Abstract
Cough is frequently self-limiting, but may persist longer in certain individuals. Most of previous studies on the epidemiology of chronic cough have only measured period prevalence, and thus have afforded limited information on the burden and natural course. We aimed to investigate the epidemiology of chronic cough by using a point prevalence measure in a large-scale general population.
We analyzed cross-sectional data collected from 18,071 adults who participated in the Korean National Health and Nutrition Examination Survey 2010–2012. Presence and duration of current cough was ascertained by structured questionnaires, and cough was classified into acute (<3 weeks), subacute (3–8 weeks), or chronic cough (≥8 weeks). Demographic and clinical parameters were examined in relation to chronic cough.
The point prevalences of acute, subacute, and chronic cough were 2.5 ± 0.2%, 0.8 ± 0.1% and 2.6 ± 0.2%, respectively. The proportion of current cough showed a steep decrease after 1 week of duration. However, 2 peaks in the prevalence of current cough were revealed; cough durations of less than 1 week and longer than 1 year were most common (31.1% and 27.7% of current cough, respectively). Subacute and chronic cough were more prevalent in the elderly (≥65 years); the positive associations with older age were independent of other confounders, including current smoking and comorbidities.
This is the first report on the epidemiology of cough using a point prevalence measure in a nationally representative population sample. Our findings indicate a high burden of chronic cough among adults with current cough in the community. The dual-peak of cough duration suggested that the pathophysiology of acute and chronic cough may differ. The preponderance of elderly people in the prevalence of chronic cough warrants further investigation. In addition, more sophistication and validation of tools to define chronic cough will help our understanding of the epidemiology.
Keywords: adult, chronic cough, epidemiology, point prevalence
1. Introduction
Cough is the principal reflex defense mechanism protecting the lower airway from foreign, noxious, or infectious agents,[1] but cough is also a common medical problem that leads many patients to seek medical advice.[2] In adult clinical guidelines, cough is classified into 3 subtypes by duration: acute (<3 weeks), subacute (3–8 weeks), and chronic (≥8 weeks); this classification is clinically helpful in the differential diagnosis of cough triggers.[3,4] Acute cough is self-limiting in nature and is frequently caused by respiratory viral infections.[5] Subacute cough is mostly post-infectious cough, and spontaneous resolution is common.[6] Chronic cough has been suggested to be caused principally by asthma, eosinophilic bronchitis, rhinitis/rhinosinusitis, or gastroesophageal reflux disease.[3,4] However, many patients with chronic cough (12–42%) have been reported to exhibit no evident underlying cause.[7] Thus, treatment of chronic cough remains a major unmet clinical need.[8–10]
We recently conducted a systematic review and meta-analysis to examine the prevalence of chronic cough in general adult populations.[11] The pooled prevalence was approximately 9.6% worldwide but varied widely among continents, being considerably higher in Western than Asian countries.[11] However, several limitations were evident. First, the questionnaire used in most previous studies was not developed to explore chronic cough but rather chronic bronchitis.[12] Accordingly, chronic cough in most previous epidemiological studies was defined as cough that persisted for ≥3 months[13]; this differs from the criterion of international clinical guidelines (≥8 weeks).[3,4] Second, most studies reported the period prevalence (e.g., the 12-month prevalence) but not the point prevalence (e.g., at the time of the survey).[13] Point prevalence has the advantage of being less subject to recall bias. Furthermore, considering that cough is frequently self-limiting,[14] the measurement of point prevalence measure could be more appropriate for studying cough epidemiology. Point prevalence data on current cough status and duration, if available, could allow evaluation of the natural course of cough and would also make it possible to investigate risk factors for chronic cough. Third, nationwide prevalence data obtained from large-scale population surveys are available for only a few countries in terms of the period prevalence of chronic cough or bronchitis.[15–19]
The Korean National Health and Nutrition Examination Survey (KNHANES) is a nationwide population survey conducted by the Korea Centers for Disease Control and Prevention, and it aims to obtain nationally representative information on the health, behavior, and nutritional status of the Korean population. Using the protocols of KNHANES 2010–2012, it is possible to extract cross-sectional data on current cough prevalence and duration. Herein, we investigated the point prevalence of cough and cough subtypes (acute, subacute, and chronic cough) by duration and identified the underlying factors for chronic cough (vs no current cough) in a nationally representative sample of the Korean adult population.
