Abstract
The aim of this study was to evaluate sex difference in the prevalence and risk factors for asymptomatic cholelithiasis in Korean health screening examinees.
Examinees who underwent examination through health promotion center at 5 hospitals of Daegu-Gyeongbuk province in 2014 were analyzed retrospectively. All examinees were checked for height, weight, waist circumference, and blood pressure, and underwent laboratory tests and abdominal ultrasound. Diagnosis of cholelithiasis was made by ultrasound.
Of the total of 30,544 examinees, mean age was 47.3 ± 10.9 years and male to female ratio was 1.4:1. Asymptomatic cholelithiasis was diagnosed in 1268 examinees with overall prevalence of 4.2%. In age below 40 years, females showed higher prevalence of asymptomatic cholelithiasis than males (2.7% vs. 1.9%, P = 0.020), whereas prevalence of asymptomatic cholelithiasis was higher in males than females older than 50 years (6.2% vs. 5.1%, P = 0.012). Multiple logistic regression analysis revealed age (≥50 years), obesity, and high blood pressure as risk factors for asymptomatic cholelithiasis in males and age, obesity, hypertriglyceridemia, and chronic hepatitis B infection in females (P < 0.05).
Overall prevalence of asymptomatic cholelithiasis was 4.2% in Korean health screening examinees. Females showed higher prevalence of asymptomatic cholelithiasis than males younger than 40 years, whereas it was higher in males older than 50 years. Age and obesity were risk factors for asymptomatic cholelithiasis in both sexes. Males had additional risk factors of high blood pressure and females had hypertriglyceridemia and chronic hepatitis B infection.
Keywords: cholelithiasis, gender, prevalence, risk factor
1. Introduction
Cholelithiasis is a major health problem easily encountered in clinics and 120,000 Koreans visited the hospital for gallstone diseases in 2012 with an annual increase rate of 7.3% from 2007 to 2012. Health burden related to cholelithiasis in Korea has been great with costs of nearly $1.6 billion in 2012 and an annual increase rate of 8.6% from 2007 to 2012.[1] The increasing prevalence and health burden of cholelithiasis should be addressed.
The prevalence and risk factors of asymptomatic cholelithiasis vary with race, diet, culture, and geographic differences. Prevalence of 13.3% to 50.5% has been reported for asymptomatic cholelithiasis in Western countries, including United States and Europe,[2,3] whereas the prevalence of asymptomatic cholelithiasis in Eastern countries, including Korea, has been reported to be lower than that of Western countries with 2.0% to 10.7%.[4–6] Age, female sex, obesity, dyslipidemia, diabetes mellitus, metabolic syndrome, rapid weight loss, total parenteral nutrition, chronic disease including Crohn disease, cystic fibrosis, and chronic liver disease, and drugs including octreotide, ceftriaxone, and statin were previously identified as risk factors for asymptomatic cholelithiasis.[7]
However, previous studies reported conflicting results for some risk factors of asymptomatic cholelithiasis. In some reports from Eastern countries, female sex was not found to be a risk factor for asymptomatic cholelithiasis and the prevalence of asymptomatic cholelithiasis was not significantly different between sexes or even higher in males than females.[4,6] In a study on sex differences in risk factors for asymptomatic cholelithiasis including 3573 subjects from China, a high level of fasting plasma glucose was a risk factor in males and hypertriglyceridemia or obesity in females.[5] Identifying differences in the prevalence and risk factors for asymptomatic cholelithiasis might be important in management of asymptomatic cholelithiasis and planning preventive strategies for asymptomatic cholelithiasis.
The aim of this study was to evaluate sex differences in the prevalence and risk factors for asymptomatic cholelithiasis in Korean health screening examinees.
2. Methods
2.1. Subjects and measurements
Examinees who underwent examinations through health promotion center at 5 hospitals in Daegu-Gyeongbuk province from January 2014 to December 2014 were included and analyzed retrospectively. Examinees younger than 18 years were excluded. All examinees were checked for height, weight, waist circumference (WC), and blood pressure (BP), and underwent laboratory tests after overnight fasting, including fasting plasma glucose, triglyceride, total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol concentration, hepatitis B surface antigen (HBsAg), and hepatitis C virus antibody (anti-HCV). Ultrasonography of the abdomen was performed in all examinees.
The prevalence and risk factors for asymptomatic cholelithiasis were compared between male and female. Institutional review board approval was obtained for this study (2015–07–046).
