Abstract
Introduction
Among conventional hemodialysis (CHD) patients, carbamylated serum albumin (C-Alb) correlates with urea and amino acid deficiencies and is associated with mortality. We postulated that reduction of C-Alb by intensive HD may correlate with improvements in protein metabolism and cardiac function.
Methods
One-year observational study of in-center nocturnal extended hemodialysis (EHD) patients and CHD control subjects. Thirty-three patients receiving 4-hour CHD who converted to 8-hour EHD were enrolled, along with 20 controls on CHD. Serum C-Alb, biochemistries, and cardiac MRI parameters were measured before and after 12 months of EHD.
Findings
EHD was associated with reduction of C-Alb (average EHD change −3.20mmol/mol [95% CI −4.23, −2.17] compared to +0.21 [95% CI −1.11, 1.54] change in CHD controls, P<0.001). EHD was also associated with increases in average essential amino acids (in standardized units) compared to CHD (+0.38 [0.08, 0.68 95%CI]) vs. −0.12 [−0.50, 0.27, 95% CI], P=0.047). Subjects who reduced C-Alb more than 25% were found to have reduced left ventricular mass, increased urea reduction ratio, and increased serum albumin compared to nonresponders, and % change in C-Alb significantly correlated with % change in left ventricular mass.
Discussion
EHD was associated with reduction of C-Alb as compared to CHD, and reduction of C-Alb by EHD correlates with reduction of urea. Additional studies are needed to test whether reduction of C-Alb by EHD also correlates with improved clinical outcomes.
Keywords: Carbamylated albumin, carbamylation, cardiac hypertrophy, extended duration hemodialysis, nocturnal hemodialysis, uremia
INTRODUCTION
Patients with end stage renal disease (ESRD) treated with standard duration (four hours per session, three sessions per week) facility-based conventional hemodialysis (CHD) suffer from disproportionately reduced quality of life (QOL) and mortality, a figure that has changed very little over the last decade.1,2 Increasing evidence suggests that extended (increased frequency and/or duration) hemodialysis (EHD) delivered either at home (H-EHD) or within a facility (FEHD) is associated with improved clinical outcomes for patients with ESRD including: improvements in blood pressure (BP) control, endothelial function, and left ventricular geometry.3–7 Other clinical benefits of EHD include superior anemia and phosphate control, and improvements in sleep disorders, and fertility and pregnancy outcomes.8–12 Uncontrolled data from centers in France that have been providing F-EHD (8 hours three times weekly or more) for decades show dramatically lower mortality rates compared to those observed in centers providing CHD; a finding that has been observed in other countries.13
From a mechanistic perspective, enhanced removal of uremic toxins has been one of the proposed explanations for the improved outcomes seen with the use of EHD as compared to CHD. One of the uremic toxins that is reduced by EHD is urea itself, and there is growing evidence that the mechanism of urea’s toxicity is by direct chemical modification of proteins (carbamylation).14 Protein carbamylation is the process whereby nonenzymatic binding of urea-derived cyanate occurs to free amino groups on proteins.15 As kidney function declines, the carbamylation of proteins increases, and these structural changes to proteins have been implicated in the pathogenesis and progression of various diseases via its perturbations on protein function.16 Moreover, chronically elevated urea promotes carbamylation of proteins in patients with chronic kidney disease. One specific biomarker for global protein carbamylation is carbamylated serum albumin (C-Alb). The accumulation of C-Alb has been shown to be correlated with both blood urea concentrations, 17 suggesting that this is an indicator of time-averaged urea akin to hemoglobin A1c as an indicator of time-average blood glucose concentrations. In addition to proteins, free amino acids (AA) are also targets for carbamylation and may play an important protective role in preventing protein carbamylation. Low AA levels have been shown to be correlated with higher C-Alb in ESRD patients. Animals on low protein diets increase their protein carbamylation dramatically, and patients treated with AA supplements have been shown to decrease their C-Alb.17,18 Thus it has been proposed that C-Alb measurements represent a combined indicator for both high blood urea and AA deficiencies in patients with kidney disease.14
In addition to its correlation with uremia, high C-Alb values in patients receiving CHD have also been shown to be associated with increased risk of erythropoietin resistance, heart failure, sudden cardiac death, and all-cause mortality.17,19,20 Interestingly, although high C-Alb was a risk factor for death in these studies, high blood urea and low Kt/V measures were not.
Because protein carbamylation is strongly correlated with blood urea concentrations, one might speculate that EHD could reduce overall protein carbamylation, and that measurement of a circulating carbamylated protein may be a useful therapeutic target for dialysis optimization. Moreover, better characterization of the relationship between protein carbamylation and the use of EHD may further enhance our understanding regarding the mechanisms underlying the impact of intensive HD on global improvements in patient outcomes. Therefore, in the present report, our primary objective was to understand if EHD as compared to conventional hemodialysis has an impact on protein carbamylation as measured by the changes in C-Alb. We postulated that intensification of HD would be associated with a reduction of C-Alb, and that this may be in part mediated by concomitant decreases in urea and increases in serum AA levels.
MATERIALS AND METHODS
Participants
Fifty-two week prospective observational study of 33 patients that had previously received standard duration conventional hemodialysis (CHD; 3–4 hours per session, 3 times per week) for a minimum of 90 days prior to recruitment who had elected to convert to in-center nocturnal hemodialysis (EHD; 7–8 hours per session, 3 times per week). A separate group of 20 patients who were maintained on CHD with no anticipated conversion to EHD and who were willing to undergo CMR were recruited as a nonrandomized control group. EHD was performed at St. Michael’s Hospital (Toronto, Canada) and St. Paul’s Hospital (Vancouver, Canada). In the absence of clinical practice guidelines to inform indications for EHD, primary reasons for conversion to EHD included refractory hyperphosphatemia, intradialytic hypotension on CHD limiting volume removal, labile blood pressure on CHD, and preservation of employment opportunities. The patient and treating nephrologist made the decision to convert to EHD jointly. This investigation was a substudy of a clinical trial whose primary goal was to analyze changes in left ventricular mass (LVM) associated with EHD treatment; the results of the original trial are reported in a separate manuscript. Only subjects who were willing and able to have a cardiac magnetic resonance imaging (CMR) study were eligible for the study, and exclusion criteria were serious comorbidity with life expectancy <1 year, planned kidney transplant from a live donor in the coming year, contraindications to CMR, and confirmed pregnancy. The Research Ethics Boards of each site approved the study and all study participants provided written informed consent.