2. Methods
2.1. Study population
We included Korean adults aged 18 years and older who participated in the KNHANES during 2010–2012. The survey protocol included an interview on health and nutrition, a physical examination, and blood tests. The institutional review board at the Korea Centers for Disease Control and Prevention approved the study protocol, and all participants signed informed consent forms. Of the original 25,534 participants, a total of 18,071 who completed cough questionnaires were finally included in the analysis (Fig. 1). The baseline demographic characteristics did not differ significantly between those who did and did not complete the cough questionnaire. The KNHANES adopted the stratified multistage cluster sampling design by using the rolling-survey sampling method. This rolling sampling allows the sample for each year to be representative of the general population and also to be homogenous and independent from each other.[20] The details of the KNHANES can be accessed online at https://knhanes.cdc.go.kr/knhanes/.
Figure 1.
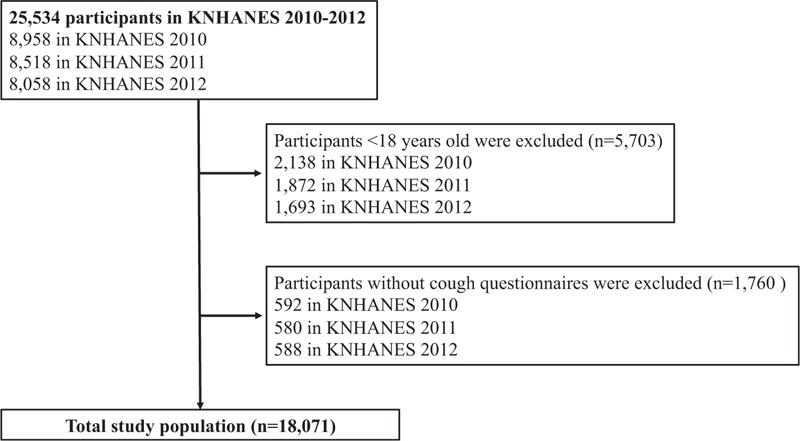
Flowchart of the study population.
2.2. Outcome definitions
The questionnaire exploring current cough was a component of the survey of respiratory disease conditions. The participants were asked about the presence of cough and its duration by trained interviewers using structured questionnaires, as follows: “Do you have cough currently?” and if “yes,” “How many days have you had this cough?” We classified current cough into “acute” (< 3 weeks), “subacute” (3–8 weeks), and “chronic” (>8 weeks), using the criteria of international clinical guidelines.[3,4]
We extracted demographic parameters including body mass index (BMI), smoking status, occupation, and household income. Height and weight were measured and BMI was calculated as the weight divided by the height squared (kg/m2). Other demographic variables were surveyed using structured questionnaires. Cigarette smoking status was classified as “never,” participants who had never smoked or had smoked fewer than 100 cigarettes ever; as “former,” those who had stopped smoking for 6 months or more but who had smoked more than 100 cigarettes; and as “current,” those who currently smoked or had quit within the past 6 months. Occupation was categorized using the Korean Standard Classification of Occupation as “white-collar,” managers, professionals, clerks, service/sales workers, the unemployed, the retired, and students and housewives; and as “blue-collar,” workers in agriculture, forestry, fisheries, and craft and related trades, plant and machine operators, assemblers, and simple laborers.[21] Household income was dichotomized as “low” or “high” at the 50th percentile.
Clinical parameters included in the analysis were chest x-ray findings, chronic rhinosinusitis (CRS), and several other comorbid conditions. Chest x-rays were interpreted by radiologists and were classified as “abnormal” if they suggested any of the following conditions: active pulmonary tuberculosis (TB), suspected TB, inactive TB, cardiac disease, pneumonia, emphysema, pneumothorax, pulmonary nodules, lung cancer, mediastinal disease, or other lung disease. Otherwise, chest x-rays were classified as “normal.” CRS was determined by nasal endoscopic findings and the presence of chronic nasal symptoms as previously described[22]; briefly, CRS was defined positive if nasal polyps were observed, or if 2 or more of the symptoms such as anterior/posterior nasal drip, nasal obstruction, facial pain/pressure, and olfactory dysfunction were present for longer than 3 months (anterior/posterior nasal drip or nasal obstruction should be included). Other comorbid conditions were determined by the questions on self-reported physician diagnosis of the following type “Have you ever been diagnosed with (a specific disease) by physician?” We selected following conditions on the basis of previous reports on the relationships with chronic cough; asthma, pulmonary TB, allergic rhinitis, diabetes mellitus, and depression.[23–25]
2.3. Statistical analysis
To obtain unbiased national estimates of current cough in the general Korean population, we applied the KNHANES sampling weights to accommodate the complex sample design of the survey. The sampling weights were extracted to account for the complex survey design, response rate, and poststratification. Briefly, sampling was reflected by sampling weights with the inverse of selection probability for primary sampling units and households. Then the inverse probability of selection was adjusted for household and individual nonresponse. In addition, the weights were modified by adjustment for sex and age demographics.[26] All participant characteristics are summarized as weighted percentages ± standard errors (SEs) for categorical variables and as weighted means ± SEs for continuous variables. Missing rates for variables were around 5% or less. Missing data were assumed to be missing completely at random, and were regarded as valid in all analyses. Chi-squared or ANOVA tests were used to compare differences between groups; if the P value was < 0.05, the post-hoc Bonferroni test was applied to identify between-group differences. Two-sided P values < 0.05 were considered statistically significant. Generalized logit model analyses were performed to identify factors independently associated with each subtype of current cough (acute, subacute or chronic cough; vs no current cough) using all participants’ data; variables with P values < 0.05 on univariate testing for chronic cough were adjusted. All statistical analyses were performed using Stata software (ver. 14.1; Stata Corp., College Station, TX).
