Abstract
Background
The influence of lifestyle diseases on postoperative complications and long-term survival in patients with non-small cell lung cancer (NSCLC) is unclear. The aim of this study was to determine whether lifestyle diseases were significant risk factors of perioperative and long-term surgical outcomes in elderly patients with stage I NSCLC.
Methods
Between December 1995 and November 2013, 110 patients aged 65 years or older who underwent surgical resection of stage I NSCLC at Dong-A University Hospital were retrospectively studied. We assessed the presence of the following lifestyle diseases as risk factors for postoperative complications and long-term mortality: diabetes, hypertension, chronic obstructive pulmonary disease, stroke, and ischemic heart disease.
Results
The mean age of the patients was 71 years (range, 65 to 82 years). Forty-six patients (41.8%) had hypertension, making it the most common lifestyle disease, followed by diabetes (n=23, 20.9%). The in-hospital mortality rate was 0.9% (n=1). The 3-year and 5-year survival rates were 78% and 64%, respectively. Postoperative complications developed in 32 patients (29.1%), including 7 (6.4%) with prolonged air leakage, 6 (5.5%) with atrial fibrillation, 5 (4.5%) with delirium and atelectasis, and 3 (2.7%) with acute kidney injury and pneumonia. Univariate and multivariate analyses showed that the presence of a lifestyle disease was the only independent risk factor for postoperative complications. In survival analysis, univariate analysis showed that age, smoking, body mass index, extent of resection, and pathologic stage were associated with impaired survival. Multivariate analysis revealed that resection type (hazard ratio [HR], 2.20; 95% confidence interval [CI], 1.08 to 4.49; p=0.030) and pathologic stage (HR, 1.89; 95% CI, 1.02 to 3.49; p=0.043) had independent adverse impacts on survival.
Conclusion
This study demonstrated that the presence of a lifestyle disease was a significant prognostic factor for postoperative complications, but not of survival, in elderly patients with stage I NSCLC. Therefore, postoperative complications may be influenced by the presence of a lifestyle disease.
Keywords: Non-small-cell lung carcinoma, Lifestyle, Postoperative complications, Survival
Introduction
Chronic diseases, frequently classified as a major component of non-communicable diseases, usually affect middle-aged or elderly individuals after prolonged exposure to an unhealthy lifestyle, which is often related to the economic transition, rapid urbanization, and 21st-century lifestyles, with behaviors such as tobacco use, harmful consumption of alcohol, an unhealthy fast food diet, insufficient physical activity, and extended working hours. Physiologically stressed lifestyles increase the prevalence of risk factors, such as hypertension, dyslipidemia, diabetes, respiratory diseases, and obesity, which act in parallel and synergistically on various metabolic pathways in the body [1]. The chronic accumulation of these adverse effects can lead to lethal disease.
The World Health Organization suggested that around 56 million deaths occurred worldwide in the year 2012, of which 38 million (68%) were due to non-communicable diseases. If cancers are excluded, ischemic heart disease (IHD), stroke, chronic obstructive pulmonary disease (COPD), and diabetes are the chief causes of deaths [2]. In the Republic of Korea, the prevalence of hypertension, diabetes, IHD, stroke, and COPD among people aged 65 years or older are 56.7%, 22.6%, 6.8%, 6.9%, and 30.2%, respectively [3].
Lifestyle factors can cause various types of cancers. The emergence of the lung cancer epidemic in the 20th century was driven by smoking. Additionally, many other risk factors of lung cancer have been reported, including diet (cured meat or deep-fried cooking), alcohol, and insufficient exercise [4]. In some countries, a decline in cigarette smoking rates resulting from strong public health policies has reduced the incidence of lung cancer, but lung cancer remains the leading cause of cancer-related mortality around the world. Numerous studies about the impact of comorbidities on the treatment of lung cancer have presented various validated scales to assess comorbidities, such as the Charlson Comorbidity Index (CCI). However, few studies have focused specifically on lifestyle diseases.
The frequency with which early-stage lung cancers are diagnosed has increased owing to advances in high-resolution computed tomography and the greater prevalence of computed tomographic screening [5]. Therefore, we performed this study to evaluate the impact of lifestyle diseases on postoperative complications and long-term survival in elderly patients with pathologic stage I non-small cell lung cancer (NSCLC).
