Supplemental Digital Content is available in the text
Keywords: angiogenesis, meta-analysis, small-cell lung cancer, survival
Abstract
Background:
This study aimed to assess the effectiveness and safety of angiogenesis inhibitors for the treatment of patients with small cell lung cancer (SCLC) via meta-analysis.
Methods:
Electronic databases including PubMed, Embase, and Cochrane Library were searched to look for eligible studies through February 1, 2016. RCTs comprising angiogenesis inhibitors and nonangiogenesis inhibitors for SCLC patients were investigated. The extracted data including overall survival (OS), progression-free survival (PFS), and objective response rate (ORR) were summarized. In addition, the common adverse events (AEs) were also explored.
Results:
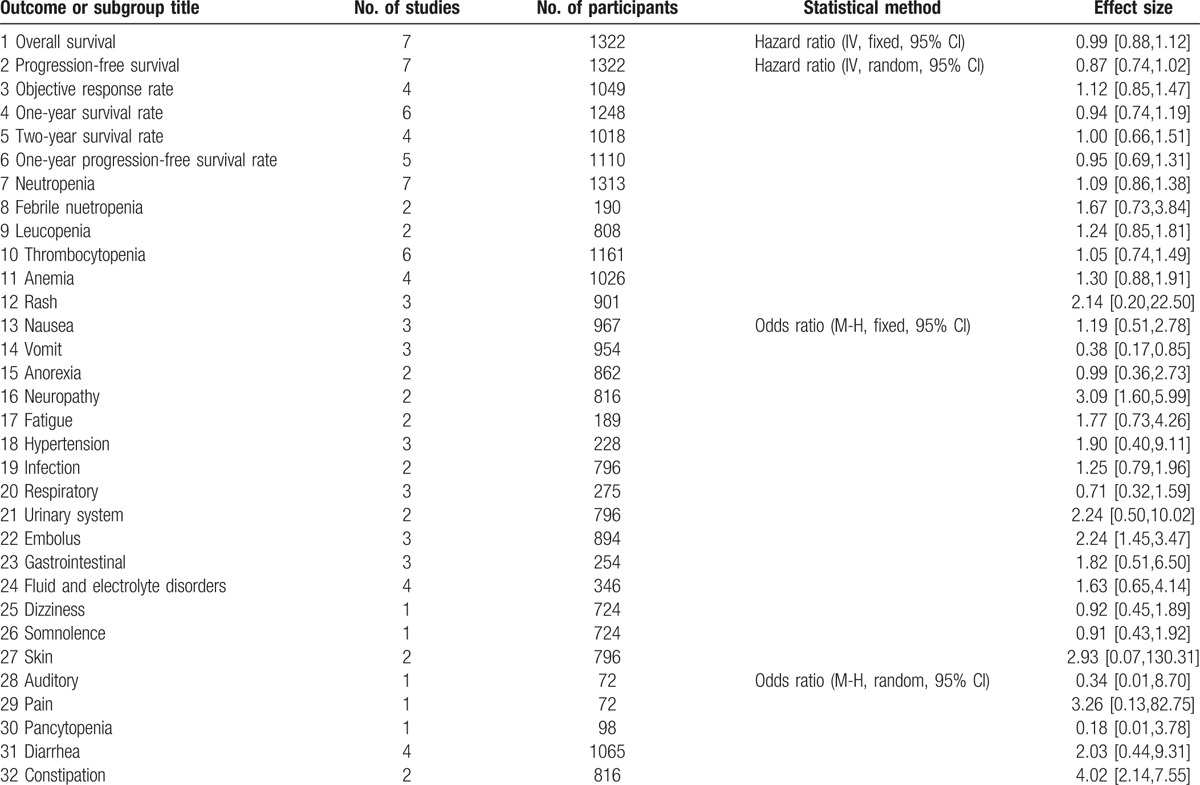
There were 7 phase II/III RCTs, encompassing 1322 SCLC patients eligible for meta-analysis. In comparison to nonangiogenesis inhibitors, angiogenesis inhibitors treatment was not associated with improvement of PFS [HR = 0.87, 95% CI (0.74–1.02), P = 0.09), OS [HR = 0.99, 95% CI (0.88–1.12), P = 0.91], or ORR [OR = 1.12, 95% CI (0.85–1.47), P = 0.41). Also, there was no improvement in 1-year survival rate [OR = 0.96, 95% CI (0.74–1.19), P = 0.63)], 2-year survival rate [OR = 1.00, 95% CI (0.66–1.51), P = 1.00)] or 1-year progression-free survival rates [OR = 0.95, 95% CI (0.69–1.31), P = 0.76)]. However, from subgroup analyses, it was observed that angiogenesis inhibitors improved ORR [HR = 1.66 (95% CI 1.02–2.71), P = 0.04] in phase II studies and bevacizumab improved PFS [HR = 0.73 (95% CI 0.42–0.97), P = 0.04]. It is important to note that angiogenesis inhibitors reduced emesis [OR = 0.38, 95% CI (0.17–0.85), P = 0.02], but increased incidence of constipation [OR = 4.02, 95% CI (2.14–7.55), P < 0.0001) and embolism [OR = 2.24, 95% CI (1.45–3.47), P = 0.0003).
Conclusion:
Adding angiogenesis inhibitors to chemotherapy did not improve PFS, OS, ORR, 1-year survival rate, 2-year survival rate or 1-year progression-free survival rate for SCLC. However, subgroup analysis revealed that bevacizumab enhanced PFS. Angiogenesis inhibitors also had a high incidence of constipation and embolism.
1. Introduction
Lung cancer is one of the most highly malignant neoplasms representing the leading cause of cancer-related death worldwide. Still worse, the morbidity and mortality continue to rise sharply.[1,2] Small cell lung cancer (SCLC) accounts for approximately 15% of cases and is considered a highly invasive form of lung cancer.[3] It is characterized by short doubling time, high recurrence rate, and early onset of dissemination, etc.[4]
At present, combination chemotherapy is the cornerstone of treatment for patients with SCLC.[5] For limited-stage disease small cell lung cancer (LD-SCLC), the response rate with first-line chemotherapy is about 80%, compared with 50% for extensive-stage disease (ED-SCLC). Despite relatively high initial response rates after first-line chemotherapy, most patients are subject to relapse within a short time.[6] Even worse, only 30% of SCLC patients present with limited disease at diagnosis and chemoresistance ensues rapidly.[7] As a consequence, the prognosis of SCLC is still dismal and the final survival time is as short as 8 to 12 weeks after relapse.[8] Therefore, exploring novel, more specific, and effective agents for SCLC is still urgently needed. Nowadays, applying targeted therapy to treat SCLC is gaining attention, especially some drugs already in clinical practice, among which anti-angiogenesis therapy may be a promising strategy. Moreover, it is widely accepted that angiogenesis is critical for the progression of SCLC.[9,10]
Currently, the overall efficacy of angiogenesis inhibitors for treating SCLC remains undetermined. So, in this work, a systematic review and meta-analysis of 7 randomized clinical trials (RCTs) was conducted to investigate the efficacy and safety of angiogenesis inhibitors for SCLC patients.
