by a microplate reader (Bio-Rad Laboratories, Hercules, CA, USA).
Apoptosis assay
Cell apoptosis was measured using Annexin V-FITC/PI apoptosis detection kit (Beijing Biosea Biotechnology, Beijing, China), according to the manufacturer’s recommendations. Transfected cells were collected and resuspended in 200 μL of binding buffer containing 10 μL Annexin-V-FITC, and incubated at room temperature for 30 minute. Subsequently, 300 μL phosphate buffer saline (PBS) and 5 μL PI was added, and apoptotic cells were discriminated by flow cytometer (Beckman Coulter, USA) immediately [16].
Real-time reverse transcription polymerase chain reaction (RT-PCR)
Transfected cells were collected and lysed in TRIzol reagent (Invitrogen) for total RNA isolation. DNA contamination was removed using DNaseI (Promega). Transcriptor First Strand cDNA Synthesis Kit (Roche, USA) was used for reverse transcription into cDNAs. RT-PCR was carried out using FastSTART Universal SYBR Green Master (ROX) (Roche, USA) and ABI Prism 7000 Sequence Detection system (Applied Biosystems, Foster City, CA, USA) [17]. Data were analyzed using 2−ΔΔCt method and were normalized to GAPDH expression. All primers were synthesized by GenePharma (Shanghai, China).
Western blot
Protein expression changes in transfected cells were determined by Western blot analysis. In brief, cells were lysed in RIPA lysis buffer (Beyotime Biotechnology, Shanghai, China), and the protein concentration was quantified using BCA™ Protein Assay Kit (Pierce, Appleton, WI, USA). The same amount of proteins were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene fluoride (PVDF) membranes. After blocking with 5% skim milk for one hour, the blots were incubated overnight with primary antibodies: Sirt1, p-inhibitory subunit of NF-κB (IκBα), t-IκBα, p-p65, t-p65, B-Cell CLL/Lymphoma 3 (Bcl-3), GAPDH (1: 1000; Santa Cruz, CA). The blots were then incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies for one hour. Finally, protein bands were visualized by the Super Signal West Femto Maximum sensitivity substrate kit (Thermo Scientific, Logan, UT, USA), and quantified using Image Pro Plus version 6 software [18].
Statistical analysis
Data were expressed as means ± standard derivations (SD) from three independent experiments. Statistical differences between the mean values of two groups were performed using GraphPad Prism 5 (GraphPad Software Inc., San Diego, CA, USA), and were analyzed by student t-test. A value of p<0.05 was considered to indicate a statistically significant result.
Results
Effects of transfection on Sirt1 expression
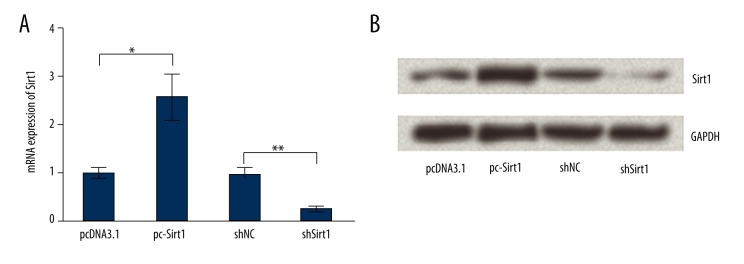
BaF3 cells were transfected with empty pcDNA3.1, pc-Sirt1, scrambled shRNA, or shRNA against Sirt1 respectively, and then the transfection efficiency was tested by RT-PCR and Western blot analysis (Figure 1A, 1B). As expected, mRNA and protein levels of Sirt1 were both significantly overexpressed by pc-Sirt when compared to its control group (p<0.05). By contrast, the expression levels of Sirt1 were significantly suppressed by Sirt1-shRNA when compared to its scramble group (p<0.01). Therefore, the transfected cells were used for the forthcoming analyses.
Figure 1.
Effects of transfection on Sirtuin 1 (Sirt1) expression. BaF3 cells were transfected with empty pcDNA3.1, pc-Sirt1, scrambled shRNA, or shRNA against Sirt1 respectively, and then the transfection efficiency was tested by RT-PCR (A) and Western blot analysis (B). GAPDH was used as an internal control. * p<0.05; ** p<0.01.
