Abstract
The relationship of oestrogen receptor with benign prostatic hyperplasia (BPH) and prostate cancer (PC) is not clear at present. This study aimed to investigate the molecular mechanism underlying the occurrence and development of BPH and prostate.
Two hundred forty-four PC cases, 260 BPH patients, and 222 healthy men were recruited from Han people in China, and the oestrogen receptor alpha (ESRα) gene polymorphism (rs2234693 [PvuII] and rs9340799 [XbaI]) on intron 1 was determined. The relationship of gene polymorphism with PC and BPH was evaluated with Logistic regression, and the linkage disequilibrium and haplotyping were assessed with SHEsis software.
The risk for PC in BPH patients with PvuII C allele was higher (OR = 1.437, 95% CI: 1.110–1.859), but the differentiation degree of cancer cells was relatively better in PC patients with PvuII C allele (OR = 0.419, 95% CI: 0.285–0.616), and most of them are circumscribed (OR = 0.706, 95% CI: 0.485–1.02). There was significant linkage disequilibrium between PvuII and XbaI. The genotype TTAG not only induced BPH (OR = 6.260, 95% CI: 1.407–27.852), but increased the risk for PC (OR = 6.696, 95% CI: 1.504–29.801). However, the genotype TTAG in BPH patients had no relationship with the risk for PC (P > 0.05). Furthermore, men with haplotype TG were more likely to suffer PC (OR = 9.168, 95% CI: 2.393–35.119), but men with haplotype TA and enlarged prostate had a low risk for PC (OR = 0.708, 95% CI: 0.551–0.912).
These results show the relationship between ESRα gene polymorphism and susceptibility to PC and BPH in Chinese men, and the ethnic and regional difference as well.
Keywords: benign prostatic hyperplasia, gene polymorphisms, oestrogen receptor
1. Introduction
Prostate cancer (PC) is a common malignancy of male urinary system and its incidence varies significantly between ethnic groups and regions worldwide. The statistics from American Association for Cancer Research showed there were 241,740 new cases of PC and 28,170 patients died of PC in 2012. Among male tumors, PC is the second most common and its incidence in African Americans (241/100,000) is far higher than in Caucasians (149/100,000). In Asia, its incidence is relatively low (21/100,000 in Shanghai, China in 2005). However, the incidence of PC is increasing over year. Though a variety of studies have been conducted to investigate the aetiology of PC, the molecular mechanism underlying its pathogenesis is still poorly understood. Considering the similarity in the incidence between PC and benign prostatic hyperplasia (BPH) in the field of morbid physiology, BPH may be an alarm signal of PC in the early period.[1–3] However, BPH and PC are 2 absolutely separate diseases, and epidemiological studies fail to show the significant relationship between BPH and PC either.[4,5] Generally, PC is derived from BPH.[6] Thus, to deeply investigate the epidemiological features of BPH will be helpful for the illustration of the pathogenesis of PC and BPH.
In recent years, studies have reported that oestrogen and its receptor play important roles in the etiology of PC and BPH.[7–9] About 30% of oestrogen in males is directly released by the sertoli cells of the testes, and 70% is as a result of conversion of androgen released by the adrenal gland and testes under the catalysis of aromatase. Thereafter, studies indicate that, age brings a gradual diminution of testes which causes a decrease of blood testosterone, but the oestrogen remains at the same level over age, leading to the increase in the ratio of oestrogen to androgen. Thus, oestrogen has been regarded as a major pathogenic factor of BPH and PC.[10–12] Oestrogen regulates and controls the growth and proliferation of prostatic cells by binding to the specific intranuclear receptor, oestrogen receptor (ESR).[13] ESR is one of the members of nuclear factor superfamily. After binding, the receptor is activated. ESR is divided into 2 types: ESR-α and ESR-β. ESR-α locates in the gap of epithelial cells and basilar membrane of the prostate, and ESR-β locates between epithelial gaps of the prostate.[14] Immunohistochemistry shows that, though ESRα is not expressed in epithelium of the normal prostate, it is strongly expressed in the prostate cells of BPH, PC tissues, LNCaP, and JCA-1 cells,[15–17] which implies that ESRα has essential relationship with the occurrence of BPH and PC.[18,19] Thus, to investigate the oestrogen acceptor may be helpful to elucidate the molecular mechanism of BPH and PC to a certain extent.