3. Results
3.1. Prevalence of current cough and cough subtype by duration
The estimated prevalence of current cough in the Korean general adult population (aged ≥18 years) was 5.9 ± 0.3%. In terms of cough duration, the prevalences of acute (<3 weeks), subacute (3–8 weeks), and chronic cough (≥8 weeks) were 2.5 ± 0.2%, 0.8 ± 0.1%, and 2.6 ± 0.2%, respectively (Table 1). The distributions of cough duration (in weeks) among subjects with current cough are shown in Fig. 2. The proportions of subjects with current cough durations of less than 1 week, 1–2 weeks, and 2–3 weeks were 31.1%, 8.3%, and 4.4%, respectively. The proportion of chronic cough among subjects with current cough was 44.1%. Cough persisting for longer than 1 year had a prevalence of 1.6 ± 0.1% and constituted 27.7% of those with current cough.
Table 1.
Baseline characteristics of the KNHANES 2010–2012 participants according to current status of cough (n = 18,071).
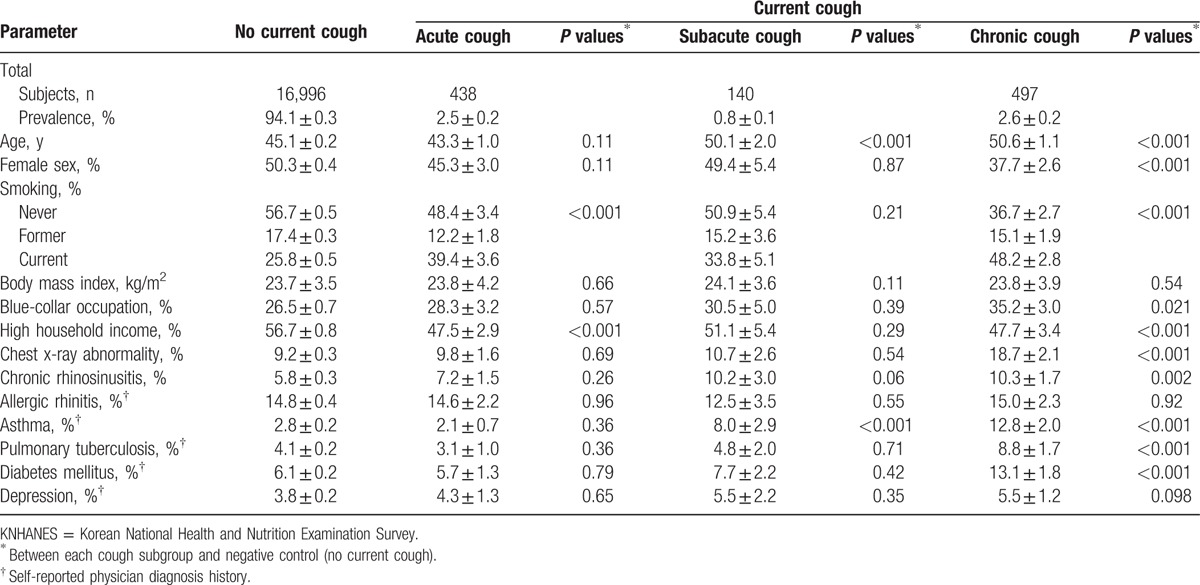
Figure 2.
