Abstract
This study aimed to investigate prospective memory impairment in patients with breast cancer with different expression of hormone receptors, including the estrogen receptor (ER) and the progesterone receptor (PR).
A total of 120 patients with breast cancer who underwent chemotherapy following surgery were divided into 2 groups. The A group included 60 patients with ER−/PR− status, and the B group included 60 patients with ER+/PR+ status. After 6 cycles of postoperative adjuvant chemotherapy, all patients were administered neuropsychological and prospective memory tests, such as the Mini-Mental State Examination (MMSE), verbal fluency test (VFT), and digit span test (DST), as well as examination of event-based prospective memory (EBPM) and time-based prospective memory (TBPM).
As the neuropsychological background test results showed, there were no significant differences in MMSE, DST, and TBPM scores (∗:P > 0.05) between patients with breast cancer in the ER−/PR− and ER+/PR+ groups, while the VFT and EBPM scores were significantly greater in patients with breast cancer with ER+/PR+ status than in those with ER−/PR− status (∗∗: P < 0.01), indicating that patients with ER−/PR− status have significant impairment in EBPM, although not in TBPM.
The results of the present study indicate that different hormone receptor expression in patients with breast cancer may be associated with heterogeneity of chemotherapy-induced prospective memory impairment.
Keywords: breast cancer, hormone receptor, neuropsychological tests, prospective memory
1. Introduction
In the clinical setting, a majority of patients with breast cancer frequently experience cognitive deficits following chemotherapy, and their memory, attention, concentration, reasoning, executive control, and visuospatial skills are commonly affected.[1–3] As several studies have shown, memory impairment is the most prominent presentation of chemotherapy-induced cognitive impairment (CICI) in patients with breast cancer,[2,4,5] and acts as an important factor affecting the long-term quality of life in these patients.[6]
Memory can be divided into retrospective memory (RM) and prospective memory (PM). PM is defined as the ability to remember to perform a future plan or to recall a planned intention, which is the type of memory most closely related to daily life in humans. According to the nature of the cues associated with the planned intention, PM can be classified into 2 subtypes, event-based prospective memory (EBPM) and time-based prospective memory (TBPM). EBPM is the memory to perform a particular action when some external event occurs, such as remembering to post a letter when you pass the postbox, and has the characteristics of a delay between the encoding and execution stages, engagement in other activities during the delay, absence of an external reminder, and self-initiation. On the other hand, TBPM is the memory to perform an action at a certain future time, such as remembering an appointment at 8 am, and the characteristics of TBPM are the same as those of EBPM.[7] PM is typically assessed with a dual-task paradigm (a PM task and an ongoing task),[8] and as our previous study demonstrated, patients with breast cancer who had undergone adjuvant chemotherapy showed deficits in EBPM tasks, but not in TBPM tasks.[9]
Molecular classification of breast cancer has become one of the most important directions of research in the field of breast cancer. Based on developments in molecular biology, several intrinsic breast cancer subtypes have been classified on the gene level, including the luminal subtypes, which are characterized by the expression of hormone receptor-related genes, the subtypes with overexpression of human epidermal growth factor receptor 2 (HER2), and the basal-like subtypes (the majority of basal-like cancers are also triple-negative breast cancer [TNBC]).[10] TNBC refers to any breast cancer that lacks expression of estrogen receptors (ER), progesterone receptor (PR), and HER2, a phenotype associated with special clinical features, risk factors, and prognostic outcomes. TNBC accounts for approximately 12% to 17% of all breast cancer cases.[11] ERs are mainly distributed in the prefrontal cortex, hippocampus, and amygdala of the brain.[12] By combining with estrogen, ERs participate in the regulation of cognitive function, including memory.[12] It is shown that estrogen could affect the cognitive performance of premenopausal and postmenopausal women. In general, work performance concerning working and verbal memory [13] as well as execution ability[14] could be enhanced by higher levels of estrogen. On the contrary, the estrogen decrease along with natural menopause is correlated with cognitive function decrease.[15] Association exists between the reduced level of estrogen and the worse memory function and verbal fluency.[16,17] A previous study showed that there is heterogeneity within CICI among breast cancer survivors.[18] The increased awareness of chemotherapy-related cognitive impairment is reflected by a growing number of recent review papers focusing on the structural and functional changes in the human brain concomitant with chemotherapy.[19] However, it remains unknown whether there are differences in PM impairment after chemotherapy between patients with breast cancer with ER or PR-positive and negative status.