At present, the association of ESRα gene polymorphism with the risk for PC and BPH has not been reported in Chinese patients. In the present study, PCR-RFLP was employed to detect the single nucleotide polymorphisms (SNP) of ESRα gene (rs2234693 [PvuII] and rs9340799 [XbaI]) at intron 1, and their relationship with PC and BPH was further evaluated. Our results showed that, the unit point gene or allelic genes, composite gene with 2 sites or haplotype gene, of ESRα genes had a relationship with the occurrence and development of PC and BPH in Chinese patients. Therefore, our findings explain the differences of PC and BPH among ethnics and regions to a certain extent and provide evidence for the molecular mechanism underlying the pathogenesis of PC and BPH.
2. Methods
2.1. Subjects
A total of 244 PC patients, 260 BPH patients, and 222 healthy men were recruited between January 2012 and December 2014 in Zhejiang province and the peripheral blood was sampled. The age ranged from 47 to 90 years (median: 71.77 years) in PC patients, from 52 to 89 years (median: 71.28 years) in BPH patients, and from 48 to 83 years (median: 66.61 years) in healthy men. The diagnosis of PC and BPH was all confirmed by pathological results of the resected specimen (Table 1). According to Tumor Node Metastasis staging system developed by the American Joint Committee on Cancer Staging, PC was classified as nonmetastatic and metastatic PC. The pathological grade of PC was evaluated with Gleason score [20]: 2 to 6, intermediately or well differentiated adenocarcinoma; 7 to 10, poorly differentiated adenocarcinoma. For healthy men, the serum prostate-specific antigen (PSA) was <4.0 μg/μL, prostate was normal as shown by ultrasound examination and there were no clinical manifestations of PC and BPH.
Table 1.
Clinical and demographic characteristics of participants at baseline.
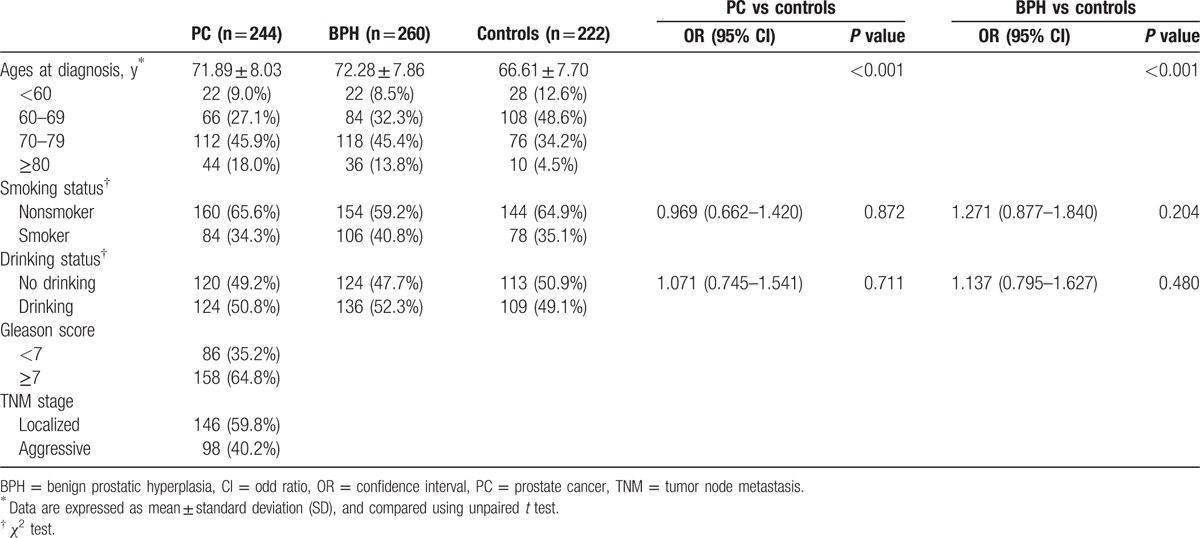
The whole study was approved and authorized by the Ethical Committee of Luqiao Division in Affiliated Taizhou Hospital of Wenzhou Medical College, Taizhou City. Written informed consent was obtained from each subject before recruitment.