Overexpression of Sirt1 increased cell viability while suppressed apoptosis
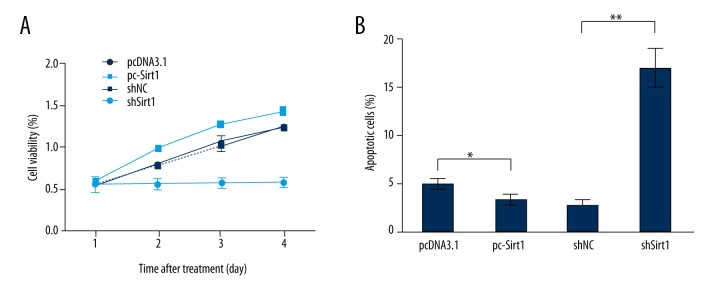
In order to investigate the biological effects of Sirt1 on BaF3 cells, the expression of Sirt1 was dysregulated by Sirt1 vector or Sirt1-shRNA transfection, and then cell viability and apoptosis were detected by MTT (Figure 2A) analysis and flow cytometry (Figure 2B). Results showed that Sirt1 overexpression significantly promoted cell viability after the transfected cells were cultured for 2–4 days (p<0.05 or p<0.01), while Sirt1 suppression significantly suppressed cell viability at the same time points (p<0.05, p<0.01 or p<0.001). In addition, Sirt1 overexpression significantly decreased apoptotic cells rate (p<0.05), while Sirt1 suppression significantly induced apoptosis (p<0.01). These data revealed that Sirt1 might be a vital regulator in BaF3 cells proliferation and apoptosis.
Figure 2.
Overexpression of Sirtuin 1 (Sirt1) increased cell viability and suppressed apoptosis. After BaF3 cells were transfected with empty pcDNA3.1, pc-Sirt1, scrambled shRNA, or Sirt1-shRNA, cell viability was measured by MTT (A) and apoptosis was determined by flow cytometry (B). * p<0.05; ** p<0.01; *** p<0.001.
Overexpression of Sirt1 upregulated the expression of pro-inflammatory cytokines while downregulated the expression of p53
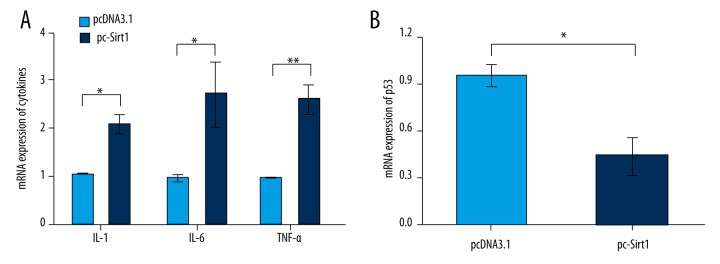
To explore the effects of Sirt1 on pro-inflammatory cytokine production in BaF3 cells, the mRNA level expressions of pro-inflammatory cytokines were determined by RT-PCR (Figure 3A). Results showed that the expression level of Interleukin 1 (IL-1), IL-6, and tumor necrosis factor-α (TNF-α) were significantly upregulated by Sirt1 overexpression (p<0.05 or p<0.01). To further explore the underling mechanism that allows Sirt1 to affect BaF3 cells apoptosis, the mRNA level expression of p53 was also determined (Figure 3B). The expression of p53 was significantly downregulated by Sirt1 overexpression (p<0.05). These results suggested that Sirt1 could contribute to pro-inflammatory cytokine production, and the expression of p53 might be involved in the suppressive effects of Sirt1 on BaF3 cells apoptosis.
Figure 3.
Overexpression of Sirtuin 1 (Sirt1) upregulated the expression of inflammatory cytokines and downregulated the expression of p53. Cells were first transfected with empty pcDNA3.1 or pc-Sirt1, and then the mRNA level expressions of pro-inflammatory cytokines (A) and p53 (B) in transfected cells were determined by RT-PCR. * p<0.05; ** p<0.01.
