Abstract
The aim of this study was to define factors associated with HIV-infected versus uninfected patients with invasive nontyphoidal Salmonella (iNTS) and factors associated with mortality, which are inadequately described in Africa.
Laboratory-based surveillance for iNTS was undertaken. At selected sentinel sites, clinical data (age, sex, HIV status, severity of illness, and outcome) were collected.
Surveillance was conducted in Gauteng, South Africa, from 2003 to 2013. Clinical and microbiological differences between HIV-infected and uninfected patients were defined and risk factors for mortality established.
Of 4886 iNTS infections in Gauteng from 2003 to 2013, 3106 (63.5%) were diagnosed at sentinel sites. Among persons with iNTS infections, more HIV-infected persons were aged ≥5 years (χ2 = 417.6; P < 0.001) and more HIV-infected children were malnourished (χ2 = 5.8; P = 0.02). Although 760 (30.6%) patients died, mortality decreased between 2003 [97/263 (36.9%)] and 2013 [926/120 (21.7%)]. On univariate analysis, mortality was associated with patients aged 25 to 49 years [odds ratio (OR) = 2.2; 95% confidence interval (CI) = 1.7–2.7; P < 0.001 and ≥50 years (OR = 3.0; 95% CI = 2.2–4.1; P < 0.001) compared with children < 5 years, HIV-infected patients (OR = 2.4; 95% CI = 1.7–3.4; P < 0.001), and severe illness (OR = 5.4; 95% CI = 3.6–8.1; P < 0.001). On multivariate analysis, mortality was associated with patients aged ≥50 years [adjusted OR (AOR) = 3.6, 95% CI = 2.1–6.1, P < 0.001] and severe illness (AOR = 6.3; 95% CI = 3.8–10.5; P < 0.001).
Mortality due to iNTS in Gauteng remains high primarily due to disease severity. Interventions must be aimed at predisposing conditions, including HIV, other immune-suppressive conditions, and malignancy.
Keywords: AIDS, HIV, invasive nontyphoidal Salmonella, malnutrition, mortality, severity of illness, South Africa
1. Introduction
Invasive nontyphoidal Salmonella (iNTS) infections are an important cause of morbidity and mortality in Africa. According to recent WHO Foodborne diseases Epidemiology Reference Group (FERG) estimates, there were approximately 600,000 iNTS infections, resulting in 63,000 deaths, globally in 2010[1]; FERG estimated that 78% of iNTS infections occurred in the WHO Africa Region.
Recent studies in several countries in Africa report high numbers of iNTS infections, commonly in association with malaria and HIV.[2–6] The multicenter Typhoid Fever Surveillance in Africa Program (TSAP) study concluded that malaria is associated with a 5 times higher odds of iNTS disease.[6] A number of studies have highlighted the high health burden of iNTS in children, frequently associated with malaria and malnutrition.[3,5] Much work has also focused on microbiological aspects of iNTS, including serotype and multidrug resistance (MDR),[4,5] or iNTS disease burden.[7] Several have called for development of nontyphoidal Salmonella vaccines.[8] Although there have been reports of decreasing incidence of iNTS infections in some parts of Africa,[7,9] mortality rates among persons with iNTS infections remain high[4,5,9] and factors associated with mortality among persons infected with iNTS are not well understood. A better understanding of disability and associated mortality of iNTS infections will inform decisions for control and prevention of iNTS infections.[8]
This study was undertaken to describe factors associated with mortality among persons infected with iNTS in Gauteng Province, South Africa, a largely industrialized urban population in a malaria-free area, with an HIV seroprevalence of 11%.[10] We also explored the differences in clinical and microbiological features of iNTS infections in HIV-uninfected versus HIV-infected individuals, as these data may inform further management of iNTS disease.
2. Methods
2.1. Laboratory-based surveillance for invasive nontyphoidal Salmonella
Between 2003 and 2013, the Centre for Enteric Diseases (CED) conducted active laboratory-based surveillance for iNTS in all clinical diagnostic laboratories in Gauteng Province, South Africa. Invasive disease was defined as the isolation of nontyphoidal Salmonella from a normally sterile body site. Laboratory audits for additional iNTS cases in Gauteng Province were conducted by reviewing data in the Central Data Warehouse (CDW) of the National Health Laboratory Service (NHLS), which stores results for all microbiology tests done by the NHLS.[11] In selected sites, patients with iNTS infections had additional clinical information collected regarding HIV status, other comorbid conditions [prematurity, protein-energy malnutrition (PEM) in children, malignancy, autoimmune disorders, severe hepatic or renal disease], antimicrobial management, severity of illness [utilizing the Pitt bacteremia score (PBS) ≥4 to define severe infection],[12] and outcome (recovery vs death). Informed consent was obtained from adult patients capable of providing clinical information or by parents or guardians in the case of minors. Where record reviews were conducted, consent was obtained for the hospital superintendent. If the patient hospitalized for another reason acquired iNTS infection more than 48 hours during the current admission, the iNTS infection was defined as a nosocomial-acquired infection. For HIV-infected patients, we additionally documented cotrimoxazole prophylaxis, antiretroviral therapy (ART), and CD4+ lymphocyte counts. Laboratories were requested to submit all Salmonella isolated from normally sterile sites to CED for serotyping and antimicrobial susceptibility testing. All Salmonella isolates received were serotyped following standard operating procedures (Mast Group, Merseyside, UK; BioRad, Marnes-la-Coquette, France; Remel, Kent, UK; Statens Serum Institute, Copenhagen, Denmark). Minimum inhibitory concentrations (MICs) were determined for ampicillin, chloramphenicol, trimethoprim-sulfamethoxazole (cotrimoxazole), tetracycline, ciprofloxacin, and ceftriaxone, using E-test strips, according to the manufacturer's instructions (BioMérieux, Marcy-l’Étoile, France). MDR was defined as resistance to 3 or more of these antimicrobials.[13] Extended-spectrum β-lactamase (ESBL) production was measured using double disk testing, according to the manufacturer instructions (MAST Diagnostics, Bootie, UK). Data were recorded in an Access 2007 database (Microsoft Corporation, Redmond).