2.2. Genotyping
Whole Blood Genome Extraction Kit (Fastagen Biotech, Shanghai, China) was employed to extract genomic DNA from peripheral blood, which was then restored at 4°C. Following primers were used: PvuII: Forward: 5′-CTG CCA CCC TAT CTG TAT CTT TTC CTA TTC TCC-3′, Reverse: 5′-TCT TTC TCT GCC ACC CTG GCG TCG ATT ATC TGA-3′; XbaI: Forward: 5′-CTG CCA CCC TAT CTG TAT CTT TTC CTA TTC TCC-3′, Reverse: 5′-TCT TTC TCT GCC ACC CTG GCG TCG ATT ATC TGA-3′. The reaction mixture of PCR included 5 μL of 10 × Ex Taq Buffer, 4 μL of 25 mM MgCl2, 4.0 μL of 2.5 mM dNTP, 0.5 μL of 10 mM primers, 80 to 200 ng of DNA template, 0.2 μL of 5 U/μL Ex Taq DNA polymerases (Fetemens), and double distilled water (total volume: 50 μL). The conditions of PCR were as follows: predenaturation at 94°C for 5 minutes, denaturation at 94°C for 30 seconds, annealing for 45 seconds, 72°C for 60 seconds, and a final extension at 72°C for 5 minutes; the concentration and purity of PCR products were determined after 2.0% agarose gel electrophoresis. Then, 10 μL of PCR products was digested with restriction enzymes for 4 hours, followed by 2.0% agarose gel electrophoresis. Sequencing verification of genotype results will be done by randomization. For quality control, samples (100) were randomly selected for validation by genotyping and sequencing. The variation of T and C bases occurs in PvuII, while the variation of A and G bases occurs in XbaI. In the present study, only wild homozygous genes were used as reference genotypes (PvuII TT and XbaI AA) for comparison.
2.3. Statistical analysis
SPSS version 13.0 was used for statistical analysis, and Hardy–Weinberg equilibrium was used to evaluate the reliability of collected information. Logistic regression was employed to analyze the frequency of genotype and alleles, and then the odd ratio (OR) and 95% confidence interval (CI) were calculated after adjustment for age. χ2 test and Fisher exact test were used to assess the distribution of composite genotype in case-control study. SHEsis was used to analyze the Linkage disequilibrium effect and haplotyping between sites.[21,22] A value of P < 0.05 was considered statistically significant.
3. Results
The frequency of each ESRα genotype in the collected case group and control group accorded with Hardy–Weinberg equilibrium (P > 0.05). Genotyping showed that the frequency of TT, CT, and CC genotypes of PvuII was 38.5% (94/244), 41.8% (102/244), and 19.7% (48/244), respectively, in PC patients, 46.2% (120/260), 43.1% (112/260), and 10.8% (28/260), respectively, in BPH patients, and 41.4%, 43.2%, and15.3%, respectively, in healthy controls (Table 2). In both PC patients and BPH patients, there was no significant difference in the distribution frequency between PvuII genes after adjustment for age (P > 0.05). However, the genotype frequency of PvuII varied between regions and ethnics. According to previous reports on healthy Chinese men,[23] the frequency of TT genotype ranges from 41.4% to 42.1%, which is higher than in Caucasia (30.5%) and Japan (25.4%).[24,25] The frequencies of genotype AA, AG, and GG of XbaI were comparable between PC patients (60.7%, 34.4%, and 4.9%) or BPH patients (69.9%, 29.2%, and 3.8%) patients and healthy controls (64.0%, 31.5%, and 4.5%) (P > 0.05). The frequency of AA genotype in Chinese men (57.1–64.0%) was similar to that in Caucasia (51.1%).[23,26]
Table 2.
Frequencies of ESR-α genotypes and alleles in BPH patients, PC patients, and healthy controls.