NF-κB pathway was involved in the effects of Sirt1 on NaF3 cells
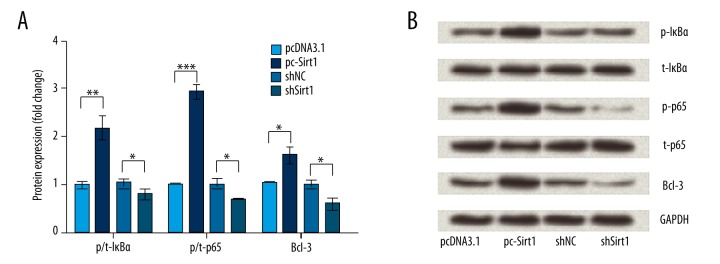
Next, we determined the protein expression changes of p/t-IκBα, p/t-p65, and Bcl-3 in transfected cells to ask whether the NF-κB pathway was involved in the effects of Sirt1 on BaF3 cells. Results in Figure 4A and 4B show that IκBα and p65 were significantly activated and phosphorylated by Sirt1 overexpression (p<0.01 or p<0.001). Meanwhile, the protein expression of Bcl-3 was significantly upregulated by Sirt1 overexpression (p<0.05). Thus, we inferred that the NF-κB pathway might be involved in the effects of Sirt1 on NaF3 cells.
Figrue 4.
Nuclear factor-kappa B (NF-κB) pathway was involved in the effects of Sirtuin 1 (Sirt1) on NaF3 cells. After BaF3 cells were transfected with empty pcDNA3.1, pc-Sirt1, scrambled shRNA, or Sirt1-shRNA, the protein expressions of p/t-inhibitory subunit of NF-κB (IκBα), p/t-p65, and B-Cell CLL/Lymphoma 3 (Bcl-3) were detected by Western blot analysis (A, B). GAPDH was used as an internal control. * p<0.05; ** p<0.01; *** p<0.001.
Discussion
SLE is an autoimmune disease which is characterized by B lymphocyte hyperactivity, and B lymphocytes play a crucial role in the pathogenesis of SLE [1,13]. In the present study, mouse B lymphocyte BaF3 was transfected with Sirt1 vector or shRNA against Sirt1 to investigate the potential role of Sirt1 in SLE. We found that Sirt1 overexpression significantly promoted cell viability while suppressing apoptosis. In addition, in Sirt1 overexpressed cells, the expression levels of three pro-inflammatory cytokines, i.e., IL-1, IL-6 and TNF-α, were significantly upregulated. However, the expression level of apoptosis-related factor p53 was significantly downregulated. Further, IκBα and p65 were significantly activated and phosphorylated by Sirt1 overexpression, and the protein expression of Bcl-3 was significantly upregulated.
Currently, Sirt1 has been identified as a vital regulator in cell proliferation and apoptosis [19]. Sirt1 could promote cell proliferation in myoblast cells [20], apical papilla and periodontal ligament stem cells [21], and human epithelial cancer cells [22]. In addition, Sirt1 could protect cells from p53-mediated apoptosis. Zhang et al. found that knockdown of Sirt1 expression resulted in leukemia cells apoptosis induction [23]. Zheng et al. revealed that Sirt1 protected human lens epithelial cells against oxidative stress by inhibiting p53-dependent apoptosis [24]. Consistent with these previous studies, our study revealed that Sirt1 promoted cell proliferation and suppressed apoptosis in BaF3 cells.
Pro-inflammatory cytokines, such as TNF-α, IL-1, and IL-6, play pivotal roles in inflammation and the maturation of B cells, and have been proven to be closely related to the pathophysiology of SLE [25,26]. IL-6 is produced by TNF-α and supposedly upregulates the number of inflammatory cells [27]. In the current study, these three cytokines were all upregulated by Sirt1 overexpression in BaF3 cells, suggesting that Sirt1 expression might contribute to pro-inflammatory cytokine production. Similarly, Niederer et al. observed high expression of Sirt1 directly upregulated the expressions of IL-6, IL-8, and TNF-α in rheumatoid arthritis synovial fibroblasts [28].