2. Methods
2.1. Ethical approval
The requirement for ethical approval was waived because this study included neither confidential personal patient data nor interventions with patients.
2.2. Search strategy
The meta-analysis adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.[11] Eligible RCTs were systematic searched by 2 authors independently, using PubMed, Embase, and Cochrane library databases through February 1, 2016. These were limited to studies in English. The following search terms were conducted: (“Small Cell Lung Carcinoma”, OR “Small Cell Lung Cancer” OR “Oat Cell Lung Cancer” OR “Small Cell Cancer the Lung” OR “Carcinoma, Small Cell Lung” OR “Oat Cell Carcinoma of Lung”) AND (“Randomized controlled trial” OR “controlled clinical trial” OR “randomized” OR “placebo” OR “drug therapy” OR “randomly” OR “trial” OR “groups”) AND (“Angiogenesis” OR “Angiogenesis inhibitors” OR “targeted therapy” OR “bevacizumab” OR “aflibercept” OR “ramucirumab” OR “sorafenib” OR “sunitinib” OR “nintedanib” OR “pazopanib” OR “motesanib” OR “vandetanib,” OR “cediranib” OR “endostatin”). To acquire relevant RCTs, we also manually examined some unavailable data, such as meeting abstracts and so on. What is more, the references of identified studies were also manually reviewed to obtain additional articles. Any disagreements were double-checked and arbitrated by a second reviewer.
2.3. Study selection
Studies that were eligible had to include the following criteria: patients with SCLC confirmed by pathological evidence; phase II or III RCTs that investigated angiogenesis inhibitors versus nonangiogenesis inhibitors in treating SCLC; RCTs showing sufficient data regarding the hazard ratio (HR) with 95% confidence interval (95% CI) of progression-free survival (PFS), overall survival (OS), or objective response rate (ORR); with regard to the duplicate data, the most complete trials were included.
Exclusion criteria were as follows: articles not be written in English language; nonrandomized studies; single-armed studies; retrospective studies; duplicate data; phase II studies; insufficient information about outcomes; reviews, letters, case reports, editorials, or expert opinion.
2.4. Data extraction and quality assessment
According to the standardized data-abstraction forms, 2 reviewers extracted the data from the eligible trials independently. When the 2 reviewers had discrepancies, these were identified and settled by consensus. The following data were extracted: first author's name and year of publication; trial phase; study population characteristics (patient stage, sample size, median age, sex, race); study design (RCT or not); targeted treatment; outcome measures (PFS, OS, ORR, 1-year survival rate, 2-year survival rate, 1-year PFS rate) and HR and 95% CI; common adverse events (AEs)
The Jadad scores were used to evaluate the quality of each eligible study (Table S1) and RCTs were assessed based on the following criteria: whether studies performed sequence generation (scores 0–2); whether studies performed allocation concealment (scores 0–2); whether studies used a suitable blind method (scores 0–2); whether studies performed appropriate dropouts (scores 0–1). Trials with a score of 4 to 7 were considered to be of high quality.
2.5. Statistical analysis
Review Manger (version 5.3 for Windows; Cochrane Collaboration, Oxford, UK) and Stata 12.0 (Stata Corporation, College Station, TX) were used for statistical analyses. The arm of using angiogenesis inhibitors was considered the experimental arm, and the nonangiogenesis inhibitors arm was defined as the control arm. The principal summary measurement of PFS and OS was reported as HR with 95% CI and P values. The odds ratio (OR) and 95% CI were used to measure ORRs, risk of AEs, 1-year PFS rate,1- and 2-year survival rates. The 95% CI no overlap with 1 and/or 2-tailed P < 0.05 were deemed to be statistically significant. All results were delineated as forest plots. For the heterogeneity between the RCTs, inconsistency statistic (I2) and forest plot were used for assessment. When P < 0.05 and/or I2 >50%, the heterogeneity was statistically significant, and a random-effects model was used. Otherwise, a fix-effects model was applied.[12] Publication bias was estimated with Egger and Begg funnel plot test.[13,14]
3. Results
3.1. Study characteristics
3.1.1. Results of the search
The flow chart of eligible RCTs selection is outlined in Fig. 1. In total, 2531 references were identified from the initial electronic search. After scanning the titles and abstracts, 1013 duplicates and 951 directly irrelevant studies were excluded. In order to further assessment, 16 potentially eligible studies were retrieved for full text, while 9 trials were excluded because of irrelevant survival information[15,16] or were single-armed studies.[17–23] Finally, 7 eligible studies[24–30] met the inclusion criteria, and were used for this meta-analysis.
Figure 1.
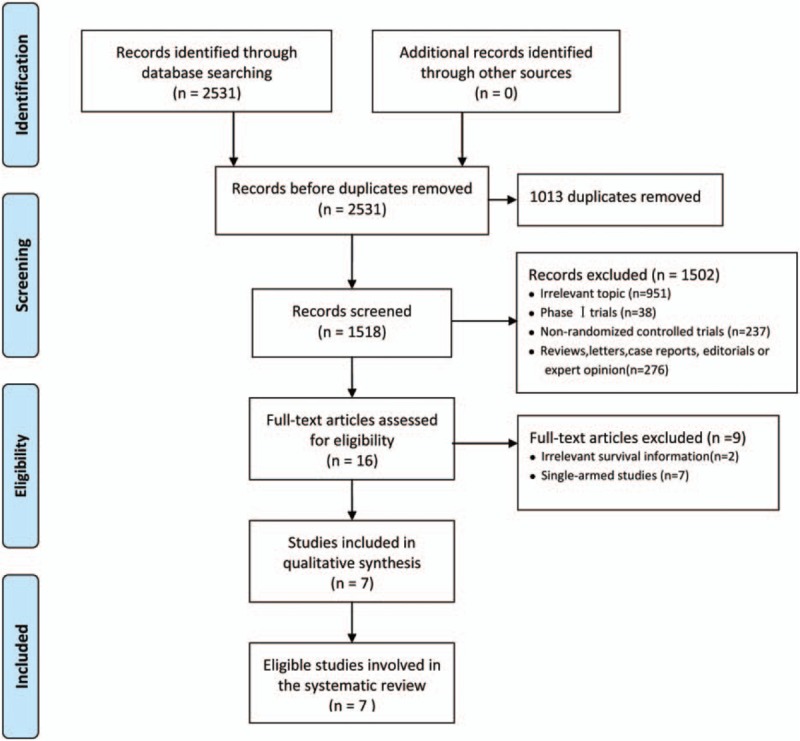
Flowchart of meta-analysis.
