Abstract
Background:
In some studies, gum-chewing was demonstrated to have a beneficial effect on resumption of bowel function; however, other contradictory findings in other studies refute the effects of gum-chewing on peristaltic movements and digestive system stimulation. In addition, most previous studies were after colorectal or gynecology surgery, whereas few reports focused on the effect of gum-chewing after gastrectomy. The aim of this randomized controlled trial was to assess the effectiveness of gum-chewing on postoperative bowel function in patients who had undergone laparoscopic gastrectomy.
Methods:
From March 2014 to March 2016, 75 patients with gastric cancer received elective laparoscopic surgery in Shanghai Tongji hospital and were postoperatively randomly divided into 2 groups: 38 in a gum-chewing (Gum) group and 37 in a control (No gum) group. The patients in the Gum group chewed sugarless gum 3 times daily, each time for at least 15 minutes, until the day of postoperative exhaust defecation.
Results:
The mean time to first flatus (83.4 ± 35.6 vs. 79.2 ± 24.2 hours; P = 0.554) and the mean time to first defecation (125.7 ± 41.2 vs. 115.4 ± 34.2 hours; P = 0.192) were no different between the no gum and Gum groups. There was also no significant difference in the incidence of postoperative ileus (P = 0.896) and postoperative hospital stay (P = 0.109) between the 2 groups. The postoperative pain score at 48 hours (P = 0.032) in the Gum group was significantly higher than in the no gum group. There was no significant difference between the 2 groups in regards to patient demographics, comorbidities, duration of surgery, complications, and nausea/vomiting score.
Conclusion:
Gum-chewing after laparoscopic gastrectomy did not hasten the return of gastrointestinal function. In addition, gum-chewing may increase patient pain on the second postoperative day.
Keywords: gastric cancer, gum-chewing, laparoscopic, Sham feeding
1. Introduction
For the past few decades, promoting the recovery of postoperative gastrointestinal function has been an issue needing urgent improvement. Patients undergoing abdominal surgery experience reduced gastrointestinal peristalsis owing to extensive dissection, postoperative exhaust, and long duration of anesthesia. Postoperative ileus (POI) is referred to as delayed defecation, lasting for 3 to 5 days, prolonging the resumption of regular bowel movements following abdominal surgery. Extended hospital stays increase the risk of hospital-acquired infections, deep vein thrombosis, pulmonary compromise, and total hospital costs.[1] Traditional interventions to prevent POI or restore bowel function after surgery include decompression of the stomach with a nasogastric tube, adequate pain control,[1] early mobilization of the patient to stimulate bowel function, epidural anesthesia,[2] and drugs such as metoclopramide, erythromycin, neostigmine, alvimopan, among others.
Recent studies aimed at shortening the period of POI have revealed that chewing gum can stimulate gastrointestinal motility, thereby reducing POI.[3,4,5] However, contradictory findings in other studies[6,7] refute the effects of gum-chewing on peristaltic movements and digestive system stimulation. In addition, most previous studies were after colorectal or gynecology surgery, whereas few reports focused on the effect of gum-chewing after gastrectomy[8,9].
The aim of this randomized controlled trial was to assess the effectiveness of gum-chewing on restoring postoperative bowel function in patients who received laparoscopic gastrectomy.
2. Methods
2.1. Patients and study design
This study was a prospective, single-center, randomized, and controlled clinical trial. The aim of the study was to evaluate the effectiveness of gum-chewing on postoperative bowel function and included consecutive adult patients with gastric cancer receiving elective laparoscopic surgery in Shanghai Tongji hospital from March 2014 to March 2016. The study was reviewed and approved by the Shanghai Tongji Hospital Review Board and the Ethics Committee of Shanghai Tongji Hospital. It was registered with the Chinese Clinical Trial Registry (Protocol ChiCTR-TRC-14004287).
For identification of cases, patient inclusion criteria were as follows: age ≥18 years; satisfactory consciousness (i.e., alertness) and cooperativeness toward chewing; underwent laparoscopic radical gastrectomy (including conversion to open surgery); any gender; any BMI; and informed consent.
Exclusion criteria for the study participation included the following: age <18 years; unconsciousness after surgery; no teeth or defective or incomplete chewing movement; need of long-term fasting and having received total parenteral nutrition; pyloric obstruction; remnant of gastric cancer; recurrence of gastric cancer; palliative surgery for advanced gastric cancer; refusal to participate in the trial; muscular and neurological disorders; history of drug addiction, especially opioids; and severe water and electrolyte disturbances.