2.2. Statistical analysis
Incidence was calculated using population denominators derived from data published annually by the National Department of Statistics (www.statssa.gov.za). To estimate risk factors for mortality due to iNTS, we calculated odds ratios (ORs), 95% confidence intervals (CIs), and P values for age group, sex, HIV status, PBS, other comorbidities, serotype, MDR, and ESBL production. For multivariate analysis, we used logistic regression to calculate adjusted ORs (AORs). Patients with missing data were excluded from the analyses. The χ2 test was used to compare clinical and microbiological features of HIV-uninfected with HIV-infected patients with iNTS. Two-sided P values of < 0.05 were considered significant for all analyses. All statistical analyses were performed using STATA (College Station, Texas, USA) version 13 software.
2.3. Ethical review
Ethics approvals were obtained from the University of the Witwatersrand Human Research Ethics Committee (HREC) (M110601, granted June 24, 2011).
3. Results
We recorded 4886 laboratory-confirmed iNTS infections in Gauteng Province from January 2003 to December 2013, of which 334 (6.8%) were identified using CDW data. Of 4728 (96.8%) iNTS-infected patients for whom sex was recorded, 2478 (52.4%) were male; 4661 (95.4%) patients had age recorded, ranging from 0 days (newborn) to 93 years, with a median of 32 years (Fig. 1). The highest incidence of iNTS infection was in children aged <5 years, averaging 10.9 per 100,000 population per year during 2003 to 2013. The lowest incidence rate was in children aged 5 to 14 years (averaging 0.9 per 100,000 population per year).
Figure 1.
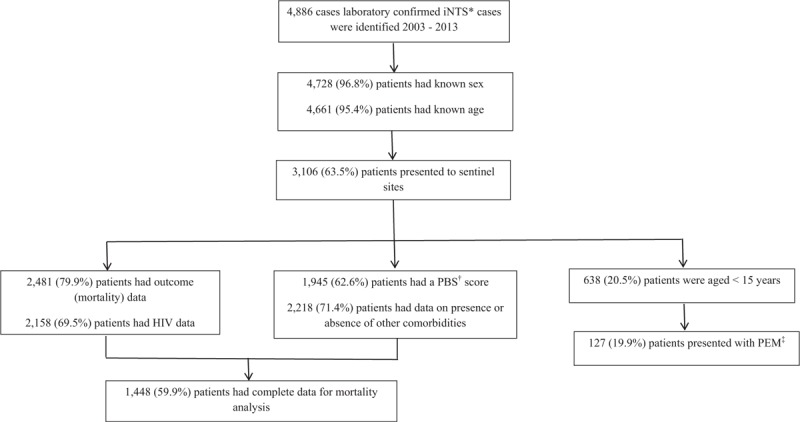
Flow chart indicating the breakdown of number of cases available for analysis and primary data for analysis. iNTS = invasive nontyphoidal Salmonella, PBS = Pitt bacteremia score, ‡PEM = protein energy malnutrition.
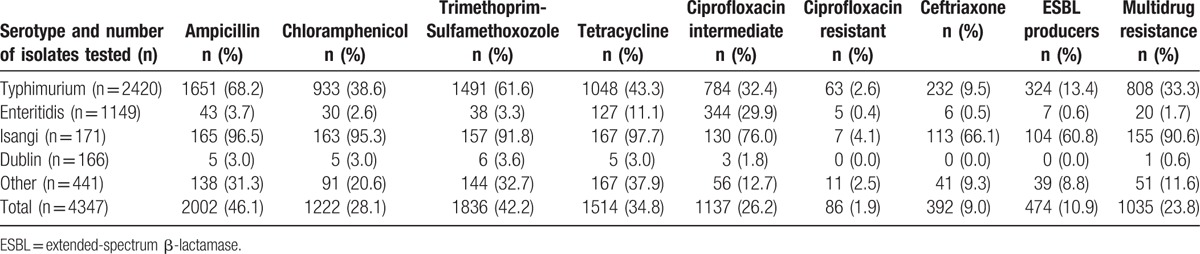
Serotyping was completed on 4459 Salmonella isolates: the most common serotypes were Salmonella enterica serovar Typhimurium (Salmonella Typhimurium) [2469 (55.4%)]; Salmonella Enteritidis [1156 (25.9%)]; Salmonella Isangi [175 (3.9%)]; and Salmonella Dublin [170 (3.8%)]. Antimicrobial susceptibility testing results were available for 4347 (97.5%) isolates: 1035 (23.8%) isolates were MDR, of which 381 (36.8%) were ESBL producers (Table 1). Salmonella Isangi [155/171 (90.6%)] had the highest prevalence of MDR among the most common serotypes, followed by Salmonella Typhimurium [808/2240 (36.1%)]. MDR among Salmonella Enteritidis (20/1149 (1.7%]) and Salmonella Dublin [1/165 (0.6%)] isolates was low.
Table 1.
Serotyping and antimicrobial resistance for invasive nontyphoidal Salmonella isolates (N = 4347), Gauteng Province, South Africa, 2003–2013.
