Abstract
Background:
The association between facial flushing after alcohol consumption and the risk of cancer remains controversial. The aim of this study was to evaluate the relation between facial flushing and cancer risk.
Methods:
PubMed, EMBASE, and Cochrane Library were searched for relevant literature. The patients’ baseline characteristics and estimated risks were extracted. Odds ratios (ORs) with 95% confidence intervals (CIs) were pooled to estimate the risk of facial flushing in cancer, and subgroup analysis was performed.
Results:
Ten studies with 89,376 participants from East Asia were included. The pooled OR of facial flushing in all cancers was 1.43 (95% CI, 1.08–1.91), with the pooled ORs of 1.94 (95% CI, 1.33–2.83) and 0.95 (95% CI, 0.80–1.12) in men and women, respectively. The pooled ORs were also estimated in different cancer types.
Conclusion:
Our results showed that facial flushing response to alcohol was associated with higher cancer risk in men in East Asia, especially in esophageal squamous cell carcinoma, yet facial flushing was not significantly associated with cancer risk among women.
Keywords: alcohol, cancer, esophageal squamous cell carcinoma, facial flushing, meta-analysis
1. Introduction
After alcohol consumption, alcohol is first oxidized to acetaldehyde. Acetaldehyde is then metabolized to acetate by aldehyde dehydrogenase enzymes, mainly aldehyde dehydrogenase 2 (ALDH2).[1] People with inactive ALDH2 exhibit facial flushing after drinking alcohol resulting from acetaldehydemia, which is more prevalent among East Asians than Western populations.[2–4] So, facial flushing is regarded as a predictor of inactive ALDH2.[4] Since acetaldehyde is recognized as a highly toxic, mutagenic, and carcinogenic agent, more acetaldehyde in body is associated with higher cancer risk.[5] So, if someone experiences facial flushing after drinking alcohol, he probably has much acetaldehyde accumulating in the blood due to ALDH2 deficiency. As a result, he had a higher risk of developing cancer. So far, many researchers hypothesized that facial flushing after drinking alcohol and cancer risk incidence were related, and have investigated the association between them.[1,6–8] These studies mainly focused on upper aerodigestive tract cancer (UATC), including oral cavity, pharyngeal, and laryngeal cancer (OPLC), and esophageal squamous cell carcinoma (ESCC).[1,6–12] Some researchers found that facial flushing response to alcohol intake put individuals at higher risk of UATC.[6,8,11,13] But others did not come to such a conclusion.[1,9] Besides, some investigators examined the risk of facial flushing in breast cancer and endometrial cancer, and the results were also inconclusive.[14,15] Due to the controversial conclusion, we aimed to perform a meta-analysis to summarize the available evidence on the association between facial flushing and cancer risk.
2. Methods
2.1. Search strategy
Since this is a meta-analysis, ethical approval was not necessary. We followed the developed guidelines for the systematic review and meta-analysis in performing our study.[16] PubMed, EMBASE, and Cochrane Library were searched to identify potentially relevant literature (last search on October 22, 2016), using the following search strategy: “flushing AND (ethanol OR alcohol) AND (neoplasm OR tumor OR cancer OR carcinoma).” Reference lists of relevant articles were also checked for any additional studies. No language restrictions were used.
2.2. Study selection
Two reviewers (JZ and YS) independently determined study eligibility. Disagreements were resolved by consensus.
The studies were included based on the following criteria: the study reported the facial flushing response to alcohol in patients with cancer and cancer-free controls; the study reported the odds ratio (OR) with 95% confidence interval (CI), or data were provided to calculate the OR with 95% CI. We exclude unrelated articles, reviews, case reports, and articles without sufficient data.
2.3. Data extraction
Two investigators extracted the data from the selected studies, with any discrepancies being discussed. The primary data were OR with 95% CI. Adjusted ORs were extracted if adjusted ORs and unadjusted ORs both existed. Additional data included first author, publication year, country, number of cases and controls, mean age, tumor type, and other study characteristics.