Administration of dialysis therapies
EHD was administered three times per week, 7 to 8 hours per session. Blood flow was 300 mL/min and dialysate flow 500 mL/min. Dialysis machines (Phoenix Hemodialysis Systems from Gambro, Inc., Richmond Hill, ON were used at St. Michael’s Hospital; Dialog+ Hemodialysis Systems from B. Braun, Inc., Bethlehem, PA were used at St. Paul’s Hospital.) and dialyzers (Xenium 210, Baxter Healthcare Corp., McGraw Park, IL used at St. Michael’s Hospital; and Rexeed 21S, Asahi, Memphis, TN used at St. Paul’s Hospital) were not changed following EHD conversion. All patients in the CHD arm continued on their previous dialysis prescription. All fundamental aspects of HD care conformed to prevailing guidelines and did not differ by study arm or by study site.
Study follow-up and data collection
Each patient was followed for 52 weeks, which commenced on the date of the first EHD session and the date of CMR for those remaining on CHD. The 52-week follow-up period was preceded by a 12-week “baseline period” during which all patients were on CHD. During this time, comorbidities were characterized, medications and erythropoietin regimen was recorded, baseline and follow-up CMR measurements were performed, and blood was drawn and plasma was isolated and frozen at −80°C at baseline and end of study for research purposes. All CMR examinations were performed with a 1.5 T scanner using a phased-array cardiac coil and retrospective vectorocardiographic gating. A standardized protocol which was used for measurement of LV mass has been previously published.21 All CMR postprocessing and LVM measurements were performed offline by a blinded experienced reader (ATY). The average values for all of the following parameters were recorded during the baseline period from samples drawn as part of routine clinical care: hemoglobin, phosphate, calcium, albumin, intact parathyroid hormone, and percent reduction of urea. Dialysis session duration, blood flow, dialysate composition, predialysis systolic and diastolic blood pressure, ultrafiltration volume and interdialytic weight gain were captured for each dialysis session and averaged over the 12-week baseline and end-of-study periods, respectively, for each patient.
Study outcomes
The primary outcome for this study was the change in carbamylated albumin (C-Alb) between baseline and end of study. Exploratory outcomes included change in serum amino acid concentrations (AAs) and the correlation between the change in C-Alb and change in left ventricular mass measured. Serum C-Alb, and free AA concentrations were measured by isotope-dilutional high performance liquid chromatography and tandem mass spectrometry using previously described methods.17 In order to account for individual differences in serum albumin concentrations and to conform to the International System of Units (SI),22 C-Alb is reported in terms of mmoles of carbamylated albumin per mole of total albumin (mmole/mole). Absolute concentrations of C-Alb (in micromoles/L) were calculated from C-Alb mmol/mol ratio values multiplied by serum total albumin concentrations measured by colorimetric assay. C-Alb assay coefficient of variation was 1.9%. Fifty-three patients with available baseline and end-of-study period C-Alb values were included in the final analysis. Patient predialysis serum samples collected at baseline and after completion of the 52 week were also analyzed for serum free AAs in order to compare the effects of EHD vs. CHD on changes in blood urea, AA levels, and protein carbamylation. Predialysis and postdialysis serum samples collected at baseline and 12 months were analyzed for blood urea concentrations; approximate estimates of time-averaged concentrations of urea (TAC urea) were calculated by taking the average of predialysis and postdialysis urea concentrations from baseline and 12 month visits. All CMR examinations were performed with a 1.5 T scanner using a phased-array cardiac coil and retrospective vectorocardiographic gating. A published standardized protocol21 performed by a blinded experienced reader (ATY) was used for measurements of LVM.
Statistical analysis
Patients were analyzed according to their initially assigned treatment group irrespective of subsequent changes between EHD and CHD (intention to treat); analyses included all patients enrolled who had samples available at baseline and at 12 months. During the study, two patients assigned to CHD opted to convert to EHD and two other patients assigned to EHD reverted to CHD; these patients were analyzed according to their assigned treatment group. Baseline characteristics of the patients were compared according to treatment group with the use of independent samples t-tests or Mann-Whitney U tests for continuous variables and Fisher’s exact tests and chi-squared tests for categorical variables. For calculation of aggregate average essential and nonessential AA, standardized units (Z-scores) were calculated for individual AA concentrations by subtracting the overall mean AA concentration value from the raw value for each AA, and dividing that result by the standard deviation of the AA for all patients. Changes in standardized AA concentrations were then calculated by subtracting baseline Z-score values from end-of-study Z-score values, and mean change in all essential and nonessential AA were calculated for each subject. Univariate general linear models (GLM) were utilized to analyze differences in blood urea and AA values as well as C-Alb. The results of GLM are summarized as means and 95% confidence intervals. For models predicting absolute change in C-Alb, additional terms were added to multivariable GLMs as follows: (1) baseline albumin, and (2) baseline albumin, baseline C-Alb, age, length of dialysis/vintage, cause of ESRD, and history of coronary artery disease (CAD). Pearson correlation coefficients were used to examine the associations between baseline and end of study C-Alb and blood urea concentrations, percent change in C-Alb and LVM, and baseline and percent change in C-Alb. For analysis of change in C-Alb versus change in LVM, as the interaction between treatment group and percent change C-Alb was not significant (P=0.21), only the overall association is shown. Statistical analyses were conducted with the use of SAS software, version 9.4 (SAS Institute). Two-tailed P values of less than 0.05 were considered to indicate statistical significance.
RESULTS
Baseline characteristics of subjects are shown in Table 1. Compared to the control patients receiving CHD, a greater proportion of patients converting to EHD had diabetes mellitus as their cause of kidney disease, more EHD patients were being dialyzed via central venous catheter access instead of a permanent AV access, and EHD patients had a shorter dialysis vintage. EHD subjects also had lower baseline urea reduction rates and lower serum albumin levels.
Table 1.