Distribution of cough duration among adult subjects with current cough in the KNHANES 2010–2012 survey. The dotted vertical line indicates 50 weeks, and the rectangle to the right of the dotted line indicates the proportion of current cough that lasted for over 1 year. KNHANES = Korean National Health and Nutrition Examination Survey.
3.2. Prevalence of current cough subtypes by age group
The prevalences of cough subtype by duration and age group (18–39 years, 40–64 years, and ≥65 years) are depicted in Fig. 3A; the distributions of subtypes differed significantly among the various age groups (Pearson's chi-squared test; P< 0.001). The prevalence of chronic cough increased significantly with age (likelihood-ratio chi-squared test: P< 0.001), whereas acute or subacute cough did not.
Figure 3.
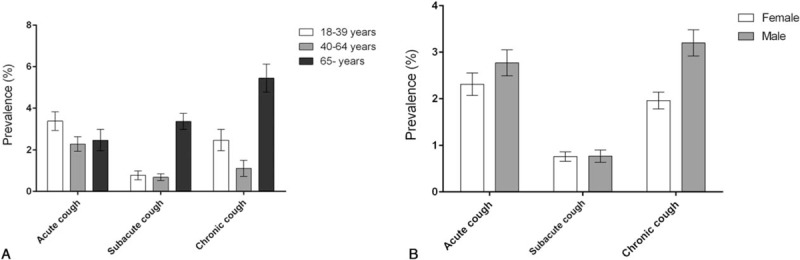
The prevalence of acute, subacute, and chronic cough by (A) age group and (B) sex. Bars with error bars are means with standard errors.
3.3. Prevalence of current cough subtypes by sex
Males had a significantly higher prevalence of current cough than did females (6.7 ± 0.5 vs 5.0 ± 0.3%; P< 0.001). The cough subtype prevalence also differed between males and females (Fig. 3B); in particular, chronic cough was significantly more prevalent in males than females (3.3 ± 0.3 vs 2.0 ± 0.2%; P < 0.001).
3.4. Socioeconomic and clinical characteristics of cough subtypes
Baseline characteristics were compared between those with and without current cough (Table 1). Compared to subjects without current cough, those with acute cough were more current smokers and had lower household income; the prevalence of comorbid conditions did not differ. Subjects with subacute cough were significantly older, and more likely to have asthma than those without cough. However, the subjects with chronic cough showed distinct characteristics from negative controls in various ways; chronic cough (vs no current cough) was significantly associated with older age, male sex, smoking history, blue-collar occupation, low household income, a chest x-ray abnormality, CRS and several comorbidities (self-reported physician diagnosis of asthma, pulmonary TB, and diabetes mellitus).
3.5. Determinants of current cough (acute, subacute, or chronic cough vs no current cough)
Generalized logit model analyses were performed to identify determinants of each subtype of current cough (vs no current cough; Table 2). Current smoking was a significant determinant factor for all subtypes of current cough. Household income status was significantly related to acute cough only, whereas older age (particularly aged ≥65 years) had significant associations with subacute and chronic cough. Among comorbid conditions, CRS and asthma showed positive relationships with subacute and chronic cough. Meanwhile, chest x-ray abnormality and diabetes mellitus were only associated with chronic cough.
Table 2.
Determinants for current cough subtypes (vs no current cough).