2.4. Study quality assessment
The Newcastle–Ottawa scale criteria were used to assess the quality of each study.[17] This scale assessed 3 aspects of the study: subject selection, 0 to 4; comparability of subject, 0 to 2; and exposure (for case–control studies)/outcome (for cohort studies), 0 to 3. The Newcastle–Ottawa scale scores ranged from 0 to 9, and those studies with 7 scores or more were graded as high-quality ones.
2.5. Statistical analysis
The logOR and variance were used for aggregation of the risk of cancer. For the studies without OR or 95% CI, the data were calculated by RevMan 5.1 (Cochrane collaboration, Oxford, UK). Then the data were pooled together by STATA 11.0 (STATA Corporation, College Station, TX). And forest plots were used to estimate the correlation between facial flushing response to alcohol and the risk of cancer. The pooled OR would be considered to be significant with the P value less than 0.05. We also did subgroup analysis based on different clinical characteristics, for example, tumor type and sex. Heterogeneity was assessed, with P < 0.10 or I2 > 50% indicating significant heterogeneity. If heterogeneity was present, a random effect model was used. Publication bias was assessed by inspection of the funnel plot with Begg rank correlation, and P > 0.05 was considered that there was no potential publication bias. Heterogeneity and publication bias were assessed by STATA 11.0.
3. Results
3.1. Literature research
The initial literature search retrieved 265 articles. After removing duplicates, 241 articles were screened. Then 213 articles were excluded for the following reasons: unrelated articles (n = 106), reviews (n = 96), or case reports (n = 11). The rest 28 articles were reviewed in full text, and 18 were further excluded due to unrelated or lack of enough data. Finally, 10 studies[1,6–12,14,15] were included in our study. The study selection process was shown in Fig. 1.
Figure 1.
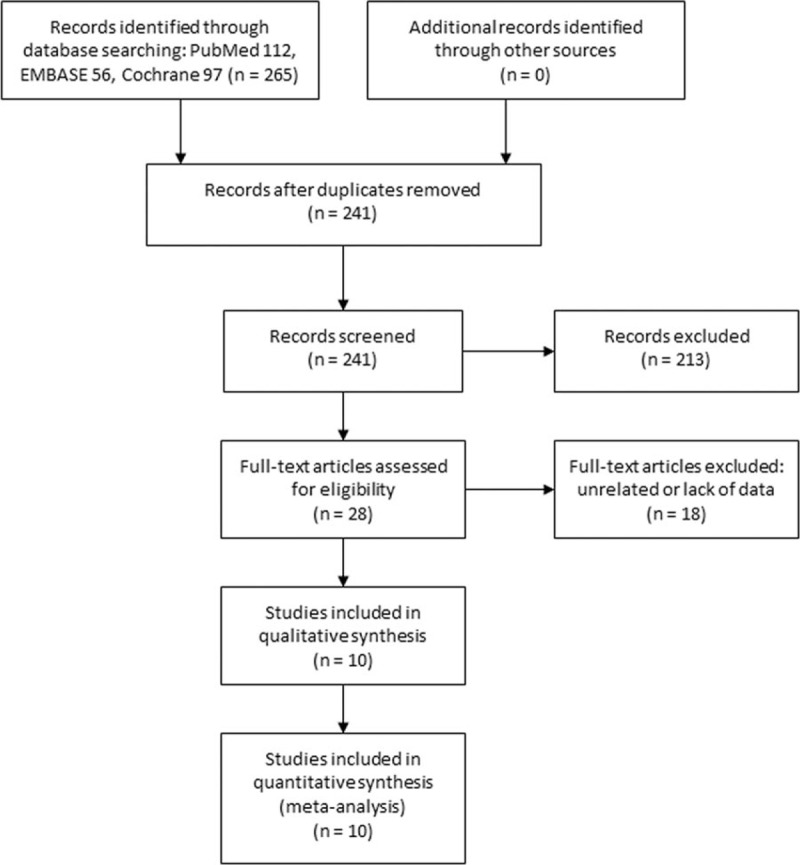
Selection process of studies.