Baseline characteristics of the study population
| EHD (n=33) | Controls (n=20) | P value | |
|---|---|---|---|
| Demographics | |||
| Age (years) | 57.8±11.0 | 53.7±12.8 | 0.22 |
| Male gender (%) | 17 (51.5) | 12 (60.0) | 0.58 |
| Ethnicity (%) | ND | ND | 0.48 |
| Caucasian | 13 (40.6) | 4 (20.0) | ND |
| African-Canadian | 6 (18.8) | 5 (25.0) | ND |
| Asian | 4 (12.5) | 4 (20.0) | ND |
| Other | 9 (28.1) | 7 (35.0) | ND |
| Cause of ESRD diabetes (%) | 16 (48.5) | 4 (20.0) | 0.046* |
| Baseline access type line (%) | 18 (54.6) | 5 (25.0) | 0.048* |
| Length of dialysis (months) | 19.9 (8.4, 56.1) | 50.8 (24.4, 109.3) | 0.01* |
| Height (cm) | 167.8±11.7 | 164.5±10.5 | 0.33 |
| Weight (kg) | 80.6±21.2 | 69.9±18.4 | 0.09 |
| Body mass index (kg/m2) | 28.4±6.1 | 25.5±5.0 | 0.11 |
| Erythropoietin use (%) | 32 (97.0) | 17 (85.0) | 0.15 |
| Left ventricular mass (g/m2.7) | 136.2±42.2 | 115.6±30.9 | 0.06 |
| Urea reduction ratio (%) | 72±9 | 77±6 | 0.04* |
| Medical History | |||
| Coronary artery disease (%) | 10 (30.3) | 2 (10.0) | 0.10 |
| Cardiovascular disease (%) | 2 (6.1) | 2 (10.0) | 0.63 |
| Cancer (%) | 4 (12.5) | 3 (15.0) | 1.00 |
| Diabetes mellitus (%) | 19 (57.6) | 6 (30.0) | 0.09 |
| Peripheral vascular disease (%) | 5 (15.2) | 3 (15.0) | 1.00 |
| Transplant (%) | 3 (9.1) | 6 (30.0) | 0.07 |
| Medications | |||
| Antiplatelet agents | 20 (54.1) | 19 (63.3) | 0.44 |
| ACE inhibitor/ARB | 27 (73.0) | 22 (73.3) | 0.97 |
| Calcium channel blocker | 18 (48.7) | 6 (20.0) | 0.02 |
| β-Blocker | 18 (48.7) | 17 (56.7) | 0.51 |
| Statin | 19 (51.4) | 12 (40.0) | 0.35 |
| Calcium-containing phosphate binder | 28 (75.7) | 23 (76.7) | 0.92 |
| Sevelamer | 8 (21.6) | 8 (26.7) | 0.63 |
| Biochemical | |||
| Urea reduction ratio (%) | 72±9 | 77±6 | 0.04 |
| Serum albumin (g/L) | 34 (28, 40) | 38 (30, 43) | 0.002* |
| Parathyroid hormone (pmol/L) | 36.1 (19.8, 49.0) | 55.2 (26.7, 95.5) | 0.13 |
| Alkaline phosphatase (U/L) | 87.3 (67.0, 137.0) | 102.5 (65.3, 149.8) | 0.71 |
| Ferritin (ug/L) | 339.0 (218.0, 517.5) | 390.0 (295.0, 815.0) | 0.27 |
| Transferrin saturation | 0.19 (0.16, 0.26) | 0.23 (0.21, 0.26) | 0.28 |
| Hemoglobin A1c (%) | 6 (6, 8) | 6 (5, 7) | 0.06 |
ARB=angiotensin II receptor blocker; ESRD=end stage renal disease; ND=no data obtained.
Table presents subjects’ characteristics as mean±standard deviation or median (quartile 1, quartile 3) or n (%);
Significant at P<0.05.
Table 2 shows average values for C-Alb and other uremic indices amongst subjects assigned to EHD compared to controls measured at baseline and 12 months, as well as the average 12 month changes within individual subjects. As shown, baseline C-Alb, predialysis urea concentrations, and all other uremic indicators were equivalent between the two treatment groups. Predialysis and postdialysis blood urea, time-averaged concentrations of urea, and C-Alb values (both absolute concentrations and proportional concentrations of carbamylated albumin per mole total albumin) all decreased significantly after 12 months of EHD, whereas controls remained relatively unchanged during the 12 months study. Furthermore, as shown in Table 3, cross-sectional measurements of C-Alb and blood urea concentrations were strongly correlated at baseline and 12 months in both treatment groups. More importantly, amongst the patients on EHD it was found that the 12 month changes in blood urea concentrations were strongly correlated with changes in C-Alb values. Note that the decreases in C-Alb associated with EHD and their correlation to blood urea concentrations were observed for both absolute concentrations of C-Alb (in micromoles/L) as well as C-Alb values reported as mmoles of carbamylated albumin per mole of total albumin (Tables 2 and 3). Lastly, patients on EHD also experienced increases in many essential and nonessential AAs, whereas patients on CHD tended to decrease their AA concentrations (Table 4). No significant change in total serum albumin was seen in either group. Note that 3 of the subjects assigned to EHD reverted to conventional HD, and two of the control subjects changed to EHD during the study. When the results were reanalyzed after grouping subjects according to how they were actually treated, the changes in blood urea and C-Alb in subjects in EHD compared to control groups were essentially unchanged whether using as-treated or intention to treat analysis (Supporting Information Table S1).
Table 2.
C-Alb and Blood urea nitrogen concentrations before and after 12 months of EHD treatment
| EHD | Controls | P value | |
|---|---|---|---|
| Baseline values | |||
| C-Alb (mmol per mole total albumin) | 11.0±4.3 | 9.8±3.0 | 0.26 |
| C-Alb (absolute conc. in μmol/L) | 5.7±2.2 | 5.7±1.9 | 0.94 |
| Pre-HD urea | 68.4±15.7 | 68.1±15.6 | 0.96 |
| Post-HD urea | 19.4±8.8 | 15.2±5.6 | 0.095 |
| TAC Urea | 43.9±11.5 | 41.7±10.0 | 0.52 |
| nPCR | 1.03±0.18 | 1.10±0.21 | 0.28 |
| 12 month values | |||
| C-Alb (mmol per mole total albumin) | 7.8±2.7 | 10.0±2.7 | 0.010 |
| C-Alb (absolute conc. in μmol/L) | 4.2±1.8 | 5.8±1.8 | 0.005 |
| Pre-HD urea | 61.0±16.9 | 72.6±18.7 | 0.037 |
| Post-HD urea | 9.9±6.3 | 16.8±6.7 | 0.001 |
| TAC Urea | 35.4±10.4 | 44.7±11.9 | 0.009 |
| nPCR | 1.07±0.25 | 1.14±0.25 | 0.31 |
| 12 month changes | |||
| C-Alb (mmol per mole total albumin) | −3.2±3.3 | 0.2±2.1 | <0.001 |
| C-Alb (absolute conc. in μmol/L) | −1.5±1.4 | 0.1±1.2 | <0.001 |
| Pre-HD urea | −7.7±18.0 | 5.8±14.5 | 0.015 |
| Post-HD urea | −10.4±8.8 | 1.6±3.4 | <0.001 |
| TAC Urea | −9.0±12.3 | 3.7±8.6 | 0.001 |
| nPCR | 0.04±0.23 | 0.07±0.20 | 0.70 |
Controls=standard duration HD; EHD=in-center HD; nPCR=normalized protein catabolic rate. Table shows mean±standard deviation. BUN units reported in mg/dL.
Table 3.