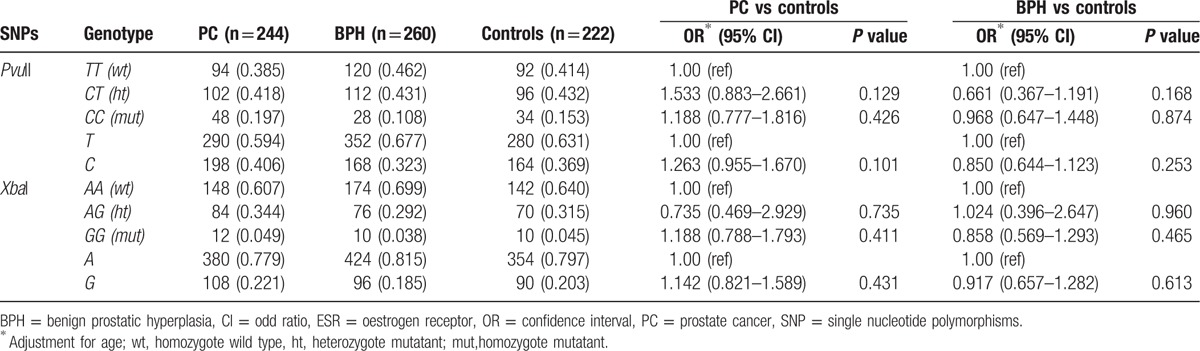
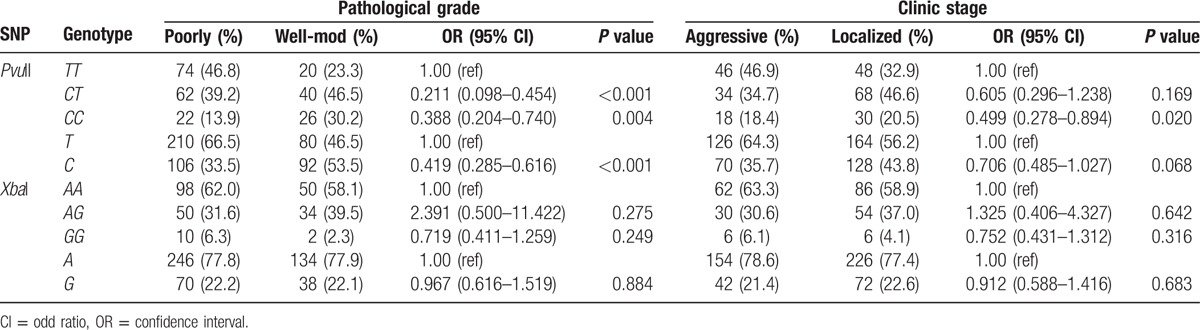
The relationship of each genotype of ESRα gene with pathological grades and clinical stages was further evaluated. Results showed that, compared with wild homozygous TT genotype of PvuII, mutant CC and CT genotypes of C allele were seldom found in poorly differentiated adenocarcinoma patients with the OR of 0.388 (0.204–0.740) and 0.211 (0.098–0.454), respectively, after adjustment for age (Table 3). Compared with T allele, C allele was also seldom observed in poorly differentiated PC patients (OR = 0.419, 95% CI: 0.285–0.616, P < 0.001). Additionally, the comparison between mutant CC and TT genotypes of PvuII showed patients with mutant CC genotype seldom developed metastasis to other sites (OR = 0.499, 95% CI: 0.278–0.894, P = 0.020). However, there was no relationship of XbaI SNP with pathological grades and clinical stages (P > 0.05).
Table 3.
Correlation of genotypes with pathological grades and clinical stages after adjustment for age.
Considering the distinct linkage disequilibrium between PvuII and XbaI of ESRα in control group (D’ = 0.958, r2 = 0.398), composite and haplotype genes were further analyzed (Table 4). Results showed that, compared with healthy men (0.9%), the frequency of TTAGI was significantly higher in PC patients (5.7%, OR = 6.696, 95% CI: 1.504–29.801, P = 0.004) and BPH patients (5.4%, OR = 6.260, 95% CI: 1.407–27.852, P = 0.006), which indicates that TTAG increases the risk for PC and BPH. The frequency of TG haplotype in PC patients was 4.7% compared with 0.5% in healthy controls, showing significant difference (OR = 9.168, 95% CI: 2.393–35.119, P < 0.001). The frequency of TA haplotype in PC patients was 54.7%, which was markedly lower than in healthy controls (60.3%, OR = 0.711, 95% CI: 0.547–0.924, P = 0.011). However, the frequency of CA haplotype in PC patients was 23.1%, which was significantly higher than in healthy controls (OR = 9.168, 95% CI: 2.393–35.119, P < 0.001). The frequency of CG haplotype in PC patients was 54.7%, which was markedly lower than in healthy controls (63.1%, OR = 0.708, 95% CI: 0.551–0.912, P = 0.007).
Table 4.
Multi-genotypes and haplotype frequencies for ESR-α polymorphisms in PC patients, BPH patients and healthy controls.