3.1. Sentinel site data
Additional data from the sentinel sites were available for 3106 (63.5%) iNTS-infected patients (Fig. 1). Outcome data were available for 2481 (79.9%) iNTS-infected patients: 760 (30.6%) patients died (Table 2), 268 (35.3%) within 2 days of admission. Mortality decreased over the time period [2003: 97/263 (36.9%); 2004: 114/301 (37.9%); 2005: 107/302 (35.4%); 2006: 113/311 (36.3%); 2007: 61/248 (24.6%); 2008: 90/304 (29.6%); 2009: 54/192 (28.1%); 2010: 36/170 (21.2%); 2011: 39/147 (26.5%); 2012: 23/123 (18.7%); 2013: 26/120 (21.7%)]. Comparing 2003 to 2005 with 2006 to 2013, there was a significant decline in mortality among iNTS-infected persons [2003–2005: 318/866 (36.7%) vs 2006–2013: 442/1615 (27.3%); χ2 = 18.6; P < 0.001].
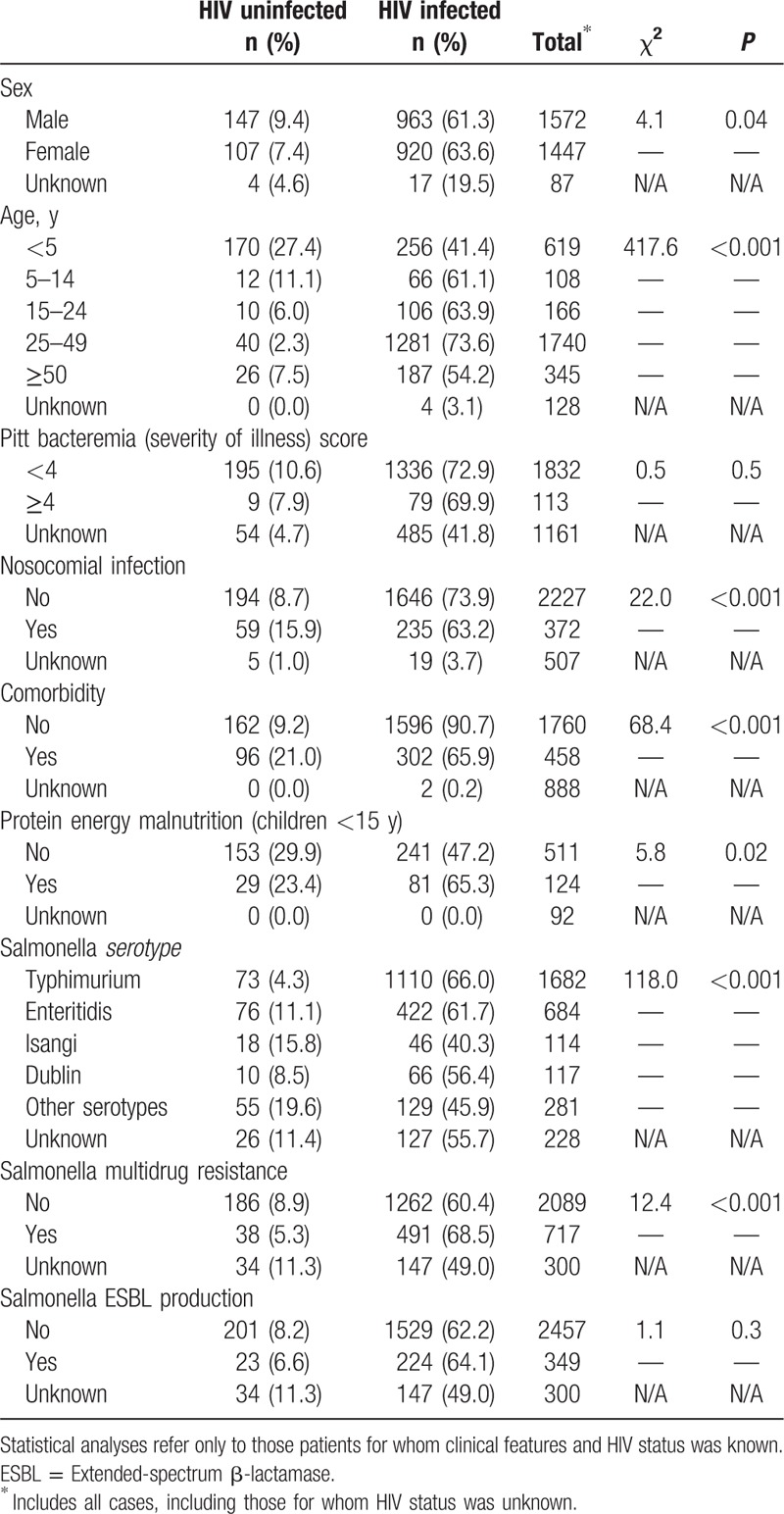
Table 2.
Clinical and microbiological features associated with HIV infection and invasive salmonellosis in Gauteng province South Africa, 2003–2013 in patients presenting to sentinel sites (N = 3106).
Of the patients with iNTS infections with available data, 1900 of 2158 (88.0%) were HIV-infected, 113 of 1945 (5.8%) had a PBS ≥4, 372 of 2599 (14.3%) had a nosocomial-acquired iNTS infection, 458 of 2218 (20.6%) presented with other comorbidities, and 127 of 638 (19.9%) children presented with PEM (Fig. 1). Among 1900 patients with iNTS infections who were HIV-infected, CD4+ counts were available for 1308 (68.8%) patients: 663 patients (50.7%) had a CD4+ count between 0 and 50 cells/mm3, 217 (16.6%) had a CD4+ count between 51 and 100 cells/mm3, 239 (18.3%) had a CD4+ count between 101 and 200 cells/mm3, and 93 (7.1%) had a CD4+ count between 201 and 350 cells/mm3; 293 of 1457 (20.1%) patients had a previous or current history of ART use, including perinatal antiretrovirals [15 patients (5.1%)] and 426 of 1354 (31.5%) patients were on cotrimoxazole.