Pearson correlations between C-Alb and blood urea measurements and their absolute changes after 12 months of EHD treatment
| EHD | Controls | |||
|---|---|---|---|---|
|
|
|
|||
| r | P value | r | P value | |
| Baseline C-Alb (mmol/mol) vs. baseline pre-HD urea | 0.60 | <0.001 | 0.75 | <0.001 |
| Baseline C-Alb (absolute conc. in μmol/L) vs. baseline pre-HD urea | 0.56 | <0.001 | 0.80 | <0.001 |
| Baseline C-Alb (mmol/mol) vs. baseline TAC Urea | 0.61 | <0.001 | 0.71 | <0.001 |
| 12 month C-Alb (mmol/mol) vs. 12 month pre-HD urea | 0.33 | 0.09 | 0.63 | 0.006 |
| 12 month C-Alb (absolute conc. in μmol/L) vs. 12 month pre-HD urea | 0.20 | 0.26 | 0.50 | 0.02 |
| 12 month C-Alb (mmol/mol) vs. 12 month TAC urea | 0.32 | 0.074 | 0.60 | 0.004 |
| 12 month C-Alb (mmol/mol) vs. 12 month pre-HD urea | 0.33 | 0.09 | 0.63 | 0.006 |
| 12 month change in C-Alb (absolute conc. in μmol/L) vs. change in pre-HD urea | 0.60 | <0.001 | 0.17 | 0.45 |
| 12 month change in C-Alb (mmol/mol) vs. change in TAC Urea | 0.57 | 0.001 | −0.9 | 0.71 |
EHD=in-center HD; Controls=standard duration HD. BUN units reported in mg/dL. Except where noted, all C-Alb analyses were in terms of units of mmole of carbamylated albumin per mole total albumin.
Table 4.
Average changes in clinical indicators and serum biochemistries by EHD treatment group
| EHD (n=33) | Controls (n=20) | P value* | |
|---|---|---|---|
| Left ventricular mass (g) | −8.2 (−18.5, 2.1) | +13.6 (0.3, 27.0) | 0.01* |
| Albumin (g/L) | +0.55 (−0.84, 1.94) | −0.40 (−2.18, 1.38) | 0.40 |
| Amino acids | |||
| Valine (μmol/L) | +28.6 (10.2, 47.0) | −2.9 (−26.5, 20.8) | 0.04* |
| Isoleucine (μmol/L) | +24.2 (9.4, 39.0) | +0.1 (−19.0, 19.1) | 0.049* |
| Methionine (μmol/L) | +4.6 (−0.01, 9.3) | −2.0 (−7.9, 4.0) | 0.09 |
| Threonine (μmol/L) | +29.6 (−4.9, 64.2) | −26.7 (−71.1, 17.7) | 0.049* |
| Lysine (μmol/L) | −12.5 (−49.7, 24.6) | −26.1 (−73.8, 21.6) | 0.65 |
| Leucine (μmol/L) | +14.7 (2.6, 26.8) | +0.2 (−15.3, 15.7) | 0.15 |
| Histidine (μmol/L) | +8.2 (−2.5, 18.9) | +1.6 (−12.2, 15.3) | 0.45 |
| Phenylalanine (μmol/L) | +7.1 (−1.8, 15.9) | −4.5 (−15.8, 6.8) | 0.11 |
| Glutathione (μmol/L) | +0.103 (−0.014, 0.220) | −0.145 (−0.295, 0.005) | 0.01* |
| Average essential AAsa | +0.38 (0.08, 0.68) | −0.12 (−0.50, 0.27) | 0.047* |
| Average nonessential AAsa | +0.31 (0.04, 0.67) | −0.20 (−0.54, 0.14) | 0.02* |
AAs= amino acids; BP=blood pressure, EHD=in-center HD; HD=hemodialysis, table shows average 12-month change in values (95% confidence intervals)
To calculate average changes in essential and nonessential AA, absolute AA concentrations were normalized according to their z-scores, then the normalized concentrations of all baseline and 12 month essential and nonessential AAs were averaged for each subject, then the change between baseline and 12 month average AAs were calculated for each subject. Finally, the average change in average essential and nonessential AA were calculated by treatment group.
Significant at P<0.05
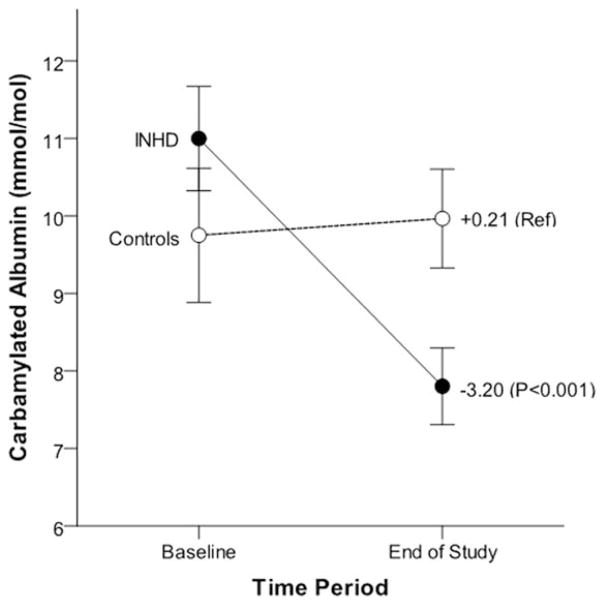
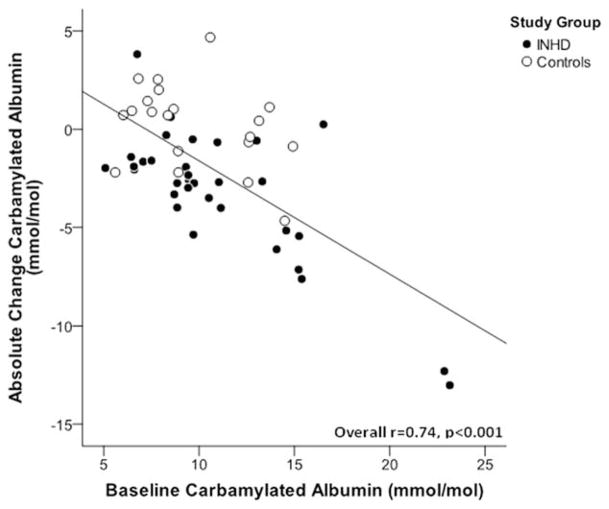
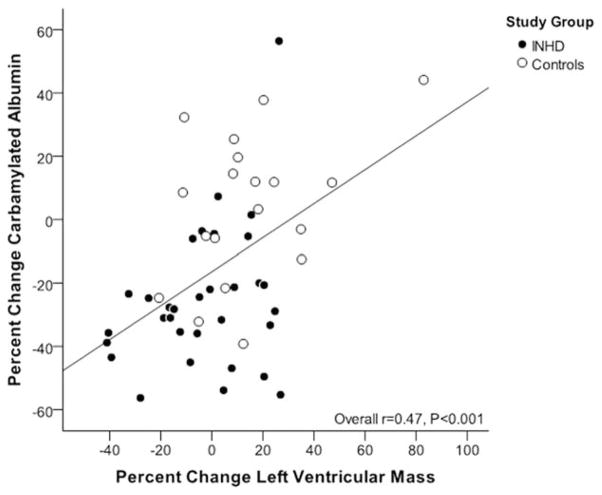
After 52 weeks of extended duration dialysis, EHD patients demonstrated significant decreases in C-Alb levels (average decrease of −29% from baseline), whereas patients receiving conventional HD generally remained at their original baseline values (Figure 1). Interestingly, there was a strong correlation between baseline C-Alb values and 12-month decreases in C-Alb in subjects treated with EHD (r=0.74, P<0.001, Figure 2), suggesting that patients with higher baseline C-Alb values experienced a greater treatment effect from EHD compared to patients with lower initial C-Alb values. Interestingly, when subjects on EHD were stratified by change in C-Alb after 12 months of intensive dialysis, it was found that subjects who responded with greater than 25% reduction in C-Alb had also decreased their left ventricular mass, decreased their predialysis urea concentrations, increased their urea reduction ratios, and increased total serum albumin (Table 5); in contrast, “non-responders” who did not decrease C-Alb when provided EHD did not display these beneficial changes in cardiac and dialytic parameters. Last, we found that there was an intriguing correlation between 12-month changes in C-Alb and changes in left ventricular mass (r=0.47, P<0.001) (Figure 3). Note that the main analysis of 12-month changes in LVM associated with EHD treatment are not included here because they are the subject of a separate study.23
Figure 1.