2.2. Definition of variables
Diagnosis of cholelithiasis was made by abdominal ultrasound if there was the presence of echogenic densities casting distal acoustic shadow or mobile upon postural change with or without distal acoustic shadowing.[8] Overweight was defined if body mass index (BMI) score was between 23 and 25 kg/m2 and obesity as BMI score >25 kg/m2 in both sexes according to definition suggested by World Health Organization criteria for the Asia Pacific region.[9] Central obesity was defined as WC >90 cm in male and >80 cm in female[10] or waist-to-height ratio (WHtR) >0.5.[11] High BP was defined as systolic BP >140 mmHg or diastolic BP >90 mmHg. Hypertriglyceridemia was defined as a triglyceride level ≥150 mg/dL, hypercholesterolemia as a total cholesterol level ≥200 mg/dL, low HDL-cholesterol level as a HDL-cholesterol level <40 mg/dL in male and <60 mg/dL in female and high LDL-cholesterol as LDL-cholesterol level ≥130 mg/dL. High fasting glucose was defined as fasting plasma glucose level ≥110 mg/dL. Chronic hepatitis B infection was defined if examinee showed positive result for HBsAg and chronic hepatitis C if positive for anti-HCV.
2.3. Statistical analysis
Categorical data were presented as the number of cases and percentages. Continuous variables were shown as mean ± standard deviation. Differences were tested for statistical significance using the Student t test and the Pearson χ2 test. A Cox regression analysis was performed for identification of risk factors for asymptomatic cholelithiasis. Regardless of the statistical tests, the level of significance was defined as P < 0.05. Statistical analyses of the data were performed using SPSS 20 (IBM SPSS, Chicago, IL).
3. Results
3.1. Baseline characteristics and prevalence of asymptomatic cholelithiasis
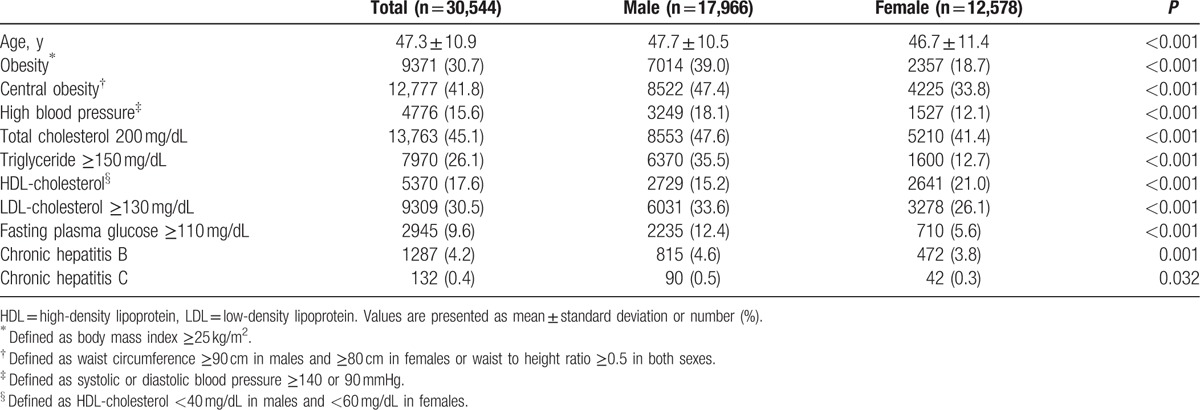
Among a total of 30,556 examinees who underwent health screening examination, 12 examinees under age 18 years were excluded. Mean age of the 30,544 examinees included in this study was 47.3 ± 10.9 years and male to female ratio was 1.4:1 (17,966:12,578). Asymptomatic cholelithiasis was diagnosed in 1268 examinees with overall prevalence of 4.2%. No significant difference in overall prevalence of asymptomatic cholelithiasis was observed between male and female (4.3% vs. 4.0%, P = 0.159).
In both sexes, the prevalence of asymptomatic cholelithiasis increased with age (P < 0.05). The prevalence of asymptomatic cholelithiasis in females and males in their 20s, 30s, 40s, 50s, 60s, and over 70 s was 2.2% vs. 1.4%, 2.9% vs. 2.0%, 3.8% vs. 3.5%, 4.0% vs. 5.5%, 6.1% vs. 7.0%, and 8.8% vs. 9.1%, respectively (Fig. 1). The average increase in the prevalence of asymptomatic cholelithiasis every decade of age was 1.46-fold in males and 1.32-fold in females. In age <40 years, significantly higher prevalence of asymptomatic cholelithiasis was observed in females compared with males (2.7% vs. 1.9%, P = 0.020), whereas males showed significantly higher prevalence than females at age over 50 years (6.2% vs. 5.1%, P = 0.012) (Fig. 2).
Figure 1.