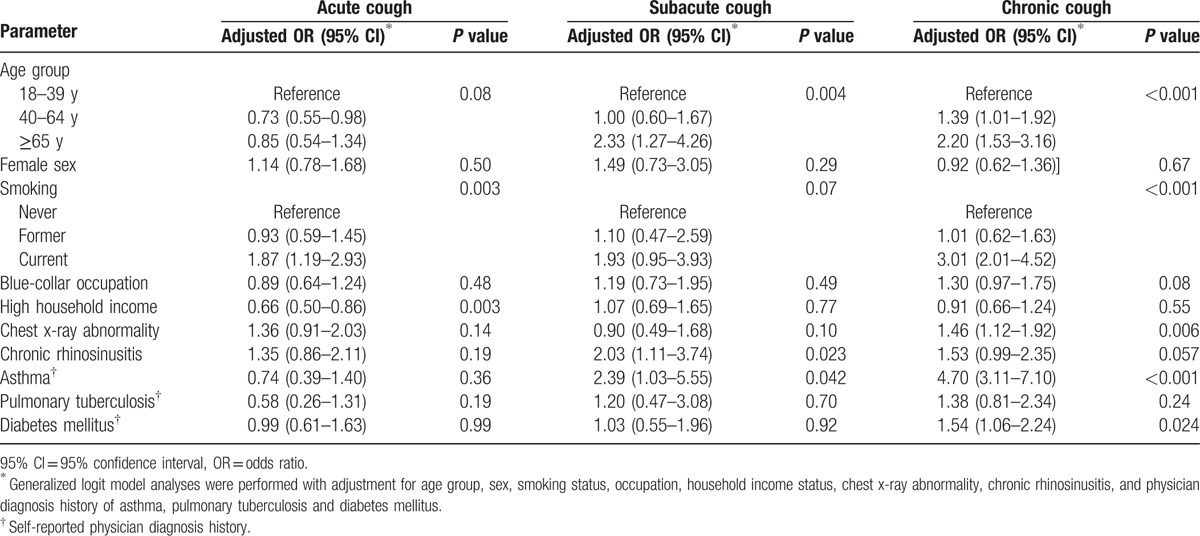
3.6. Male sex and smoking associations with chronic cough
Meanwhile, the positive association between male sex and chronic cough evident in univariate analysis became statistically insignificant after adjustment for smoking status (for males: chronic cough vs no current cough, odds ratio [OR] 1.02, 95% confidence interval [95% CI] 0.72–1.43; chronic cough vs acute cough, OR 0.82, 95% CI 0.57–1.19; Table not shown), suggesting that the male sex associations frequently observed in general population studies[13] are likely to reflect current smoking.
4. Discussion
In the present study, we sought to obtain a cross-sectional overview of the prevalence of current cough, and subtypes thereof, by cough duration and to identify the determinants of current cough (vs no current cough). We evaluated a nationally representative sample of the general Korean adult population. We found that a considerable proportion of subjects with current cough had chronic cough (44% of current cough subjects), and that 2 subtypes of current cough, subacute and chronic cough, were notably more frequent in the elderly (aged ≥65 years). On multivariate analyses, older age, current smoking, and clinical parameters indicative of upper/lower airway pathology were significant determinants of chronic cough.
The literature contains several studies on the prevalence of chronic cough in general adult populations.[11] However, most previous studies reported the period prevalence (such as chronic cough during the past year).[13] The study by Fujimura[27] is one of the very few reports to explore the prevalence of current cough; the cited authors asked the question: “At present, do you have cough?” Of the Japanese population evaluated, 10.2% had a current cough and 2.3% a current chronic cough (≥8 weeks). However, a limitation of the study was that it was conducted via the Internet using a panel of volunteers registered with a research company. In a Swedish population-based survey (n = 1387),[28] cough was explored by asking: “Are you bothered with cough?”; however, chronic cough was defined only as “daily or frequent coughing” and not by cough duration. Chen et al[29] explored the prevalence of cough in young college students (n = 1087) in Guangzhou, China, and found that 10.9% had current cough and 3.3% chronic cough (≥8 weeks). However, to the best of our knowledge, no epidemiological survey to date has examined the point prevalence of chronic cough on a nationally representative sample. In this regard, our present work is new addition to previous knowledge.
Our present cross-sectional analysis showed that approximately 90% of current cough was acute or chronic (43.9% acute and 44.1% chronic). Cough persisting for less than 1 week formed the largest proportion of current cough (31.1%; Fig. 2). The proportion of current cough that lasted for longer than 1 week decreased steeply, suggesting that the cough was self-limiting. However, the proportion of current cough lasting for longer than 1 year constituted the second largest proportion (27.7%; Fig. 2). This dual peak in the prevalence of current cough may suggest that self-limiting and chronic cough are intrinsically different. Such findings warrant longitudinal, observational cohort studies exploring the natural course of cough.
Interestingly, we found that the prevalence of subacute and chronic cough had clear tendency to increase with age, unlike acute cough. Such an age-related increase in chronic cough was also observed in an internet-based survey conducted in Japan.[27] In our multivariate analysis, the relationships of subacute or chronic cough with older age was independent of smoking and several clinical parameters indicative of upper/lower respiratory conditions. In a recent multinational survey on chronic cough patients visiting cough specialist clinics, the most frequent age bracket of presentation was 60 to 69 years.[30] In our previous clinic-based study,[31] we suggested that heightened cough sensitivity, particularly in females, was relevant to the preponderance of cough in old age. However, this hypothesis has not been tested in general populations to date.
