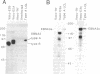Abstract
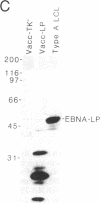
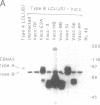
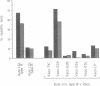
The potentially pathogenic effects of infection with Epstein-Barr virus (EBV), a B-lymphotropic agent with cell growth-transforming potential, are contained in healthy virus carriers by virus-specific cytotoxic T-lymphocyte (CTL) surveillance. The target antigens against which such CTL responses are directed are yet undefined, but the antigens probably derived from one or more of the EBV "latent" proteins constitutively expressed in virus-transformed B cells. We have analyzed target specificity of CTL responses from two EBV-immune donors that are preferentially reactive against autologous cells transformed with type A but not with type B virus isolates. Coding sequences for four EBV latent proteins with allelic polymorphism between A and B virus types--namely, the EBV nuclear antigens (EBNAs) EBNA 2, EBNA 3a, EBNA 3c, and EBNA leader protein--have been introduced into vaccinia virus vectors under control of vaccinia promoter P7.5 and used to express relevant EBNA proteins in appropriate target cells. Thus the CTL response from one donor has been mapped to type A EBNA 2 protein and from a second donor to type A EBNA 3a protein. Thereafter, a series of recombinant vaccinia viruses were constructed that carried specific internal deletions within the EBNA 2 type A coding sequence; by using these vectors, the above EBNA 2 type A-specific CTL response was shown to be directed against an epitope within a 100-amino acid fragment near the N terminus of the protein. This work clearly shows human CTL recognition of virus-coded nuclear antigens in the EBV system; moreover, it establishes an experimental approach that can be extended to all EBV latent proteins and to the more common CTL responses that cross-react against type A and type B virus isolates.
Full text
PDF
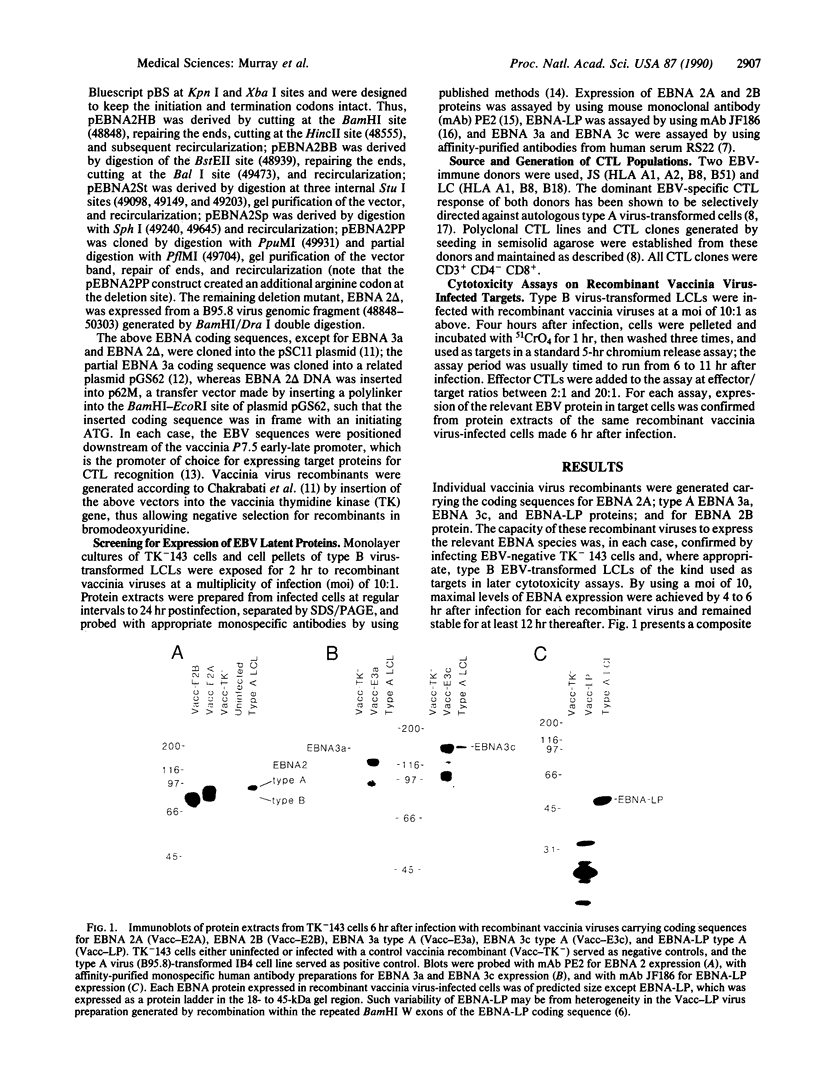
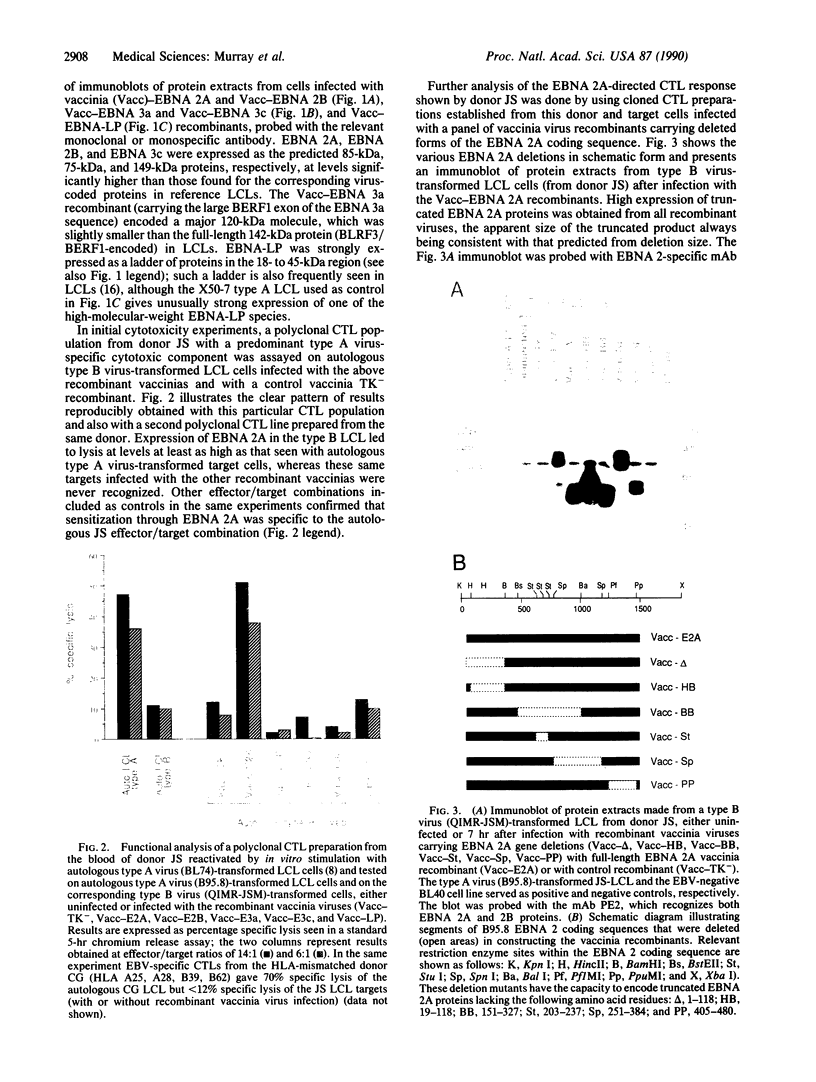

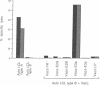
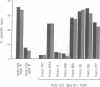
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burrows S. R., Sculley T. B., Misko I. S., Schmidt C., Moss D. J. An Epstein-Barr virus-specific cytotoxic T cell epitope in EBV nuclear antigen 3 (EBNA 3). J Exp Med. 1990 Jan 1;171(1):345–349. doi: 10.1084/jem.171.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S., Brechling K., Moss B. Vaccinia virus expression vector: coexpression of beta-galactosidase provides visual screening of recombinant virus plaques. Mol Cell Biol. 1985 Dec;5(12):3403–3409. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coupar B. E., Andrew M. E., Both G. W., Boyle D. B. Temporal regulation of influenza hemagglutinin expression in vaccinia virus recombinants and effects on the immune response. Eur J Immunol. 1986 Dec;16(12):1479–1487. doi: 10.1002/eji.1830161203. [DOI] [PubMed] [Google Scholar]