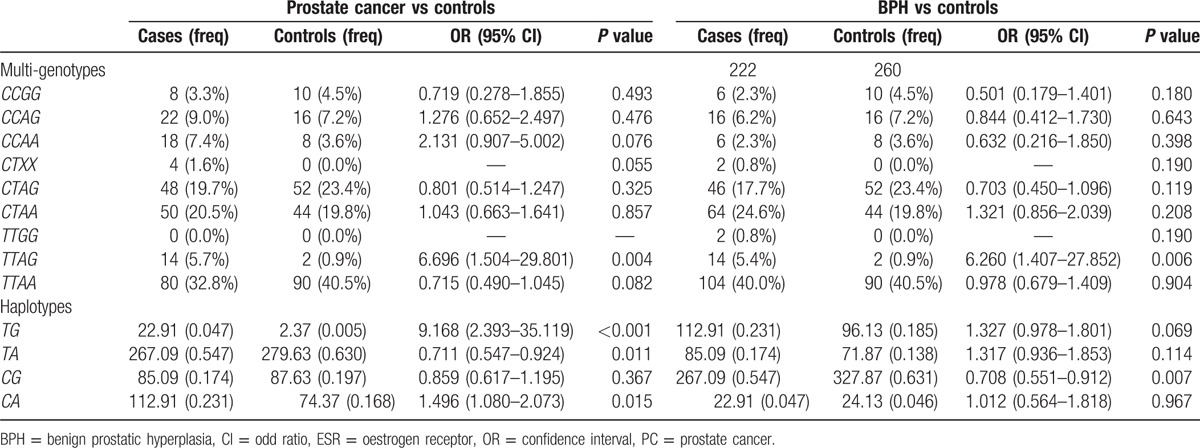
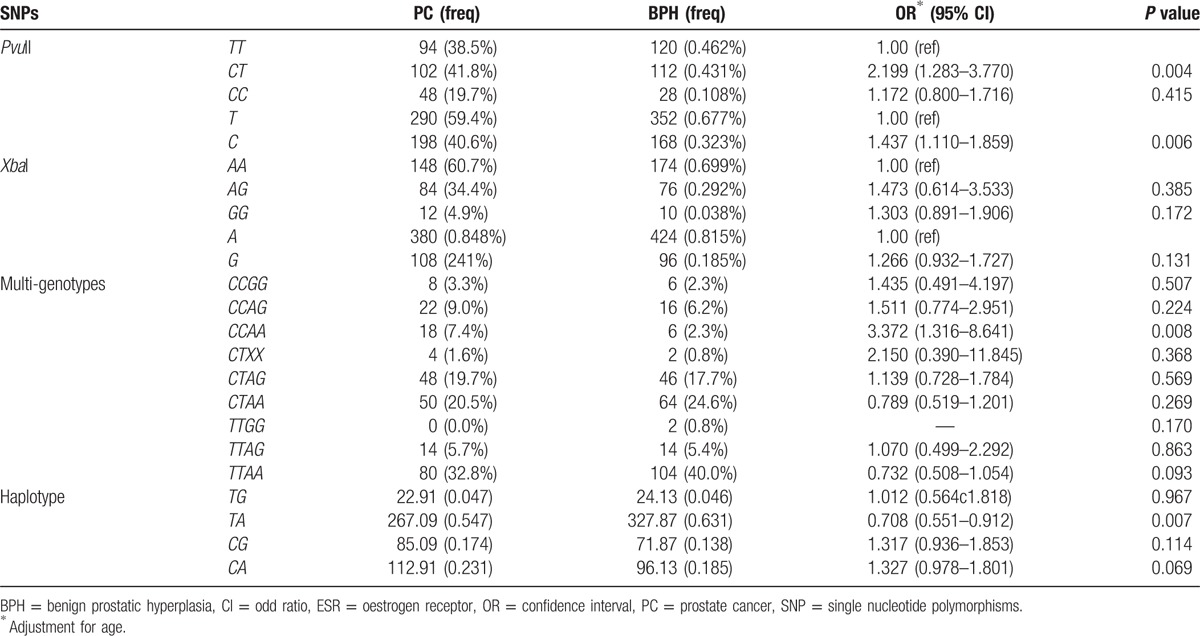
Table 5 displays the relationship between PC and BPH. Compared with T allele, BPH patients with C allele of PvuII had a higher risk for PC (OR = 1.437, 95% CI: 1.110–1.859, P = 0.006). Similarly, compared with TT genotype, BPH patients with heterozygous mutant CT genotype of PvuII had a higher risk for PC (OR = 2.199, 95% CI: 1.283–3.770, P = 0.004). Moreover, the frequency of CCAA genotype in PC group was 7.4%, which was higher than in BPH group (2.3%), indicating that it has a high risk for PC (OR, 3.372 [1.316–8.641]). Analysis with SHEsis showed that BHP patients with TA haplotype had a lower risk for PCI, and the risk was 0.7 times higher than that in patients without TA haplotype.