In addition to age, multivariate analysis showed that chest x-ray abnormality and self-reported physician diagnosis of asthma were positively associated with chronic cough (vs no current cough). These findings are plausible; cough is a frequent symptom of chronic lower respiratory tract conditions including asthma. These findings also indicate that cough persistence is common in subjects with pre-existing lower respiratory diseases. However, our findings on asthma should be viewed with caution since a self-reported history of physician diagnosed asthma may be prone to diagnostic error, the physician erroneously ascribing the chronic cough as due to asthma when other less frequently considered conditions may be the true cause. Whether respiratory conditions such as asthma or chronic obstructive pulmonary disease could underlie the age-related increase of chronic cough warrants further investigation.
Gastrointestinal conditions could also potentially influence the prevalence of chronic cough. Gastroesophageal reflux disease (GERD) is a frequent comorbidity in patients with chronic cough.[1] The irritable bowel syndrome was significantly associated with cough in the community-based adult population survey conducted in Yorkshire, UK.[32] In the elderly community population survey in Korea, constipation was found to have positive relationships with cough.[23] Unfortunately, these conditions were not included in the original protocols of the KNAHNES 2010–2012.
We observed that history of diabetes mellitus was positively related to chronic cough (vs no current cough; OR 1.54, 95% CI 1.06–2.24, P = 0.025; Table 2). Interestingly, we previously had observed that diabetes mellitus history or an indicator of uncontrolled diabetes (hemoglobin A1c≥8%) had significant relationships with frequent cough or chronic persistent cough (vs control without recent cough) in the elderly population cohort, and also the associations remained statistically significant even after excluding the subjects with history of angiotensin enzyme inhibitor (ACEi) medication.[23] The present analysis could not assess the effects of ACEi medication on these associations, due to the lack of parameter on recent medication history. However, our repetitive observations may warrant further investigations for the roles of diabetes mellitus in the epidemiology of chronic cough.
Unlike the female predominance observed in recent clinic-based surveys,[30,31] chronic cough was more frequent among males both in earlier studies on general populations[13] and in the present study. Several possible reasons may be advanced, including sex difference in health-seeking behaviors.[33] However, current smoking may well underlie the male predominance of chronic cough in general population studies, as shown in the present analysis. The effects of smoking cessation were reported in a population-based interventional study involving 2408 daily smokers in Denmark; cessation of or reduction in tobacco smoking led to significant reductions in self-reported cough symptoms (OR of 14.2 or 3.7, respectively).[34]
In Korea, 2 previous studies reported the period prevalence of chronic cough among middle-aged or elderly subjects living in certain areas. In the Korean Health and Genome study conducted on middle-aged adults (40–69 years) living in 2 cities, the period prevalence of chronic cough (cough on most days for ≥3 months per year for at least 2 successive years) was 3.7%.[35] In the Korean Longitudinal Survey on Health and Aging conducted on elderly subjects (≥65 years) living in an urban area, the period prevalence of chronic persistent cough (cough on most days for ≥3 months during the year) was 4.6%.[23] Direct comparisons between the studies are not possible, as the definitions of cough differed. However, collectively, these data afford an overview of the prevalence of chronic cough in Korean adults.
Several major limitations need to be considered in interpreting our findings. First, cough was defined by simple questionnaire (on the presence and duration), which was not validated before. A simple binary questionnaire was the usual method in previous surveys of cough prevalence (i.e., the presence of cough for at least 3 months of the year[36]), and the lack of clinical validation was the common limitation in previous surveys published so far.[13] As several tools have been recently validated to characterize cough,[37] we suggest more sophisticated tools to be introduced in future epidemiological surveys of cough. Second, the cross-sectional design of the work rendered it impossible to identify causal relationships between cough and potential risk factors. Third, the principal outcomes on demographics and comorbid conditions were mostly self-reported. Thus, recall bias and potential errors need to be considered in interpreting our analyses. In addition, we could not fully examine the comorbidity relationships, because gastrointestinal disorders, such as GERD, were not included in the original protocol of the KNHANES (2010–2012) survey. However, in East Asian populations, reflux-related cough is reported to be less prevalent (1–5%) compared with Western populations (20%).[4,38,39] Fourth, our definition of acute cough may not be equal to self-limiting cough, although the distribution plot (Fig. 2) suggested that the cough was considerably self-limiting. Fifth, influences of seasonal factors on cough could not be examined; however, the surveys were conducted in random order all over 3 years (2010–2012). Finally, our participants’ responses on cough duration are subject to recall bias; we observed several spikes in the distribution of cough duration (Fig. 2), particularly at monthly intervals. The subjects had been asked to answer “in day scale” in the study protocol, but may have had difficulty in calculating the “exact days” of cough duration, particularly in cases of longstanding cough. However, given the clear dual-peak of cough duration, we believe that this would not have strongly biased our conclusions. Because of these limitations, our cross-sectional data on cough duration may not be entirely sound in understanding the etiology of the natural history of cough. However, all information was obtained by exploring data from random subjects in a large-scale population sample, using a per-protocol approach.