3.2. Study characteristics
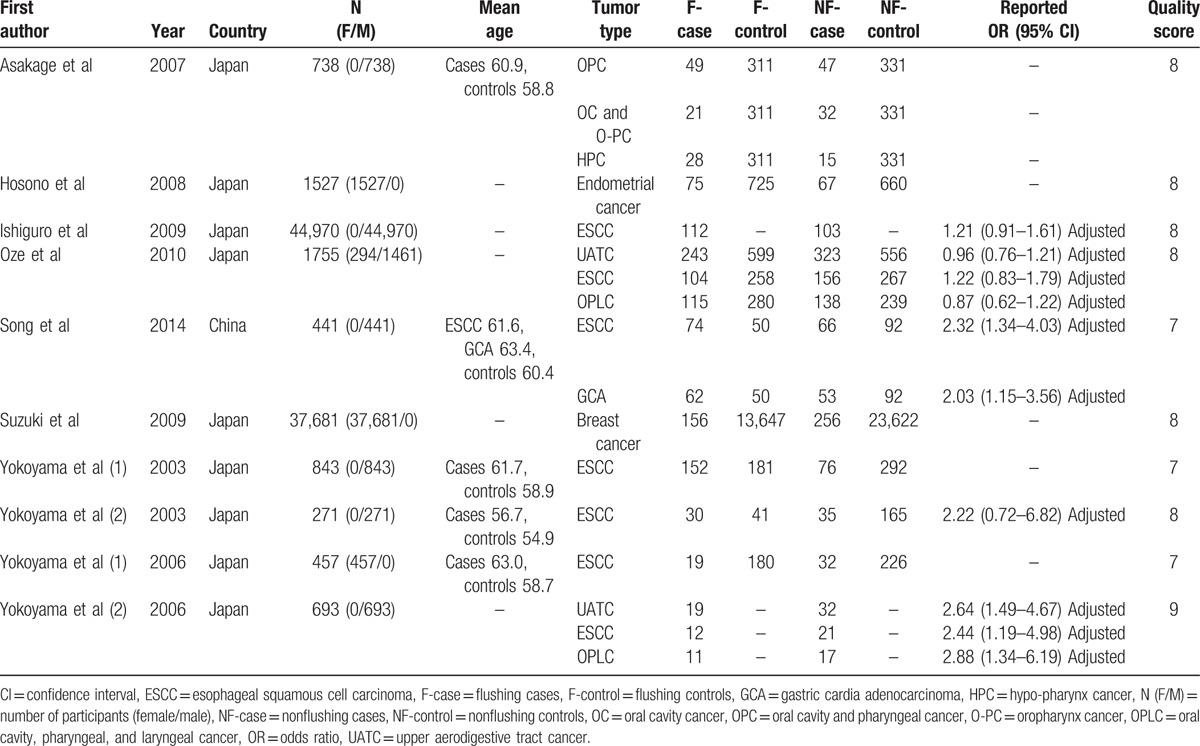
The detailed characteristics of the 10 included studies were shown in Table 1. All of the studies were from East Asia, with 9 of them from Japan and 1 from China. The total number of participants was 89,376 (range 271–44,970). Six studies included only men and 3 only women. The rest study included both men and women. The tumor types were ESCC, OPLC, gastric cardia adenocarcinoma, breast cancer, and endometrial cancer. Five of them reported OR with 95% CI while the other 5 did not. All of the studies got 7 scores or more in quality assessment, which meant they were high-quality ones.
Table 1.
Characteristics of the included studies.