Table 5.
Frequencies of ESR-α genotypes, alleles, and haplotypes in BPH and PC patients.
4. Discussion
Oestrogen receptor plays an important role in accommodating the diseases relevant with hormone. Therefore, to study the oestrogen receptor gene polymorphism will be helpful for the elucidation of molecular mechanism underlying the occurrence and development of diseases related to this hormone. At present, though a variety of cancers (such as endometrial carcinoma and mammary cancer) have been reported to have relationship with the polymorphisms of PvuII and XbaI of ESRα in Chinese people, the correlation of their polymorphisms with the pathogenesis of PC is still poorly understood.[27,28] The present study was undertaken to investigate the relationship of SNP of ESRα with PC and BPH in Han Chinese men recruited from East China. Our results showed that there is no distinct relationship between PvuII and XbaI sites and risk of PC or BPH. At present, no study has been conducted to investigate the relationship between PvuII polymorphism and BPH, although results from experiments about the relationship of PvuII polymorphism with PC are in accordant with those in Japanese and USA men.[29,30] A study on Iran men showed that the C allele (CT or CC) of PvuII increased the risk for PC. Compared with wild homozygous TT genotype, mutant CT and CC increased the risk for PC by 3.12 and 4.73 times, respectively.[31] Our results indicated that, for healthy men with C allele of PvuII, the risk for PC and BHP was not increased. However, in BHP patients, the risk for PC increased by 1.4 times in the presence of C allele of PvuII. This indicates that C allele of PvuII may be a predisposing factor of PC. However, our results were inconsistent with that from a Japanese study.[29] Their results showed that, compared with the healthy men with CC genotype of PvuII, the risk for PC increased by 3.44 times in healthy men with TT genotype of PvuII. They also found that, compared with CC genotype, TT genotype will increase the risk for PC,[25] which was consistent with our finding. Even if in Chinese men with C allele or CT or CC genotype suffering PC, the differentiation degree of cancer cells was higher, and the cancer cells seldom metastasized into other sites, which indicates that the prognosis of patients with C allele is better. As to XbaI, our results showed no relationship with the risk for PC, which was similar to the findings from a Japanese study,[25,29] but not with those from the European and USA studies. In a study on black and white people in the United States, Hernandez et al[30] found that the risk for PC in American black people with AG genotype and G allele (AG + GG) increased by 2.25 and 2.14 times, respectively. However, a recent study on Iran men showed that the risk for PC in men with AG genotype increased by 4.36 times as compared with those with AA genotype.[31] The results on PvuII and XbaI from different regions and ethnics are quite different, which may reflect the influence of genetic backgrounds, diet habits, life style, and even sunlight exposure on the risk for BPH and PC.[32]
However, it is still not clear how PvuII and XbaI influence the occurrence and development of PC and BPH. PvuII and XbaI are the 2 most common sites of polymorphism, and all the polymorphisms occur in the intron 1 which contains promoter, enhancer, and other important regulatory sequences, and thus its polymorphisms may affect the expression and function of ESRα.[13] T allele of PvuII changes to T genes, and then other sites, B-myb will bind to myb transcription factor on the gene order, increasing the transcription of downstream report gene. Therefore, C allele may increase the transcriptional activity of ESRαto a large extent. It is not clear whether XbaI has a separate effect on oestrogen receptor. Of note, the distance between Xbal and PvuII is only 50 bp, which exists strong linkage disequilibrium and may have a negative effect on the function of PvuII, or regulate and control target genes by forming composite genes with PvuII or haplotype.
Thus, the synergistic action or inhibitory action expressed by the above 2 sites in the form of composite genes or haplotype was further investigated in this study. Results showed that, haplotype TG or CA increased the risk for PC in healthy men, but haplotype TA decreased the risk for PC in both healthy men and BPH patients. Additionally, haplotype CG decreased the risk for BPH. Analysis of composite genes indicated that TTAG increased the risk for both BPH and PC. However, in case of BPH, the composite genes had no influence on the risk for PC. CCAA genes increased the risk for PC in BPH patients.