In conclusion, we investigated the point prevalence and distribution pattern of current cough, and subtypes thereof, in terms of duration. Chronic cough constituted a considerably high proportion of current cough, and more in the elderly. The 2 peaks of cough duration suggest that the pathophysiology of acute and chronic cough differ. These findings warrant further longitudinal population cohort studies and also more sophistication and validation of tools to define chronic cough.
Footnotes
Abbreviations: 95% CI = 95% confidence interval, BMI = body mass index, CRS = chronic rhinosinusitis, KNHANES = Korean National Health and Nutrition Examination Survey, OR = odds ratio, SE = standard error, TB = tuberculosis.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Chung KF, Pavord ID. Prevalence, pathogenesis, and causes of chronic cough. Lancet 2008;371:1364–74. [DOI] [PubMed] [Google Scholar]
- [2].Morice AH. Epidemiology of cough. Pulm Pharmacol Ther 2002;15:253–9. [DOI] [PubMed] [Google Scholar]
- [3].Irwin RS, Baumann MH, Bolser DC, et al. Diagnosis and management of cough executive summary: ACCP evidence-based clinical practice guidelines. Chest 2006;129(1 suppl):1S–23S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Morice AH, Fontana GA, Sovijarvi AR, et al. The diagnosis and management of chronic cough. Eur Respir J 2004;24:481–92. [DOI] [PubMed] [Google Scholar]
- [5].Abdullah H, Heaney LG, Cosby SL, et al. Rhinovirus upregulates transient receptor potential channels in a human neuronal cell line: implications for respiratory virus-induced cough reflex sensitivity. Thorax 2014;69:46–54. [DOI] [PubMed] [Google Scholar]
- [6].Kwon NH, Oh MJ, Min TH, et al. Causes and clinical features of subacute cough. Chest 2006;129:1142–7. [DOI] [PubMed] [Google Scholar]
- [7].McGarvey LP. Does idiopathic cough exist? Lung 2008;186:78–81. [DOI] [PubMed] [Google Scholar]
- [8].Gibson PG, Vertigan AE. Management of chronic refractory cough. BMJ 2015;351:h5590. [DOI] [PubMed] [Google Scholar]
- [9].Morice AH, Millqvist E, Belvisi MG, et al. Expert opinion on the cough hypersensitivity syndrome in respiratory medicine. Eur Respir J 2014;44:1132–48. [DOI] [PubMed] [Google Scholar]
- [10].Song W-J, Chang Y-S, Morice AH. Changing the paradigm for cough: does’ cough hypersensitivity’aid our understanding? Asia Pac Allergy 2014;4:3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Song WJ, Chang YS, Faruqi S, et al. The global epidemiology of chronic cough in adults: a systematic review and meta-analysis. Eur Respir J 2015;45:1479–81. [DOI] [PubMed] [Google Scholar]
- [12].Council MR. Definition and classification of chronic bronchitis for clinical and epidemiological purposes. Lancet 1965;1:775–9. [PubMed] [Google Scholar]
- [13].Song WJ, Chang YS, Faruqi S, et al. Defining chronic cough: a systematic review of the epidemiological literature. Allergy Asthma Immunol Res 2016;8:146–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Irwin RS, Rosen MJ, Braman SS. Cough: a comprehensive review. Arch Intern Med 1977;137:1186. [DOI] [PubMed] [Google Scholar]
- [15].Brown CA, Crombie IK, Smith WC, et al. The impact of quitting smoking on symptoms of chronic bronchitis: results of the Scottish Heart Health Study. Thorax 1991;46:112–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Cerveri I, Accordini S, Corsico A, et al. Chronic cough and phlegm in young adults. Eur Respir J 2003;22:413–7. [DOI] [PubMed] [Google Scholar]
- [17].Cheng Y, Guo Y-L, Yeh W-Y. A national survey of psychosocial job stressors and their implications for health among working people in Taiwan. Int Arch Occup Environ Health 2001;74:495–504. [DOI] [PubMed] [Google Scholar]
- [18].Huchon G, Vergnenegre A, Neukirch F, et al. Chronic bronchitis among French adults: high prevalence and underdiagnosis. Eur Respir J 2002;20:806–12. [DOI] [PubMed] [Google Scholar]