3.2. HIV-infected versus HIV-uninfected patients
Among persons infected with iNTS, comparing clinical features of HIV-uninfected with HIV-infected individuals, there were significant differences in age range: a significantly smaller proportion of HIV-infected patients were aged <5 years [HIV-infected: 256/1896 (13.5%) vs HIV-uninfected: 170/258 (65.9%); χ2 = 417.6; P < 0.001] (Table 2). HIV-infected individuals were less likely to present with nosocomially acquired iNTS infection [HIV-infected: 235/1881 (12.5%) vs HIV-uninfected: 59/253 (23.3%); χ2 = 22.0; P < 0.001] and were less likely to have other comorbid conditions [HIV-infected: 302/1898 (15.9%) vs HIV-uninfected: 96/258 (37.2%); χ2 = 68.4; P < 0.001] (Table 2). HIV-infected children presenting with iNTS were also more likely to be diagnosed with PEM [HIV-infected: 81/322 (25.2%) vs HIV-uninfected: 29/182 (15.9%); χ2 = 5.8; P = 0.02]. Among persons infected with iNTS, there was no significant difference, however, between severity of illness (PBS score ≥4) between the 2 groups [HIV-infected: 79/1415 (5.6%) vs HIV-uninfected: 9/204 (4.4%); χ2 = 0.5; P = 0.5] (Table 2). HIV-infected patients were also more likely to present with invasive Salmonella Typhimurium than other serotypes [HIV-infected: 1110/1773 (62.6%) vs HIV-uninfected: 73/232 (31.5%); χ2 = 118.0, P < 0.001] or to present with MDR Salmonella isolates [HIV-infected: 491/1753 (28.0%) vs HIV-uninfected: 38/224 (16.9%); χ2 = 12.4; P < 0.001] (Table 2). HIV-infected individuals were less likely to be infected with invasive Salmonella Enteritidis [HIV-infected: 422/1773 (23.8%) vs HIV-uninfected: 76/232 (32.8%); χ2 = 8.8, P = 0.003].
3.3. Risk factors for mortality
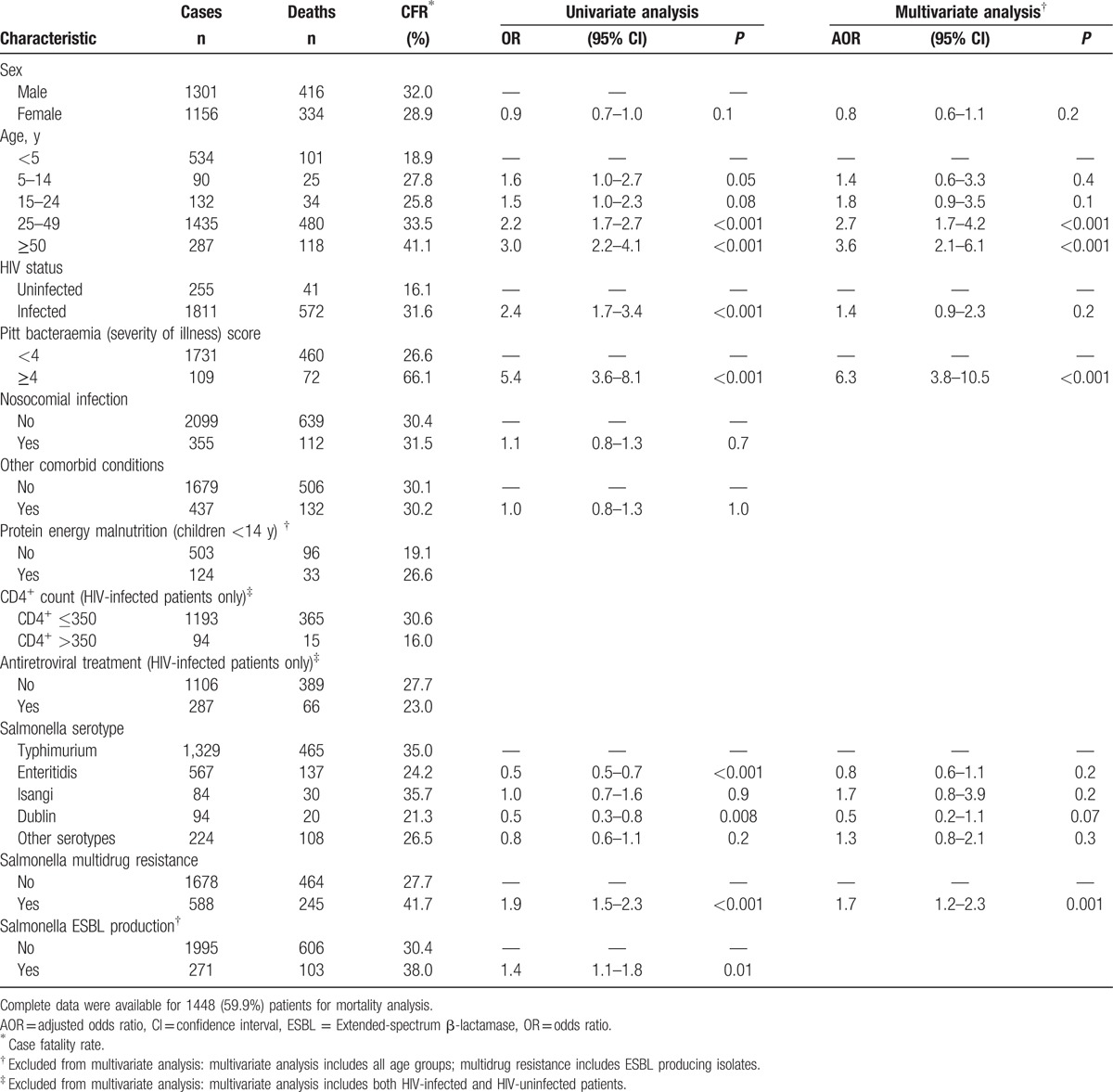
Complete clinical and microbiological data were available for 1448 of 2481 (59.9%) patients for mortality analysis. On univariate analysis, mortality among persons with iNTS infection was associated with patients aged 25 to 49 years compared with children aged <5 years (OR = 2.2; 95% CI = 1.7–2.7; P < 0.001) and with those ≥50 years of age compared with children aged <5 years (OR = 3.0; 95% CI = 2.2–4.1; P < 0.001), HIV-infected patients (OR = 2.4; 95% CI = 1.7–3.4; P < 0.001) compared with HIV-uninfected patients, more severe illness (PBS ≥4) (OR = 5.4; 95% CI = 3.6–8.1; P < 0.001), and infection with a MDR Salmonella (OR = 1.9; 95% CI = 1.5–2.3; P < 0.001) (Table 3). On multivariate analysis, mortality among iNTS-infected persons was associated with patients ≥50 years of age [adjusted OR (AOR) = 3.6, 95% CI = 2.1–6.1, P < 0.001], PBS ≥4 (AOR = 6.3; 95% CI = 3.8–10.5; P < 0.001), and infection with MDR Salmonella (AOR = 1.7, 95% CI = 1.2–2.3, P = 0.001), but not associated with being HIV-infected (AOR = 1.4; 95% C = 0.9–2.3; P = 0.2) (Table 3). Analyzing data on PEM in children, these patients were not significantly more likely to die than children who did not have PEM (OR = 1.5; 95% CI = 1.0–2.4; P = 0.07) (Table 3). Among HIV-infected patients, those with CD4+ counts ≤350 were significantly more likely to die (OR = 0.4; 95% CI = 0.2–0.7; P = 0.004), but a history of ART use did not significantly impact outcome (OR = 0.8; 95% CI = 0.6–1.1, P = 0.1)
Table 3.
Risk factors for mortality among persons with invasive nontyphoidal Salmonella with known outcome (N = 2481) in Gauteng Province, South Africa, 2003–2013.
Reviewing information on antimicrobial management of patients, 2138 of 3106 (68.8%) of all sentinel site patients and 575 of 760 (75.7%) of patients who died had data collected. Individual review of clinical case report forms revealed inconsistent data collection regarding prescribing and administration of antimicrobials. Many patients were prescribed multiple antibiotics and prescriptions were changed during the admission.
4. Discussion
Invasive salmonellosis remains a challenge in Africa, due to a number of significant predisposing factors, including malaria, PEM in children, and HIV infection.[5,14,15] In South Africa, the major contributing factor to invasive salmonellosis is HIV infection.[16] However, factors associated with mortality in patients infected with iNTS have not previously been well described.