In control group, healthy men were recruited. However, in the Japanese studies,[25,30] both BPH patients and healthy men were included in control group, which may bias the results, especially the relationship between BPH and PC. Therefore, our study not only illustrated the mechanism of the occurrence and development of PC which provides a method to screen the subjects with high risk for PC, but also provided evidence on the relationship between BPH and PC to a certain extent, which may assist the early detection and diagnosis of PC and BPH. However, there were limitations in this study: the sample size was small, shortage of gene sites, and horizontal analysis on different ethnical people and zones. In our future studies, we will investigate the SNPs in subjects with different genetic background and retrospectively analyze studies on this issue to clarify the influence of ESR gene polymorphisms on the occurrence and development of PC and BPH. During the transformation from BPH to malignance, there are prostatic intraepithelial neoplasm and proliferative inflammatory atrophy, which might be the keys to inducing canceration. However, due to sample sizes and difficulties in clinical diagnosis, these samples were not analyzed. Future studies will focus on these cases.
In summary, our study shows the close relationship between ESRα gene polymorphism and risk for PC and BPH in Chinese men, which varies between ethnics and regions.
Footnotes
Abbreviations: BPH = benign prostatic hyperplasia, CI = confidence interval, ESR = oestrogen receptor, OR = odd ratio, PC = prostate cancer, PSA = prostate-specific antigen, SNP = single nucleotide polymorphisms.
The authors have no conflicts of interest to disclose.
References
- [1].Haas GP, Sakr WA. Epidemiology of prostate cancer. CA Cancer J Clin 1997;47:273–87. [DOI] [PubMed] [Google Scholar]
- [2].Guess HA. Benign prostatic hyperplasia and prostate cancer. Epidemiol Rev 2001;23:152–8. [DOI] [PubMed] [Google Scholar]
- [3].Levi F, Lucchini F, Negri E, et al. Recent trends in mortality from benign prostatic hyperplasia. Prostate 2003;56:207–11. [DOI] [PubMed] [Google Scholar]
- [4].Luo J, Duggan DJ, Chen Y, et al. Human prostate cancer and benign prostatic hyperplasia: molecular dissection by gene expression profiling. Cancer Res 2001;61:4683–8. [PubMed] [Google Scholar]
- [5].De Marzo AM, Coffey DS, Nelson WG. New concepts in tissue specificity for prostate cancer and benign prostatic hyperplasia. Urology 1999;53(3 suppl 3a):29–39. [DOI] [PubMed] [Google Scholar]
- [6].Dai X, Fang X, Ma Y, et al. Benign prostatic hyperplasia and the risk of prostate cancer and bladder cancer: a meta-analysis of observational studies. Medicine (Baltimore) 2016;95:e3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Matsubara Y, Murata M, Kawano K, et al. Genotype distribution of estrogen receptor polymorphisms in men and postmenopausal women from healthy and coronary populations and its relation to serum lipid levels. Arterioscler Thromb Vasc Biol 1997;17:3006–12. [DOI] [PubMed] [Google Scholar]
- [8].Li LC, Chui R, Nakajima K, et al. Frequent methylation of estrogen receptor in prostate cancer: correlation with tumor progression. Cancer Res 2000;60:702–6. [PubMed] [Google Scholar]
- [9].Sobti RC, Gupta L, Singh SK, et al. Role of hormonal genes and risk of prostate cancer: gene-gene interactions in a North Indian population. Cancer Genet Cytogenet 2008;185:78–85. [DOI] [PubMed] [Google Scholar]
- [10].Suzuki K, Inaba S, Takeuchi H, et al. [Endocrine environment of benign prostatic hyperplasia—relationships of sex steroid hormone levels with age and the size of the prostate]. Nihon Hinyokika Gakkai Zasshi 1992;83:664–71. [DOI] [PubMed] [Google Scholar]
- [11].Cunha GR, Wang YZ, Hayward SW, et al. Estrogenic effects on prostatic differentiation and carcinogenesis. Reprod Fertil Dev 2001;13:285–96. [DOI] [PubMed] [Google Scholar]
- [12].Gupta L, Thakur H, Sobti RC, et al. Role of genetic polymorphism of estrogen receptor-alpha gene and risk of prostate cancer in north Indian population. Mol Cell Biochem 2010;335:255–61. [DOI] [PubMed] [Google Scholar]