Change in carbamylated albumin by treatment group. Figure shows unadjusted mean baseline and end of study (1 year) carbamylated albumin levels±stand errors in EHD patients (closed circle) and controls (open circle); mean values did not differ significantly between treatment groups at baseline (P=0.26). Values listed in figure are mean 12-month change in carbamylated albumin by treatment group and associated P value.
Figure 2.
EHD reduces C-Alb in proportion to baseline C-Alb values. Figure shows association between change in carbamylated albumin over the 1-year study period and baseline C-Alb values. EHD patients are represented with closed circle and controls with open circles. Line shows linear association amongst EHD-treated subjects (r=0.74, P<0.001).
Table 5.
Average changes in clinical and biochemical indicators in subjects who reduced carbamylated albumin more than 25% compared to nonresponders
| Responders (n=21) | Nonresponders (n=32) | P value* | |
|---|---|---|---|
| Left ventricular mass (g) | −12.2 (−25.4, 0.9) | +7.8 (−2.8, 18.4) | 0.02* |
| Pre-HD Urea (mg/dL) | −13.2 (−20.5, −5.9) | +5.3 (−1.1, 11.8) | <0.001* |
| Urea reduction ratio (%) | +11.2 (7.3, 15.1) | +5.3 (2.2, 8.4) | 0.02* |
| Albumin (g/L) | +1.9 (0.3, 3.6) | −1.0 (−2.3, 0.4) | 0.008* |
Table shows average 12 month change in values (95% confidence intervals)
Significant at P<0.05.
Figure 3.
Correlation between change in carbamylated albumin and change in left ventricular mass. Figure shows correlation (r=0.47, P<0.001) between percent change carbamylated albumin and percent change left ventricular mass over the 1-year study period. EHD patients are represented with closed circle and controls with open circles. After adjusting for treatment group this correlation was attenuated but remained statistically significant (r=0.32, P=0.02).
DISCUSSION
In this study, we have demonstrated that intensification of dialysis through extended duration thrice-weekly EHD is associated with significant decreases in C-Alb.17 There is growing evidence of a strong association between serum carbamylated protein levels and mortality in patients with ESRD, and there are a number of studies that implicate carbamylation as a contributor to the pathologic sequelae of kidney disease.17,19,24–36 Our previous study have shown that among ESRD patients, C-Alb levels are strongly associated with risk of sudden cardiac death and heart failure,20 suggesting a possible pathophysiologic connection between protein carbamylation and progressive uremic cardiomyopathy. It is therefore interesting that we observed that decreases in C-Alb associated with intensive HD correlated with improvements in uremia-associated left ventricular hypertrophy.
Together, our results suggest that extending dialysis duration is an effective means of reducing protein carbamylation, and that this effects is mediated primarily by reduction of average blood urea concentrations. Given the strong correlation between C-Alb and blood urea concentrations, it could be argued that instead of measurement of C-Alb, response to EHD might instead be effectively monitored using blood urea, urea reduction ratios, or equilibrated Kt/V measurements. There are several pieces of evidence from our data to suggest that C-Alb may represent a better indicator of the efficacy of hemodialysis than these standard measures of uremia and dialysis adequacy, however. We have previously shown that C-Alb is strongly associated with mortality in both incident and prevalent hemodialysis patients, however we found no mortality risk associated with predialysis blood urea concentrations, urea reduction ratios, or equilibrated Kt/V values in this study.17 The risk associated with C-Alb was also still present after adjusting for URR and Kt/V in these multivariable models. There are several possible explanations for why C-Alb is more strongly associated with risk and may be a better biomarker than these other measures of uremia. First, the relationship between carbamylated albumin and urea is analogous to that of hemoglobin A1c and blood glucose: C-Alb is representative of time-averaged urea concentrations, and thus may be a more accurate indicator of uremia than are single-sample measurements of blood urea. Second, C-Alb is not affected by “urea rebound,” which confounds the ability of URR and Kt/V measurements to accurately measure efficacy of dialysis.38 Third, protein carbamylation is correlated with both high urea concentrations and amino acid deficiencies, and therefore C-Alb measurements integrate two pathologic processes into one indicator.
The findings from our study which suggest that EHD reduces C-Alb primarily by reducing average urea concentrations include the following: (1) The strong correlation between baseline C-Alb and blood urea seen in Table 2 suggest that the subset of patients with high baseline C-Alb suffer from “hypercarbamylation” associated with chronically elevated urea levels. (2) The significant correlation between reduction of blood urea concentrations and reduction of C-Alb seen in Table 2, as well as the overall average decreases of blood urea and C-Alb values associated with EHD therapy seen in Table 3 and Figure 1 suggest that extended duration HD is an effective means of reducing C-Alb and protein carbamylation in general. (3) The correlation between high baseline C-Alb and greater decreases in C-Alb after initiation of EHD seen in Figure 2 suggest that CHD patients with high C-Alb may be “under-dialyzed” and may benefit more from extended duration HD than patients on CHD with lower C-Alb values.