The participants were given a thorough description of the research approach before entering the study. After eligibility had been established and patients provided written informed consent, patients were randomly allocated by a 1:1 ratio to the gum-chewing (Gum) or control (No gum) groups using a computer-generated (www.random.org) randomization sequence in our coordinating office. The sequence was then provided to the participating nurses by telephone after the operation. The same surgical group, to ensure technical replication, performed all the operations. All patients remained enrolled until the end of the study.
2.2. Sample size calculation
The required sample size in each group was calculated using G∗Power software (University of Kiel, Germany). The time to first bowel movement was used for power analysis because it was more accurate than the time to flatus. For this purpose, the medical records of patients who had undergone laparoscopic gastrectomy between January 2012 and January 2013 were reviewed. The mean time to first bowel movement was estimated to be 122 ± 40 hours. The few previous studies on the effect of gum-chewing after gastric surgery showed conflicted results.[8,9] Therefore, we assumed a 20% reduction of time to bowel movement for the gum-chewing group, according to a previous meta-analysis,[10] whose results were mostly from colectomy studies predicting a 98-hour mean time to bowel movement for the gum-chewing group, with a clinically relevant difference of 40 hours. A minimum sample size of 36 patients per randomization arm was estimated to obtain a power of 80% for detecting a difference at the 5% level.
3. Interventions and data collection
The protocol was carried out as follows: patients in the Gum group chewed sugarless gum for at least 15 minutes at 7:00, 12:00, and 18:00 from the first postoperative day (POD)-1 and continued until the day of exhaust defecation (up to 7 days). The patients in the No gum group received medical interventions with standardized ward care, thus minimizing confounding variables, to permit comparison for a placebo-like control for gum-chewing (i.e., sham feeding) alone. Although the patients, ward nurses, and the research assistant could not be blinded, all other investigators were blinded. Patients or their relatives completed their own confidential questionnaires to prevent bias and subjectivity.
Specific elements of the traditional enhanced recovery after surgery (ERAS) were incorporated, including preoperative and intraoperative warming. Other ERAS elements included use of patient-controlled analgesia, early removal of urinary catheters for most cases, and early ambulation, beginning on POD 1.
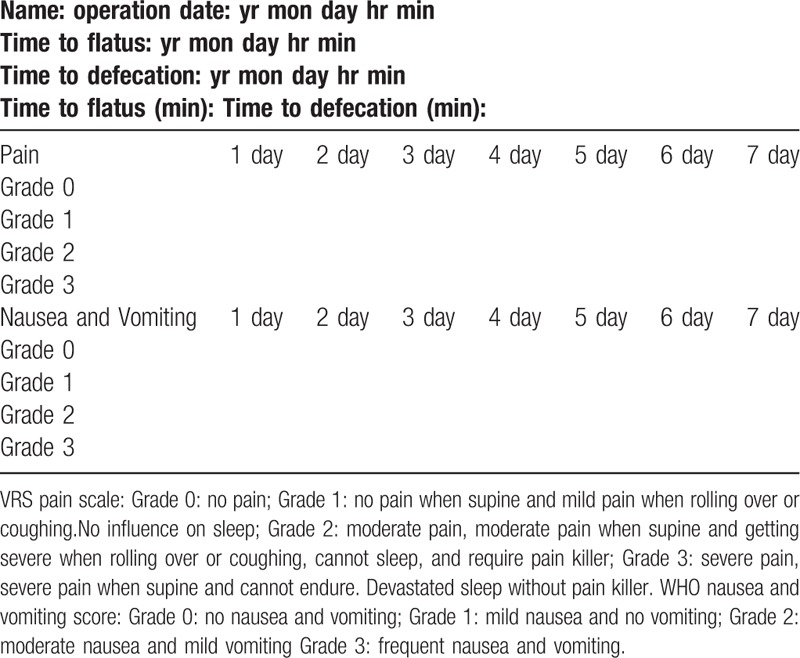
We followed the strategy for removing the nasogastric tube within 24 hours after surgery.[11] Patients were subsequently allowed to receive a clear-liquid diet. The drain was removed when the aspirate was minimal or nonpurulent, usually within 3 to 4 days. Using 24-hour durations as time points after operation, we recorded the occurrence of first flatus and defecation, the incidence of POI, pain scores, nausea, and vomiting scores (Table 1), analgesic drug use, and complication data. Adynamic or paralytic ileus that persisted for >3 days following surgery was termed POI.[12] Complications were graded and reported using the Clavien–Dindo (CD) classification.[13] Complications of grades I and II were defined as minor complications, and grades III and higher were defined as major complications. The data-collecting instruments included the interview form, questionnaires, and the examination of subjects. In addition, age, sex, comorbidity, American Society of Anesthesiologists (ASA) grade, duration of the operation, need for postoperative analgesics, morbidity, mortality and postoperative hospital stay were also recorded. At our hospital, discharge from the department was performed when 3 conditions were fulfilled: normal body temperature for at least 24 hours, normal leukocyte count, and no apparent surgical site infection.