- [13].Mangan FR, Neal GE, Williams DC. The effects of diethylstilboestrol and castration on the nucleic acid and protein metabolism of rat prostate gland. Biochem J 1967;104:1075–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bonkhoff H, Fixemer T, Hunsicker I, et al. Estrogen receptor expression in prostate cancer and premalignant prostatic lesions. Am J Pathol 1999;155:641–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ito T, Tachibana M, Yamamoto S, et al. Expression of estrogen receptor (ER-alpha and ER-beta) mRNA in human prostate cancer. Eur Urol 2001;40:557–63. [DOI] [PubMed] [Google Scholar]
- [16].Latil A, Bieche I, Vidaud D, et al. Evaluation of androgen, estrogen (ER alpha and ER beta), and progesterone receptor expression in human prostate cancer by real-time quantitative reverse transcription-polymerase chain reaction assays. Cancer Res 2001;61:1919–26. [PubMed] [Google Scholar]
- [17].Bodker A, Bruun J, Balslev E, et al. Estrogen receptors in the human male prostatic urethra and prostate in prostatic cancer and benign prostatic hyperplasia. Scand J Urol Nephrol 1999;33:237–42. [DOI] [PubMed] [Google Scholar]
- [18].Chen GG, Zeng Q, Tse GM. Estrogen and its receptors in cancer. Med Res Rev 2008;28:954–74. [DOI] [PubMed] [Google Scholar]
- [19].Prins GS, Korach KS. The role of estrogens and estrogen receptors in normal prostate growth and disease. Steroids 2008;73:233–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gleason DF, Mellinger GT. Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. 1974. J Urol 2002;167(2 pt 2):953–8. [PubMed] [Google Scholar]
- [21].Shi YY, He L. SHEsis, a powerful software platform for analyses of linkage disequilibrium, haplotype construction, and genetic association at polymorphism loci. Cell Res 2005;15:97–8. [DOI] [PubMed] [Google Scholar]
- [22].Li Z, Zhang Z, He Z, et al. A partition-ligation-combination-subdivision EM algorithm for haplotype inference with multiallelic markers: update of the SHEsis (http://analysis.bio-x.cn). Cell Res 2009;19:519–23. [DOI] [PubMed] [Google Scholar]
- [23].Long JR, Zhang YY, Liu PY, et al. Association of estrogen receptor alpha and vitamin D receptor gene polymorphisms with bone mineral density in Chinese males. Calcif Tissue Int 2004;74:270–6. [DOI] [PubMed] [Google Scholar]
- [24].Allcroft LC, Varanasi SS, Dimopoulos D, et al. Mutational and polymorphic analysis of the estradiol receptor-alpha gene in men with symptomatic vertebral fractures. Calcif Tissue Int 2002;71:400–5. [DOI] [PubMed] [Google Scholar]
- [25].Suzuki K, Nakazato H, Matsui H, et al. Genetic polymorphisms of estrogen receptor alpha, CYP19, catechol-O-methyltransferase are associated with familial prostate carcinoma risk in a Japanese population. Cancer 2003;98:1411–6. [DOI] [PubMed] [Google Scholar]
- [26].Lorentzon M, Lorentzon R, Backstrom T, et al. Estrogen receptor gene polymorphism, but not estradiol levels, is related to bone density in healthy adolescent boys: a cross-sectional and longitudinal study. J Clin Endocrinol Metab 1999;84:4597–601. [DOI] [PubMed] [Google Scholar]
- [27].Wedren S, Lovmar L, Humphreys K, et al. Estrogen receptor alpha gene polymorphism and endometrial cancer risk—a case-control study. BMC Cancer 2008;8:322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gold B, Kalush F, Bergeron J, et al. Estrogen receptor genotypes and haplotypes associated with breast cancer risk. Cancer Res 2004;64:8891–900. [DOI] [PubMed] [Google Scholar]
- [29].Fukatsu T, Hirokawa Y, Araki T, et al. Genetic polymorphisms of hormone-related genes and prostate cancer risk in the Japanese population. Anticancer Res 2004;24:2431–7. [PubMed] [Google Scholar]
- [30].Hernandez J, Balic I, Johnson-Pais TL, et al. Association between an estrogen receptor alpha gene polymorphism and the risk of prostate cancer in black men. J Urol 2006;175:523–7. [DOI] [PubMed] [Google Scholar]
- [31].Safarinejad MR, Safarinejad S, Shafiei N, et al. Estrogen receptors alpha (rs2234693 and rs9340799), and beta (rs4986938 and rs1256049) genes polymorphism in prostate cancer: evidence for association with risk and histopathological tumor characteristics in Iranian men. Mol Carcinog 2012;51Suppl 1:E104–17. [DOI] [PubMed] [Google Scholar]
- [32].Bai Y, Yu Y, Yu B, et al. Association of vitamin D receptor polymorphisms with the risk of prostate cancer in the Han population of Southern China. BMC Med Genet 2009;10:125. [DOI] [PMC free article] [PubMed] [Google Scholar]