3.3. Pooled results
3.3.1. Facial flushing and the risk of all cancers
In the 10 studies, 11 datasets were pooled to estimate the risk of all types of cancer mentioned above. The pooled OR was 1.43 (95% CI, 1.08–1.91), suggesting higher cancer risk in patients who experienced facial flushing response to alcohol (Fig. 2). The between-study heterogeneity was significant (I2 = 84.2%, P < 0.001) and random effect model was used.
Figure 2.
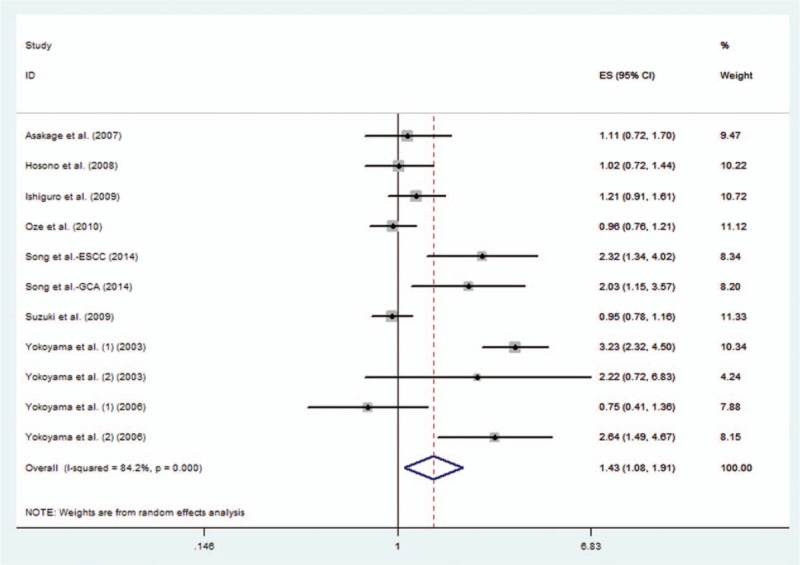
Pooled odds ratio (OR) of facial flushing response to alcohol in all cancers.
3.3.2. Facial flushing and the risk of all cancers in men and women
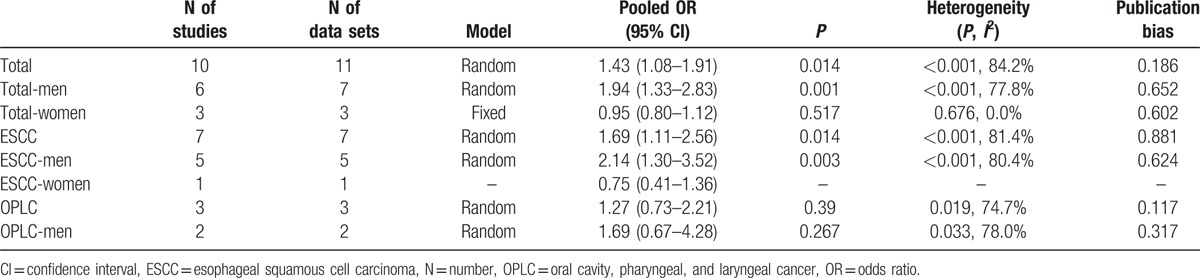
After pooling the results in the 6 studies those only included men, the OR was 1.94 (95% CI, 1.33–2.83). However, for the 3 studies that only included women, the pooled OR was 0.95 (95% CI, 0.80–1.12).
3.3.3. Facial flushing and the risk of ESCC
Seven studies[1,6–11] examined facial flushing and the risk of ESCC, and the pooled OR for ESCC was 1.69 (95% CI, 1.11–2.56).
3.3.4. Facial flushing and the risk of ESCC in men and women
After pooling the results in the 5 studies those only included men, the OR was 2.14 (95% CI, 1.30–3.52). One study only included women, the OR was 0.75 (95% CI, 0.41–1.36).
3.3.5. Facial flushing and the risk of OPLC
Three studies investigated the risk of OPLC, and the pooled OR was 1.27 (95% CI, 0.73–2.21). Two studies only included men, and the pooled OR of the 2 studies was 1.69 (95% CI, 0.67–4.28).