Methods
The medical records of 110 patients who underwent curative resection for stage I NSCLC at a single institute between December 1995 and November 2013 were reviewed. All of them were 65 years or older, and underwent lobectomy or pneumonectomy with mediastinal lymph node dissection. We excluded patients with a history of other malignant disease, neoadjuvant treatment, sublobar resection, or uncommon histologic subtypes other than adenocarcinoma or squamous cell carcinoma. The pathologic stage of all tumors was assessed according to the seventh edition of the TNM (tumor-node-metastasis) classification of the International Union Against Cancer.
In all patients, the preoperative diagnostic workup included a medical history, physical examination, plain chest radiography, electrocardiography, routine laboratory tests, pulmonary function test, and computed tomography of the chest. If the tissue diagnosis was confirmed, additional staging procedures, such as a bone scan, brain magnetic resonance imaging, and positron emission tomography-computed tomography were performed.
Patients were followed with regular visits to the outpatient clinic or through telephone contact. Follow-up was completed in all patients through June 2015. The median follow-up time was 40.4 months (range, 0.4 to 222.3 months). Overall survival was measured from the date of surgery to the date of death or the last follow-up contact.
Postoperative complications were defined as any deviation from the normal postoperative course occurring in the initial 30 days after surgery. These complications included air leakage, atrial fibrillation, delirium, atelectasis, acute kidney injury (AKI), pneumonia, and acute respiratory distress syndrome (ARDS). Air leakage was defined as a leak that persisted for more than 7 days after surgery.
The relationships of the following variables with postoperative complications and overall survival were evaluated: age, sex, smoking, each individual lifestyle disease, the presence of a lifestyle disease, forced expiratory volume in 1 second, body mass index (BMI), surgical technique, operative time, tumor size, tumor site, extent of resection, pathologic stage, and histologic subtype. The following highly prevalent lifestyle-related and chronic diseases were analyzed: hypertension, diabetes, IHD, stroke, and COPD. The variable ‘presence of a lifestyle disease’ applied to patients with 1 or more of the above 5 lifestyle diseases.
Categorical data were analyzed using the chi-square test or the Fisher exact test. The Student t-test was used to analyze continuous variables. Survival curves were estimated by the Kaplan-Meier method, and compared using the log-rank test. Univariate and multivariate analyses for postoperative complications and survival were performed by means of a binary logistic regression model and a Cox proportional hazard model. Variables that had p-values <0.2 or <0.05 in the univariate analysis were selected for inclusion in the multivariate analysis of postoperative complications and overall survival, respectively. All statistical analyses were carried out in IBM SPSS Statistics ver. 20.0 (IBM Corp., Armonk, NY, USA). p-values <0.05 were considered to indicate statistical significance.
Results
Among 110 patients, there were 76 males and 34 females. Sixty-three patients (57.3%) had lifestyle diseases, and 36 patients (32.7%) were non-smokers. Their mean age was 70.7 years (range, 65 to 82 years). Adenocarcinoma (61.8%) was more frequent than squamous cell carcinoma (38.2%). Seventy-one patients (64.5%) had stage IA lung cancer, and 39 patients (35.5%) were diagnosed with stage IB disease. The following types of pulmonary resection were performed: lobectomy (87.3%) and bilobectomy or pneumonectomy (12.7%). Forty-five patients (40.9%) underwent video-assisted thoracoscopic surgery (VATS). Upper-lobe (including middle-lobe) tumors were more common (58.2%) than lower-lobe tumors (41.8%). Right-sided tumors occurred in 70 cases (63.6%), and left-sided tumors in 40 cases (36.4%). The median tumor size was 2.8 cm (range, 0.5 to 5.0 cm). Further details of the patients’ baseline characteristics are shown in Table 1.
Table 1.