We examined disease burdens of iNTS in the predominantly urbanized population of Gauteng between 2003 and 2013, recording a significant decrease in the numbers of iNTS cases, following the roll out of ART in 2004.[11] Incidence for iNTS was highest among children aged <5 years and adults aged 25 to 49 years, highlighting the vulnerability of the former and the predominance of HIV-associated infections in the latter, supporting data from previous studies and systematic analyses.[15,17] Similar to other African studies, the predominant serotypes were Salmonella Typhimurium and Salmonella Enteritidis. We additionally identified increased numbers of Salmonella Isangi and Salmonella Dublin. Salmonella Enteritidis and Salmonella Dublin isolate were predominantly susceptible to the antimicrobials tested, but in common with other African studies, MDR was common in Salmonella Typhimurium[4,5] and Salmonella Isangi. Reports from Mali indicate that MDR occurred in >90% and 40% of Salmonella Typhimurium and Salmonella Enteritidis, respectively.[4] Conversely, reports from Kenya suggest that 36% to 77% of invasive Salmonella Typhimurium[5,18] and 30% of invasive Salmonella Enteritidis are multidrug resistant.[18] In the Democratic Republic of the Congo, MDR occurs in >80% of these 2 serotypes.[19] Interestingly, MDR was low in Salmonella Dublin in Mali,[4] similar to our findings. The predominance of Salmonella Typhimurium and Salmonella Enteritidis in Africa has highlighted these pathogens as primary serotypes for Salmonella vaccine development. Rapidly burgeoning MDR is making vaccine development an important imperative.
There were additional differences between HIV-infected and HIV-uninfected individuals presenting with iNTS. Children aged less than 5 years with iNTS infections were less likely to be HIV infected.[20] In addition, more HIV-uninfected patients with iNTS had comorbidities including chronic organ failure or malignancy, highlighting noncommunicable diseases as potential risk factor for iNTS infection. Although HIV-uninfected patients presented more frequently with nosocomial-acquired iNTS infections, this study did not examine the role of HIV clinics in patients with iNTS. Data on long-term care facilities were also not always forthcoming; thus, this finding may not be truly reflective, as some HIV-infected patients in particular may have acquired iNTS while in these care facilities. Nosocomial salmonellosis is well described in South Africa, often affecting children[21,22] and nonetheless warrants careful monitoring. In contrast to invasive shigellosis,[23] we did not see an excess of adult women presenting with iNTS: intrinsic characteristics of Salmonella, in HIV-infected patients in particular, appear to be at play in the context of invasive disease, beyond human behavioral factors. This has already been shown for Salmonella Typhimurium ST313[24,25] and Salmonella Enteritidis.[4,26] Further differences between HIV-infected versus uninfected individuals included Salmonella serotype: a significantly higher proportion of HIV-uninfected individuals presented with invasive Salmonella Enteritidis. This lesser association of Salmonella Enteritidis with HIV infection may partly explain why mortality due to this pathogen was significantly lower than Salmonella Typhimurium. This contrasts with observations of Tapia et al,[4] who found a significantly higher case fatality of 27.8% associated with Salmonella Enteritidis than Salmonella Typhimurium in Mali, possibly in relation to a highly virulent clone in that country.[4] HIV-infected patients in our series were more likely to be infected with multidrug-resistant pathogens. This could be ascribed to their multiple exposures to antibiotics associated with management of other opportunistic infections.[27] Severity of illness (PBS ≥4), however, was comparable between the 2 groups: irrespective of the primary illness, immune-suppressive conditions predispose patients to iNTS.