- Dambaugh T., Hennessy K., Chamnankit L., Kieff E. U2 region of Epstein-Barr virus DNA may encode Epstein-Barr nuclear antigen 2. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7632–7636. doi: 10.1073/pnas.81.23.7632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finke J., Rowe M., Kallin B., Ernberg I., Rosén A., Dillner J., Klein G. Monoclonal and polyclonal antibodies against Epstein-Barr virus nuclear antigen 5 (EBNA-5) detect multiple protein species in Burkitt's lymphoma and lymphoblastoid cell lines. J Virol. 1987 Dec;61(12):3870–3878. doi: 10.1128/jvi.61.12.3870-3878.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory C. D., Murray R. J., Edwards C. F., Rickinson A. B. Downregulation of cell adhesion molecules LFA-3 and ICAM-1 in Epstein-Barr virus-positive Burkitt's lymphoma underlies tumor cell escape from virus-specific T cell surveillance. J Exp Med. 1988 Jun 1;167(6):1811–1824. doi: 10.1084/jem.167.6.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss D. J., Misko I. S., Burrows S. R., Burman K., McCarthy R., Sculley T. B. Cytotoxic T-cell clones discriminate between A- and B-type Epstein-Barr virus transformants. Nature. 1988 Feb 25;331(6158):719–721. doi: 10.1038/331719a0. [DOI] [PubMed] [Google Scholar]
- Moss D. J., Rickinson A. B., Wallace L. E., Epstein M. A. Sequential appearance of Epstein-Barr virus nuclear and lymphocyte-detected membrane antigens in B cell transformation. Nature. 1981 Jun 25;291(5817):664–666. doi: 10.1038/291664a0. [DOI] [PubMed] [Google Scholar]
- Petti L., Sample J., Wang F., Kieff E. A fifth Epstein-Barr virus nuclear protein (EBNA3C) is expressed in latently infected growth-transformed lymphocytes. J Virol. 1988 Apr;62(4):1330–1338. doi: 10.1128/jvi.62.4.1330-1338.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickinson A. B., Moss D. J., Wallace L. E., Rowe M., Misko I. S., Epstein M. A., Pope J. H. Long-term T-cell-mediated immunity to Epstein-Barr virus. Cancer Res. 1981 Nov;41(11 Pt 1):4216–4221. [PubMed] [Google Scholar]
- Rowe M., Rowe D. T., Gregory C. D., Young L. S., Farrell P. J., Rupani H., Rickinson A. B. Differences in B cell growth phenotype reflect novel patterns of Epstein-Barr virus latent gene expression in Burkitt's lymphoma cells. EMBO J. 1987 Sep;6(9):2743–2751. doi: 10.1002/j.1460-2075.1987.tb02568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe M., Young L. S., Cadwallader K., Petti L., Kieff E., Rickinson A. B. Distinction between Epstein-Barr virus type A (EBNA 2A) and type B (EBNA 2B) isolates extends to the EBNA 3 family of nuclear proteins. J Virol. 1989 Mar;63(3):1031–1039. doi: 10.1128/jvi.63.3.1031-1039.