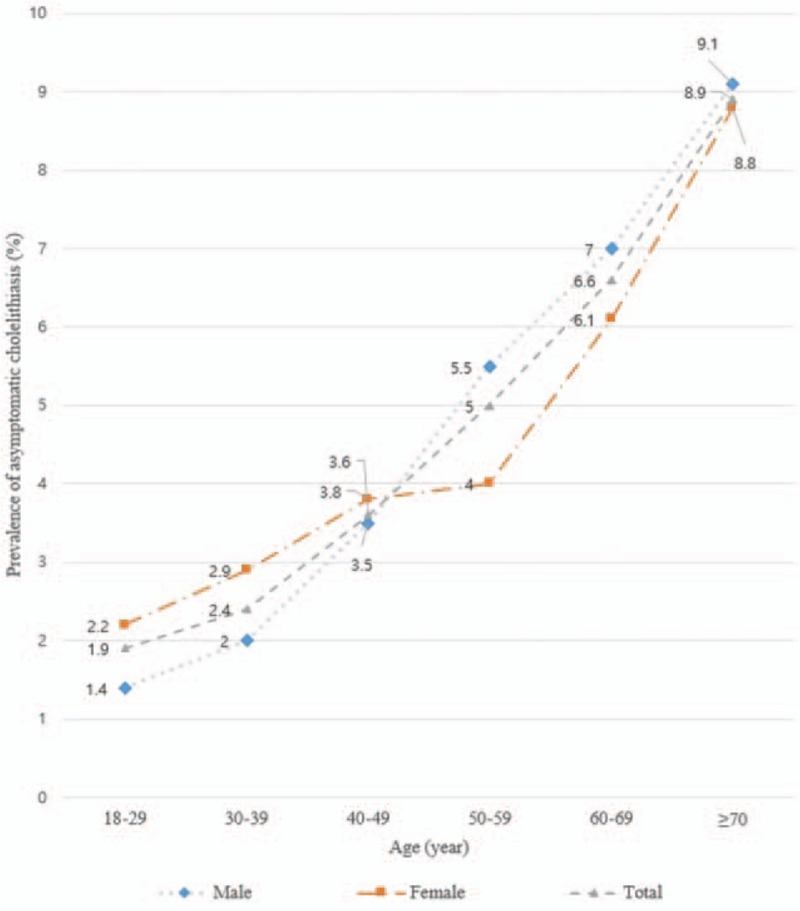
Sex difference in the prevalence of asymptomatic cholelithiasis according to age.
Figure 2.
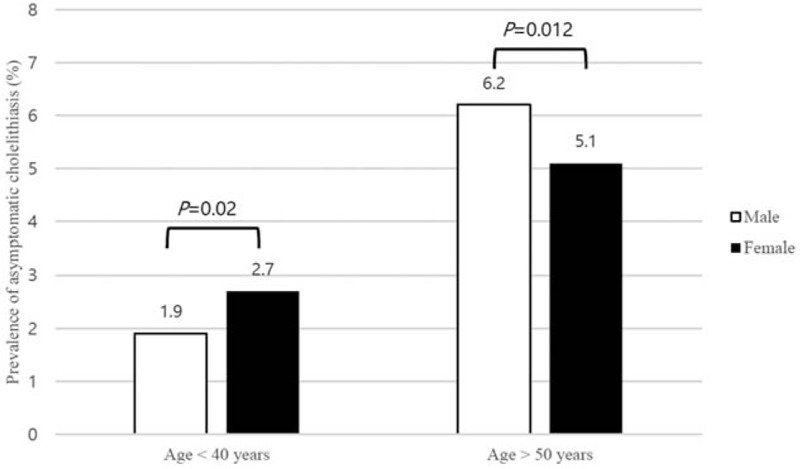
Prevalence of asymptomatic cholelithiasis according to age groups and sex.
The mean age of males and females was 47.7 ± 10.5 years and 46.7 ± 11.4 years, respectively (P < 0.001). Proportion of obesity was 39.0% (7014/17,966) in male examinees and 18.7% (2357/12,578) in female examinees (P < 0.001). Central obesity was observed in 8522 (47.4%) male examinees and 4225 (33.8%) female examinees (P < 0.001). Hypertriglyceridemia was observed in 6370 (35.5%) male examinees and 1600 (12.7%) female examinees (P < 0.001). High fasting glucose was observed in 2235 (12.4%) males and 710 (5.6%) females (P < 0.001). Chronic hepatitis B infection was observed in 815 (4.6%) male examinees and 472 (3.8%) in female examinees (P = 0.001). Chronic hepatitis C infection was observed in 90 (0.5%) males and 42 (0.3%) females (P = 0.032) (Table 1).
Table 1.
Baseline characteristics of examinees.