3.1.2. Included studies
The baseline characteristics of the 7 eligible studies are summarized in Table 1. There were 4 phase II,[24,26,29,30] 2 phase III,[25,27] and 1 phase II–III trials.[26] These studies enrolled 1322 subjects (669 received angiogenesis inhibitors and 653 received nonangiogenesis inhibitors). There were 5 kinds of angiogenesis inhibitors: bevacizumab (Bev),[28,30] thalidomide,[25,27] vandetanib,[24] sunitinib,[29] and endostatin[26] with comparable data. All 7 trials used antiangiogenesis drugs as maintenance and first-line therapies were platinum-based chemotherapy. Among these investigations, Spigel et al[30] for the first time evaluated the effects of Bev on ED-SCLC. In their study, angiogenesis inhibitors group contained Bev (15 mg/kg), etoposide and cisplatin/carboplatin (EP/EC), with placebo and EP/EC being the control group. In Pujol investigation,[28] the initial chemotherapy was EP/cisplatin-cyclophosphamide-epidoxorubicin-etoposide (PCDE), and the angiogenesis inhibitors group used Bev (7.5 mg/kg) after 2 additional cycles of PCDE. In the study by Lee et al, the experimental group contained thalidomide (100–200 mg/d) with chemotherapy (EP/EC) and the control group included placebo and chemotherapy (EP/EC). Pujol et al[27] explored the effects of thalidomide on treating ED-SCLC. The initial chemotherapy was PCDE, and after 2 cycles, the experimental group was treated with PCDE and thalidomide (400 mg/d); the control group received PCDE plus placebo. Arnold et al[24] chose LD-SCLC and ED-SCLC patients to carry out their study, in which the experimental group was treated with vandetanib (300 mg/d) and the control group was given placebo after patients had achieved complete response (CR) or partial response (PR) with chemotherapy. In the study by Ready et al[29] ED-SCLC patients were treated with chemotherapy (EP/EC). After 4 to 6 cycles, patients exhibiting no progression were randomly classified into 2 groups, where 1 group was assigned to placebo and the other with sunitinib (37.5 mg per day) until disease progression; cross-over after progression was allowed. Lu et al[26] conducted a multicenter, open-label, randomized phase II study that selected ED-SCLC patients. Their experimental group was treated with EC and rh-endostatin and the control group was given only EC. Sample size of the studies varied from 74 to 724, and among the 1322 patients there were 801 males and 521 females. The median or mean age ranged from 56 to 65 years. Furthermore, the patients were mostly Caucasian (86% to 98.1%), and most had ED-SCLC (68.7%). PFS and OS were reported in all 7 trials and corresponding HR with 95% CI were acquired directly. According to the Jadad score instrument, all studies were qualified enough with a score varying from 4 to 7 except the study 26 (Table S1). As shown in Fig. 2, there was no potential bias in the 7 studies. The overall methodological quality of the included trials was generally good and fair.
Table 1.
Characteristics of all 7 included randomized controlled trials.
Figure 2.
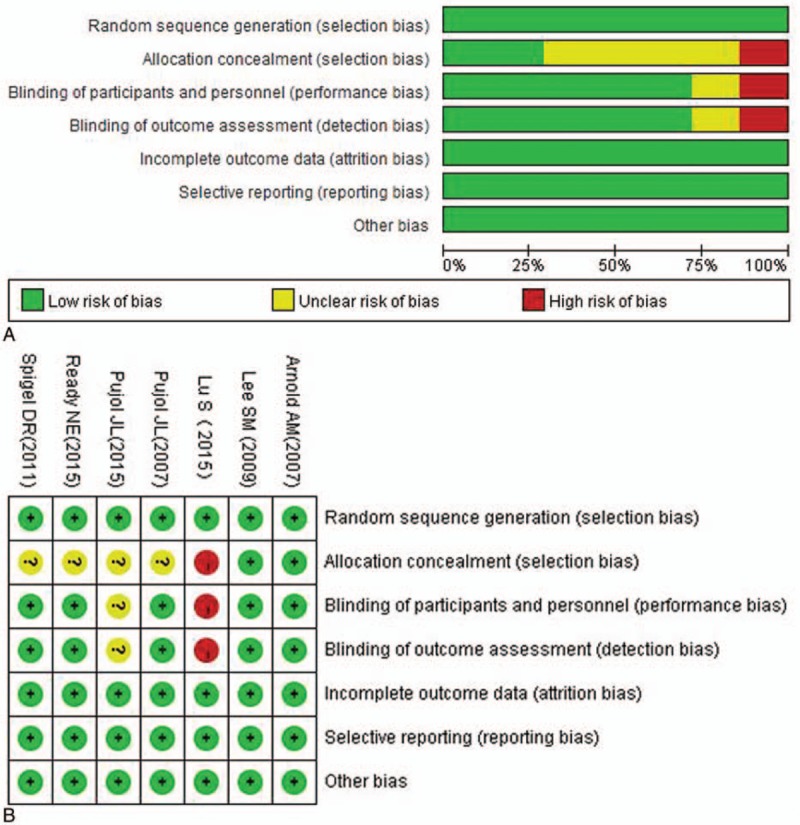
Appraisal of risk of bias of included trials using Cochrane risk-of-bias tool.