Characteristics of the 110 patients with completely resected stage I non-small cell lung cancer
| Characteristic | Value |
|---|---|
| Sex | |
| Male | 76 (69.1) |
| Female | 34 (30.9) |
| Age (yr) | 70.73±3.78 |
| 65–69 | 47 (42.7) |
| 70–74 | 44 (40.0) |
| ≥75 | 19 (17.3) |
| Presence of a lifestyle disease | |
| No | 47 (42.7) |
| Yes | 63 (57.3) |
| Smoking | |
| Non | 36 (32.7) |
| Current/past | 74 (67.3) |
| FEV1, (L) | 2.21±0.59 |
| FEV1 (%) | 95.0±18.8 |
| Body mass index (kg/m2) | 23.2±2.9 |
| Surgical technique | |
| Thoracotomy | 65 (59.1) |
| Video-assisted thoracoscopic surgery | 45 (40.9) |
| Operative time (min) | 239 (95–495) |
| Tumor location | |
| Upper lobe (including middle lobe) | 64 (58.2) |
| Lower lobe | 46 (41.8) |
| Tumor laterality | |
| Right-sided | 70 (63.6) |
| Left-sided | 40 (36.4) |
| Resection type | |
| Lobectomy | 96 (87.3) |
| Bilobectomy/pneumonectomy | 14 (12.7) |
| Tumor size (cm) | 2.8 (0.5–5.0) |
| Pathologic stage | |
| IA | 71 (64.5) |
| IB | 39 (35.5) |
| Histologic type | |
| Adenocarcinoma | 68 (61.8) |
| Squamous cell carcinoma | 42 (38.2) |
Values are presented as number (%), mean±standard deviation, or mean (range).
FEV1, forced expiratory volume in the first second.
Sixty-three patients (57.3%) had lifestyle diseases. The most common lifestyle disease was hypertension (n=46, 41.8%), and the second most frequent disease was diabetes (n=23, 20.9%), followed by IHD (13.6%), COPD (9.1%), and stroke (5.5%) (Table 2).
Table 2.
Details of lifestyle diseases
| Type of lifestyle disease | No. of patients (%) |
|---|---|
| Hypertension | 46 (41.8) |
| Diabetes | 23 (20.9) |
| Ischemic heart disease | 15 (13.6) |
| Chronic obstructive pulmonary disease | 10 (9.1) |
| Stroke | 6 (5.5) |
The 30-day postoperative mortality rate was 0.9% (n=1). The cause of death was pneumonia. The number of postoperative cases of mortality was too low to identify any statistically significant associations. The details of the postoperative complications are shown in Table 3. The most common complications were air leakage (6.4%) and atrial fibrillation (5.5%), followed by delirium (4.5%), atelectasis (4.5%), AKI (2.7%), pneumonia (2.7%), and ARDS (1.8%).
Table 3.
Details of postoperative complications
| Type of complication | No lifestyle disease (n=47) | Lifestyle disease (n=63) | No. of total patients (%) |
|---|---|---|---|
| Air leak | 4 | 3 | 7 (6.4) |
| Atrial fibrillation | - | 6 | 6 (5.5) |
| Delirium | 1 | 4 | 5 (4.5) |
| Atelectasis | 1 | 4 | 5 (4.5) |
| Acute kidney injury | 1 | 2 | 3 (2.7) |
| Pneumonia | 1 | 2 | 3 (2.7) |
| Acute respiratory distress syndrome | 1 | 1 | 2 (1.8) |
| Asthma aggravation | - | 2 | 2 (1.8) |
| Wound infection | 1 | - | 1 (0.9) |
| Pulmonary edema | - | 1 | 1 (0.9) |
| No. of total patients (%) | 9 (19.1) | 23 (36.5) | 32 (29.1) |
Some patients had more than one complication.
In the univariate and multivariate analyses of postoperative complications, no lifestyle disease showed any association with postoperative complications (Table 4). Instead, the presence of a lifestyle disease was related with an increased incidence of postoperative complications (odds ratio, 2.49; 95% confidence interval [CI], 1.02 to 6.07; p=0.045). No other factors were found to be significantly related with postoperative complications.
Table 4.