NF-κB is a family of five transcription factors: p50, p52, p65 (RelA), c-Rel, and RelB [29]. Activation and regulation of the NF-κB pathway are tightly controlled by its natural biological inhibitor IκB [30]. IκBα and Bcl-3 are two family members of IκB family proteins; activation and phosphorylation of these two factors, as well as phosphorylation of p65, are implicated in the activation of NF-κB [31]. It is well known that the NF-κB pathway is primarily involved in mounting effective inflammatory responses [32]. NF-κB regulates a number of cytokines, such as TNF-α, IL-1, and IL6, and also contributes to tumor-promoting inflammation [33]. A recent study found that Bcl-3 could interact with p50, and identified Bcl-3 was an effective anti-inflammatory peptide [34]. This pathway also contributes to the modulation of cell proliferation and apoptosis [30,35]. An in vivo study found that p65 could stimulate ovine fetal pulmonary vascular smooth muscle cell proliferation [36]. Moreover, p65 has been implicated in apoptosis of embryonic liver cells and chicken bone marrow cells [37].
In the current study, IκBα and p65 were remarkably phosphorylated, and the protein expression of Bcl-3 was upregulated by Sirt1 overexpression, implying that the NF-κB pathway was involved in the effects of Sirt1 on BaF3 cells. Actually, Sirt1 is widely investigated as a suppressor of NF-κB-dependent transcription through the deacetylation of the p65 subunits [38,39]. Schug et al. found Sirt1 could increase the expression of pro-inflammatory cytokines via inhibiting the NF-κB pathway [39]. Busch et al. found that Sirt1 inhibition stimulated phosphorylation of IκB-α and p65, and correlated with upregulation of NF-κB-regulated gene products involved in cell proliferation, inflammation, and apoptosis [40]. However, this study provided the first insight into the effects of Sirt1 on BaF3 cells proliferation, apoptosis, and inflammatory response via modulating the NF-κB pathway.
Conclusions
Our findings revealed Sirt1 overexpression could promote BaF3 cells proliferation, inhibit apoptosis, and upregulate pro-inflammatory cytokines, indicating that Sirt1 might be a potential risk factor of SLE. Further investigations still need to confirm these hypotheses.
Footnotes
Conflict of interests
The authors declare that they do not have competing interests.
Source of support: The work was supported by Science Research Foundation for Excellent Youth Scholars of Shandong Province (BS2009YY037)
References
- 1.da Silva LS, Almeida BL, de Melo AK, et al. IgA nephropathy in systemic lupus erythematosus patients: Case report and literature review. Rev Bras Reumatol (Rio J) 2016;56:270–73. doi: 10.1016/j.rbre.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 2.D’Cruz DP, Khamashta MA, Hughes GR. Systemic lupus erythematosus. Lancet. 2007;369:587–96. doi: 10.1016/S0140-6736(07)60279-7. [DOI] [PubMed] [Google Scholar]
- 3.Tsokos GC. Systemic lupus erythematosus. N Engl J Med. 2011;365:2110–21. doi: 10.1056/NEJMra1100359. [DOI] [PubMed] [Google Scholar]
- 4.Lisnevskaia L, Murphy G, Isenberg D. Systemic lupus erythematosus. Lancet. 2014;384:1878–88. doi: 10.1016/S0140-6736(14)60128-8. [DOI] [PubMed] [Google Scholar]
- 5.Wallace DJ. The evolution of drug discovery in systemic lupus erythematosus. Nat Rev Rheumatol. 2015;11:616–20. doi: 10.1038/nrrheum.2015.86. [DOI] [PubMed] [Google Scholar]
- 6.Huang KP, Zhang ZH, Li RM, Chen X. The therapeutic effects of the chinese herbal medicine, lang chuang fang granule, on lupus-prone MRL/lpr mice. Evid Based Complement Alternat Med. 2016;2016:8562528. doi: 10.1155/2016/8562528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Consiglio CR, Juliana da Silveira S, Monticielo OA, et al. SIRT1 promoter polymorphisms as clinical modifiers on systemic lupus erythematosus. Mol Biol Rep. 2014;41:4233–39. doi: 10.1007/s11033-014-3294-3. [DOI] [PubMed] [Google Scholar]