Many patients with dialysis and nondialysis dependent chronic kidney disease suffer from low serum albumin and low serum free AA levels.39–42 In addition to reducing urea and C-Alb, we found that EHD treatment was associated with increases in serum essential and nonessential AAs, but with no change in average normalized protein catabolic rate (Tables 2 and 4). Increasing dialysis dose through use of increased frequency hemodialysis strategies has been previously shown to improve appetite and increase protein intake, increase normalized protein catabolic rates, and increase free amino acids.43–48 In contrast to other studies of nocturnal dialysis, our results suggest that patients on EHD did not increase their total protein intake, raising the possibility that EHD increases AAs by other mechanisms such as reduction systemic inflammation.47,49
The rise in AAs associated with EHD in our study may also have significance with regards to effects on protein carbamylation. Free AAs are natural scavengers for carbamylation, 34 and we have previously demonstrated that low free AA levels in dialysis patients are significantly correlated with high C-Alb levels.17 We have also shown that inducing AA deficiencies results in accelerated protein carbamylation,17 and we recently reported results of a pilot clinical trial in HD patients that found that postdialysis intravenous administration of a select combination of essential AAs over 8 weeks produced significant reductions of C-Alb levels.18 Together these findings raise the question whether increased circulating AAs could also contribute to the reduction of protein carbamylation associated with EHD.
Left ventricular hypertrophy (LVH) is a common consequence of ESRD. Multiple studies have shown that patients transitioned to more intensive longer hours HD often experience reduction of left ventricular mass,4,50 including those converted to EHD,50,51 but there is significant inter-individual variability in the amount of LVM reduction, and to date no study has shown whether regression of LVH via dialysis intensification is also associated with improved survival. Our previous studies have demonstrated a strong association between C-Alb levels and risk of death from heart failure.20 It was therefore interesting to note that reductions in LVM were also strongly correlated with simultaneous reductions of C-Alb levels in this 1-year study (Figure 3). Although this correlation is intriguing, before any causal relationships or clinical significance can be evaluated, larger randomized trials that adjust for effects of blood pressure, ultrafiltration volume, and other important hemodynamic variables are needed in order to assess the effects of EHD on C-Alb and the subsequent association between changes in C-Alb and cardiac outcomes.
This study has several limitations. Although the effect sizes we observed were significant, the number of subjects included was relatively modest, and the subjects were not randomized to treatment and the results should be considered exploratory in nature. Furthermore, subjects in each treatment arm were not matched and there were several differences in baseline characteristics between patients on EHD versus controls: subjects on EHD had lower urea reduction ratios, were more likely to have kidney failure secondary to diabetes mellitus, a greater proportion of subjects on EHD had line access instead of permanent AV access, shorter average times on dialysis, and had slightly higher baseline C-Alb levels. Although it is possible that these baseline differences could have influenced the 1-year changes in C-Alb levels and how they compared between patients on EHD vs. CHD, it is important to note that the 1-year changes in C-Alb in the patients who received EHD were still present after adjusting for the effects of age, albumin, length of dialysis, history of diabetes mellitus, and history of coronary artery disease (Table 4).
In addition to these limitations, it might be speculated that EHD reduces C-Alb by directly removing carbamylated albumin in the dialysate. However, this is unlikely for the following reasons: (1) We did not see any significant change in total albumin in EHD patients (Table 1), so it is also unlikely that EHD increased albumin removal to a significant degree. (2) C-Alb is reported as the ratio of carbamylated albumin vs. total albumin. Carbamylation of albumin results in a very small increase in the molecular weight of albumin (+43 atomic mass units), and thus any removal of protein by extended dialysis would affect both carbamylated and noncarbamylated albumin equally, and thus any effects would be automatically accounted for and the C-Alb ratio would be unaffected.
Notwithstanding these limitations, we have demonstrated for the first time that intensification of HD via conversion to EHD is associated with significantly reduced levels of C-Alb, and our data suggests that this effect was mediated via the impact of EHD on urea and AA levels. While these preliminary findings should be confirmed in larger randomized controlled studies using extended-duration and other dialysis intensification regimens, our results suggest that measurement of C-Alb may represent a novel area of investigation for therapeutic monitoring of dialysis optimization among patients with ESRD, both to assess clinical responsiveness to intensive HD and to identify patients who may stand to gain maximal clinical benefits from HD intensification.
Supplementary Material
Footnotes
Conflict of Interest: All authors declare that they have no conflict of interest.
Disclosure of grants or other funding: Provisional applications for U.S. and International patents related to carbamylated albumin as a biomarker have been filed by AHB, SAK, RT, and their affiliated institutions. RT is a consultant to Fresenius Medical Care North America. JP has received speaking honoraria from Baxter Healthcare, Amgen Canada, DaVita Healthcare Partners, and has consulting fees from Amgen, Canada Baxter Healthcare, Otsuka, Janssen Ortho Shire and Takeda and has previously received research support from Baxter Healthcare. A. Yan is supported by the Heart and Stroke Foundation of Ontario, CIHR grant MOP 89982. A. Karumanchi received research funding from Howard Hughes Medical Institute. R. Thadhani is supported by award K24 DK094872 and R01 DK094486 from the National Institutes of Health. A. Berg is supported by award K08 HL121801 from the National Institutes of Health and by American Diabetes Association Innovation award 1-15-IN-02. JP receives salary support from Arbor Research Collaborative for Health. None of the entities who provided research support had any role in the study design, collection of data, analysis and interpretation of results, or writing of the manuscript.
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site: Table S1 C-Alb and Blood urea nitrogen concentrations before and after 12 months in subjects grouped by treatment protocol used (as-treated analysis).