Univariate and multivariate analysis of postoperative complications in 110 patients
| Variable | Postoperative complication (n=32) | ||
|---|---|---|---|
|
| |||
| Univariate | Multivariate | ||
|
|
|
||
| p-value | Odds ratio (95% confidence interval) | p-value | |
| Sex | 0.960 | ||
|
| |||
| Age | 0.777 | ||
|
| |||
| Diabetes (yes) | 0.721 | ||
|
| |||
| Hypertension (yes) | 0.265 | ||
|
| |||
| Chronic obstructive pulmonary disease (yes) | 0.151 | 0.356 | |
|
| |||
| Stroke (yes) | 0.670 | ||
|
| |||
| Ischemic heart disease (yes) | 0.130 | 0.378 | |
|
| |||
| Presence of a lifestyle disease | 0.047 | ||
|
| |||
| No | Reference | ||
|
| |||
| Yes | 2.490 (1.022–6.068) | 0.045 | |
|
| |||
| Smoking | 0.832 | ||
|
| |||
| Forced expiratory volume in the first second | 0.119 | 0.210 | |
|
| |||
| Body mass index | 0.617 | ||
|
| |||
| Surgical technique | 0.214 | ||
|
| |||
| Operation time | 0.292 | ||
|
| |||
| Tumor location | 0.062 | 0.086 | |
|
| |||
| Tumor laterality | 0.552 | ||
|
| |||
| Resection type | 1.000 | ||
|
| |||
| Tumor size | 0.929 | ||
|
| |||
| Pathologic stage | 0.880 | ||
|
| |||
| Histologic type | 0.338 | ||
Variables with a p-value of<0.2 were entered into the multivariate analysis.
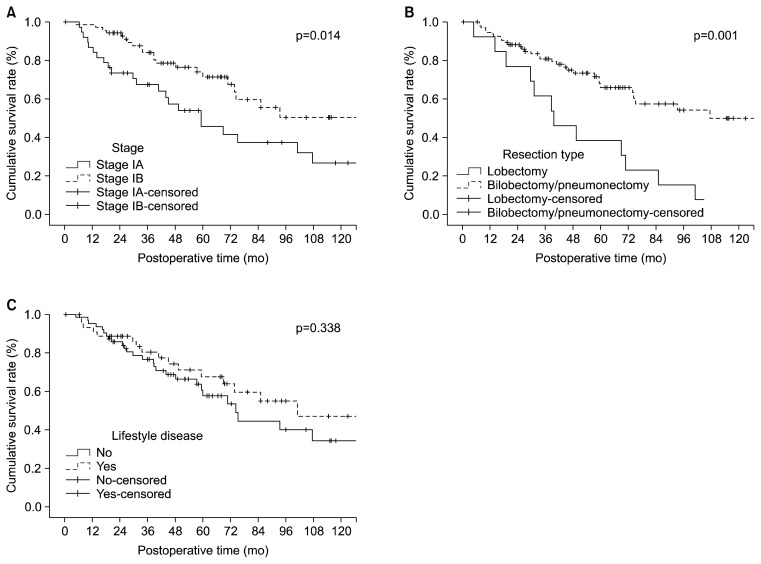
The 3-year and 5-year overall survival rates were 78.3% and 63.6%, respectively. When evaluating risk factors for overall survival in the univariate analysis, age, smoking, BMI, resection type, and stage were significantly associated with impaired survival. In the multivariate analysis, the resection type (hazard ratio [HR], 2.20; 95% CI, 1.08 to 4.49; p=0.030) and pathologic stage (HR, 1.89; 95% CI, 1.02 to 3.49; p=0.043) were independent poor prognostic factors. All of the individual lifestyle diseases and the presence of a lifestyle disease were not factors that significantly affected overall survival (Table 5). The Kaplan-Meier curves showed differences in the survival rate according to the resection type (p=0.001) and pathologic stage (p=0.014) (Fig. 1A, B). However, the overall survival curves according to the presence of a lifestyle disease showed no significant difference (p=0.338) (Fig. 1C).
Table 5.