3.2. Effects of interventions
3.2.1. Progression-free survival
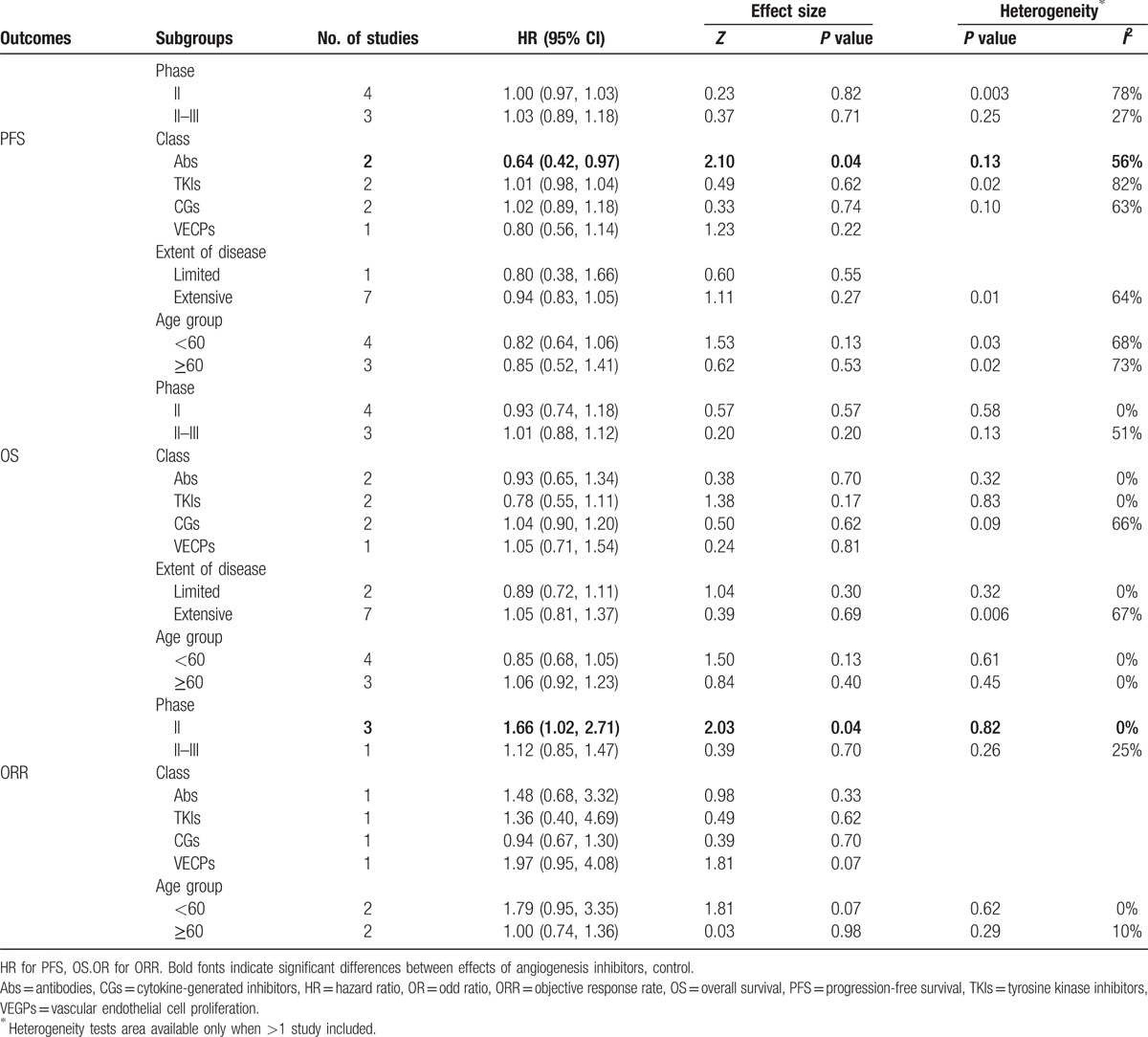
All of the 7 studies reported available data concerning PFS. Median PFS of angiogenesis inhibitor and control arms varied from 2.7 to 7.6 months, 2.1 to 7.6 months, respectively. HR (angiogenesis inhibitors versus control) varied from 0.53 (95% CI 0.33–0.86) to 1.10 (95% CI 0.48–2.50). Pooled HR was 0.87 (95% CI 0.74–1.02, P = 0.09), which indicated that there was no significant difference between the 2 groups and angiogenesis inhibitors did not prolong PFS (Fig. 3, Table S2). Significant heterogeneity was detected (P = 0.01, I2 = 64%), so random-efforts model was performed for the pooled HR. Remarkably, as shown Table 2, subgroup analyses stratified by different drug class indicated that significant PFS benefit was found in antibodies (Abs) group [HR = 0.73 (95% CI 0.42–0.97), P = 0.04].
Figure 3.
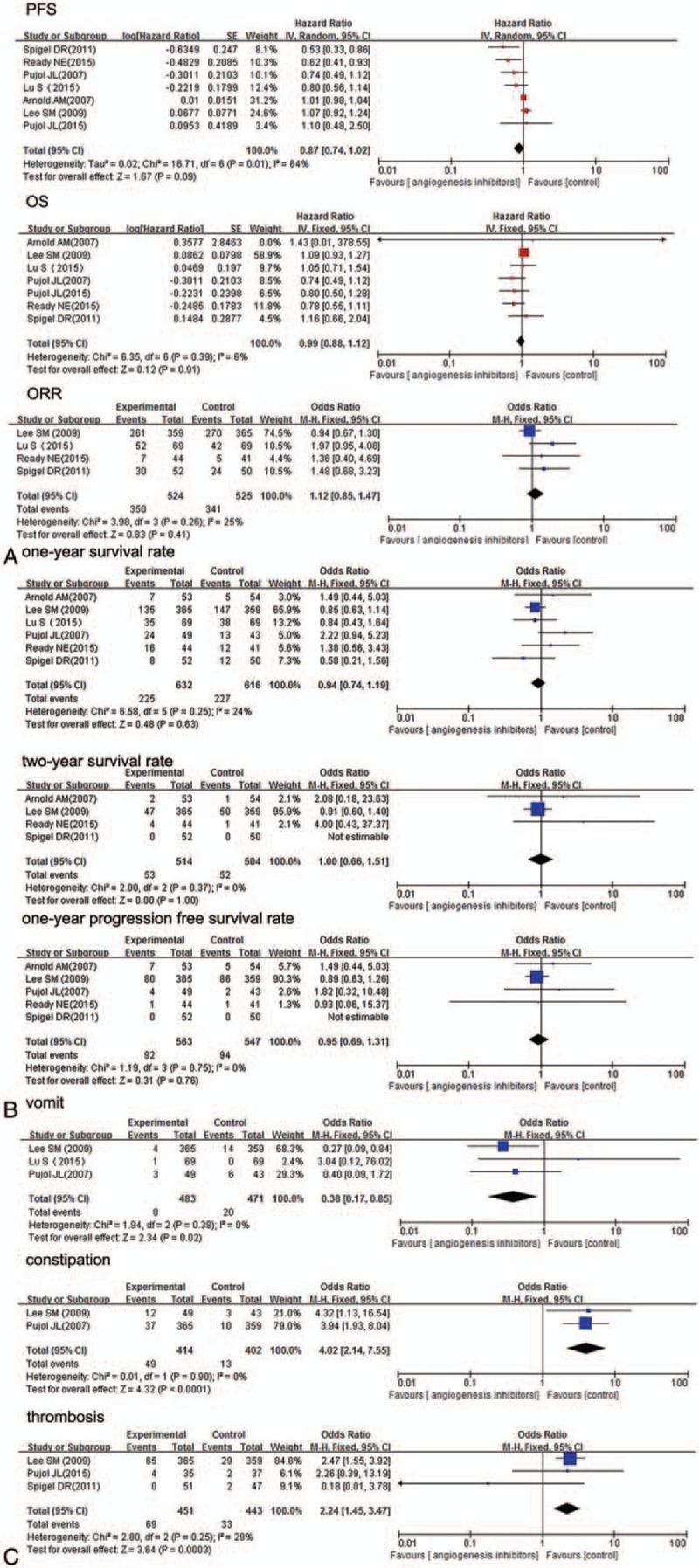
Forest plot of merged analyses for (A) HR with 95% CI for OS and PFS, OR with 95% CI for ORR; (B) 1- and 2-year survival rate and 1-year progression-free survival rate; (C) the incidence of adverse effects for SCLC patients treated with angiogenesis inhibitors versus control group. HR = hazard ratio, OR = odds ratio, ORR = objective response rate, OS = overall survival, PFS = progression-free survival, SCLC = small-cell lung cancer, TKIs = tyrosine kinase inhibitors.