Table 1.
VRS pain scale and WHO Nausea and vomiting grade.
4. End points
The primary end points were time to flatus, time to defecation, and the incidence of POI. The secondary end points were length of postoperative hospital stay, pain score, and nausea/vomiting scores.
4.1. Statistical analysis
Summarized data were analyzed using SPSS (version 19.0; SPSS Inc, Chicago, IL). Continuous variables, such as age, duration of surgery, analgesic drug consumption, time to first flatus, and defecation, were presented as the mean ± standard deviation. Categorical variables, such as sex, ASA grade, comorbidities, postoperative complications, pain scores, and nausea and vomiting scores were expressed as frequencies. Student t tests were used to compare the means of continuous variables with normal distribution, whereas Mann-Whitney U tests were used for those with nonparametric distribution. Categorical variables were compared using the χ2 test. For small samples, we used Yate correction for continuity, as appropriate. A probability value ≤0.05 (P ≤ 0.05) was considered significant.
5. Results
Between March 2014 and March 2016, 85 patients participated in this trial. After 10 patients were excluded before randomization (see flowchart), a total of 75 patients were randomly assigned to either the Gum (n = 38) or No gum (n = 37) group.
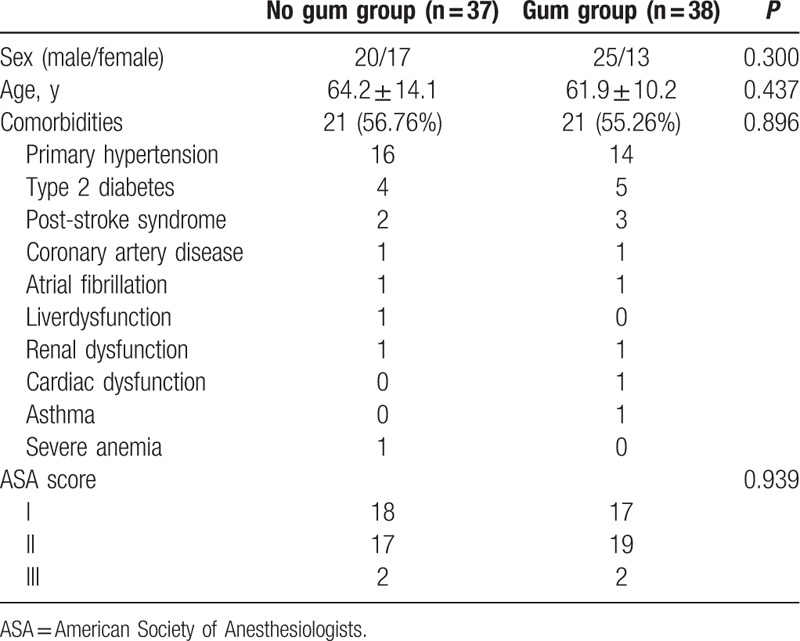
Baseline characteristics were similar between the 2 groups (Table 2). There were no differences in sex, age, comorbidities, and ASA grade. Twenty-one patients in the No gum group had comorbidities before their operations, as did 21 patients in the Gum group. The most common comorbidities included primary hypertension, type 2 diabetes mellitus, post-stroke syndrome, and coronary artery disease.
Table 2.
Baseline characteristics.