The present study examined neuropsychological tests and PM changes in patients with breast cancer with ER+/− or PR+/− status who were matched for age, education, and general intelligence before and after chemotherapy, and therefore, this study aimed to investigate chemotherapy-induced PM impairment in patients with breast cancer with different hormone receptor expression.
2. Methods
2.1. Patients and groups
A total of 120 patients with breast cancer, who were hospitalized from June 2013 to December 2015 in the Department of Oncology, The Affiliated Second Hospital of Anhui Medical University, were recruited, and divided into 2 groups according to the positive and negative expression of estrogen receptor (ER+/−) and progesterone receptor (PR+/−), including 60 patients with ER−/PR− status (Group A) and 60 patients with ER+/PR+ status (Group B). Patients suffering from breast cancer were selected according to the following inclusion standards: Denovo breast cancer diagnosed according to postoperative pathology; standard-dose chemotherapy treatment with paclitaxel, doxorubicin, fluorouracil, and cyclophosphamide, except for the hormone therapy; normal cognitive function with a Mini-Mental State Examination (MMSE) score which is not lower than 24; normal activities of daily life with a Karnofsky Performance Scale score that is not lower than 80; no damage to hearing, vision, or linguistic competence; and normal results of magnetic resonance imaging and brain computer tomography. The test selected the breast cancer patients satisfying the following conditions: distant metastasis and cachexia; hormonal treatment; psychiatric symptoms such as depression and anxiety; diseases causing cognitive disorder; previous cognitive therapy and dependence on drug or alcohol; and serious functional disorder in kidney, heart, brain, liver, or the hematopoietic system. The study was approved by the Research Ethics Committee of The Second Affiliated Hospital of Anhui Medical University, and informed consent was obtained from all subjects.
2.2. Neuropsychological background tests
To evaluate general memory and cognitive functions to the above grouping of patients with breast cancer, related tests on neuropsychological backgrounds were carried out for 4 weeks, which is divided into 2 stages: before the chemotherapy and after 6 rounds of postoperative adjuvant chemotherapy. Based on the MMSE, cognitive functions such as short-term memory, space-time orientation, computation, visuospatial skills, and linguistic competence were evaluated. In the verbal fluency test (VFT), testees were required to name as many animals as possible in 1 minute. The digit span test (DST) aimed to measure the short-term memory, wherein the testees were required to recall a string of numbers heard by them in a random sequence. The total score depended on the quantity of numbers recalled with a correct sequence. Likewise, PM tasks were then carried out.
2.3. Event-based prospective memory (EBPM) task
Subjects were initially instructed to tap the desk whenever they found the 2 animal words (target events). Next, the testees needed to tell the examiner their telephone numbers after the tests. Then, they joined a task of word selection using 30 question cards printed with 12 Chinese words respectively. Ten words among the 12 words were classified into 1 type, and the other 2 words were classified into another type. The testees selected the 2 words of 1 type different from the left 10 words. The tester presented each card to the testees. Then, they needed to give verbal answers at personal pace. On the 5th, 10th, 15th, 20th, 25th, and 30th cards in the word selection task, the target events of PM task took place. An approach similar to the method applied in the report of McDaniel and Einstein was used to record performance of the testees in the word selection. One point was marked for the correct response to each target event (there were 6 target events in total). Correct telling of the telephone number after the test was marked by 2 points. Wrong incorrect response to a target event or failure to tell their telephone numbers obtained zero point. The highest EBPM score was 8 points.
2.4. Time-based prospective memory (TBPM) task
The testees were required to dab the desk at an interval of 5 minutes from the start time (namely, dabbed the desk at the time points of 5, 10, and 15 minutes). In the test, subjects could check time by a digital clock. To eliminate visible cues, the clock was put 1 m away behind the right shoulder of the testee. Hence, the testees could check time only after turning head to the clock. At the very beginning, 00:00:00 (hour, minute, second) was displayed by the clock. After the clock started working, the testees started executing the number selection task involving 100 cards printed with 12 two-digit numbers respectively. The testees were instructed to select the maximum and minimum numbers on each card. The exact time for the testees to make a response by dabbing the desk was recorded. When 17 minutes was displayed by the clock, the number selection task could be stopped. Two points were marked if the testees made a response between the 10 seconds before the target time and 10 seconds after the target time. One point was marked if the testees made a response between 30 seconds before the target time and 30 seconds after the target time. The maximum TBPM score was 6 points.