- [19].Zemp E, Elsasser S, Schindler C, et al. Long-term ambient air pollution and respiratory symptoms in adults (SAPALDIA study). The SAPALDIA Team. Am J Respir Crit Care Med 1999;159(4 pt 1):1257–66. [DOI] [PubMed] [Google Scholar]
- [20].Cheng HM, Kim S, Park G-H, et al. Low vitamin D levels are associated with atopic dermatitis, but not allergic rhinitis, asthma, or IgE sensitization, in the adult Korean population. J Allergy Clin Immunol 2014;133:1048–55. [DOI] [PubMed] [Google Scholar]
- [21].Korean Standard Classification of Occupations (KSCO) 2007. http://kostat.go.kr/kssc. [Google Scholar]
- [22].Lee WH, Wee JH, Kim DK, et al. Prevalence of subjective olfactory dysfunction and its risk factors: Korean National Health and Nutrition Examination Survey. PLoS One 2013;8:e62725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Song WJ, Morice AH, Kim MH, et al. Cough in the elderly population: relationships with multiple comorbidity. PLoS One 2013;8:e78081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Dicpinigaitis PV, Tso R, Banauch G. Prevalence of depressive symptoms among patients with chronic cough. Chest 2006;130:1839–43. [DOI] [PubMed] [Google Scholar]
- [25].Song WJ, Faruqi S, Klaewsongkram J, et al. Chronic cough: an Asian perspective. Part 1: Epidemiology. Asia Pac Allergy 2015;5:136–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kweon S, Kim Y, Jang MJ, et al. Data resource profile: the Korea National Health and Nutrition Examination Survey (KNHANES). Int J Epidemiol 2014;43:69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Fujimura M. Frequency of persistent cough and trends in seeking medical care and treatment-results of an internet survey. Allergol Int 2012;61:573–81. [DOI] [PubMed] [Google Scholar]
- [28].Bende M, Millqvist E. Prevalence of chronic cough in relation to upper and lower airway symptoms; the Skovde population-based study. Front Physiol 2012;3:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chen RC, Lai KF, Liu CL, et al. An epidemiologic study of cough in young college students in Guangzhou. Zhonghua Liu Xing Bing Xue Za Zhi 2006;27:123–6. [PubMed] [Google Scholar]
- [30].Morice AH, Jakes AD, Faruqi S, et al. A worldwide survey of chronic cough: a manifestation of enhanced somatosensory response. Eur Respir J 2014;44:1149–55. [DOI] [PubMed] [Google Scholar]
- [31].Song W-J, Kim J-Y, Jo E-J, et al. Capsaicin cough sensitivity is related to the older female predominant feature in chronic cough patients. Allergy Asthma Immunol Res 2014;6:401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ford AC, Forman D, Moayyedi P, et al. Cough in the community: a cross sectional survey and the relationship to gastrointestinal symptoms. Thorax 2006;61:975–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Thorson A, Hoa N, Long N. Health-seeking behaviour of individuals with a cough of more than 3 weeks. Lancet 2000;356:1823–4. [DOI] [PubMed] [Google Scholar]
- [34].Pisinger C, Godtfredsen NS, Jorgensen T. Smoking reduction and cessation reduce chronic cough in a general population: the Inter99 study. Clin Respir J 2008;2:41–6. [DOI] [PubMed] [Google Scholar]
- [35].Shin C, Lee S, Abbott RD, et al. Respiratory symptoms and undiagnosed airflow obstruction in middle-aged adults: the Korean Health and Genome Study. Chest 2004;126:1234–40. [DOI] [PubMed] [Google Scholar]
- [36].Iversen L, Hannaford PC, Price DB, et al. Is living in a rural area good for your respiratory health? Results from a cross-sectional study in Scotland. Chest 2005;128:2059–67. [DOI] [PubMed] [Google Scholar]
- [37].Birring SS, Spinou A. How best to measure cough clinically. Curr Opin Pharmacol 2015;22:37–40. [DOI] [PubMed] [Google Scholar]
- [38].Lai K, Chen R, Lin J, et al. A prospective, multicenter survey on causes of chronic cough in China. Chest 2013;143:613–20. [DOI] [PubMed] [Google Scholar]
- [39].Kang SY, Kim GW, Song WJ, et al. Chronic cough in Korean adults: a literature review on common comorbidity. Asia Pac Allergy 2016;6:198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]