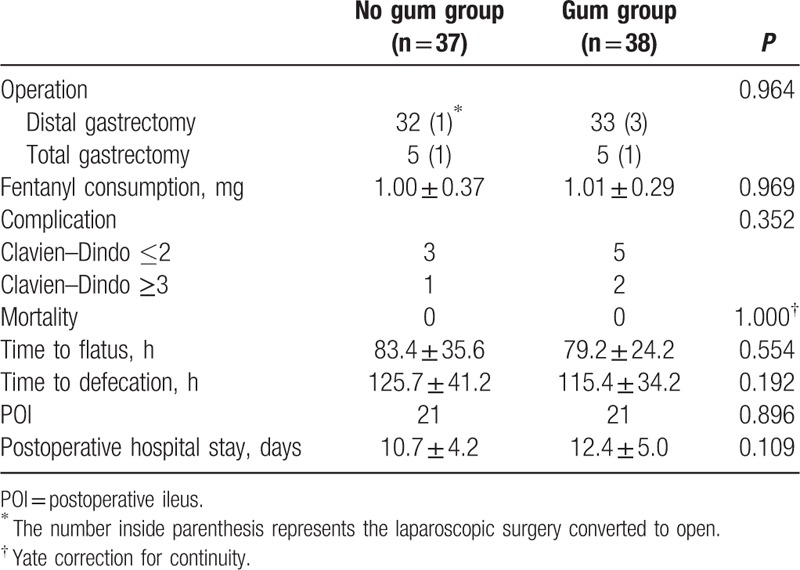
The operation outcomes for both groups are shown in Table 2. Two cases in the No gum group and 4 cases in the Gum group were converted to open surgery. There was no significant difference in the duration of operation between the 2 groups. The rates of POI of the 2 groups did not significantly differ. In the No gum group, 1 patient developed CD grade I complications: wound infection requiring dressing change. Grade II complications occurred in 2 patients: pneumonia requiring antibiotics. One patient developed a grade III complication: pleural effusion and atelectasis requiring thoracocentesis under local anesthesia. In the Gum group, 1 patient developed CD grade I complications: wound infection requiring dressing change. Grade II complications occurred in 4 patients: 2 developed pneumonia requiring antibiotics, 1 developed liver abscess requiring antibiotics, and 1 developed congestive heart failure requiring cardiac glycosides and diuretics. Two patients developed a grade III complication: pleural effusion and atelectasis requiring thoracocentesis under local anesthesia. There was no perioperative mortality in this series.
Patient-controlled analgesia (PCA) with fentanyl was administered to all the patients. There was no significant difference in fentanyl consumption between the 2 groups (P = 0.969).
As shown in Table 3, there was no significant difference in the mean time to the onset of gas passage (P = 0.554) or defecation (P = 0.192) between the 2 groups. There was also no significant difference in the incidence of POI (P = 0.896) and postoperative hospital stay (P = 0.109) between the 2 groups.
Table 3.
Operative outcomes.
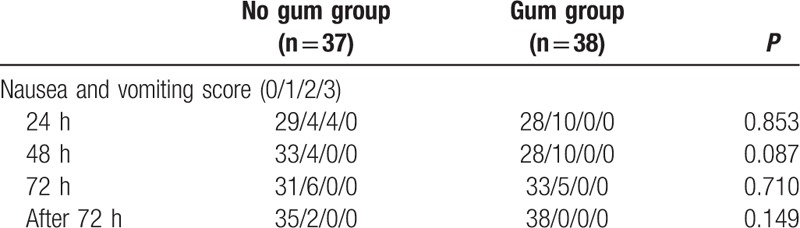
Pain scores after operation are listed in Table 4. We found that the 48-hour postoperative pain scores in the Gum group were significantly higher (P = 0.032). However, the 24-, 72-, and 72-hour-after pain scores were not significantly different between the 2 groups. We evaluated nausea and vomiting scores 24, 48, 72, and after 72 hours in the patients (Table 5). Between the 2 groups, 24 hours, 48 hours, 72 hours, and 72 hours-after nausea and vomiting scores were not significantly different.
Table 4.
Pain score after operation.
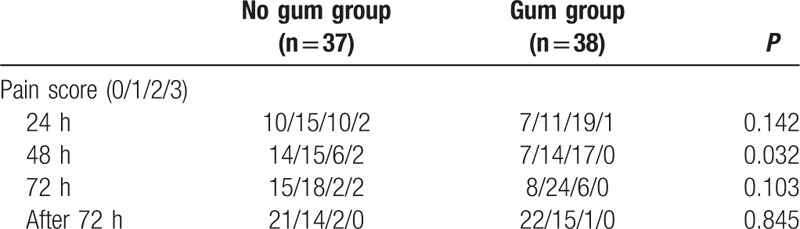
Table 5.