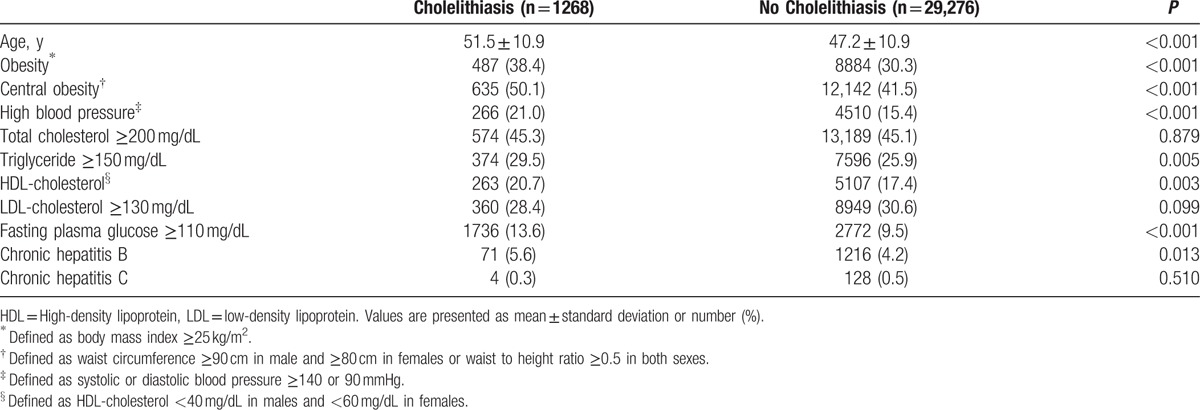
Age, serum triglyceride and HDL-cholesterol, fasting plasma glucose, and proportion of obesity, central obesity, chronic hepatitis B infection, and high BP were significantly higher in examinees with asymptomatic cholelithiasis compared to examinees without asymptomatic cholelithiasis (P < 0.05) (Table 2).
Table 2.
Comparison of baseline characteristics of examinees according to presence of asymptomatic cholelithiasis.
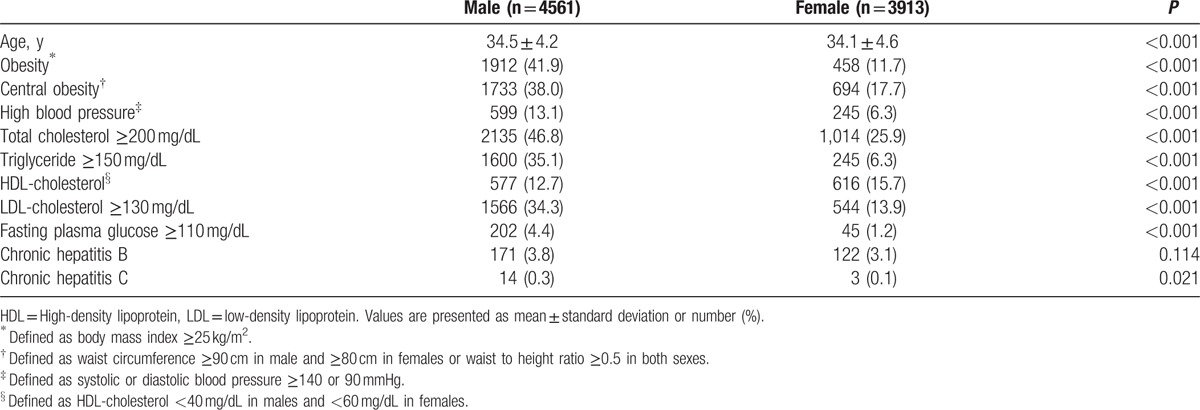
In examinees older than 50 years, higher fasting plasma glucose and proportion of obesity and central obesity were observed in males than females, whereas higher age, total cholesterol, HDL-cholesterol, and LDL-cholesterol were observed in females than males (P < 0.05) (Table 3). In examinees younger than 40 years, males had higher age, total cholesterol, triglyceride, LDL-cholesterol, fasting plasma glucose, and proportion of obesity, central obesity, chronic hepatitis C infection, and high BP than females and higher HDL-cholesterol was observed in females than in males (P < 0.05) (Table 4).
Table 3.
Baseline characteristics of examinees older than 50 years.
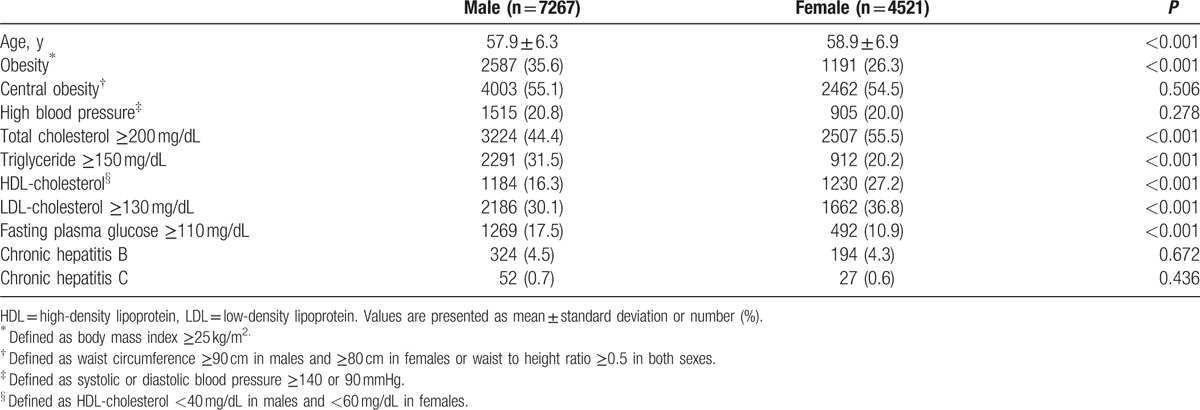
Table 4.