All the pooled results above were shown in Table 2.
Table 2.
Summary of meta-analysis results.
3.3.6. Facial flushing and cancer risk among different drinking statuses
We also attempted to find out the relationship between facial flushing and cancer risk among subjects with different drinking statuses. However, the studies used diverse classification models to define drinking statuses, thus the results could not be pooled.
3.4. Publication bias
No publication bias was found in this meta-analysis. The plot of publication bias of the 10 included studies is shown in Fig. 3.
Figure 3.
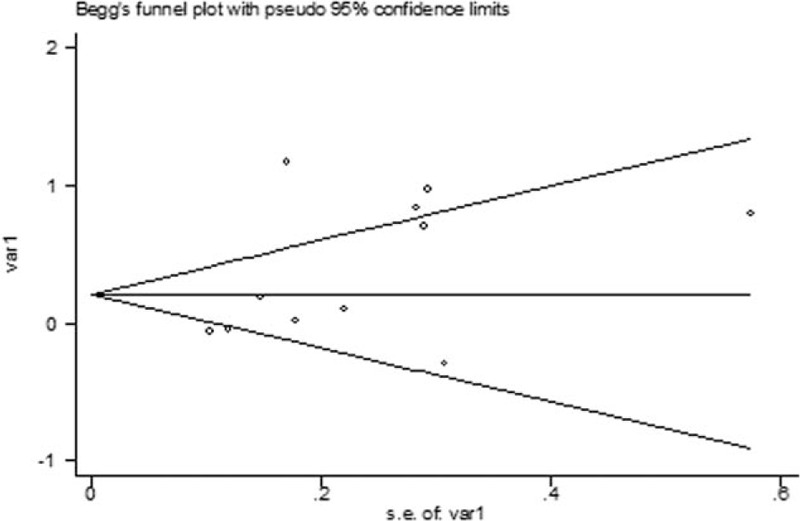
The Begg publication bias plot of the 10 studies that reported facial flushing and the risk of all cancers.
4. Discussion
4.1. Implications
This study emphasized in examining the association between facial flushing after alcohol consumption and cancer risk. We performed a meta-analysis to summarize the existing evidence. Our results showed that, in East Asia, facial flushing response to alcohol was associated with higher cancer risk in men, especially in ESCC, and that facial flushing was not significantly associated with cancer risk among women.
Andrici et al[13] performed a meta-analysis on the association of facial flushing and the risk of ESCC. They included 7 studies with a total size of 50,566. They found a positive relationship between the flushing response and ESCC, which was consistent with our analysis. Our study included 10 studies with a total size of 89,376. Besides ESCC, we also included patients with OPLC, gastric cardia adenocarcinoma, breast cancer, and endometrial cancer. We did subgroup analysis among men and women, and found that the relationship was positive in men and negative in women, especially in ESCC.
After pooling all the ORs in the 10 studies, we found a positive relationship between facial flushing and cancer risk. However, after subgroup analysis sorted by sex, we found that the conclusion was still positive among men, but it was not the same among women (OR 0.95, 95%CI 0.80–1.12). Since some researchers found that facial flushing was related to cancer among moderate and heavy drinkers,[1,13] one possible explanation is that women drink much less alcohol than men. Another reason maybe that the 3 studies[9,14,15] in the women's group included 3 distinct types of cancer (ESCC, breast cancer, and endometrial cancer). Furthermore, the study number in the women's group was limited. So, more studies focused on women on this topic are warranted.
In East Asia, more than 90% of esophageal cancer is ESCC, while in western countries adenocarcinoma is more common.[18,19] All the included studies in this meta-analysis were from East Asia and all focused on ESCC when they were investigating esophageal cancer. In the subgroup analysis, we found that facial flushing was associated with higher ESCC risk in men. One study only included women and the OR was not statistically significant. Thus, more studies on women are needed to draw a conclusion.