Table 2.
Subgroup analysis for PFS, OS, ORR.
3.2.2. Overall survival
Concerning OS, 6 studies reported available HR and 95% CI data. Median OS of angiogenesis inhibitors arms varied from 9.0 to 12.1 months, and control arm from 6.9 to 12.4 months. Thus, anti-angiogenesis therapy displayed no improvement in OS. Furthermore, as shown in Fig. 3 (Table S2), HR for OS (angiogenesis inhibitors versus control) ranged from 0.74 (95% CI 0.49–1.12) to 1.43 (95% CI 0.01–378.55). Pooled HR was 0.99 (95% CI 0.88–1.12, P = 0.91), without statistical significance between the 2 groups. The pooled model showed angiogenesis inhibitors did not prolong OS. Apparent heterogeneity was not observed among the trials (P = 0.39, I2 = 6%). Therefore, we used fixed-effects model. Exploratory subgroup analyses were conducted according to trials phase, drug class, and extent of disease or patient age. As shown in Table 2, these variables did not alter the effects of angiogenesis inhibitors on OS.
3.2.3. Objective response rate
Among the 7 studies, OR for ORR were available in 4. The ORR of angiogenesis inhibitors arm varied from 16% to 73%, and the control arm from 12% to 74%. As shown in Fig. 3 (Table S2), the OR for ORR (angiogenesis inhibitors versus control) ranged from 0.94 (95% CI 0.67–1.30) to 1.97 (95% CI 0.95–4.08). The combined OR (1.12, 95% CI = 0.85–1.47, P = 0.41) demonstrated that angiogenesis inhibitors did not improve objective response rate. Statistical heterogeneity was not observed among the studies (P = 0.26, I2 = 25%); therefore, a fixed-effects model was used. From subgroup analyses (Table 2), it was observed that angiogenesis inhibitors substantially improved the ORR in phase II studies, in which the HR was 1.66 (95% CI 1.02–2.71, P = 0.04).
3.2.4. One-year survival rate
Six trials evaluated ORs for 1-year survival rate. The 1-year survival rates of angiogenesis inhibitors and control varied from 13% to 50%, 9% to 55%, respectively. As seen in Fig. 3 (Table S2), OR for survival at 1 year (angiogenesis inhibitors versus control) ranged from 0.58 (95% CI 0.21–1.56) to 2.22 (95% CI 0.94–5.23), and the pooled OR was 0.94 (95% CI 0.74–1.19, P = 0.63). The pooled results showed that angiogenesis inhibitors did not improve survival rate at 1 year. There was no significant heterogeneity (P = 0.25, I2 = 24%) among the studies, so a fixed-effects model was used for this analysis.
3.2.5. Two-year survival rate
Four studies among the 7 RCTs reported available data concerning the 2-year survival rate. The survival rate of the angiogenesis inhibitors group varied from 0% to 13%, with the control arm ranging from 0% to 14%. The OR for survival at 2 years (angiogenesis inhibitors vs control) ranged from 0.91 (95% CI 0.60–1.40) to 3.28 (95% CI 0.54–19.78). The pooled OR (1.00, 95% CI = 0.66–1.51, P = 1.00) indicated that anti-angiogenesis therapy had no obvious effect on improving 2-year survival (Fig. 4). We did not observe any statistical heterogeneity (P = 0.37, I2 = 0%), so a fixed-effects model was used.
Figure 4.
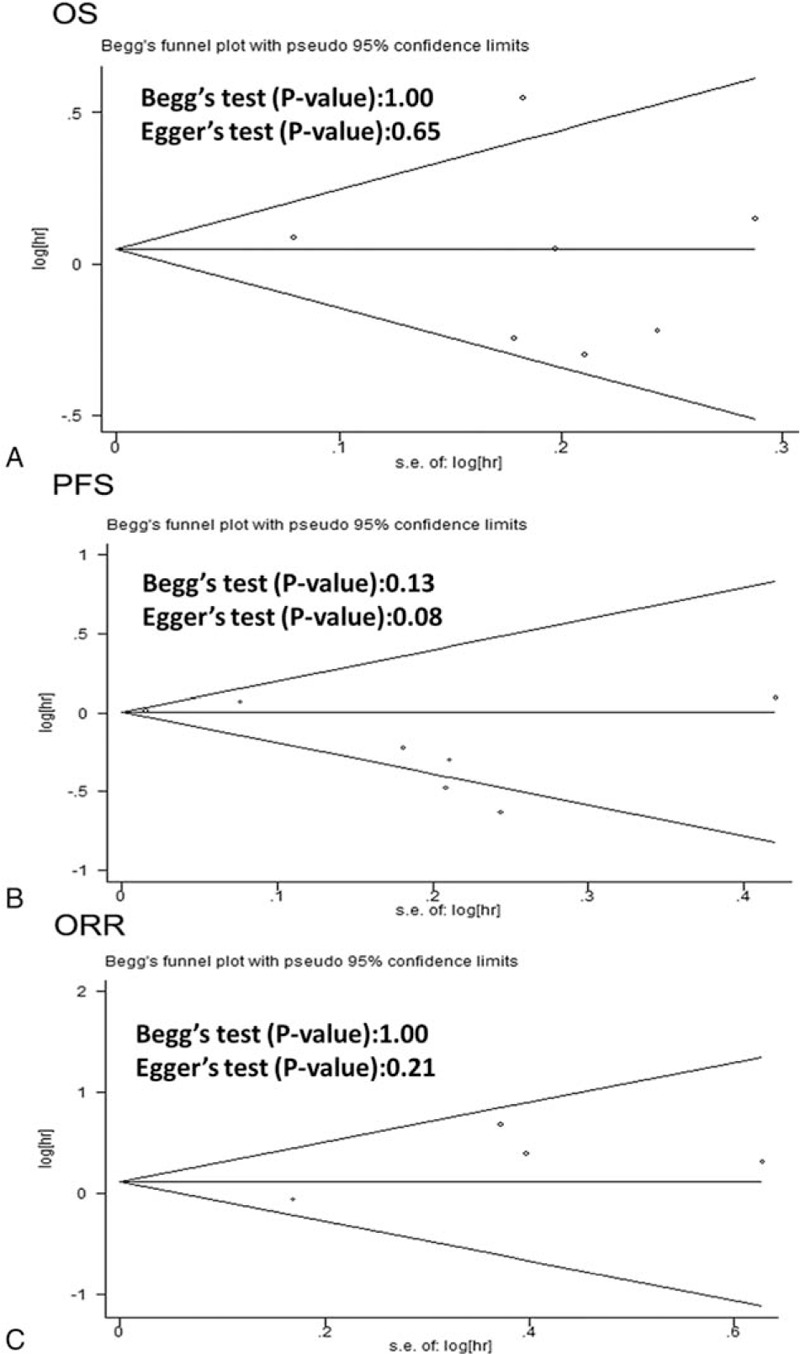
Publication bias analysis by funnel plot graphic. A, OS. B, PFS. C, ORR.