Baseline characteristics of examinees younger than 40 years.
3.2. Risk factors for asymptomatic cholelithiasis
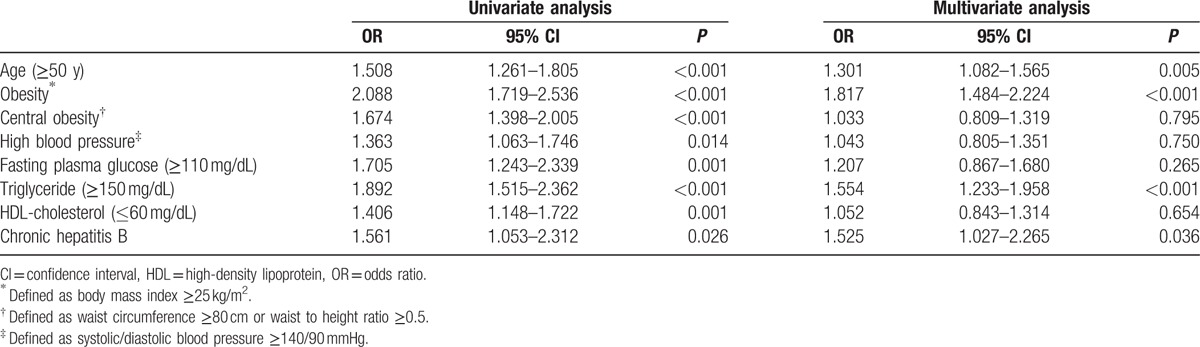
In univariate analysis, age (≥50 years), obesity, central obesity, high fasting glucose, high BP, and high LDL-cholesterol were risk factors for asymptomatic cholelithiasis in male examinees, and age, obesity, central obesity, high fasting glucose, high BP, hypertriglyceridemia, low HDL-cholesterol, and chronic hepatitis B infection were risk factors for females.
Multiple logistic regression analysis indicated that age (odds ratio [OR] 2.139, 95% confidence interval [CI] 1.839–2.488, P < 0.001), obesity (OR 1.171, 95% CI 1.009–1.359, P = 0.038) and high BP (OR 1.361, 95% CI 1.146–1.617, P < 0.001) were risk factors for asymptomatic cholelithiasis in males, whereas age (OR 1.301, 95% CI 1.082–1.565, P = 0.005), obesity (OR 1.817, 95% CI 1.484–2.224, P < 0.001), hypertriglyceridemia (OR 1.554, 95% CI 1.233–1.958, P < 0.001), and chronic hepatitis B infection (OR 1.525, 95% CI 1.027–2.265, P = 0.036) in females (Tables 5 and 6).
Table 5.
Univariate and multivariate analysis for risk factors of asymptomatic cholelithiasis in male examinees.
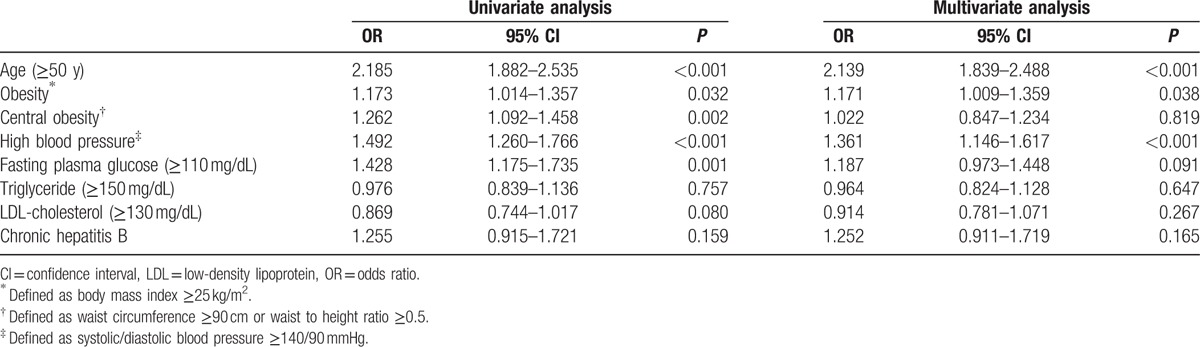
Table 6.
Univariate and multivariate analysis for risk factors of asymptomatic cholelithiasis in female examinees.
In stratification of examinees as normal, overweight, and obese according to the level of BMI, positive correlation was observed between the degree of BMI and prevalence of asymptomatic cholelithiasis with statistical significance in males and females (3.9% vs. 4.2% vs. 4.7%, P = 0.025 vs. 3.3% vs. 3.5% vs. 6.7%, P < 0.001).