As to OPLC, the pooled OR was not statistically significant, although 2[11,12] of the 3 studies in this group concluded that facial flushing was associated with a higher risk of OPLC. More studies were needed to solve this controversy. Moreover, it might be better to investigate oral cavity cancer, pharyngeal cancer, and laryngeal cancer separately in the future studies.
Although the ORs could not be pooled among subjects with different drinking statuses, we could find some information in common in the included studies. Ishiguro et al[7] and Oze et al[1] found that heavy drinkers who experienced facial flushing were at higher risk of developing ESCC compared with heavy drinkers who did not, yet for light drinkers or nondrinkers the ORs were not significant. Asakage et al[12] found that heavy drinkers who experienced facial flushing had higher oral and pharynx cancer risk compared to the heavy drinkers who did not. This phenomenon was also not significant among ex-drinkers, light drinkers, or nondrinkers. Based on their findings and the fact that increased alcohol intake is an established risk factor for cancer,[20] heavy drinkers with facial flushing should be much more cautious.
Significant between-study heterogeneity was present in our meta-analysis. To minimize heterogeneity, subgroup analysis and sensitivity analysis were performed. In each subgroup, heterogeneity was still present except in the all cancers analysis among women. Thus, sex might be a source of heterogeneity. However, heterogeneity still existed in the men's group. Sensitivity analysis identified 2 studies[8,11] to be potential contributors to the heterogeneity. In the all cancers analysis and ESCC group, after excluding the 2 studies, the heterogeneity shrinked to less than 60% but still above 50%, and the pooled ORs remained almost the same. Only 1[11] of the 2 studies was in the OPLC group, after exclusion, the heterogeneity became 0.0% but the pooled OR was still not statistically significant.
4.2. Limitations
There were some limitations in our study. First, this study was based on a limited number of studies, especially in the women's group. Second, significant heterogeneity was found, which may be attributed to sex and other factors. Random effects models were used for more conservative estimates. Besides, 5 studies did not report the OR, and our calculated data were not adjusted and may not be accurate with the original study. Moreover, although no publication bias was found in our study, publication bias could not be completely excluded and was a major concern for all meta-analyses.
4.3. Future research
In conclusion, our results showed that facial flushing response to alcohol was associated with higher cancer risk in men in East Asia, especially in ESCC, yet facial flushing was not significantly associated with cancer risk among women. Based on the findings, health providers could use a simple facial flushing questionnaire to quickly predict individual risk of cancer, especially among heavy drinkers. Moreover, education about the association can help prevent alcohol related cancer among high risk populations. However, much more well-designed studies were needed to address this issue, especially in women.
Acknowledgments
The authors thank the reviewers for their constructive comments.
Footnotes
Abbreviations: ALDH2 = aldehyde dehydrogenase 2, CI = confidence interval, ESCC = esophageal squamous cell carcinoma, OPLC = oral cavity, pharyngeal, and laryngeal cancer, OR = odds ratio, UATC = upper aerodigestive tract cancer.
JZ, SZ, YS, and GM contributed equally to this work.
Funding/support: This work was supported by National Natural Science Foundation of China (NSFC81572850).
The authors have no conflicts of interest to disclose.