References
- 1.Foley RN, Parfrey PS, Harnett JD, et al. Mode of dialysis therapy and mortality in end-stage renal disease. J Am Soc Nephrol. 1998;9:267–276. doi: 10.1681/ASN.V92267. [DOI] [PubMed] [Google Scholar]
- 2.Gudex CM. Health-related quality of life in endstage renal failure. Qual Life Res. 1995;4:359–366. doi: 10.1007/BF01593889. [DOI] [PubMed] [Google Scholar]
- 3.Culleton BF, Walsh M, Klarenbach SW, et al. Effect of frequent nocturnal hemodialysis vs conventional hemodialysis on left ventricular mass and quality of life: A randomized controlled trial. JAMA. 2007;298:1291–1299. doi: 10.1001/jama.298.11.1291. [DOI] [PubMed] [Google Scholar]
- 4.Chan CT, Floras JS, Miller JA, Richardson RM, Pierratos A. Regression of left ventricular hypertrophy after conversion to nocturnal hemodialysis. Kidney Int. 2002;61:2235–2239. doi: 10.1046/j.1523-1755.2002.00362.x. [DOI] [PubMed] [Google Scholar]
- 5.Walsh M, Culleton B, Tonelli M, Manns B. A systematic review of the effect of nocturnal hemodialysis on blood pressure, left ventricular hypertrophy, anemia, mineral metabolism, and health-related quality of life. Kidney Int. 2005;67:1500–1508. doi: 10.1111/j.1523-1755.2005.00228.x. [DOI] [PubMed] [Google Scholar]
- 6.Walsh M, Manns BJ, Klarenbach S, Quinn R, Tonelli M, Culleton BF. The effects of nocturnal hemodialysis compared to conventional hemodialysis on change in left ventricular mass: Rationale and study design of a randomized controlled pilot study. BMC Nephrol. 2006;7:2. doi: 10.1186/1471-2369-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuen DA, Kuliszewski MA, Liao C, Rudenko D, Leong-Poi H, Chan CT. Nocturnal hemodialysis is associated with restoration of early-outgrowth endothelial progenitor-like cell function. Clin J Am Soc Nephrol. 2011;6:1345–1353. doi: 10.2215/CJN.10911210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barua M, Hladunewich M, Keunen J, Pierratos A, McFarlane P, Sood M, Chan CT. Successful pregnancies on nocturnal home hemodialysis. Clin J American Soc Nephrol. 2008;3:392–396. doi: 10.2215/CJN.04110907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanly PJ, Pierratos A. Improvement of sleep apnea in patients with chronic renal failure who undergo nocturnal hemodialysis. N Engl J Med. 2001;344:102–107. doi: 10.1056/NEJM200101113440204. [DOI] [PubMed] [Google Scholar]
- 10.McFarlane PA. Nocturnal hemodialysis: Effects on solute clearance, quality of life, and patient survival. Curr Opin Nephrol Hypertens. 2011;20:182–188. doi: 10.1097/MNH.0b013e3283437046. [DOI] [PubMed] [Google Scholar]
- 11.Perl J, Chan CT. Home hemodialysis, daily hemodialysis, and nocturnal hemodialysis: Core Curriculum 2009. Am J Kidney Dis. 2009;54:1171–1184. doi: 10.1053/j.ajkd.2009.06.038. [DOI] [PubMed] [Google Scholar]
- 12.Rioux JP, Chan CT. Nocturnal home hemodialysis and its impact on erythropoietin responsiveness. Clin Nephrol. 2010;74:167–172. doi: 10.5414/cnp74167. [DOI] [PubMed] [Google Scholar]
- 13.Charra B, Calemard E, Ruffet M, et al. Survival as an index of adequacy of dialysis. Kidney Int. 1992;41:1286–1291. doi: 10.1038/ki.1992.191. [DOI] [PubMed] [Google Scholar]
- 14.Kalim S, Karumanchi SA, Thadhani RI, Berg AH. Protein carbamylation in kidney disease: Pathogenesis and clinical implications. Am J Kidney Dis. 2014;64:793–803. doi: 10.1053/j.ajkd.2014.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stark GR. Reactions of cyanate with functional groups of proteins. 3. Reactions with amino and carboxyl groups. Biochemistry. 1965;4:1030–1036. doi: 10.1021/bi00882a008. [DOI] [PubMed] [Google Scholar]
- 16.El-Gamal D, Rao SP, Holzer M, et al. The urea decomposition product cyanate promotes endothelial dysfunction. Kidney Int. 2014;86:923–931. doi: 10.1038/ki.2014.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berg AH, Drechsler C, Wenger J, et al. Carbamylation of serum albumin as a risk factor for mortality in patients with kidney failure. Sci Transl Med. 2013;5:175ra129. doi: 10.1126/scitranslmed.3005218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalim S, Ortiz-SanJuan G, Trottier CA, et al. The effects of parenteral amino acid therapy on protein carbamylation in maintenance hemodialysis patients. J Ren Nutr. 2015;25:388–932. doi: 10.1053/j.jrn.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalim S, Tamez H, Wenger J, et al. Carbamylation of serum albumin and erythropoietin resistance in end stage kidney disease. Clin J Am Soc Nephrol. 2013;8:1927–1934. doi: 10.2215/CJN.04310413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drechsler C, Kalim S, Wenger J, et al. Protein carbamylation is associated with heart failure and mortality in diabetic patients with end stage renal disease. Kidney Int. 2015;87:1201–1208. doi: 10.1038/ki.2014.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jakubovic BD, Wald R, Goldstein MB, et al. Comparative assessment of 2-dimensional echocardiography vs cardiac magnetic resonance imaging in measuring left ventricular mass in patients with and without endstage renal disease. Can J Cardiol. 2013;29:384–390. doi: 10.1016/j.cjca.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 22.International Federation of Clinical C, Laboratory Medicine ISD. Nordin G, Dybkaer R. Recommendation for term and measurement unit for “HbA1c”. Clin Chem Lab Med. 2007;45:1081–1082. doi: 10.1515/CCLM.2007.245. [DOI] [PubMed] [Google Scholar]
- 23.Wald R, Goldstein MB, Perl J, et al. The Association between conversion to in-centre nocturnal hemodialysis and left ventricular mass regression in patients with endstage renal disease. Can J Cardiol. 2016;32:369–377. doi: 10.1016/j.cjca.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 24.Tang WH, Shrestha K, Wang Z, et al. Protein carbamylation in chronic systolic heart failure: Relationship with renal impairment and adverse long-term outcomes. J Card Fail. 2013;19:219–224. doi: 10.1016/j.cardfail.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koeth RA, Kalantar-Zadeh K, Wang Z, Fu X, Tang WH, Hazen SL. Protein carbamylation predicts mortality in ESRD. J Am Soc Nephrol. 2013;24:853–861. doi: 10.1681/ASN.2012030254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Z, Nicholls SJ, Rodriguez ER, et al. Protein carbamylation links inflammation, smoking, uremia and atherogenesis. Nat Med. 2007;13:1176–1184. doi: 10.1038/nm1637. [DOI] [PubMed] [Google Scholar]