4. Discussion
The overall prevalence of asymptomatic cholelithiasis in Korean health screening examinees was 4.2% in this study, comparable with previous reports of Eastern countries. Lower prevalence of asymptomatic cholelithiasis compared to Western countries despite recent westernized dietary changes in Korea suggests that other factors such as genetic or environmental factors might be important in the formation of asymptomatic cholelithiasis. Reasons for this difference in prevalence of asymptomatic cholelithiasis might be diverse. First, the proportion of pigment cholelithiasis is higher in Eastern countries than that of cholesterol stones, which is more predominant in Western countries. A study conducted in Korea reported a higher proportion of pigment stones compared to cholesterol stones in patients who underwent cholecystectomy owing to cholelithiasis.[12] Second, obesity is less prevalent in Korean females compared with Western countries, but similar in males. A study conducted in the United States using data from the National Health and Nutrition Examination Survey (2007–2012) reported obesity of 36.8% in females and 35.0% in males, whereas obesity was reported in 30.5% of females and 34.9% of males in Korea.[13,14] Similarity in proportion of obesity in males and difference in females can explain in part the higher prevalence of asymptomatic cholelithiasis in males compared with females in Korea.
Age is a well-known risk factor for asymptomatic cholelithiasis and the prevalence of asymptomatic cholelithiasis increased with age in many studies. This study showed that the risk of asymptomatic cholelithiasis increased with age in both sexes showing consistent results with previous reports.[15] Increased formation of cholelithiasis with age is suggested to be related to a longer period of exposure to various risk factors for cholelithiasis and gallbladder dysmotility secondary to sedentary activity in old age.[16,17]
Female sex is an important risk factor for asymptomatic cholelithiasis, and most previous studies reported a higher prevalence of asymptomatic cholelithiasis in female than male.[18] In the present study, females showed significantly higher prevalence of asymptomatic cholelithiasis than males only in age below 40 years. This increased risk of cholelithiasis in females is related to the estrogen effect, pregnancy, use of oral contraceptives, or hormonal replacement therapy.[18,19]
Interestingly, in this study, average increase in the prevalence of asymptomatic cholelithiasis every decade of age was 1.46-fold in males and 1.32-fold in females. As a result, the prevalence of asymptomatic cholelithiasis showed a greater increase in males than females older than 50 years, and at age above 50 years, significantly higher prevalence of asymptomatic cholelithiasis was observed in males than females. Higher prevalence of asymptomatic cholelithiasis in females younger than 40 years was likely to be related to estrogen effect or pregnancy, whereas higher prevalence of asymptomatic cholelithiasis in males than females older than 50 years suggests that decreased estrogen effect or pregnancy diminished the formation of cholelithiasis in females, whereas increase in other lithogenic factors led to the formation of asymptomatic cholelithiasis in males. In this study, males older than 50 years had higher BMI, triglyceride level, and fasting plasma glucose than females, and these differences presumably influenced higher formation of asymptomatic cholelithiasis in males than females older than 50 years.
Obesity, another well-known risk factor for asymptomatic cholelithiasis in the general population, is mainly related to cholesterol stone formation. Mechanisms of cholelithiasis formation in obesity include loss of gallbladder contractility, increased level of cholesterol secretion from liver, and bile supersaturation by increase in biliary secretion of cholesterol. A large prospective study of obese women reported a strong linear association between BMI and the incidence of cholelithiasis.[20] This study also showed statistically significant positive correlation between the level of BMI and prevalence of asymptomatic cholelithiasis when examinees were stratified as normal, overweight, and obese according to the level of BMI.
WC, WHtR, and waist-to-hip ratio indicate the degree of central obesity, whereas BMI represents the parameter of obesity. BMI is known to be related to risk of cholelithiasis in females but not in males because BMI poorly reflects central obesity in males.[3] However, in this study, BMI was a risk factor for asymptomatic cholelithiasis in both sexes. Although waist-to-hip ratio is known to reflect central obesity more than BMI, in this study hip circumference was not measured due to retrospective design. In this study, central obesity was not found to be a risk factor for asymptomatic cholelithiasis in both sexes. Owing to the retrospective nature of the present study, measurement of WC might have been slightly different between hospitals and this might have influenced the association between central obesity and asymptomatic cholelithiasis. Central obesity is associated with formation of cholesterol cholelithiasis and these differences in association between parameters of obesity and asymptomatic cholelithiasis in the present study might be because of higher proportion of pigment cholelithiasis than cholesterol stones in Eastern countries.
Among lipid profiles, hypertriglyceridemia, a known risk factor for cholesterol stones,[5] was a risk factor for asymptomatic cholelithiasis in females only. Proportion of examinees with hypertriglyceridemia was not significantly different between examinees with and without asymptomatic cholelithiasis in males (34.9% vs. 35.5%, P = 0.757). The mechanisms of increased risk of cholelithiasis formation related to hypertriglyceridemia are increase in the activity of HMG CoA reductase and subsequent increase in biliary cholesterol saturation and decrease in gallbladder sensitivity to cholecystokinin and contractility. Conflicting result of hypertriglyceridemia as risk factor for asymptomatic cholelithiasis between sexes in this study might be related to more frequent alcohol consumption in Korean male than female resulting in higher incidence of alcohol-induced hypertriglyceridemia in males compared to females.