Nausea and vomiting score after operation.
6. Discussion
Paralytic ileus is the most common postoperative complication after abdominal surgery. POI can result in pain, vomiting, and abdominal distension; this can delay the speed of a patient's recovery after major gastrointestinal surgery. In recent years, reducing the burden of surgery, postoperative complications, and facilitating rehabilitation have been the focus of patient management. Gum-chewing is one of the treatments for POI, but there is no consensus on its efficacy for lessening the time to flatus and defecation.
A meta-analysis published in the Cochrane database in 2015 provided some support for a benefit of postoperative gum-chewing in improving recovery of gastrointestinal function.[14] This documentation reviewed 4053 records, of which only 81 met the inclusion criteria, including 10 studies of gastrointestinal surgery, whereas the rest regarded cesarean or other surgeries. Of those 10 studies, 6[15,16,9,17,18,19] found no significant difference in time to flatus and defecation after gastrointestinal operation between gum-chewing and control groups. However, 4 studies[20,21,22,23] found that gum-chewing could hasten the recovery of flatus and defecation. Nine of these 10 randomized control trials studied surgery for colorectal disease (except Bonventre research,[9] which included gastric, colon and cholecystectomy.) There were only 4 documentations about laparoscopic surgery[16,9,17,20] and the rest were about open colonectomy. More recent meta-analysis suggested that gum-chewing in the immediate postoperative period after a cesarean section is a well tolerated intervention that enhances early recovery of bowel function.[24] However, as to the effect of gum-chewing after colorectal resection, the results remained inconsistent based on the latest studies[25,26].
Our research was a prospective randomized control study of POI management after laparoscopic gastrectomy that included 75 gastric cancer patients. As mentioned above, little research has focused on the effect of gum-chewing on return of gastrointestinal function after laparoscopic surgery, especially for gastric cancer. Our results were essentially the same as most previous studies,[15,16,9,17,18,19] suggesting that gum-chewing may not enhance gastrointestinal recovery after laparoscopic surgery for gastric cancer in the ERAS program.[27] A promising intervention to enhance gastrointestinal recovery is early postoperative feeding, hypothesized to activate the cephalic-vagal reflex.[28,29] Cephalic phase hormonal release occurs through the activation of vagal-efferent fibers in response to food-related sensory stimuli. Gum-chewing stimulates the person to eat and increases peristaltic bowel movements, hastening ileus recovery owing to the cephalic-vagal reflex. Therefore, there are 3 reasons that might explain why gum-chewing could not enhance gastrointestinal recovery in the present study. First, the vagus nerve trunks were divided during gastrectomy, especially in total gastrectomy, which may block the cephalic-vagal response and make gum-chewing ineffective theoretically. Second, the effect in promoting the bowel movements by sham feeding was likely offset by the early feeding routine set forth in the ERAS program. In consideration of the fact that most patients in our study were submitted to laparoscopic treatment, another possible reason is that the weak improvement owing to gum-chewing is not significant enough to observe after laparoscopy.
It was slightly surprising to find that patients had significantly higher pain scores in the Gum group on POD 2. According to patient feedback, gum-chewing may exhaust them after operations, lowering their pain threshold, whereas Fitzgerald[30] proposed that some ingredients of gum may aggravate the feeling of pain. Although more pain was seen in the Gum group, there were no significant differences in analgesic drug consumption that would indicate the pain was considerably less endurable in the Gum group compared to the No gum group.
There are several limitations to our trial. First, our study was limited by the fact that all of the patients were treated at a single hospital. Second, this was a single-blind randomized controlled trial, and the fact that the patients were not blinded to their group assignments could lead to selection bias. Third, although we documented the time to flatus and defecation as the primary endpoint of our study, these measurements were not very accurate, partially because of patients’ frequent omission of their first flatus; thus, these indicators were too subjective. In future studies, we would improve these limitations by using multichannel luminal manometry or electrical recordings.[31,32] Fourth, we only applied part of the ERAS program to the management of our patients as standard care. ERAS is a multimodal perioperative care pathway designed to attenuate the stress response during a patient's journey through a surgical procedure, facilitating the maintenance of preoperative bodily compositions and organ function, to better permit achievement of early recovery.[27] This process was initially thought to be a radical move away from tradition and dogma to a fundamental change in the (more effective) perioperative management of patients, and this process has struggled to gain wider acceptance.[33] Moreover, the contents of ERAS have also changed with time. Although our trial protocol was partly consonant with ERAS, we also followed traditional surgical practice for gastric tube decompression, with routine placement of drainage tubes and urinary catheters. Therefore, more complete ERAS adoption should be applied to future studies.