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sample J., Hummel M., Braun D., Birkenbach M., Kieff E. Nucleotide sequences of mRNAs encoding Epstein-Barr virus nuclear proteins: a probable transcriptional initiation site. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5096–5100. doi: 10.1073/pnas.83.14.5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw S., Luce G. E., Quinones R., Gress R. E., Springer T. A., Sanders M. E. Two antigen-independent adhesion pathways used by human cytotoxic T-cell clones. Nature. 1986 Sep 18;323(6085):262–264. doi: 10.1038/323262a0. [DOI] [PubMed] [Google Scholar]
- Smith G. L., Levin J. Z., Palese P., Moss B. Synthesis and cellular location of the ten influenza polypeptides individually expressed by recombinant vaccinia viruses. Virology. 1987 Oct;160(2):336–345. doi: 10.1016/0042-6822(87)90004-3. [DOI] [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Mann K. P. Early events in Epstein-Barr virus infection provide a model for B cell activation. J Exp Med. 1985 Jul 1;162(1):45–59. doi: 10.1084/jem.162.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torsteinsdottir S., Masucci M. G., Ehlin-Henriksson B., Brautbar C., Ben Bassat H., Klein G., Klein E. Differentiation-dependent sensitivity of human B-cell-derived lines to major histocompatibility complex-restricted T-cell cytotoxicity. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5620–5624. doi: 10.1073/pnas.83.15.5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend A. R., Rothbard J., Gotch F. M., Bahadur G., Wraith D., McMichael A. J. The epitopes of influenza nucleoprotein recognized by cytotoxic T lymphocytes can be defined with short synthetic peptides. Cell. 1986 Mar 28;44(6):959–968. doi: 10.1016/0092-8674(86)90019-x. [DOI] [PubMed] [Google Scholar]
- Wallace L. E., Young L. S., Rowe M., Rowe D., Rickinson A. B. Epstein-Barr virus-specific T-cell recognition of B-cell transformants expressing different EBNA 2 antigens. Int J Cancer. 1987 Mar 15;39(3):373–379. doi: 10.1002/ijc.2910390317. [DOI] [PubMed] [Google Scholar]
- Wang F., Gregory C. D., Rowe M., Rickinson A. B., Wang D., Birkenbach M., Kikutani H., Kishimoto T., Kieff E. Epstein-Barr virus nuclear antigen 2 specifically induces expression of the B-cell activation antigen CD23. Proc Natl Acad Sci U S A. 1987 May;84(10):3452–3456. doi: 10.1073/pnas.84.10.3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L., Alfieri C., Hennessy K., Evans H., O'Hara C., Anderson K. C., Ritz J., Shapiro R. S., Rickinson A., Kieff E. Expression of Epstein-Barr virus transformation-associated genes in tissues of patients with EBV lymphoproliferative disease. N Engl J Med. 1989 Oct 19;321(16):1080–1085. doi: 10.1056/NEJM198910193211604. [DOI] [PubMed] [Google Scholar]