- 8.Cui Y, Li J, Zheng F, et al. Effect of SIRT1 gene on epithelial-mesenchymal transition of human prostate cancer PC-3 cells. Med Sci Monit. 2016;22:380–86. doi: 10.12659/MSM.895312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qin W, Xie W, Yang X, et al. Inhibiting microRNA-449 attenuates cisplatin-induced injury in NRK-52E cells possibly via regulating the SIRT1/P53/BAX Pathway. Med Sci Monit. 2016;22:818–23. doi: 10.12659/MSM.897187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stein S, Lohmann C, Schafer N, et al. SIRT1 decreases Lox-1-mediated foam cell formation in atherogenesis. Eur Heart J. 2010;31:2301–9. doi: 10.1093/eurheartj/ehq107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sequeira J, Boily G, Bazinet S, et al. sirt1-null mice develop an autoimmune-like condition. Exp Cell Res. 2008;314:3069–74. doi: 10.1016/j.yexcr.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 12.Hu N, Long H, Zhao M, et al. Aberrant expression pattern of histone acetylation modifiers and mitigation of lupus by SIRT1-siRNA in MRL/lpr mice. Scand J Rheumatol. 2009;38:464–71. doi: 10.3109/03009740902895750. [DOI] [PubMed] [Google Scholar]
- 13.Rao H, Zeng Q, Liang Y, et al. Correlation between TLR9 expression and cytokine secretion in the clinical diagnosis of systemic lupus erythematosus. Mediators Inflamm. 2015;2015:710720. doi: 10.1155/2015/710720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo Q, Huang Z, Ye J, et al. PD-L1-expressing neutrophils as a novel indicator to assess disease activity and severity of systemic lupus erythematosus. Arthritis Res Ther. 2016;18:47. doi: 10.1186/s13075-016-0942-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu L, Li C, Li D, et al. Cryptotanshinone inhibits human glioma cell proliferation by suppressing STAT3 signaling. Mol Cell Biochem. 2013;381:273–82. doi: 10.1007/s11010-013-1711-x. [DOI] [PubMed] [Google Scholar]
- 16.Pan XW, Zhao XH. In vitro proliferation and anti-apoptosis of the papain-generated casein and soy protein hydrolysates towards osteoblastic cells (hFOB1.19) Int J Mol Sci. 2015;16:13908–20. doi: 10.3390/ijms160613908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jang KY, Noh SJ, Lehwald N, et al. SIRT1 and c-Myc promote liver tumor cell survival and predict poor survival of human hepatocellular carcinomas. PLoS One. 2012;7:e45119. doi: 10.1371/journal.pone.0045119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang J, Zhu Y, Xie K, et al. The role of the AMOP domain in MUC4/Y-promoted tumour angiogenesis and metastasis in pancreatic cancer. J Exp Clin Cancer Res. 2016;35:91. doi: 10.1186/s13046-016-0369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li S, Hong H, Lv H, et al. SIRT 1 overexpression is associated with metastasis of pancreatic ductal adenocarcinoma (PDAC) and promotes migration and growth of PDAC cells. Med Sci Monit. 2016;22:1593–600. doi: 10.12659/MSM.896697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang L, Zhang T, Xi Y, et al. Sirtuin 1 promotes the proliferation of C2C12 myoblast cells via the myostatin signaling pathway. Mol Med Rep. 2016;14(2):1309–15. doi: 10.3892/mmr.2016.5346. [DOI] [PubMed] [Google Scholar]
- 21.Zhang QB, Cao W, Liu YR, et al. Effects of Sirtuin 1 on the proliferation and osteoblastic differentiation of periodontal ligament stem cells and stem cells from apical papilla. Genet Mol Res. 2016;15(1) doi: 10.4238/gmr.15015234. [DOI] [PubMed] [Google Scholar]
- 22.Ford J, Jiang M, Milner J. Cancer-specific functions of SIRT1 enable human epithelial cancer cell growth and survival. Cancer Res. 2005;65:10457–63. doi: 10.1158/0008-5472.CAN-05-1923. [DOI] [PubMed] [Google Scholar]