2.5. Statistical analysis
All data are expressed as the mean ± standard deviation. Statistical analysis was performed with 1-way analysis of variance using SPSS software (version 22.0, http://spss.en.softonic.com/; Chicago, IL), and Student t tests were performed in a group of 2 samples, and P < 0.05 and P < 0.01 were considered to indicate significant differences and highly significant differences, respectively.
3. Results
3.1. Basic clinical information for patients with breast cancer with ER−/PR− and ER+/PR+ status
There were no significant differences in age or years of education in patients with breast cancer in group A, with ER−/PR− status, and those in group B, with ER+/PR+ status (49.45 ± 10.93 vs 47.92 ± 10.53 and 9.93 ± 3.15 vs 9.95 ± 3.38, respectively). In group A, the pathological pattern manifested as 55 cases of invasive breast carcinoma, nospecial type; 2 cases of invasive breast carcinoma, special type; and 3 cases of carcinoma in situ. Similarly, the pathological pattern of group B manifested as 58 cases of invasive breast carcinoma, no special type; 1 case of carcinoma in situ; and 1 case of microinvasive carcinoma (Table 1).
Table 1.
Basic clinical information of breast cancer patients with ER−/PR− and ER+/PR+ status.

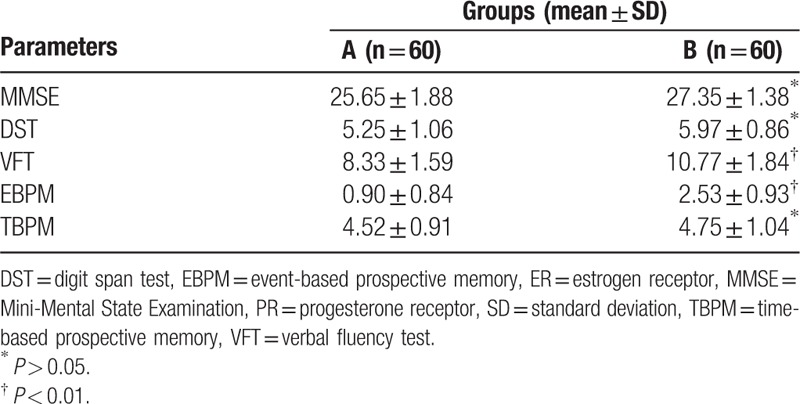
3.2. Neuropsychological background tests of breast cancer patients with ER−/PR− and ER+/PR+ status
According to the neuropsychological background tests results, the MMSE, DST, and TBPM scores of breast cancer patients with ER−/PR− and ER+/PR+ status were as follows: MMSE: 27.35 ± 1.38 versus 25.65 ± 1.88; DST: 5.25 ± 1.06 versus 5.97 ± 0.86; and TBPM:4.75 ± 1.04 versus 4.52 ± 0.91, respectively, with no significant difference (∗: P > 0.05). However, the VFT and EBPM scores were significantly greater in group B than in group A (10.77 ± 1.84 vs 8.33 ± 1.59 and 2.53 ± 0.93 vs 0.90 ± 0.84, respectively; ∗∗: P < 0.01) (Table 2).
Table 2.
Neuropsychological background tests in patients with breast cancer with ER−/PR− and ER+/PR+ status.
4. Discussion
In the present study, we demonstrated that breast cancer patients with different hormone receptor expression have different levels of CICI. Specifically, patients with breast cancer with ER-/PR-status have poorer scores on neuropsychological background tests measuring chemotherapy-induced impairment than patients with ER+/PR+ status. In addition, patients with breast cancer with ER-/PR-status have greater chemotherapy-induced event PM impairment than patients with ER+/PR+ status.
Memory impairment is the most prominent aspect of CICI, which has been receiving increased attention as an identifiable psychological cognitive change.[20,21] Previous research has shown that cognitive dysfunction in patients with breast cancer after chemotherapy affects their daily lives and reduces their quality of life.[22] The influence of CICI on the long-term survival of patients with breast cancer has been found to be even more significant than that of recurrence and metastasis of the tumor itself.[23] Furthermore, the severity of CICI in patients with breast cancer is affected by many factors, including pathological classification.[24] In our study, 120 patients with breast cancer treated with chemotherapy were found to demonstrate decreases in cognitive function. We also found that the neuropsychological background tests in patients with ER- and PR-positive status showed more significant decreases than those of patients with ER- and PR-negative status after chemotherapy.