3.2.6. One-year progression-free survival rate
Five trials reported available data about the 1-year PFS rate. The OR for PFS at 1 year (angiogenesis inhibitors vs control) ranged from 0.89 (95% CI 0.63–1.26) to 1.77 (95% CI 0.34–9.20). As shown in Fig. 3 (Table S2), the pooled OR was 0.95 (95% CI 0.69–1.31, P = 0.76), without statistical significance between 2 arms, which suggested antiangiogenesis therapy has no obvious effect on improving 1-year progression-free survival rate. We used fixed-effects model to analyze pooled data due to no heterogeneity (P = 0.75, I2 = 0%).
3.2.7. Serious adverse event
All 7 trials evaluated grade 3 to 5 AEs. The correspondingly pooled OR is shown in Table 3. There were 6 types of hematological AEs and 20 types of nonhematological AEs. Angiogenesis inhibitor-related deaths were not reported. As shown in Fig. 3 (Table S2), antiangiogenesis therapy group patients had reduced emesis [OR 0.38, 95% CI (0.17–0.85), P = 0.02], but an increased incidence of constipation [OR 4.02, 95% CI (2.14–7.55), P <0.0001], embolism [OR 2.24, 95% CI (1.45–3.47), P = 0.0003].
Table 3.
Summary of results: pooled HRs/ORs with 95% CI.
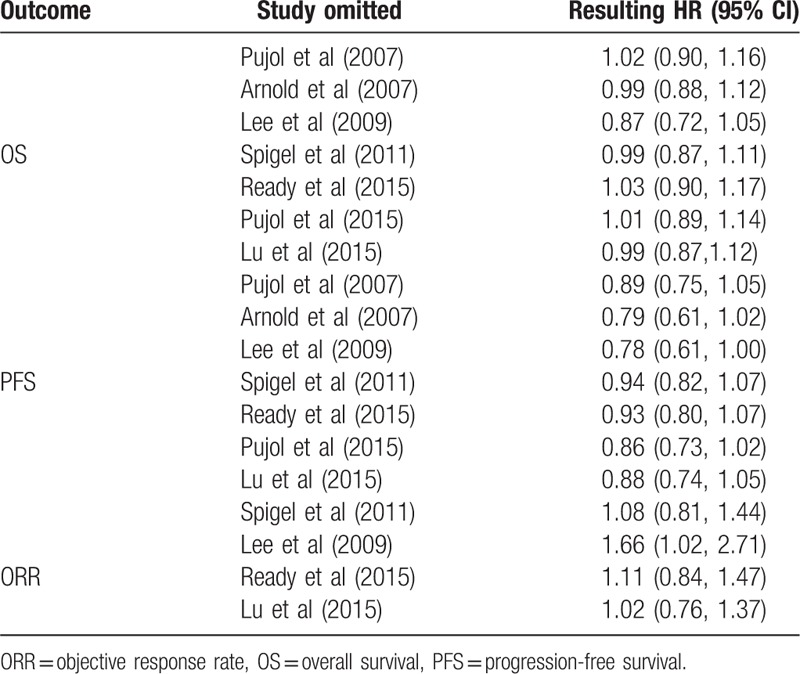
3.3. Sensitivity analyses
In order to evaluate the stability of our result, sensitivity analyses were carried out by sequentially removing single trials. As seen in Table 4, no individual studies altered the pooled results for PFS, OS, or ORR, indicating that outcomes were stable enough for this meta-analysis.
Table 4.
Sensitive analyses for OS, PFS, ORR.
3.4. Publication bias
Funnel plots were performed on all 7 studies investigating PFS, OS, and ORR to evaluate the reliability of our results. As shown in Fig. 4, Funnel plots showed symmetry, and no evidence was observed to reveal publication bias (all P > 0.05).
4. Discussion
SCLC is still a major challenge in clinical practice. Currently, the combination of platinum-based doublet with etoposide is the globally accepted standard of treatment.[31] However, most patients relapse soon after discontinuing chemotherapy, and the median survival, even for limited-stage patients, is no more than 2 years. In addition, trials of new chemotherapy regimens in recent 3 decades have failed to significantly improve survival.[32] Numerous methods have been attempted to enhance the therapeutic effectiveness, such as dose intensification, bone marrow transplantation, and maintenance therapy with both chemotherapy as well as other agents. However, unfortunately none of these strategies has achieved a significant effect on survival.[33,34] In contrast, several trials have shown that added therapy was only more toxic, with sometimes negative impact on quality of life.[35] Thus, therapeutic progress in SCLC is long overdue and new therapies and drugs are highly desired.
Angiogenesis is critical for carcinogenesis. Research has shown that SCLC exhibits a higher microvessel density than nonsmall cell lung cancer (NSCLC).[36] Targeted therapy against angiogenesis is well established in NSCLC.[37] Previous studies have revealed that SCLC patients express functional vascular endothelial growth factor receptor (VEGFR)-2 and VEGFR-3 and elevated the levels of serum vascular endothelial growth factor (VEGF).[38,39] Blocking angiogenesis could, therefore, inhibit the growth, invasion, and metastasis of tumors. So, angiogenesis inhibitors seem promising to treat SCLC. However, studies of angiogenesis inhibitors, including bevacizumab and thalidomide, in SCLC have produced mixed results.
To the best of our knowledge, few of previous researches are investigating the effect of angiogenesis inhibitors on patients with SCLC.[40] In this study, the pooled results revealed that angiogenesis inhibitors did not improve OS [HR = 0.99 (95% CI 0.88–1.12), P = 0.91], PFS [HR = 0.87 (95% CI 0.74–1.02), P = 0.09] or ORR [HR = 0.94 (95% CI 0.74–1.19), P = 0.89].
However, when we conducted subgroup analysis by trial phase, it is noteworthy that angiogenesis inhibitors can enhance ORR [HR = 1.66 (95% CI 1.02–2.71), P = 0.04] in phase II studies. According to different drug targets, the investigated 7 randomized controlled trials were classified into 4 subgroups, including 2 Abs(bevacizumab), 2 with tyrosine kinase inhibitors (TKIs) (vandetanib, sunitinib), 2 with cytokine-generated inhibitors (CGIs) (thalidomide), and 1 with an anti-vascular endothelial cell proliferation drug (VECPs) (endostatin). Compared with control, Abs were demonstrated to improve the PFS [HR = 0.64 (95% CI 0.42–0.97), P = 0.04]. In order to eliminate the influences of different disease extents and patient ages, stratified analyses were performed. However, neither of these variables altered the results. Upon close analysis, we found that anti-angiogenesis inhibitors failed to improve the outcome of 1-year OS rate [OR = 0.94 (95% CI 0.74–1.19), P = 0.63], 2-year OS rate [OR = 1.00 (95% CI 0.66–1.51), P = 1.00], or 1-year PFS rate [OR = 0.95 (95% CI 0.69–1.31), P = 0.76]. Antiangiogenesis therapy increased adverse effects including constipation [OR = 4.02 (95% CI 2.14–7.55), P < 0.0001] and occurrence of embolism [OR = 2.24 (95% CI 1.45–3.47), P = 0.0003]. However, in general, angiogenesis inhibitors proved to be safe with mild toxicity.