Other researchers have highlighted the importance of underlying immunosuppressive disease, other than HIV, as the most important risk factor in adult patients for iNTS. Older patients, neonates, and isolation of invasive serotypes were identified as risk factors for iNTS infection in HIV-uninfected patients.[28] Malaria and malnutrition are important risk factors for iNTS in children.[9] The excessive numbers of children in our series with iNTS who were HIV-infected with PEM is concerning. HIV infection has been associated with undernourishment in children aged <5 years in South Africa,[29,30] and clearly remains problematic. This may be directly due to an HIV-associated effect in our study or due to other factors such as maternal illness or death.[30] This may also be indicative of ongoing failures in nutritional programs in primary health care.[31] Feasey et al[9] have clearly shown that iNTS rates decrease when childhood nutrition programs are introduced and a greater emphasis may be needed on such programs in South Africa.
We elected to analyze data for children aged less than 5 years separately from those aged 5 to 14 years to correspond to incidence data that have been calculated for iNTS and ART programs in Gauteng Province, South Africa.[11] First, the younger group is predisposed to higher mortality due to foodborne diseases, including salmonellosis.[1] Second, differences in the HIV rates and presentation with iNTS between these 2 age groups may impact analyses: HIV-infected children surviving beyond 5 years of age was an unusual observation before the ART roll-out. Many of these patients in our series had associated malignancies or other predisposing immune-suppressive conditions as opposed to HIV infection. We have shown that iNTS incidence rates in children aged <5 years correlate poorly with ART treatment programs.[11] (Keddy et al, submitted). Additional factors, such as HIV exposure in HIV-uninfected infants born to HIV-infected mothers, may also be at play, as has been postulated for neonatal Salmonella meningitis.[20]
We characterized risk factors for mortality due to iNTS, with a view to highlighting interventions to decrease mortality rates. Mortality among persons infected with iNTS has decreased significantly after the introduction of ART, in other African studies[32] and we have observed a similar trend, but clinical risk factors needed clarification. HIV infection on univariate analysis, and severity of illness (PBS ≥4), adults ≥25 years, and isolation of MDR Salmonella on multivariate analysis, were associated with excessive mortality. Older age and disease severity were the most significant predictors of a poor outcome. Excessive mortality occurs in patients with severely immunosuppressive conditions predisposing to iNTS, besides HIV infection, including malignancy, organ failure, and prematurity.[28] In HIV-infected persons, we found that mortality was associated with patients with a low CD4+ count, who also bore the greatest burden of illness, but not with ART use, supporting global initiatives for earlier initiation of ART.[33]
This study had some limitations. First, although we attempted to comprehensively identify the majority of the Gauteng iNTS cases during the study period, it is possible that some were not identified by our surveillance system, which focused primarily on patients presenting to public hospitals. Indications and use of blood cultures are unlikely to have changed over the period, however, and the majority of cases (∼60%) were identified in sentinel surveillance hospitals. This study also did not look at the impact of delayed or inappropriate antimicrobial therapy on mortality, as these data were not sufficiently or were inconsistently collected and over 30% of patients died within 48 hours of admission, before culture results and susceptibilities would have become available. Complete clinical information was not available for all cases at sentinel hospitals, but total case numbers were large. This should minimize the effect on the statistical analysis. Similarly, missing isolates constituted <10% of those characterized; we believe that the serotyping and drug resistance data are representative and it is unlikely those iNTS cases that were identified on audit would have significantly altered our findings.
In conclusion, we showed although mortality due to iNTS in South Africa has decreased, it remains high irrespective of the patients’ HIV status. We additionally noted a concerning increase in invasive cases of Salmonella Enteritidis across all age groups,[11] for reasons that are not well understood and needing further elucidation. These observations, in combination with the prevalence of multidrug-resistant Salmonella Typhimurium in Africa, support the need for the development of vaccines for these serotypes. As ART becomes even more readily available in South Africa, with more patients and older patients accessing antiretroviral programs, we may see a change in disease patterns of iNTS to more closely resemble those of the developed world.[15]
Acknowledgments
We would like to thank our many partners in the GERMS-SA (previously Group for Enteric, Respiratory and Meningeal Disease Surveillance in South Africa), National Institute for Communicable Diseases (NICD), a division of the NHLS, for submission of isolates to CED.
Footnotes
Abbreviations: AOR = Adjusted odds ratio, ART = Antiretroviral therapy, CDW = Central Data Warehouse, CED = Centre for Enteric Diseases, CI = Confidence interval, ESBL = Extended-spectrum beta lactamase, FERG = Foodborne diseases Epidemiology Reference Group, HIV = Human immunodeficiency virus, iNTS = Invasive nontyphoidal Salmonella, MDR = Multidrug resistance, MIC = Minimum inhibitory concentrations, NHLS = National Health Laboratory Service, OR = Odds Ratio, PBS = Pitt bacteremia score, PEM = Protein energy malnutrition, TSAP = Typhoid fever surveillance in Africa program.