In addition, a growing amount of evidence has demonstrated the importance of estrogen and progesterone in modulating cognition. It is well known that estrogen is the primary female sex hormones and that it has many effects in the brain, such as neurogenerative, neurotrophic, cholinergic neurotransmission, and synaptic plasticity, which are crucial for modulating cognition function.[25,26] In the clinical setting, estrogen can be useful during menopause and in the treatment of age-related cognitive decline.[27,28] Estrogen exerts its effects by binding to ERs and plays a pivotal role in cognitive function, especially in memory. Progesterone is involved in the modulation of neurotrophin expression and promotion of cell survival. When progesterone binds to its receptor, it can activate signal transduction pathways, which, in turn, trigger relevant cellular events that are key to neuroprotection.[29] It is clear that PRs could mediate neuroprotective actions of progesterone in the central nervous system, especially on the release of brain-derived neurotrophic factor, which is broadly and abundantly expressed in the brain.[30,31] PRs are widely expressed in the human brain and play an important role in cognitive function. In the present study, patients with ER−/PR− status were found to have more significant damage in EBPM than patients with ER+/PR+ status.
The present results may provide valuable evidence regarding the differences in chemotherapy-induced PM impairment between 2 groups of patients with breast cancer with different ER and PR receptor expression. However, additional studies should be performed to examine the mechanisms underlying PM impairment in patients with breast cancer with different hormone receptor expression. Because of the limited sample size, the validity and reliability of our findings on PM impairment should be validated by studies including large samples of patients with breast cancer in the future.
5. Conclusion
This study indicated that different hormone receptor expression in patients with breast cancer may be associated with heterogeneity in chemotherapy-induced PM impairment. Larger studies are warranted to confirm these results.
Footnotes
Abbreviations: CICI = chemotherapy-induced cognitive impairment, DST = digit span test, EBPM = event-based prospective memory, ER = estrogen receptor, MMSE = mini-mental state examination, PM = prospective memory, PR = progesterone receptor, RM = retrospective memory, TBPM = time-based prospective memory, TNBC = triple-negative breast cancer, VFT = verbal fluency test.
This research was supported by the National Natural Science Foundation of China (No. 81372487).
The authors have no conflicts of interest to disclose.
References
- [1].Lawrence JA, Griffin L, Balcueva EP, et al. A study of donepezil in female breast cancer survivors with self-reported cognitive dysfunction 1 to 5 years following adjuvant chemotherapy. J Cancer Surviv 2016;10:176–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Park JH, Bae SH, Jung YS, et al. [Prevalence and characteristics of chemotherapy-related cognitive impairment in patients with breast cancer]. J Korean Acad Nurs 2015;45:118–28. [DOI] [PubMed] [Google Scholar]
- [3].Pullens MJ, De Vries J, Van Warmerdam LJ, et al. Chemotherapy and cognitive complaints in women with breast cancer. Psychooncology 2013;22:1783–9. [DOI] [PubMed] [Google Scholar]
- [4].Ng T, Teo SM, Yeo HL, et al. Brain-derived neurotrophic factor genetic polymorphism (rs6265) is protective against chemotherapy-associated cognitive impairment in patients with early-stage breast cancer. Neurooncology 2016;18:244–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Rey D, Bouhnik AD, Mancini J, et al. Self-reported cognitive impairment after breast cancer treatment in young women from the ELIPPSE40 cohort: the long-term impact of chemotherapy. Breast J 2012;18:406–14. [DOI] [PubMed] [Google Scholar]
- [6].Pinto AC, de Azambuja E. Improving quality of life after breast cancer: dealing with symptoms. Maturitas 2011;70:343–8. [DOI] [PubMed] [Google Scholar]
- [7].McDaniel MA, Einstein GO. The neuropsychology of prospective memory in normal aging: a componential approach. Neuropsychologia 2011;49:2147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Scullin MK, McDaniel MA, Shelton JT, et al. Framework: evidence from prospective memory with contextual variability. Cogn Psychol 2013;67:55–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cheng H, Yang Z, Dong B, et al. Chemotherapy-induced prospective memory impairment in patients with breast cancer. Psychooncology 2013;22:2391–5. [DOI] [PubMed] [Google Scholar]
