Abstract
Kimura disease (KD) is an immune-mediated chronic inflammatory disease of unknown etiology. KD has many complications associated with hypereosinophilia, including various forms of allergic reactions and eosinophilic lung disease. Additionally, hypereosinophilia is associated with hypercoagulability, which may lead to thromboembolic events. A 36-year-old man with KD presented with acute limb ischemia and coronary artery occlusion. He underwent thrombectomy, partial endarterectomy of both popliteal arteries, and coronary artery stent insertion. KD is a systemic disease that affects many organs and presents with thromboembolism and vasculitis. In a patient with KD, physicians should evaluate the vascular system, including the coronary arteries.
Keywords: Angiolymphoid hyperplasia with eosinophilia, Acute limb ischemia, Coronary artery stenosis
Case report
A 36-year-old man visited the emergency department of Inje University Haeundae Paik Hospital with severe pain in both lower limbs for 1 day. A physical examination revealed pale, pulseless, and cold lower limbs. He had sensory changes and foot drop of the right lower limb.
One year ago, he had been diagnosed with Kimura disease (KD) after excision of a right upper limb mass (Fig. 1) at another hospital. He had been taking oral steroids but stopped doing so 6 months ago.
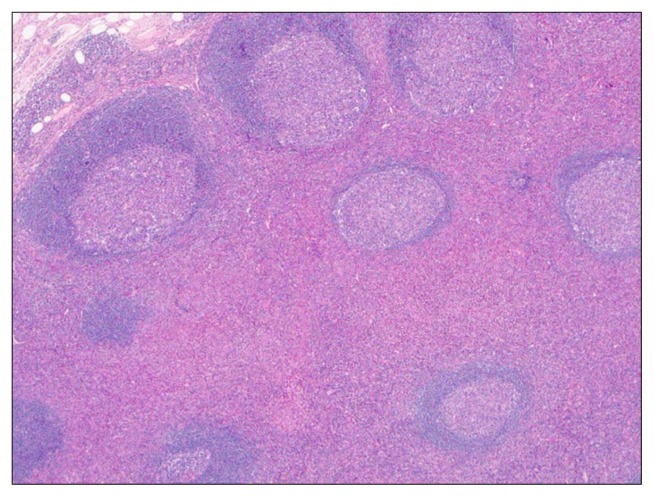
Fig. 1.
Lymphoid follicles with germinal centers are surrounded by a prominent eosinophilic infiltration (H&E, ×40).
The initial laboratory findings were as follows: leukocyte count of 45.5×109/L with 74% eosinophils, elevated serum fibrinogen degradation product (FDP) level (18.7 μg/mL), elevated D-dimer level (5.1 μg/mL), and extremely elevated serum immunoglobulin (Ig) E (>2,500 IU/dL). A computed tomography (CT) angiogram showed total occlusion of both popliteal arteries (PAs) (Fig. 2A).
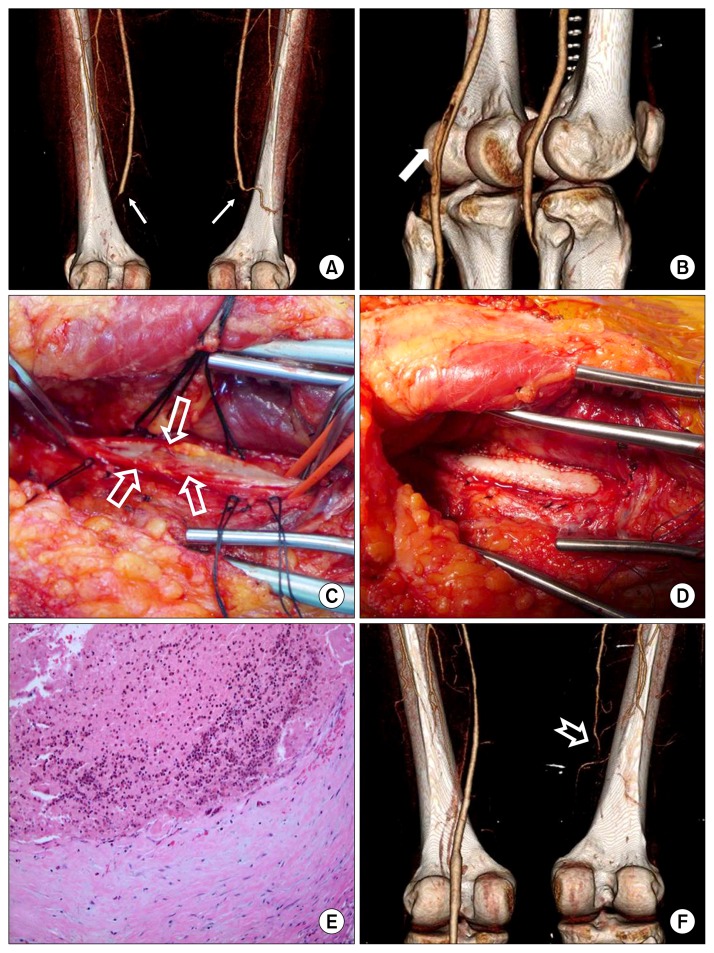
Fig. 2.