- 27.Pietrement C, Gorisse L, Jaisson S, Gillery P. Chronic increase of urea leads to carbamylated proteins accumulation in tissues in a mouse model of CKD. PloS One. 2013;8:e82506. doi: 10.1371/journal.pone.0082506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Apostolov EO, Ok E, Burns S, Nawaz S, Savenka A, Shah S, Basnakian AG. Carbamylated-oxidized LDL: Proatherosclerotic effects on endothelial cells and macrophages. J Atheroscler Thromb. 2013;20:878–892. doi: 10.5551/jat.14035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Apostolov EO, Basnakian AG, Ok E, Shah SV. Carbamylated low-density lipoprotein: Nontraditional risk factor for cardiovascular events in patients with chronic kidney disease. J Ren Nutr. 2011;22:134–138. doi: 10.1053/j.jrn.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 30.Apostolov EO, Ray D, Alobuia WM, et al. Endonuclease G mediates endothelial cell death induced by carbamylated LDL. Am J Physiol Heart Circ Physiol. 2011;300:H1997–2004. doi: 10.1152/ajpheart.01311.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Apostolov EO, Shah SV, Ray D, Basnakian AG. Scavenger receptors of endothelial cells mediate the uptake and cellular proatherogenic effects of carbamylated LDL. Arterioscler Thromb Vasc Biol. 2009;29:1622–1630. doi: 10.1161/ATVBAHA.109.189795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Apostolov EO, Shah SV, Ok E, Basnakian AG. Carbamylated low-density lipoprotein induces monocyte adhesion to endothelial cells through intercellular adhesion molecule-1 and vascular cell adhesion molecule-1. Arterioscler Thromb Vasc Biol. 2007;27:826–832. doi: 10.1161/01.ATV.0000258795.75121.8a. [DOI] [PubMed] [Google Scholar]
- 33.Ok E, Basnakian AG, Apostolov EO, Barri YM, Shah SV. Carbamylated low-density lipoprotein induces death of endothelial cells: A link to atherosclerosis in patients with kidney disease. Kidney Int. 2005;68:173–178. doi: 10.1111/j.1523-1755.2005.00391.x. [DOI] [PubMed] [Google Scholar]
- 34.Kraus LM, Kraus AP., Jr Carbamoylation of amino acids and proteins in uremia. Kidney Int Suppl. 2001;78:S102–107. doi: 10.1046/j.1523-1755.2001.59780102.x. [DOI] [PubMed] [Google Scholar]
- 35.Apostolov EO, Ray D, Savenka AV, Shah SV, Basnakian AG. Chronic uremia stimulates LDL carbamylation and atherosclerosis. J Am Soc Nephrol. 2010;21:1852–1857. doi: 10.1681/ASN.2010040365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bro S. Cardiovascular effects of uremia in apolipoprotein E-deficient mice. Dan Med Bull. 2009;56:177–192. [PubMed] [Google Scholar]
- 37.Eknoyan G, Beck GJ, Cheung AK, Daugirdas JT, Greene T, Kusek JW, et al. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med. 2002;347:2010–2019. doi: 10.1056/NEJMoa021583. [DOI] [PubMed] [Google Scholar]
- 38.Daugirdas JT, Greene T, Depner TA, et al. Factors that affect postdialysis rebound in serum urea concentration, including the rate of dialysis: Results from the HEMO Study. J Am Soc Nephrol. 2004;15:194–203. doi: 10.1097/01.asn.0000103871.20736.0c. [DOI] [PubMed] [Google Scholar]
- 39.Divino Filho JC, Barany P, Stehle P, Furst P, Bergstrom J. Free amino-acid levels simultaneously collected in plasma, muscle, and erythrocytes of uraemic patients. Nephrol Dial Transplant. 1997;12:2339–2348. doi: 10.1093/ndt/12.11.2339. [DOI] [PubMed] [Google Scholar]
- 40.Duranton F, Lundin U, Gayrard N, et al. Plasma and urinary amino acid metabolomic profiling in patients with different levels of kidney function. Clin J Am Soc Nephrol. 2013;9:37–45. doi: 10.2215/CJN.06000613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bergstrom J, Alvestrand A, Furst P. Plasma and muscle free amino acids in maintenance hemodialysis patients without protein malnutrition. Kidney Int. 1990;38:108–114. doi: 10.1038/ki.1990.174. [DOI] [PubMed] [Google Scholar]
- 42.Lindholm B, Alvestrand A, Furst P, Bergstrom J. Plasma and muscle free amino acids during continuous ambulatory peritoneal dialysis. Kidney Int. 1989;35:1219–1226. doi: 10.1038/ki.1989.113. [DOI] [PubMed] [Google Scholar]
- 43.Sikkes ME, Kooistra MP, Weijs PJ. Improved nutrition after conversion to nocturnal home hemodialysis. J Ren Nutr. 2009;19:494–499. doi: 10.1053/j.jrn.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 44.Ipema KJ, van der Schans CP, Vonk N, et al. A difference between day and night: Protein intake improves after the transition from conventional to frequent nocturnal home hemodialysis. J Ren Nutr. 2011;22:365–372. doi: 10.1053/j.jrn.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 45.Spanner E, Suri R, Heidenheim AP, Lindsay RM. The impact of quotidian hemodialysis on nutrition. Am J Kidney Dis. 2003;42(Suppl 1):30–35. doi: 10.1016/s0272-6386(03)00535-3. [DOI] [PubMed] [Google Scholar]
- 46.Raj DS, Ouwendyk M, Francoeur R, Pierratos A. Plasma amino acid profile on nocturnal hemodialysis. Blood Purif. 2000;18:97–102. doi: 10.1159/000014431. [DOI] [PubMed] [Google Scholar]
- 47.David S, Kumpers P, Eisenbach GM, Haller H, Kielstein JT. Prospective evaluation of an in-centre conversion from conventional haemodialysis to an intensified nocturnal strategy. Nephrol Dial Transplant. 2009;24:2232–2240. doi: 10.1093/ndt/gfp029. [DOI] [PubMed] [Google Scholar]
- 48.Ipema KJ, Westerhuis R, van der Schans CP, et al. Effect of nocturnal haemodialysis on body composition. Nephron Clin Pract. 2014;128:171–177. doi: 10.1159/000368239. [DOI] [PubMed] [Google Scholar]
- 49.Carrero JJ, Stenvinkel P, Cuppari L, et al. Etiology of the protein-energy wasting syndrome in chronic kidney disease: a consensus statement from the International Society of Renal Nutrition and Metabolism (ISRNM) J Ren Nutr. 2013;23:77–90. doi: 10.1053/j.jrn.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 50.Wald R, Yan AT, Perl J, et al. Regression of left ventricular mass following conversion from conventional hemodialysis to thrice weekly incentre nocturnal hemodialysis. BMC Nephrol. 2012;13:3. doi: 10.1186/1471-2369-13-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Demirci MS, Celik G, Ozkahya M, et al. Effects of thrice weekly nocturnal hemodialysis on arterial stiffness. Atherosclerosis. 2011;220:477–485. doi: 10.1016/j.atherosclerosis.2011.11.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.