Authorship: All authors contributed to the writing of this manuscript and reviewed the final content. KHK, FJA, and KPK designed the study; KHK and AS developed the database; KK and AK were responsible for reviewing clinical data; AS, TN, SS, MN, and RL were responsible for the laboratory characterization of Salmonella isolates: KHK and AM completed the data analysis.
Funding/support: This research has been supported by NICD/NHLS and the President's Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention (CDC) under the terms of [5U2GPS001328] and, in part, for 2003 to 2006 by funds from the United States Agency for International Development's Antimicrobial Resistance Initiative, transferred via a cooperative agreement [number U60/CCU022088] from the Centers for Disease Control and Prevention (CDC), Atlanta, Georgia. For 2007 to 2009, it was supported by the HHS Centers for Disease Control and Prevention (CDC), National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention (NCHHSTP), Global AIDS Program (GAP) Cooperative Agreement [U62/PSO022901]. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NICD/NHLS or CDC. KHK, AS, TN, SS, MN, and RL are permanent employees of the NHLS and receive no additional funding from other institutions resulting in a conflict of interest. AK is a permanent employee of Gauteng Department of Health and receives no additional funding from other institutions resulting in a conflict of interest. AM is employed by South Africa Global Disease Detection Centre, Centers for Disease Control and Prevention, Pretoria, South Africa. FJA is staff at the Centers for Disease Control and Prevention, Atlanta, GA, and KPK is employed by the Bill and Melinda Gates Foundation, Seattle, WA.
GERMS-SA includes Carel Haummann, Patricia Hanise, Pieter Ekermans; Sandeep Vasaikar (Eastern Cape); Anwar Hoosen, Dominique Goedhals, Justyna Wojno, Madeleine Pieters (Free State); Alan Karstaedt, Caroline Maluleka, Charl Verwey, Charles Feldman, Jeannette Wadula, Kathy Lindeque, Norma Bosman, Ranmini Kularatne, Theunis Avenant, Nicolette du Plessis, Vindana Chibabhai (Gauteng); Asmeeta Burra, Constant Kapongo, Fathima Naby, Halima Dawood, Koleka Mlisana, Lisha Sookan, Praksha Ramjathan, Prasha Mahabeer, Romola Naidoo, Sumayya Haffejee, Yacoob Coovadia (Kwa-Zulu Natal); Andries Dreyer, Ken Hamese (Limpopo); Greta Hoyland, Jacob Lebudi (Mpumalanga); Dhamiran Naidoo, Eunice Weenink; Riezaah Abrahams (Northern Cape); Ebrahim Variava, Eduard Silberbauer (North West); Catherine Samuel, Preneshni Naicker (Western Cape); Adrian Brink, Charlotte Sriruttan, Inge Zietsman, Maria Botha, Peter Smith, Xoliswa Poswa (AMPATH); Chetna Govind, Keshree Pillay, Suzy Budavari, (LANCET); Marthinus Senekal (PathCare); Cynthia Whitney, Stephanie Schrag, Jennifer Verani (CDC); Ananta Nanoo, Anne von Gottberg, Cecilia Miller, Cheryl Cohen, Claire von Mollendorf, Languta Sibiya, Linda de Gouveia, Linda Erasmus, Mmakgomo Rakhudu, Marshagne Smith, Melony Fortuin-de Smidt, Nazir Ismail, Nelesh Govender, Nevashan Govender, Nireshni Naidoo, Olga Perovic, Ruth Mpembe, Sarona Lengana, Sonwabo Lindani, Penny Crowther-Gibson, Susan Meiring, Vanessa Quan (NICD).
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention (CDC).
The authors report no conflicts of interest.
References
- [1].Kirk MD, Pires SM, Black RE, et al. World Health Organization estimates of the global and regional disease burden of 22 foodborne bacterial, protozoal, and viral diseases, 2010: a data synthesis. PLoS Med 2015;12:e1001921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Feasey NA, Masesa C, Jassi C, et al. Three epidemics of invasive multidrug-resistant salmonella bloodstream infection in Blantyre, Malawi, 1998-2014. Clin Infect Dis 2015;61Suppl 4:S363–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mandomando I, Bassat Q, Sigauque B, et al. Invasive salmonella infections among children from rural Mozambique, 2001-2014. Clin Infect Dis 2015;61Suppl 4:S339–45. [DOI] [PubMed] [Google Scholar]
- [4].Tapia MD, Tennant SM, Bornstein K, et al. Invasive nontyphoidal salmonella infections among children in Mali, 2002-2014: microbiological and epidemiologic features guide vaccine development. Clin Infect Dis 2015;61Suppl 4:S332–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Muthumbi E, Morpeth SC, Ooko M, et al. Invasive salmonellosis in Kilifi, Kenya. Clin Infect Dis 2015;61Suppl 4:S290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Park SE, Pak GD, Aaby P, et al. The relationship between invasive nontyphoidal salmonella disease, other bacterial bloodstream infections, and malaria in sub-Saharan Africa. Clin Infect Dis 2016;62Suppl 1:S23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Verani JR, Toroitich S, Auko J, et al. Burden of invasive nontyphoidal salmonella disease in a rural and urban site in Kenya, 2009-2014. Clin Infect Dis 2015;61Suppl 4:S302–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Crump JA, Heyderman RS. A perspective on invasive salmonella disease in Africa. Clin Infect Dis 2015;61Suppl 4:S235–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Feasey NA, Everett D, Faragher EB, et al. Modelling the contributions of malaria, HIV, malnutrition and rainfall to the decline in paediatric invasive non-typhoidal salmonella disease in Malawi. PLoS Negl Trop Dis 2015;9:e0003979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Actuarial Society of South Africa. ASSA Provincial Output_110216. Available at: www.actuarialsociety.org.za. Accessed August 1, 2012. [Google Scholar]