In summary, our study suggests that gum-chewing after laparoscopic gastrectomy may not hasten the return of gastrointestinal function and may in fact increase pain on the second postoperative day. Therefore, gum-chewing may not be recommended in patients receiving laparoscopic gastrectomy. As to the clinical application of gum-chewing after open gastrectomy, it should be based on the results of further prospective, randomized, and controlled trials.
Acknowledgments
The authors are grateful to Zhenqing Tang (Master of Public Health) for his help with statistics.
Footnotes
Abbreviations: ASA = American Society of Anesthesiologists, CD = Clavien–Dindo classification, ERAS = enhanced recovery after surgery, PCA = patient-controlled analgesia, POD = postoperative day, POI = postoperative ileus.
BG, HZ, and RL contributed equally to this work
This project was supported by the National Natural Science Foundation of China (Grant Number81370586), Health and Family planning Commission of Shanghai (Grant No. 201540072), Shanghai Science and Technology Committee (Grant No. 15411962600), and the Fundamental Research Funds for Central Universities (Grant No. 1508219072).
The authors report no conflicts of interest.
References
- [1].Johnson MD, Walsh RM. Current therapies to shorten postoperative ileus. Cleveland Clin J Med 2009;76:641–8. [DOI] [PubMed] [Google Scholar]
- [2].Jorgensen H, Wetterslev J, Moiniche S, et al. Epidural local anaesthetics versus opioid-based analgesic regimens on postoperative gastrointestinal paralysis, PONV and pain after abdominal surgery. Cochrane Database Syst Rev 2000;4: Cd001893. [DOI] [PubMed] [Google Scholar]
- [3].Purkayastha S, Tilney HS, Darzi AW, et al. Meta-analysis of randomized studies evaluating chewing gum to enhance postoperative recovery following colectomy. Arch Surg 2008;143:788–93. [DOI] [PubMed] [Google Scholar]
- [4].Kouba EJ, Wallen EM, Pruthi RS. Gum chewing stimulates bowel motility in patients undergoing radical cystectomy with urinary diversion. Urology 2007;70:1053–6. [DOI] [PubMed] [Google Scholar]
- [5].van den Heijkant TC, Costes LM, van der Lee DG, et al. Randomized clinical trial of the effect of gum chewing on postoperative ileus and inflammation in colorectal surgery. Br J Surg 2015;102:202–11. [DOI] [PubMed] [Google Scholar]
- [6].Andersson T, Bjersa K, Falk K, et al. Effects of chewing gum against postoperative ileus after pancreaticoduodenectomy–a randomized controlled trial. BMC Res Notes 2015;8:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kobayashi T, Masaki T, Kogawa K, et al. Efficacy of gum chewing on bowel movement after open colectomy for left-sided colorectal cancer: a randomized clinical trial. Dis Colon Rectum 2015;58:1058–63. [DOI] [PubMed] [Google Scholar]
- [8].Zhang Q, Zhao P. Influence of gum chewing on return of gastrointestinal function after gastric abdominal surgery in children. Eur J Pediatr Surg 2008;18:44–6. [DOI] [PubMed] [Google Scholar]
- [9].Bonventre S, Inviati A, Di Paola V, et al. Evaluating the efficacy of current treatments for reducing postoperative ileus: a randomized clinical trial in a single center. Minerva Chir 2014;69:47–55. [PubMed] [Google Scholar]
- [10].Yin Z, Sun J, Liu T, et al. Gum chewing: another simple potential method for more rapid improvement of postoperative gastrointestinal function. Digestion 2013;87:67–74. [DOI] [PubMed] [Google Scholar]
- [11].Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric cancer: official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Livingston EH, Passaro EP., Jr Postoperative ileus. Dig Dis Sci 1990;35:121–32. [DOI] [PubMed] [Google Scholar]
- [13].Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Short V, Herbert G, Perry R, et al. Chewing gum for postoperative recovery of gastrointestinal function. Cochrane Database Syst Rev 2015;2:Cd006506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zaghiyan K, Felder S, Ovsepyan G, et al. A prospective randomized controlled trial of sugared chewing gum on gastrointestinal recovery after major colorectal surgery in patients managed with early enteral feeding. Dis Colon Rectum 2013;56:328–35. [DOI] [PubMed] [Google Scholar]