In this study, high fasting glucose was a risk factor for asymptomatic cholelithiasis in univariate analysis, although it was not statistically significant in multivariate analysis. Insulin resistance is also associated with reduced gallbladder motility, overactivation of the rate-limiting enzyme for cholesterol synthesis, and cholesterol supersaturation in the bile, which leads to cholelithiasis formation.
An interesting point of this study was that chronic hepatitis B infection was found as a risk factor for asymptomatic cholelithiasis only in female sex, and chronic hepatitis C infection was not significantly associated with the formation of asymptomatic cholelithiasis. Previous studies have reported conflicting results for chronic hepatitis B infection as a risk factor for cholelithiasis,[21,22] and previous reports suggested chronic hepatitis C as a risk factor for cholelithiasis.[23] Chronic liver inflammation increases the risk of cholelithiasis[24] and chronic inflammation caused by chronic hepatitis B or C might increase the risk of cholelithiasis. Risk of cholelithiasis was reported to show correlation with the degree of liver cirrhosis.[25] In addition, proportion of examinees with chronic hepatitis C was small and the use of anti-HCV as a surrogate for chronic hepatitis C infection may overestimate the prevalence of chronic hepatitis C infection in this study. This discrepancy between the present study and previous reports is presumably related to predominantly higher prevalence in chronic hepatitis B compared to chronic hepatitis C in Korea.
In this study, high BP was found as a risk factor of asymptomatic cholelithiasis in male sex. Some previous studies have reported on the relation between high BP and cholelithiasis.[26,27] Risk factors for high BP were age, obesity, diabetes, and dyslipidemia, and cholelithiasis shares these as risk factors.
There were several limitations in this study. First, because data were analyzed retrospectively, information about other risk factors for asymptomatic cholelithiasis such as history of pregnancy, parity, rapid weight reduction, or the use of lithogenic drugs were not investigated. Further studies including these risk factors are required to identify reasons for the differences in the prevalence of asymptomatic cholelithiasis, particularly in females younger than 40 years. Second, males were significantly older than females; thus, comparison of overall incidence of asymptomatic cholelithiasis was difficult because risk of asymptomatic cholelithiasis increased with age. However, the influence of age might be small in this study because the difference in age between males and females was only 1 year and the prevalence of asymptomatic cholelithiasis was also analyzed according to age group. Third, type of asymptomatic cholelithiasis was not evaluated and as this is important in the evaluation of prevalence and risk factors for asymptomatic cholelithiasis, studies concerning composition of asymptomatic cholelithiasis are needed. Nevertheless, this study was one of the few studies to evaluate sex differences in the prevalence and risk factors for asymptomatic cholelithiasis, particularly in a non-Western area.
In conclusion, overall prevalence of asymptomatic cholelithiasis in Korean health screening examinees was 4.2% with similar prevalence in both sexes. Females showed higher prevalence of asymptomatic cholelithiasis compared to males younger than 40 years, whereas it was higher in males older than 50 years. Age and obesity were risk factors for asymptomatic cholelithiasis in both sexes. Males had additional risk factors of high BP and females had hypertriglyceridemia and chronic hepatitis B infection.
Footnotes
Abbreviations: anti-HCV = hepatitis C virus antibody, BMI = body mass index, BP = blood pressure, CI = confidence interval, HBsAg = hepatitis B surface antigen, HDL = high-density lipoprotein, LDL = low-density lipoprotein, OR = odds ratio, WC = waist circumference, WHtR = waist-to-height ratio.
The authors have no potential conflict of interest to declare.