- [11].Keddy KH, Takuva S, Musekiwa A, et al. An association between decreasing incidence of invasive non-typhoidal salmonellosis and increased use of antiretroviral therapy, Gauteng Province, South Africa, 2003 – 2013. PLOS One 2017;12:e0173091.doi: 10.1371/journal.pone.0173091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Feldman C, Alanee S, Yu VL, et al. Severity of illness scoring systems in patients with bacteraemic pneumococcal pneumonia: implications for the intensive care unit care. Clin Microbiol Infect 2009;15:850–7. [DOI] [PubMed] [Google Scholar]
- [13].Clinical Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fifth Informational Supplement. Pennsylvania: Clinical Laboratory Standards Institute; 2015. [Google Scholar]
- [14].Feasey NA, Dougan G, Kingsley RA, et al. Invasive non-typhoidal salmonella disease: an emerging and neglected tropical disease in Africa. Lancet 2012;379:2489–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ao TT, Feasey NA, Gordon MA, et al. Global burden of invasive nontyphoidal salmonella disease, 2010(1). Emerg Infect Dis 2015;21:941–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Keddy KH, Dwarika S, Crowther P, et al. Genotypic and demographic characterization of invasive isolates of Salmonella Typhimurium in HIV co-infected patients in South Africa. J Infect Dev Ctries 2009;3:585–92. [DOI] [PubMed] [Google Scholar]
- [17].Feasey NA, Archer BN, Heyderman RS, et al. Typhoid fever and invasive nontyphoid salmonellosis, Malawi and South Africa. Emerg Infect Dis 2010;16:1448–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kariuki S, Onsare RS. Epidemiology and genomics of invasive nontyphoidal salmonella infections in Kenya. Clin Infect Dis 2015;61Suppl 4:S317–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kalonji LM, Post A, Phoba MF, et al. Invasive salmonella infections at multiple surveillance sites in the Democratic Republic of the Congo, 2011-2014. Clin Infect Dis 2015;61Suppl 4:S346–53. [DOI] [PubMed] [Google Scholar]
- [20].Keddy KH, Sooka A, Musekiwa A, et al. Clinical and microbiological features of Salmonella meningitis in a South African population, 2003-2013. Clin Infect Dis 2015;61Suppl 4:S272–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Smith AM, Mthanti MA, Haumann C, et al. Nosocomial outbreak of Salmonella enterica serovar Typhimurium primarily affecting a pediatric ward in South Africa in 2012. J Clin Microbiol 2014;52:627–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wadula J, von GA, Kilner D, et al. Nosocomial outbreak of extended-spectrum beta-lactamase-producing Salmonella Isangi in pediatric wards. Pediatr Infect Dis J 2006;25:843–4. [DOI] [PubMed] [Google Scholar]
- [23].Keddy KH, Sooka A, Crowther-Gibson P, et al. Systemic shigellosis in South Africa. Clin Infect Dis 2012;54:1448–54. [DOI] [PubMed] [Google Scholar]
- [24].Carden S, Okoro C, Dougan G, et al. Non-typhoidal Salmonella Typhimurium ST313 isolates that cause bacteremia in humans stimulate less inflammasome activation than ST19 isolates associated with gastroenteritis. Pathog Dis 2015;73:ftu023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Okoro CK, Barquist L, Connor TR, et al. Signatures of adaptation in human invasive Salmonella Typhimurium ST313 populations from sub-Saharan Africa. PLoS Negl Trop Dis 2015;9:e0003611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Feasey NA, Hadfield J, Keddy KH, et al. Distinct Salmonella Enteritidis lineages associated with enterocolitis in high-income settings and invasive disease in low-income settings. Nat Genet 2016;48:1211–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Madhi SA, Petersen K, Madhi A, et al. Increased disease burden and antibiotic resistance of bacteria causing severe community-acquired lower respiratory tract infections in human immunodeficiency virus type 1-infected children. Clin Infect Dis 2000;31:170–6. [DOI] [PubMed] [Google Scholar]
- [28].Chiu CH, Lin TY, Ou JT. Predictors for extraintestinal infection of non-typhoidal Salmonella in patients without AIDS. Int J Clin Pract 1999;53:161–4. [PubMed] [Google Scholar]
- [29].Horwood C, Butler LM, Vermaak K, et al. Disease profile of children under 5 years attending primary health care clinics in a high HIV prevalence setting in South Africa. Trop Med Int Health 2011;16:42–52. [DOI] [PubMed] [Google Scholar]
- [30].Saloojee H, De MT, Garenne ML, et al. What's new? Investigating risk factors for severe childhood malnutrition in a high HIV prevalence South African setting. Scand J Public Health Suppl 2007;69:96–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bourne LT, Hendricks MK, Marais D, et al. Addressing malnutrition in young children in South Africa. Setting the national context for paediatric food-based dietary guidelines. Matern Child Nutr 2007;3:230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Feasey NA, Houston A, Mukaka M, et al. A reduction in adult blood stream infection and case fatality at a large African hospital following antiretroviral therapy roll-out. PLoS One 2014;9:e92226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Abdool Karim SS. Overcoming impediments to global implementation of early antiretroviral therapy. N Engl J Med 2015;373:875–6. [DOI] [PubMed] [Google Scholar]