(A) A preoperative CT angiogram shows total occlusion of both PAs (line arrow). (B) CT angiogram on postoperative day 3 shows a diffuse filling defect (white arrow) in the left PA. (C) A narrowing lesion of PA (empty arrow). (D) The left PA was reconstructed using bovine pericardium (Vascu-Guard Biovascular Inc., St. Paul, MN, USA). (E) The thrombus occludes the vessel and contains a prominent number of eosinophils. The intima is thickened with a fibroblastic proliferation (H&E, ×200). (F) CT angiogram 56 days after the second operation shows the occlusion of the right superficial femoral artery and PA (notched arrow). CT, computed tomography; PA, popliteal artery.
Under the presumptive diagnosis of acute limb ischemia (ALI), the patient underwent an emergency thrombectomy of both femoral arteries using a Fogarty catheter (Edwards Laboratories, Santa Ana, CA, USA). After the operation, the pain and color changes resolved, but the right foot drop remained. To prevent thromboembolic events, he was treated with low-molecular-weight heparin followed by warfarin.
The patient was evaluated to determine the causes of ALI with hypercoagulability and eosinophilia. No personal or family history of thromboembolism or hematological disorders was reported. Immunochemical tests for autoimmune diseases, including lupus anticoagulant, anticardiolipin IgG and IgM antibodies, anti-beta2-glycoprotein IgG and IgM, and anti-phospholipid antibodies, were negative.
No parasite infections were found. The antithrombin III level and protein C activity were within reference ranges. Protein S activity was low, but the levels of the total and free protein S antigen were normal, suggesting a type-II protein S deficiency. However, this was more likely an effect of warfarin and postoperative systemic changes. An echocardiogram was performed; it revealed no evidence of a cardiac thrombus or decreased systolic function of the left ventricle (ejection fraction=47%). Under the presumptive diagnosis of KD-related ALI, the patient was administered steroid therapy.
A follow-up CT angiogram on postoperative day (POD) 3 showed a filling defect on the left PA (Fig. 2B); the ankle–brachial index (ABI) was 0.87 on the right side and 0.62 on the left. Coronary angiography (CAG) on POD 5 showed triple-vessel disease with chronic total occlusion of the left anterior descending artery (LAD), left circumflex artery (LCX), proximal obtuse marginal artery, and posterior descending artery (Fig. 3). A percutaneous coronary balloon dilatation was performed. Additionally, the patient was treated with aspirin and clopidogrel. Stent insertion for the coronary artery was delayed because we needed to observe the KD activity and plan the second operation for the left PA.
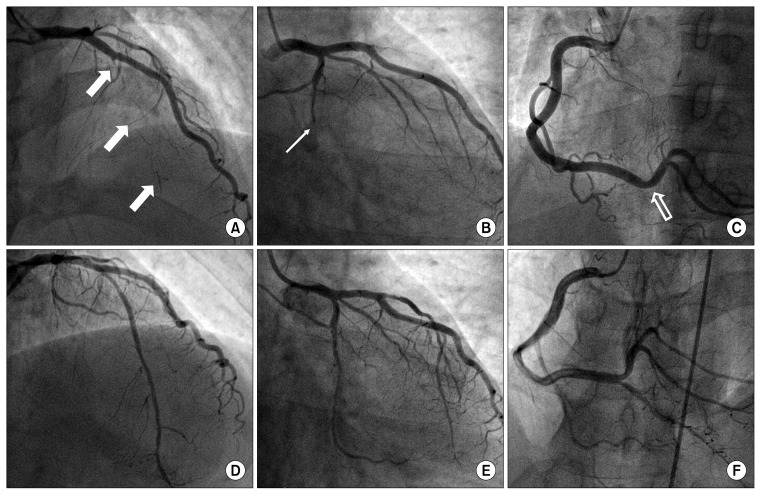
Fig. 3.
Coronary angiography. (A–C) Coronary angiography before the stent insertion and balloon dilation shows chronic total occlusion of the left anterior descending artery (white arrow), left circumflex artery (line arrow), and posterior descending artery (empty arrow). (D–F) Coronary angiography after stent insertion and balloon dilation.
We performed the second operation on POD 17 because of the low ABI of the left lower limb. The left PA was exposed via a posterior approach. A thrombus and a hypertrophic, injured intimal layer were observed after the longitudinal arteriotomy (Fig. 2C). However, unexpectedly, no atherosclerotic changes were detected in the PA. After the removal of the thrombus and the injured intimal layer, a patch angioplasty using bovine pericardium (Vascu-Guard Biovascular Inc., St. Paul, MN, USA) was performed (Fig. 2D). After 3 days, the pathologist reported the following findings (Fig. 2E): (1) Thrombosis occluding the vessel lumen and intimal thickening with fibroblastic proliferation (the intima showed reactive changes with fibroblastic proliferation) were observed. (2) The thrombotic material contained a prominent number of eosinophils, and mixed inflammatory cell infiltration composed of eosinophils and lymphocytes was observed in the vessel wall. (3) No evidence of atherosclerosis was seen.