- [16].Lim P, Morris OJ, Nolan G, et al. Sham feeding with chewing gum after elective colorectal resectional surgery: a randomized clinical trial. Ann Surg 2013;257:1016–24. [DOI] [PubMed] [Google Scholar]
- [17].Forrester DA, Doyle-Munoz J, McTigue T, et al. The efficacy of gum chewing in reducing postoperative ileus: a multisite randomized controlled trial. J Wound Ostomy Continence Nurs 2014;41:227–32. [DOI] [PubMed] [Google Scholar]
- [18].Matros E, Rocha F, Zinner M, et al. Does gum chewing ameliorate postoperative ileus? Results of a prospective, randomized, placebo-controlled trial. J Am Coll Surg 2006;202:773–8. [DOI] [PubMed] [Google Scholar]
- [19].Quah HM, Samad A, Neathey AJ, et al. Does gum chewing reduce postoperative ileus following open colectomy for left-sided colon and rectal cancer? A prospective randomized controlled trial. Colorectal Dis 2006;8:64–70. [DOI] [PubMed] [Google Scholar]
- [20].Asao T, Kuwano H, Nakamura J-i, et al. Gum chewing enhances early recovery from postoperative ileus after laparoscopic colectomy. J Am Coll Surg 2002;195:30–2. [DOI] [PubMed] [Google Scholar]
- [21].Bahena-Aponte JA, Cardenas-Lailson E, Chavez-Tapia N, et al. [Usefulness of chewing gum for the resolution of postoperative ileus in left colon resections]. Rev Gastroenterol Mex 2010;75:369–73. [PubMed] [Google Scholar]
- [22].Hirayama I, Suzuki M, Ide M, et al. Gum-chewing stimulates bowel motility after surgery for colorectal cancer. Hepatogastroenterol 2006;53:206–8. [PubMed] [Google Scholar]
- [23].Schuster R, Grewal N, Greaney GC, et al. Gum chewing reduces ileus after elective open sigmoid colectomy. Arch Surg 2006;141:174–6. [DOI] [PubMed] [Google Scholar]
- [24].Pereira Gomes Morais E, Porfírio RR, Macedo GJ, et al. Chewing gum for enhancing early recovery of bowel function after caesarean section. Cochrane Database Syst Rev 2016;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Atkinson C, Ness PC, Longman AR, et al. Randomized clinical trial of postoperative chewing gum versus standard care after colorectal resection. Br J Surg 2016;103:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Shum NF, Mak CH, Foo JC, WL, et al. Randomized clinical trial of chewing gum after laparoscopic colorectal resection. Br J Surg 2016;103:7. [DOI] [PubMed] [Google Scholar]
- [27].Melnyk M, Casey RG, Black P, et al. Enhanced recovery after surgery (ERAS) protocols: time to change practice? Can Urol Assoc J 2011;5:342–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Carr CS, Ling KD, Boulos P, et al. Randomised trial of safety and efficacy of immediate postoperative enteral feeding in patients undergoing gastrointestinal resection. BMJ 1996;312:869–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Stewart BT, Woods RJ, Collopy BT, et al. Early feeding after elective open colorectal resections: a prospective randomized trial. Aust N Z J Surg 1998;68:125–8. [DOI] [PubMed] [Google Scholar]
- [30].Fitzgerald JEF, Ahmed I. Systematic review and meta-analysis of chewing-gum therapy in the reduction of postoperative paralytic ileus following gastrointestinal surgery. World J Surg 2009;33:2557–66. [DOI] [PubMed] [Google Scholar]
- [31].Hennig GW, Costa M, Chen BN, et al. Quantitative analysis of peristalsis in the guinea-pig small intestine using spatio-temporal maps. J Physiol 1999;517(Pt 2):575–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Krauter EM, Strong DS, Brooks EM, et al. Changes in colonic motility and the electrophysiological properties of myenteric neurons persist following recovery from trinitrobenzene sulfonic acid colitis in the guinea pig. Neurogastroenterol Motil 2007;19:990–1000. [DOI] [PubMed] [Google Scholar]
- [33].Steenhagen E. Enhanced recovery after surgery: it's time to change practice!. NutritionClin Pract 2016;31:18–29. [DOI] [PubMed] [Google Scholar]