Univariate and multivariate analysis for overall survival in 109 patients
| Variable | Survival (n=109) | ||
|---|---|---|---|
|
| |||
| Univariate | Multivariate | ||
|
|
|
||
| p-value | Hazard ratio (95% confidence interval) | p-value | |
| Sex | 0.063 | ||
|
| |||
| Age | 0.042 | 0.106 | |
|
| |||
| Diabetes (yes) | 0.364 | ||
|
| |||
| Hypertension (yes) | 0.198 | ||
|
| |||
| Chronic obstructive pulmonary disease (yes) | 0.630 | ||
|
| |||
| Stroke (yes) | 0.842 | ||
|
| |||
| Ischemic heart disease (yes) | 0.555 | ||
|
| |||
| Presence of lifestyle disease (yes) | 0.340 | ||
|
| |||
| Smoking | 0.021 | 0.055 | |
|
| |||
| Forced expiratory volume in the first second | 0.380 | ||
|
| |||
| Body mass index | 0.024 | 0.244 | |
|
| |||
| Surgical technique | 0.679 | ||
|
| |||
| Operation time | 0.494 | ||
|
| |||
| Tumor location | 0.962 | ||
|
| |||
| Tumor laterality | 0.591 | ||
|
| |||
| Resection type | 0.001 | ||
|
| |||
| Lobectomy | Reference | ||
|
| |||
| Bilobectomy/pneumonectomy | 2.201 (1.079–4.490) | 0.030 | |
|
| |||
| Pathologic stage | 0.016 | ||
|
| |||
| IA | Reference | ||
|
| |||
| IB | 1.888 (1.021–3.491) | 0.043 | |
|
| |||
| Histologic type | 0.703 | ||
|
| |||
| Postoperative complication (yes) | 0.576 | ||
Fig. 1.
(A) Overall survival curves according to the pathologic stage. (B) Overall survival curves according to the resection type. (C) Overall survival curves according to the presence of a lifestyle disease.
Discussion
Recent changes in lifestyle have increased the prevalence of lifestyle diseases. The prevalence of comorbidities, including lifestyle diseases, is higher in older patients than in younger patients, and the incidence of lung cancer is also higher in older populations [6]. Various validated scales to assess comorbidities in patients with lung cancer include the CCI, the Cumulative Illness Rating Scale for Geriatrics, the Simplified Comorbidity Score, the Adult Comorbidity Evaluation, and the Older American Resources and Services Comorbidity Scale. However, these scales may not be specific to patients with cancer or may be cumbersome to use [7]. No study has yet been carried out to e valuate the relationships between lifestyle diseases and postoperative complications or survival in patients with lung cancer. Therefore, we investigated the association between lifestyle diseases and postoperative outcomes in elderly patients with stage I NSCLC.
According to various recent studies on the comorbidities of lung cancer patients, hypertension, COPD, IHD, stroke, and diabetes are highly prevalent. These diseases are closely associated with lifestyle factors, and tend to progress to chronic conditions. Therefore, in the present study, these 5 diseases were defined as lifestyle diseases, and their frequencies were as follows: hypertension (41.8%), diabetes (20.9%), IHD (13.6%), COPD (9.1%), and stroke (5.5%).
Many studies have described the prognostic impacts of comorbidities on postoperative complications after NSCLC surgery. Pei et al. [8] reported that each lifestyle disease influenced postoperative complications. Okami et al. [9] studied the risk factors for complications after surgery in patients aged 80 years or older with clinical stage I NSCLC, and reported that the presence of comorbidities (excluding hypertension) was a factor associated with operative risks. On the contrary, Shiono et al. [10] reported that the presence of comorbidities, including lifestyle diseases, did not affect postoperative complications in elderly patients. In the present study, postoperative complications were not associated with any specific lifestyle disease, but rather with the presence of a lifestyle disease. Postoperative complications have been argued to be closely associated with the postoperative systemic inflammatory response, but the correct mechanism has not yet been clearly identified [11]. The frequency of postoperative complications is also thought to increase as the postoperative systemic inflammatory response worsens due to the operation in patients with a lifestyle disease, because these diseases are chronic inflammatory diseases. However, in this study, no statistically significant associations were observed between undergoing VATS and the incidence of postoperative complications, although VATS is generally accepted as a surgical technique that can reduce the postoperative inflammatory response. An analysis with a larger sample size may show a statistically significant association.