References
- [1].Oze I, Matsuo K, Hosono S, et al. Comparison between self-reported facial flushing after alcohol consumption and ALDH2 Glu504Lys polymorphism for risk of upper aerodigestive tract cancer in a Japanese population. Cancer Sci 2010;101:1875–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Suwaki H, Ohara H. Alcohol-induced facial flushing and drinking behavior in Japanese men. J Stud Alcohol 1985;46:196–8. [DOI] [PubMed] [Google Scholar]
- [3].Wolff PH. Ethnic differences in alcohol sensitivity. Science 1972;175:449–50. [DOI] [PubMed] [Google Scholar]
- [4].Shibuya A, Yasunami M, Yoshida A. Genotype of alcohol dehydrogenase and aldehyde dehydrogenase loci in Japanese alcohol flushers and nonflushers. Hum Genet 1989;82:14–6. [DOI] [PubMed] [Google Scholar]
- [5].Acetaldehyde. IARC Monogr Eval Carcinog Risk Chem Hum. 1985; 36:101–32. [PubMed] [Google Scholar]
- [6].Song Q, Hu P, Wang J, et al. Association between gastric cardia adenocarcinoma risk and alcohol flushing response, but not alcohol consumption. Med Oncol 2014;31:858. [DOI] [PubMed] [Google Scholar]
- [7].Ishiguro S, Sasazuki S, Inoue M, et al. Effect of alcohol consumption, cigarette smoking and flushing response on esophageal cancer risk: a population-based cohort study (JPHC study). Cancer Lett 2009;275:240–6. [DOI] [PubMed] [Google Scholar]
- [8].Yokoyama T, Yokoyama A, Kato H, et al. Alcohol flushing, alcohol and aldehyde dehydrogenase genotypes, and risk for esophageal squamous cell carcinoma in Japanese men. Cancer Epidemiol Biomarkers Prev 2003;12(11 Pt 1):1227–33. [PubMed] [Google Scholar]
- [9].Yokoyama A, Kato H, Yokoyama T, et al. Esophageal squamous cell carcinoma and aldehyde dehydrogenase-2 genotypes in Japanese females. Alcohol Clin Exp Res 2006;30:491–500. [DOI] [PubMed] [Google Scholar]
- [10].Yokoyama A, Yokoyama T, Muramatsu T, et al. Macrocytosis, a new predictor for esophageal squamous cell carcinoma in Japanese alcoholic men. Carcinogenesis 2003;24:1773–8. [DOI] [PubMed] [Google Scholar]
- [11].Yokoyama A, Omori T, Yokoyama T, et al. Risk of squamous cell carcinoma of the upper aerodigestive tract in cancer-free alcoholic Japanese men: an endoscopic follow-up study. Cancer Epidemiol Biomarkers Prev 2006;15:2209–15. [DOI] [PubMed] [Google Scholar]
- [12].Asakage T, Yokoyama A, Haneda T, et al. Genetic polymorphisms of alcohol and aldehyde dehydrogenases, and drinking, smoking and diet in Japanese men with oral and pharyngeal squamous cell carcinoma. Carcinogenesis 2007;28:865–74. [DOI] [PubMed] [Google Scholar]
- [13].Andrici J, Hu SX, Eslick GD. Facial flushing response to alcohol and the risk of esophageal squamous cell carcinoma: a comprehensive systematic review and meta-analysis. Cancer Epidemiol 2015;40:31–8. [DOI] [PubMed] [Google Scholar]
- [14].Suzuki R, Iwasaki M, Inoue M, et al. Alcohol consumption-associated breast cancer incidence and potential effect modifiers: the Japan Public Health Center-based Prospective Study. Int J Cancer 2010;127:685–95. [DOI] [PubMed] [Google Scholar]
- [15].Hosono S, Matsuo K, Kajiyama H, et al. Reduced risk of endometrial cancer from alcohol drinking in Japanese. Cancer Sci 2008;99:1195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Stang A. Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [18].Bytzer P, Christensen PB, Damkier P, et al. Adenocarcinoma of the esophagus and Barrett's esophagus: a population-based study. Am J Gastroenterol 1999;94:86–91. [DOI] [PubMed] [Google Scholar]
- [19].Devesa SS, Blot WJ, Fraumeni JF., Jr Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer 1998;83:2049–53. [PubMed] [Google Scholar]
- [20].Kamangar F, Chow WH, Abnet CC, et al. Environmental causes of esophageal cancer. Gastroenterol Clin North Am 2009;38:27–57. vii. [DOI] [PMC free article] [PubMed] [Google Scholar]