Although the present meta-analysis suggested that TKIs did not enhance survival of SCLC patients, it was worth mentioning that 1 study[29] reported that sunitinib led to improvement of PFS for patients with ED-SCLC and with satisfactory safety. One explanation is that inhibition of VEGFR kinase activity is neutralized very rapidly by upregulating alternative pro-angiogenic factors, including fibroblast growth factor 1 (FGF1) and FGF2, ephrin A1 (Efna1), Efna2, and angiopoietin 1 (Angpt1).[41] Another possible reason is that the sample size was small, which could affect the results to a certain degree. In addition, the present research revealed that angiogenesis inhibitors did not significantly improve the prognosis (OS, PFS, ORR) of SCLC patients, but in detail, bevacizumab enhanced the PFS of SCLC. On the contrary, numerous studies suggested angiogenesis inhibitors were generally demonstrated to be effective to NSCLC.[41–44] Particularly, Gao et al[45] conducted a meta-analysis encompassing over 452 advanced NSCLC patients who previously received bevacizumab and found that angiogenesis inhibitors improved clinical benefits. Therefore, different subtypes of diseases should be considered when using angiogenesis inhibitors in lung cancer.
Several potential limitations should be acknowledged and some results of meta-analysis need to be cautiously interpreted. First, the number of eligible trails was limited, and the meta-analysis was not based on individual patient data. Second, we failed to evaluate the effect of the combined strategy on other meaningful endpoints such as quality of life, etc. Finally, these trials had different treatment schedules, where the chemotherapy regimens, targeted drugs, and disease stages were different. Thus, heterogeneity existed. Further, more powerful phase III trials will be necessary to further assess the effect of angiogenesis inhibitors on survival of SCLC.
5. Conclusion
In summary, despite certain limitations existing, the present results suggest that angiogenesis inhibitors do not improve PFS, OS, ORR, 1-year survival rate, or 2-year survival rates and 1-year progression-free survival rate. However, subgroup analysis did reveal that bevacizumab seemed to enhance PFS. Further, large-scale RCTs with larger samples are required for confirmation.
Supplementary Material
Footnotes
Abbreviations: 95% CI = 95% confidence intervals, Ab = antibody, AEs = adverse events, Bev = bevacizumab, CGIs = cytokine-generated inhibitors, CR = complete response, EC = etoposide and carboplatin, ED-SCLC = extensive-stage disease small cell lung cancer, EP = etoposide and cisplatin, FGF = fibroblast growth factor, HR = hazard ratio, LD-SCLC = limited-stage disease small cell lung cancer, NM = not mentioned, NSCLC = nonsmall cell lung cancer, OR = odds ratio, ORR = objective response rate, OS = overall survival, PCDE = cisplatin-cyclophosphamide-epidoxorubicin-etoposide, PFS = progression-free survival, PR = partial response, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses, RCT = randomized controlled trial, SCLC = small-cell lung cancer, TKIs = tyrosine kinase inhibitors, VECPs = anti-vascular endothelial cell proliferation, VEGFR = vascular endothelial growth factor receptor.
QL and TW contributed equally to this study.
Our research received fund support from Wu Jieping Medical Foundation Project (Grant No: 50603020).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- [2].Gironés R, López P, Chulvi R, et al. Ten years of lung cancer in a single center: gender, histology, stage and survival. J Cancer Metastasis Treat 2015;1:201–7. [Google Scholar]
- [3].Sher T, Dy GK, Adjei AA. Small cell lung cancer. Mayo Clin Proc 2008;83:355–67. [DOI] [PubMed] [Google Scholar]
- [4].Semenova EA, Nagel R, Berns A. Origins, genetic landscape, and emerging therapies of small cell lung cancer. Genes Dev 2015;29:1447–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kalemkerian GP, Akerley W, Bogner P, et al. Small cell lung cancer. National Comprehensive Cancer Network 2013;11:78–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Amarasena IU, Chatterjee S, Walters J, et al. Platinum versus non-platinum chemotherapy regimens for small cell lung cancer. Cochrane Database Syst Rev 2015;CD006849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Alvarado-Luna G, Morales-Espinosa D. Treatment for small cell lung cancer, where are we now? a review. Transl Lung Cancer Res 2016;5:26–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Carter BW, Glisson BS, Truong MT, et al. Small cell lung carcinoma: staging, imaging, and treatment considerations. Radiographics 2014;34:1707–21. [DOI] [PubMed] [Google Scholar]
- [9].Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol 2005;23:1011–27. [DOI] [PubMed] [Google Scholar]
- [10].Roth BJ, Johnson DH, Einhorn LH, et al. Randomized study of cyclophosphamide, doxorubicin, and vincristine versus etoposide and cisplatin versus alternation of these two regimens in extensive small-cell lung cancer: a phase III trial of the Southeastern Cancer Study Group. J Clin Oncol 1992;10:282–91. [DOI] [PubMed] [Google Scholar]
- [11].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 2009;151:W65–94. [DOI] [PubMed] [Google Scholar]
- [12].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Egger M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- [15].Jalal S, Bedano P, Einhorn L, et al. Paclitaxel plus bevacizumab in patients with chemosensitive relapsed small cell lung cancer: a safety, feasibility, and efficacy study from the Hoosier Oncology Group. J Thorac Oncol 2010;5:2008–11. [DOI] [PubMed] [Google Scholar]
- [16].Allen JW, Moon J, Redman M, et al. Southwest Oncology Group S0802: a randomized, phase II trial of weekly topotecan with and without ziv-aflibercept in patients with platinum-treated small-cell lung cancer. J Clin Oncol 2014;32:2463–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Spigel DR, Waterhouse DM, Lane S, et al. Efficacy and safety of oral topotecan and bevacizumab combination as second-line treatment for relapsed small-cell lung cancer: an open-label multicenter single-arm phase II study. Clin Lung Cancer 2013;14:356–63. [DOI] [PubMed] [Google Scholar]
- [18].Mountzios G, Emmanouilidis C, Vardakis N, et al. Paclitaxel plus bevacizumab in patients with chemoresistant relapsed small cell lung cancer as salvage treatment: a phase II multicenter study of the Hellenic Oncology Research Group. Lung Cancer 2012;77:146–50. [DOI] [PubMed] [Google Scholar]