In summary, to prevent postoperative complications, optimization of the patient’s preoperative condition regarding lifestyle diseases, the administration of preoperative medications to relieve inflammation, and minimally invasive surgery (VATS) can be considered. By doing this, the postoperative systemic inflammatory response will be reduced, and consequently, postoperative complications will also be decreased.
The effects of comorbid conditions, including lifestyle diseases, on survival have drawn significant research interest, although the results have been inconsistent. Some studies have shown comorbidities to be independent prognostic factors for lung cancer survival [12,13]. Battafarano et al. [14] investigated the impact of comorbidities on long-term survival after surgical resection in 451 patients with stage I NSCLC. They classified the severity of comorbidities using the modified Kaplan-Feinstein index, and confirmed that patients with moderate and severe comorbidities were approximately twice as likely to die as were patients with no comorbidities. Moro-Sibilot et al. [15] showed a significant impact of comorbidities on survival after resection of 588 patients with pathologic stage I NSCLC. They showed that independent prognostic values were associated with stroke and the CCI. According to the report of Birim et al. [16], in which comorbid conditions, the CCI, and survival were assessed in patients who underwent curative resections for NSCLC, the CCI was found to be a better prognostic factor than the individual comorbid conditions. Comorbidities potentially affect lung cancer survival in several ways. Certain conditions, including COPD, stroke, and IHD were found to have an independent negative effect on survival. In addition, comorbid conditions could camouflage cancer symptoms and cause a delay in the diagnosis. Additionally, the presence of comorbidities could prevent a complete diagnostic evaluation, leading to less accurate staging. Comorbidities also influence treatment selection, preventing patients from receiving aggressive lung cancer treatment [12,17]. However, in this study, lifestyle diseases did not show a significant relationship with the survival of lung cancer patients. This result seems to arise from the features of lifestyle diseases and lung cancer. The deleterious effects of lifestyle diseases can diminish the function of vital organ systems slowly over time [12]. In contrast, lung cancer is one of the most aggressive forms of cancers, with a generally poor prognosis. The overwhelming fatality of lung cancer might offset the effect of lifestyle diseases on survival.
The biases and limitations of this study are as follow s. First, patient selection bias may have been inevitable due to the fact that this was a single-institute retrospective study. Second, the relatively small number of cases included in the analysis could limit the accuracy of the results. Third, an assessment of comorbidity severity was not been included in this study. Considering such limitations and biases, the clinical application of the presence of a lifestyle disease as a predictive variable for postoperative complications, as proposed in this study, may be controversial.
In summary, we investigated the effect of lifestyle diseases on the morbidity and overall survival of lung cancer patients in 2 ways: (1) the morbidity rate and overall survival rate were compared in lung cancer patients with and without lifestyle diseases, and (2) the morbidity rate and overall survival rate of lung cancer patients with any 1 of the 5 lifestyle diseases were compared with the corresponding rates in patients with no lifestyle diseases. The 5 lifestyle diseases were hypertension, diabetes, IHD, stroke, and COPD. The results of this study showed that the presence of a lifestyle disease was a significant prognostic factor for postoperative complications, but not of survival rates in elderly stage I NSCLC patients. Therefore, postoperative complications may be influenced by the presence of a lifestyle disease.
Acknowledgments
This study was supported by a Grant of the Samsung Vein Clinic Network (Daejeon, Anyang, Cheongju, Cheonan; Fund No. KTCS04-067).
Footnotes
This article was presented at the 47th Annual Meeting of the Korean Society for Thoracic and Cardiovascular Surgery, Jeongseon, Korea, October 22–24, 2015.
Conflict of interest
No potential conflicts of interest relevant to this article are reported.