References
- [1].Korean National health insurance—National sample cohort.(2007–2012). [Google Scholar]
- [2].Shaffer EA. Gallstone disease: epidemiology of gallbladder stone disease. Best Pract Res Clin Gastroenterol 2006;20:981–96. [DOI] [PubMed] [Google Scholar]
- [3].Maurer KR, Everhart JE, Ezzati TM, et al. Prevalence of gallstone disease in Hispanic populations in the United States. Gastroenterology 1989;96:487–92. [DOI] [PubMed] [Google Scholar]
- [4].Chen CH, Huang MH, Yang JC, et al. Prevalence and risk factors of gallstone disease in an adult population of Taiwan: an epidemiological survey. J Gastroenterol Hepatol 2006;21:1737–43. [DOI] [PubMed] [Google Scholar]
- [5].Sun H, Tang H, Jiang S, et al. Gender and metabolic differences of gallstone diseases. World J Gastroenterol 2009;15:1886–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chung YJ, Park YD, Lee HC, et al. Prevalence and risk factors of gallstones in a general health screened population. Korean J Med DE—2007-05-01 2007;72:480–90. [Google Scholar]
- [7].Stinton LM, Myers RP, Shaffer EA. Epidemiology of gallstones. Gastroenterol Clin North Am 2010;39:157–69. vii. [DOI] [PubMed] [Google Scholar]
- [8].Cooperberg PL, Burhenne HJ. Real-time ultrasonography. Diagnostic technique of choice in calculous gallbladder disease. N Engl J Med 1980;302:1277–9. [DOI] [PubMed] [Google Scholar]
- [9].Anuurad E, Shiwaku K, Nogi A, et al. The new BMI criteria for asians by the regional office for the western pacific region of WHO are suitable for screening of overweight to prevent metabolic syndrome in elder Japanese workers. J Occup Health 2003;45:335–43. [DOI] [PubMed] [Google Scholar]
- [10].Alberti KG, Zimmet P, Shaw J. Metabolic syndrome—a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med 2006;23:469–80. [DOI] [PubMed] [Google Scholar]
- [11].Browning LM, Hsieh SD, Ashwell M. A systematic review of waist-to-height ratio as a screening tool for the prediction of cardiovascular disease and diabetes: 0.5 could be a suitable global boundary value. Nutr Res Rev 2010;23:247–69. [DOI] [PubMed] [Google Scholar]
- [12].Kim JW, Oh HC, Do JH, et al. Has the prevalence of cholesterol gallstones increased in Korea? A preliminary single-center experience. J Dig Dis 2013;14:559–63. [DOI] [PubMed] [Google Scholar]
- [13].Yang L, Colditz GA. Prevalence of overweight and obesity in the United States, 2007-2012. JAMA Intern Med 2015;175:1412–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kim CS, Ko SH, Kwon HS, et al. Prevalence, awareness, and management of obesity in Korea: data from the Korea national health and nutrition examination survey (1998–2011). Diabetes Metab J 2014;38:35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Festi D, Dormi A, Capodicasa S, et al. Incidence of gallstone disease in Italy: results from a multicenter, population-based Italian study (the MICOL project). World J Gastroenterol 2008;14:5282–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Volzke H, Baumeister SE, Alte D, et al. Independent risk factors for gallstone formation in a region with high cholelithiasis prevalence. Digestion 2005;71:97–105. [DOI] [PubMed] [Google Scholar]
- [17].Kriska AM, Brach JS, Jarvis BJ, et al. Physical activity and gallbladder disease determined by ultrasonography. Med Sci Sports Exerc 2007;39:1927–32. [DOI] [PubMed] [Google Scholar]
- [18].Youming D, Bin W, Weixing W, et al. The effect of h(1) calponin expression on gallstone formation in pregnancy. Saudi Med J 2006;27:1661–6. [PubMed] [Google Scholar]
- [19].Tierney S, Nakeeb A, Wong O, et al. Progesterone alters biliary flow dynamics. Ann Surg 1999;229:205–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Stampfer MJ, Maclure KM, Colditz GA, et al. Risk of symptomatic gallstones in women with severe obesity. Am J Clin Nutr 1992;55:652–8. [DOI] [PubMed] [Google Scholar]
- [21].Lee YC, Wu JS, Yang YC, et al. Hepatitis B and hepatitis C associated with risk of gallstone disease in elderly adults. J Am Geriatr Soc 2014;62:1600–2. [DOI] [PubMed] [Google Scholar]
- [22].Lu SN, Chang WY, Wang LY, et al. Risk factors for gallstones among Chinese in Taiwan. A community sonographic survey. J Clin Gastroenterol 1990;12:542–6. [DOI] [PubMed] [Google Scholar]
- [23].Acalovschi M, Buzas C, Radu C, et al. Hepatitis C virus infection is a risk factor for gallstone disease: a prospective hospital-based study of patients with chronic viral C hepatitis. J Viral Hepat 2009;16:860–6. [DOI] [PubMed] [Google Scholar]
- [24].Elzouki AN, Nilsson S, Nilsson P, et al. The prevalence of gallstones in chronic liver disease is related to degree of liver dysfunction. Hepatogastroenterology 1999;46:2946–50. [PubMed] [Google Scholar]
- [25].Park JH, Kim TN, Lee SH. The prevalence and risk factors of gallstones in Korean patients with liver cirrhosis. Hepatogastroenterology 2013;60:461–5. [DOI] [PubMed] [Google Scholar]
- [26].Liew PL, Wang W, Lee YC, et al. Gallbladder disease among obese patients in Taiwan. Obes Surg 2007;17:383–90. [DOI] [PubMed] [Google Scholar]
- [27].Chen LY, Qiao QH, Zhang SC, et al. Metabolic syndrome and gallstone disease. World J Gastroenterol 2012;18:4215–20. [DOI] [PMC free article] [PubMed] [Google Scholar]