- [19].Ramalingam SS, Belani CP, Mack PC, et al. Phase II study of Cediranib (AZD 2171), an inhibitor of the vascular endothelial growth factor receptor, for second-line therapy of small cell lung cancer (National Cancer Institute# 7097). J Thorac Oncol 2010;5:1279–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sharma N, Pennell NA, Halmos B, et al. Phase II trial of sorafenib in conjunction with chemotherapy and as maintenance therapy in extensive-stage small cell lung cancer (SCLC): final results. Paper presented at: ASCO Annual Meeting Proceedings 2012. [DOI] [PubMed] [Google Scholar]
- [21].Dowlati A, Subbiah S, Cooney M, et al. Phase II trial of thalidomide as maintenance therapy for extensive stage small cell lung cancer after response to chemotherapy. Lung Cancer 2007;56:377–81. [DOI] [PubMed] [Google Scholar]
- [22].Young R, Tin A, Brown N, et al. Analysis of circulating angiogenic biomarkers from patients in two phase III trials in lung cancer of chemotherapy alone or chemotherapy and thalidomide. Br J Cancer 2012;106:1153–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Han J-Y, Kim HY, Lim KY, et al. A phase II study of sunitinib in patients with relapsed or refractory small cell lung cancer. Lung Cancer 2013;79:137–42. [DOI] [PubMed] [Google Scholar]
- [24].Arnold AM, Seymour L, Smylie M, et al. Phase II study of vandetanib or placebo in small-cell lung cancer patients after complete or partial response to induction chemotherapy with or without radiation therapy: National Cancer Institute of Canada Clinical Trials Group Study BR.20. J Clin Oncol 2007;25:4278–84. [DOI] [PubMed] [Google Scholar]
- [25].Lee SM, Woll PJ, Rudd R, et al. Anti-angiogenic therapy using thalidomide combined with chemotherapy in small cell lung cancer: a randomized, double-blind, placebo-controlled trial. J Natl Cancer Inst 2009;101:1049–57. [DOI] [PubMed] [Google Scholar]
- [26].Lu S, Li L, Luo Y, et al. A multicenter, open-label, randomized phase II controlled study of rh-endostatin (Endostar) in combination with chemotherapy in previously untreated extensive-stage small-cell lung cancer. J Thorac Oncol 2015;10:206–11. [DOI] [PubMed] [Google Scholar]
- [27].Pujol JL, Breton JL, Gervais R, et al. Phase III double-blind, placebo-controlled study of thalidomide in extensive-disease small-cell lung cancer after response to chemotherapy: an intergroup study FNCLCC cleo04 IFCT 00-01. J Clin Oncol 2007;25:3945–51. [DOI] [PubMed] [Google Scholar]
- [28].Pujol JL, Lavole A, Quoix E, et al. Randomized phase II-III study of bevacizumab in combination with chemotherapy in previously untreated extensive small-cell lung cancer: results from the IFCT-0802 trialdagger. Ann Oncol 2015;26:908–14. [DOI] [PubMed] [Google Scholar]
- [29].Ready NE, Pang HH, Gu L, et al. Chemotherapy with or without maintenance sunitinib for untreated extensive-stage small-cell lung cancer: a randomized, double-blind, placebo-controlled phase II study-CALGB 30504 (Alliance). J Clin Oncol 2015;33:1660–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Spigel DR, Townley PM, Waterhouse DM, et al. Randomized phase II study of bevacizumab in combination with chemotherapy in previously untreated extensive-stage small-cell lung cancer: results from the SALUTE trial. J Clin Oncol 2011;29:2215–22. [DOI] [PubMed] [Google Scholar]
- [31].Koinis F, Kotsakis A, Georgoulias V. Small cell lung cancer (SCLC): no treatment advances in recent years. Transl Lung Cancer Res 2016;5:39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ettinger D, Johnson B. Update: NCCN small cell and non-small cell lung cancer Clinical Practice Guidelines. J Natl Compr Canc Netw 2005;3:S17. [PubMed] [Google Scholar]
- [33].Krug LM, Crapanzano JP, Azzoli CG, et al. Imatinib mesylate lacks activity in small cell lung carcinoma expressing c-kit protein. Cancer 2005;103:2128–31. [DOI] [PubMed] [Google Scholar]
- [34].Moore A, Einhorn L, Estes D, et al. Gefitinib in patients with chemo-sensitive and chemo-refractory relapsed small cell cancers: a Hoosier Oncology Group phase II trial. Lung Cancer 2006;52:93–7. [DOI] [PubMed] [Google Scholar]
- [35].Jiang J, Liang X, Zhou X, et al. A meta-analysis of randomized controlled trials comparing irinotecan/platinum with etoposide/platinum in patients with previously untreated extensive-stage small cell lung cancer. J Thorac Oncol 2010;5:867–73. [DOI] [PubMed] [Google Scholar]
- [36].Lucchi M, Mussi A, Fontanini G, et al. Small cell lung carcinoma (SCLC): the angiogenic phenomenon. Eur J Cardiothorac Surg 2002;21:1105–10. [DOI] [PubMed] [Google Scholar]
- [37].Capelletto E, Novello S, Scagliotti GV. First-line therapeutic options for advanced non-small-cell lung cancer in the molecular medicine era. Future Oncol 2014;10:1081–93. [DOI] [PubMed] [Google Scholar]
- [38].Tanno S, Ohsaki Y, Nakanishi K, et al. Human small cell lung cancer cells express functional VEGF receptors, VEGFR-2 and VEGFR-3. Lung Cancer 2004;46:11–9. [DOI] [PubMed] [Google Scholar]
- [39].Tas F, Duranyildiz D, Oguz H, et al. Serum vascular endothelial growth factor (VEGF) and interleukin-8 (IL-8) levels in small cell lung cancer. Cancer Investig 2006;24:492–6. [DOI] [PubMed] [Google Scholar]
- [40].Lin H, Li L, Luo S, et al. Efficacy and safety of angiogenesis inhibitors in small-cell lung cancer. Oncotarget 2017;8:1141–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer 2008;8:592–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Xiao YY, Zhan P, Yuan DM, et al. Chemotherapy plus multitargeted antiangiogenic tyrosine kinase inhibitors or chemotherapy alone in advanced NSCLC: a meta-analysis of randomized controlled trials. Eur J Clin Pharmacol 2013;69:151–9. [DOI] [PubMed] [Google Scholar]
- [43].Tian RH, Wu X, Liu X, et al. The role of angiogenesis inhibitors in the treatment of elderly patients with advanced non-small-cell lung cancer: A meta-analysis of eleven randomized controlled trials. J Cancer Res Ther 2016;12:571–5. [DOI] [PubMed] [Google Scholar]
- [44].Sheng J, Yang Y, Ma Y, et al. The efficacy of combining antiangiogenic agents with chemotherapy for patients with advanced non-small cell lung cancer who failed first-line chemotherapy: a systematic review and meta-analysis. PloS one 2015;10: e0127306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zhao L, Li W, Zhang H, et al. Angiogenesis inhibitors rechallenge in patients with advanced non-small-cell lung cancer: a pooled analysis of randomized controlled trials. Onco Targets Ther 2015;8:2775–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