- 23.Zhang W, Wu H, Yang M, et al. SIRT1 inhibition impairs non-homologous end joining DNA damage repair by increasing Ku70 acetylation in chronic myeloid leukemia cells. Oncotarget. 2016;7:13538–50. doi: 10.18632/oncotarget.6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng T, Lu Y. SIRT1 protects human lens epithelial cells against oxidative stress by inhibiting p53-dependent apoptosis. Curr Eye Res. 2015 doi: 10.3109/02713683.2015.1093641. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 25.Thanei S, Trendelenburg M. Anti-C1q autoantibodies from systemic lupus erythematosus patients induce a proinflammatory phenotype in macrophages. J Immunol. 2016;196:2063–74. doi: 10.4049/jimmunol.1501659. [DOI] [PubMed] [Google Scholar]
- 26.Adler S, Kolev M, Varisco PA, et al. Induction of severe systemic lupus erythematosus by TNF blockade and response to anti-IL-6 strategy. J Allergy Clin Immunol. 2013;131:1235–37. 1237.e12. doi: 10.1016/j.jaci.2012.09.034. [DOI] [PubMed] [Google Scholar]
- 27.Fernandes JC, Martel-Pelletier J, Pelletier JP. The role of cytokines in osteoarthritis pathophysiology. Biorheology. 2002;39:237–46. [PubMed] [Google Scholar]
- 28.Niederer F, Ospelt C, Brentano F, et al. SIRT1 overexpression in the rheumatoid arthritis synovium contributes to proinflammatory cytokine production and apoptosis resistance. Ann Rheum Dis. 2011;70:1866–73. doi: 10.1136/ard.2010.148957. [DOI] [PubMed] [Google Scholar]
- 29.Herrington FD, Nibbs RJ. Regulation of the adaptive immune response by the IkappaB family protein Bcl-3. Cells. 2016;5(2) doi: 10.3390/cells5020014. pii: E14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verma IM, Stevenson JK, Schwarz EM, et al. Rel/NF-kappa B/I kappa B family: Intimate tales of association and dissociation. Genes Dev. 1995;9:2723–35. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- 31.Hayashi T, Sekine T, Okamoto T. Identification of a new serine kinase that activates NF kappa B by direct phosphorylation. J Biol Chem. 1993;268:26790–95. [PubMed] [Google Scholar]
- 32.Park MH, Hong JT. Roles of NF-kappaB in cancer and inflammatory diseases and their therapeutic approaches. Cells. 2016;5(2) doi: 10.3390/cells5020015. pii: E15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rinkenbaugh AL, Baldwin AS. The NF-kappaB pathway and cancer stem cells. Cells. 2016;5(2) doi: 10.3390/cells5020016. pii: E16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Collins PE, Grassia G, Colleran A, et al. Mapping the Interaction of B Cell leukemia 3 (BCL-3) and nuclear factor kappaB (NF-kappaB) p50 identifies a BCL-3-mimetic anti-inflammatory peptide. J Biol Chem. 2015;290:15687–96. doi: 10.1074/jbc.M115.643700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guttridge DC, Albanese C, Reuther JY, et al. NF-kappaB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol Cell Biol. 1999;19:5785–99. doi: 10.1128/mcb.19.8.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ogbozor UD, Opene M, Renteria LS, et al. Mechanism by which nuclear factor-kappa beta (NF-κB) regulates ovine fetal pulmonary vascular smooth muscle cell proliferation. Mol Genet Metab Rep. 2015;4:11–18. doi: 10.1016/j.ymgmr.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beg AA, Sha WC, Bronson RT, et al. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-kappa B. Nature. 1995;376:167–70. doi: 10.1038/376167a0. [DOI] [PubMed] [Google Scholar]
- 38.Lee JH, Song MY, Song EK, et al. Overexpression of SIRT1 protects pancreatic beta-cells against cytokine toxicity by suppressing the nuclear factor-kappaB signaling pathway. Diabetes. 2009;58:344–51. doi: 10.2337/db07-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schug TT, Xu Q, Gao H, et al. Myeloid deletion of SIRT1 induces inflammatory signaling in response to environmental stress. Mol Cell Biol. 2010;30:4712–21. doi: 10.1128/MCB.00657-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Busch F, Mobasheri A, Shayan P, et al. Sirt-1 is required for the inhibition of apoptosis and inflammatory responses in human tenocytes. J Biol Chem. 2012;287:25770–81. doi: 10.1074/jbc.M112.355420. [DOI] [PMC free article] [PubMed] [Google Scholar]