- [10].Bauer KR, Brown M, Cress RD, et al. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer 2007;109:1721–8. [DOI] [PubMed] [Google Scholar]
- [11].Williams N, Harris L. Triple-negative breast cancer in the post-genomic era. Oncology (Williston Park) 2013;27:859–60. [PubMed] [Google Scholar]
- [12].Almey A, Milner TA, Brake WG. Estrogen receptors in the central nervous system and their implication for dopamine-dependent cognition in females. Horm Behav 2015;74:125–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Epperson CN, Amin Z, Ruparel K, et al. Interactive effects of estrogen and serotonin on brain activation during working memory and affective processing in menopausal women. Psychoneuroendocrinology 2012;37:372–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Daniel JM. Estrogens, estrogen receptors, and female cognitive aging: the impact of timing. Horm Behav 2013;63:231–7. [DOI] [PubMed] [Google Scholar]
- [15].Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nature reviews Cancer 2007;7:192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Moller MC, Bartfai AB, Radestad AF. Effects of testosterone and estrogen replacement on memory function. Menopause 2010;17:983–9. [DOI] [PubMed] [Google Scholar]
- [17].Rapp SR, Espeland MA, Shumaker SA, et al. Effect of estrogen plus progestin on global cognitive function in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. JAMA 2003;289:2663–72. [DOI] [PubMed] [Google Scholar]
- [18].Wefel JS, Kesler SR, Noll KR, et al. Clinical characteristics, pathophysiology, and management of noncentral nervous system cancer-related cognitive impairment in adults. CA Cancer J Clin 2015;65:123–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Simo M, Rifa-Ros X, Rodriguez-Fornells A, et al. Chemobrain: a systematic review of structural and functional neuroimaging studies. Neurosci Biobehav Rev 2013;37:1311–21. [DOI] [PubMed] [Google Scholar]
- [20].Kanaskie ML, Loeb SJ. The experience of cognitive change in women with breast cancer following chemotherapy. J Cancer Surviv 2015;9:375–87. [DOI] [PubMed] [Google Scholar]
- [21].Collins B, Mackenzie J, Tasca GA, et al. Persistent cognitive changes in breast cancer patients 1 year following completion of chemotherapy. JINS 2014;20:370–9. [DOI] [PubMed] [Google Scholar]
- [22].Chang J, Couture FA, Young SD, et al. Weekly administration of epoetin alfa improves cognition and quality of life in patients with breast cancer receiving chemotherapy. Support Cancer Ther 2004;2:52–8. [DOI] [PubMed] [Google Scholar]
- [23].Kesler S, Hadi Hosseini SM, Heckler C, et al. Cognitive training for improving executive function in chemotherapy-treated breast cancer survivors. Clin Breast Cancer 2013;13:299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jim HS, Phillips KM, Chait S, et al. Meta-analysis of cognitive functioning in breast cancer survivors previously treated with standard-dose chemotherapy. J Clin Oncol 2012;30:3578–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Baudry M, Bi X, Aguirre C. Progesterone-estrogen interactions in synaptic plasticity and neuroprotection. Neuroscience 2013;239:280–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sherwin BB, Henry JF. Brain aging modulates the neuroprotective effects of estrogen on selective aspects of cognition in women: a critical review. Front Neuroendocrinol 2008;29:88–113. [DOI] [PubMed] [Google Scholar]
- [27].Lan YL, Zhao J, Li S. Update on the neuroprotective effect of estrogen receptor alpha against Alzheimer's disease. J Alzheimers Dis 2015;43:1137–48. [DOI] [PubMed] [Google Scholar]
- [28].Foster TC. Role of estrogen receptor alpha and beta expression and signaling on cognitive function during aging. Hippocampus 2012;22:656–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Liu A, Margaill I, Zhang S, et al. Progesterone receptors: a key for neuroprotection in experimental stroke. Endocrinology 2012;153:3747–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Frye CA, Koonce CJ, Walf AA. Progesterone, compared to medroxyprogesterone acetate, to C57BL/6, but not 5alpha-reductase mutant, mice enhances object recognition and placement memory and is associated with higher BDNF levels in the hippocampus and cortex. Neurosci Lett 2013;551:53–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Su C, Cunningham RL, Rybalchenko N, et al. Progesterone increases the release of brain-derived neurotrophic factor from glia via progesterone receptor membrane component 1 (Pgrmc1)-dependent ERK5 signaling. Endocrinology 2012;153:4389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]