References
- 1.Senapati S, Bharti N, Bhattacharya A. Modern lifestyle diseases: chronic diseases, awareness and prevention. Int J Curr Res Acad Rev. 2015;3:215–23. [Google Scholar]
- 2.World Health Organization. Global status report on non-communicable diseases 2014 [Internet] Geneva: World Health Organization; 2014. [cited 2016 Sep 28]. Available from: http://apps.who.int/iris/bitstream/10665/148114/1/9789241564854_eng.pdf?ua=1. [Google Scholar]
- 3.Oh YH. The health status of older Koreans and policy considerations. Health Welf Policy Forum. 2015;223:29–39. [Google Scholar]
- 4.Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83:584–94. doi: 10.1016/S0025-6196(11)60735-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okada M, Koike T, Higashiyama M, Yamato Y, Kodama K, Tsubota N. Radical sublobar resection for small-sized non-small cell lung cancer: a multicenter study. J Thorac Cardiovasc Surg. 2006;132:769–75. doi: 10.1016/j.jtcvs.2006.02.063. [DOI] [PubMed] [Google Scholar]
- 6.Janssen-Heijnen ML, Schipper RM, Razenberg PP, Crommelin MA, Coebergh JW. Prevalence of co-morbidity in lung cancer patients and its relationship with treatment: a population-based study. Lung Cancer. 1998;21:105–13. doi: 10.1016/S0169-5002(98)00039-7. [DOI] [PubMed] [Google Scholar]
- 7.Gajra A. Assessment of comorbidity in lung cancer: how, why, and in whom? J Geriatr Oncol. 2016;7:64–7. doi: 10.1016/j.jgo.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Pei G, Zhou S, Han Y, Liu Z, Xu S. Risk factors for postoperative complications after lung resection for non-small cell lung cancer in elderly patients at a single institution in China. J Thorac Dis. 2014;6:1230–8. doi: 10.3978/j.issn.2072-1439.2014.07.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okami J, Higashiyama M, Asamura H, et al. Pulmonary resection in patients aged 80 years or over with clinical stage I non-small cell lung cancer: prognostic factors for overall survival and risk factors for postoperative complications. J Thorac Oncol. 2009;4:1247–53. doi: 10.1097/JTO.0b013e3181ae285d. [DOI] [PubMed] [Google Scholar]
- 10.Shiono S, Abiko M, Sato T. Postoperative complications in elderly patients after lung cancer surgery. Interact Cardiovasc Thorac Surg. 2013;16:819–23. doi: 10.1093/icvts/ivt034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McSorley ST, Watt DG, Horgan PG, McMillan DC. Postoperative systemic inflammatory response, complication severity, and survival following surgery for colorectal cancer. Ann Surg Oncol. 2016;23:2832–40. doi: 10.1245/s10434-016-5204-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tammemagi CM, Neslund-Dudas C, Simoff M, Kvale P. Impact of comorbidity on lung cancer survival. Int J Cancer. 2003;103:792–802. doi: 10.1002/ijc.10882. [DOI] [PubMed] [Google Scholar]
- 13.Piccirillo JF, Tierney RM, Costas I, Grove L, Spitznagel EL., Jr Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004;291:2441–7. doi: 10.1001/jama.291.20.2441. [DOI] [PubMed] [Google Scholar]
- 14.Battafarano RJ, Piccirillo JF, Meyers BF, et al. Impact of comorbidity on survival after surgical resection in patients with stage I non-small cell lung cancer. J Thorac Cardiovasc Surg. 2002;123:280–7. doi: 10.1067/mtc.2002.119338. [DOI] [PubMed] [Google Scholar]
- 15.Moro-Sibilot D, Aubert A, Diab S, et al. Comorbidities and Charlson score in resected stage I nonsmall cell lung cancer. Eur Respir J. 2005;26:480–6. doi: 10.1183/09031936.05.00146004. [DOI] [PubMed] [Google Scholar]
- 16.Birim O, Kappetein AP, Bogers AJ. Charlson comorbidity index as a predictor of long-term outcome after surgery for nonsmall cell lung cancer. Eur J Cardiothorac Surg. 2005;28:759–62. doi: 10.1016/j.ejcts.2005.06.046. [DOI] [PubMed] [Google Scholar]
- 17.Islam KM, Jiang X, Anggondowati T, Lin G, Ganti AK. Comorbidity and survival in lung cancer patients. Cancer Epidemiol Biomarkers Prev. 2015;24:1079–85. doi: 10.1158/1055-9965.EPI-15-0036. [DOI] [PubMed] [Google Scholar]