Two months after the first discharge, the patient returned to the emergency department with right lower extremity pain and a color change that had developed the day before. A CT angiogram showed total occlusion from the right superficial femoral artery to the distal PA (Fig. 2F).
We performed an emergency thrombectomy, partial endarterectomy, and patch angioplasty on the right side. The operative findings on the right side were the same as the findings on the left side. The follow-up CT angiogram showed improved vascular patency.
Follow-up CAG showed total occlusion of the LAD and the LCX. A coronary stent was inserted into the LAD, and the LCX was balloon dilated (Fig. 3).
The patient was then treated with aspirin, clopidogrel, and cilostazol for preventing in-stent thrombosis and prednisolone for KD. His peripheral blood eosinophil and IgE levels normalized. No evidence of recurrence was observed at the 6-month follow-up.
Discussion
KD is characterized by marked eosinophilia, an elevated IgE level, and recurrent subcutaneous nodules around the head and neck.
The pathogenesis of KD has not been clearly established. However, the increased serum IgE level and eosinophilia suggest an allergic disease [1–4]. Despite an unclear mechanism, hypereosinophilia can lead to a hypercoagulable condition and vasculitis according to some published reports [5–7]. A possible mechanism is that the cytokines secreted by eosinophils, including eosinophil cationic protein, eosinophil peroxidase, and major basic protein, activate platelet aggregation. Some researchers have demonstrated that human eosinophils contain various amounts of the tissue factor (TF) and have concluded that relatively high TF expression in patients with hypereosinophilia may contribute to an increased thrombotic risk [7]. In this case, KD presents with organ ischemia.
A standard treatment for KD has not been established thus far. However, KD has been treated with local mass excision, steroids for systemic inflammation, revascularization, and anticoagulation for organ ischemia [1,2,5]. Some researchers have reported that radiotherapy was a successful treatment for KD [4].
In our patient, arterial stenosis and hypercoagulability due to chronic vasculitis and hypereosinophilia were causes of ALI. We performed an emergency thrombectomy. Furthermore, we performed a partial endarterectomy and patch angioplasty for revascularization. Additionally, the patient was administered prednisolone to control the activity of KD. His serum leukocyte and eosinophil counts and FDP and serum IgE levels normalized. Many revascularization methods are available for ALI, including thrombectomy, stent insertion, patch angioplasty, and extra-anatomic bypass with a synthetic or saphenous vein graft. Patch angioplasty is more effective for removing a thrombus and widening the lumen than a stent and is easier than a bypass. CAG to evaluate other forms of vasculitis revealed coronary artery obstructive disease (CAOD), and we inserted a coronary stent instead of performing a bypass. Because of the patient’s young age, coronary artery bypass grafting was the treatment of choice for CAOD. However, he refused to undergo another operation. The mechanism of CAOD in our patient was different from that of CAOD in other cases. However, antiplatelet therapy was needed, and the patient required long-term follow-up.
In conclusion, KD is a systemic disease that affects many organs and presents with thromboembolism and vasculitis. In a patient with KD, physicians should evaluate the vascular system, including the coronary arteries.
Footnotes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
References
- 1.Ishii M. Kimura’s disease: a review of 429 cases and four new cases. Oto Rhino Laryngol Tokyo. 1982;25:407–15. [Google Scholar]
- 2.Chen H, Thompson LD, Aguilera NS, Abbondanzo SL. Kimura disease: a clinicopathologic study of 21 cases. Am J Surg Pathol. 2004;28:505–13. doi: 10.1097/00000478-200404000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Koh H, Kamiishi N, Chiyotani A, et al. Eosinophilic lung disease complicated by Kimura’s disease: a case report and literature review. Intern Med. 2012;51:3163–7. doi: 10.2169/internalmedicine.51.8600. [DOI] [PubMed] [Google Scholar]
- 4.Chang AR, Kim K, Kim HJ, Kim IH, Park CI, Jun YK. Outcomes of Kimura’s disease after radiotherapy or non-radiotherapeutic treatment modalities. Int J Radiat Oncol Biol Phys. 2006;65:1233–9. doi: 10.1016/j.ijrobp.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 5.Lee J, Hong YS. Kimura disease complicated with bowel infarction and multiple arterial thromboses in the extremities. J Clin Rheumatol. 2014;20:38–41. doi: 10.1097/RHU.0000000000000064. [DOI] [PubMed] [Google Scholar]
- 6.Davoine F, Lacy P. Eosinophil cytokines, chemokines, and growth factors: emerging roles in immunity. Front Immunol. 2014;5:570. doi: 10.3389/fimmu.2014.00570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cugno M, Marzano AV, Lorini M, Carbonelli V, Tedeschi A. Enhanced tissue factor expression by blood eosinophils from patients with hypereosinophilia: a possible link with thrombosis. PLoS One. 2014;9:e111862. doi: 10.1371/journal.pone.0111862. [DOI] [PMC free article] [PubMed] [Google Scholar]