Abstract
Background:
This study aimed to demonstrate the effect of intra-articular (IA) lumbar facet joint (LFJ) pulsed radiofrequency (PRF) for the management of LFJ pain, and to compare the effect of IA LFJ PRF to IA corticosteroid injection (ICI). Pathology in the LFJ is a common source of lower back pain (LBP). It is responsible for chronic LBP in approximately 15% to 45% of patients. It has been reported that PRF stimulation can effectively reduce refractory joint pain.
Methods:
Sixty patients with LFJ pain were recruited and randomly assigned to 1 of 2 groups: the IA PRF group and the ICI group. There were 30 patients in each group. At pretreatment, 2 weeks, 1, 3, and 6 months after treatment, we assessed the severity of LBP using a numeric rating scale (NRS).
Results:
Compared with the pretreatment NRS scores, patients in both groups showed a significant decrease in NRS scores at 2 weeks, and 1, 3, and 6 months after each treatment. Between groups, changes in the NRS scores were significantly different over time. At 2 weeks and 1 month after each procedure, the NRS score after ICI was significantly lower than that after the PRF stimulation. However, at 3 and 6 months after the procedures, the decrements of NRS scores were not significantly different between the 2 groups. Six months after treatment, about half of patients in both groups reported successful pain relief (pain relief of ≥50%).
Conclusion:
In the current study, both IA PRF stimulation and ICI into the LFJ significantly relieved LFJ pain. Their effects persisted for at least 6 months after the procedure. Thus, IA PRF is a useful therapeutic option for the management of LFJ pain.
Keywords: chronic pain, corticosteroid injection, intra-articular stimulation, lumbar facet joint pain, pulsed radiofrequency
1. Introduction
Chronic lower back pain (LBP) is 1 of the leading causes of disability.[1] Lumbar intervertebral discs, facet joints, and sacroiliac joints are the major sources of persistent LBP.[2] The prevalence of facet origin LBP is 15% to 45%.[3–5] Repeated chemical and mechanical stresses of the lumbar facet joint (LFJ) can elicit osteoarthritis,[6,7] with subsequent inflammation and stretching of the joint capsule, which leads to axial LBP.[8]
For the management of LBP related to LFJ, several therapeutic procedures have been used. The intra-articular (IA) LFJ injection or lumbar medial branch block (MBB) with corticosteroid is widely and conventionally used for the management of LBP originating in the LFJ.[9–14] However, the levels of evidence for these procedures are considered fair or moderate.[15] Furthermore, corticosteroids can have several adverse effects.[16,17] As an alternative, radiofrequency neurolysis of the lumbar medial branch has been used for management of LFJ pain.[10,18,19]
Conventional radiofrequency (CRF) treatment involves continuous stimulation and results in ablation of nerves and tissues. The ablation is the result of frictional heat from a catheter needle.[20] In contrast to CRF, pulsed radiofrequency (PRF) uses a brief stimulation, followed by a long resting phase. PRF exposes the target nerves and tissues to an electric field, and rarely damages these structures.[21] Although the mechanism of PRF has not been clearly elucidated, it has been suggested that the electrical field produced by PRF can alter pain signals and have a selective effect on small unmyelinated fibers (C-fiber).[22,23] Currently, PRF is used for various types of pain, including neuralgia, joint pain, and myofascial pain.[24–26] PRF stimulation on the lumbar medial branch has been reported to have a positive effect in the control of LFJ pain.[9,12] In addition, PRF stimulation after placement of the needle electrodes into a joint space can effectively reduce refractory joint pain.[27,28] However, little is known about the effect of IA PRF stimulation for controlling LFJ pain.
In the current study, we treated chronic LFJ pain by placing an electrode into the LFJ space and applying PRF. In addition, we compared the effect of IA LFJ PRF with that of IA LFJ corticosteroid injection.
2. Methods
2.1. Patients
We prospectively evaluated consecutive patients who presented with spontaneous onset of chronic LBP. After applying the inclusion criteria, 60 patients (mean age: 65.0 ± 10.4, range 41–79) were included in this study (Table 1). The following inclusion criteria were used: ≥6-month history of axial LBP without radicular symptoms; age between 20 and 79 years; local paraspinalis tenderness with increased pain on hyperextension, rotation, or lateral bending of the lower lumbar spine; ≥50% temporary pain relief following a diagnostic block with IA injection of 0.5 mL of 1% lidocaine; and failure to respond to physical therapy and medication (LBP of at least 4 on the numeric rating scale [NRS]). Each patient underwent lumbar spine magnetic resonance imaging (MRI). Exclusion criteria were as follows: disc herniation, lumbar spinal stenosis, spinal instability, coagulopathy, allergy to iodinated contrast, rheumatic disorders, and any uncontrolled medical or psychiatric condition. All subjects provided written informed consent before the study. The Institutional Review Board of Yeungnam University Hospital approved this study. Sixty patients with LFJ pain were randomly assigned to 1 of 2 groups. In the PRF group, 30 patients received PRF stimulation in the IA space of the LFJ. In the IA corticosteroid injection group (ICI group), 30 patients received IA LFJ corticosteroid injection. Randomization was performed using a random table. Treatment was carried out only 1 time for each patient. A putatively painful LFJ was selected on the basis of the physical examination (local tenderness site) and findings of degenerative facet pathology (osteophyte, bone sclerosis, or joint effusion) on radiographs or MR images.
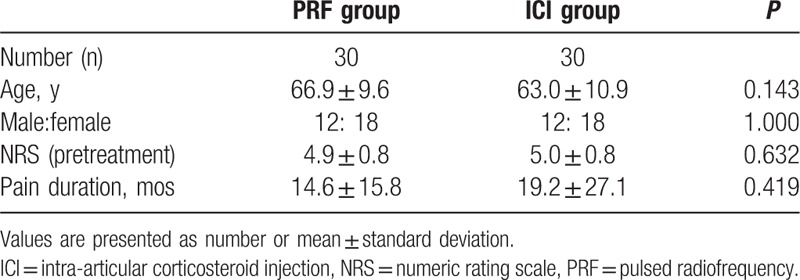
Table 1.
Demographic characteristics of patients in the PRF and ICI groups.
2.2. Procedures
In the PRF group, the treatment was performed via a posterior approach with the patient in a prone position for C-arm fluoroscopy (Siemens). Patients were positioned in the prone position with a cushion below the lower abdomen to straighten the lumbar spine. The C-arm tube was angled cephalad and rotated until it was at a tangent to the LFJ space. A 23-gauge cannula (SMK Pole needle, 100 mm with a 10 mm active tip, Cotop International BV) was inserted under fluoroscopy parallel to the C-arm beam. To confirm IA access, an arthrogram of the LFJ was obtained by injecting 0.3 mL of contrast (Fig. 1). IA access was successful in all 30 patients. In the PRF group, an electrode was connected to the cannula, and the LFJ was stimulated (Cosman G4 radiofrequency generator, Cosman Medical). PRF treatment was administered at 5 Hz, and a 5-millisesond pulsed width, for 360 seconds, at 55 V, under the condition that the electrode tip temperature did not exceed 42°C. In the ICI group, the preparation steps were identical to the PRF group. Under C-arm fluoroscopy, after confirming IA access by injecting 0.3 mL of contrast into the CFJ space, we injected 10 mg (0.25 mL) of dexamethasone mixed with 0.25 mL of 0.125% bupivacaine using a 26-gauge, 90 mm spinal needle. IA injection was successful in all 30 patients in the ICI group.
Figure 1.
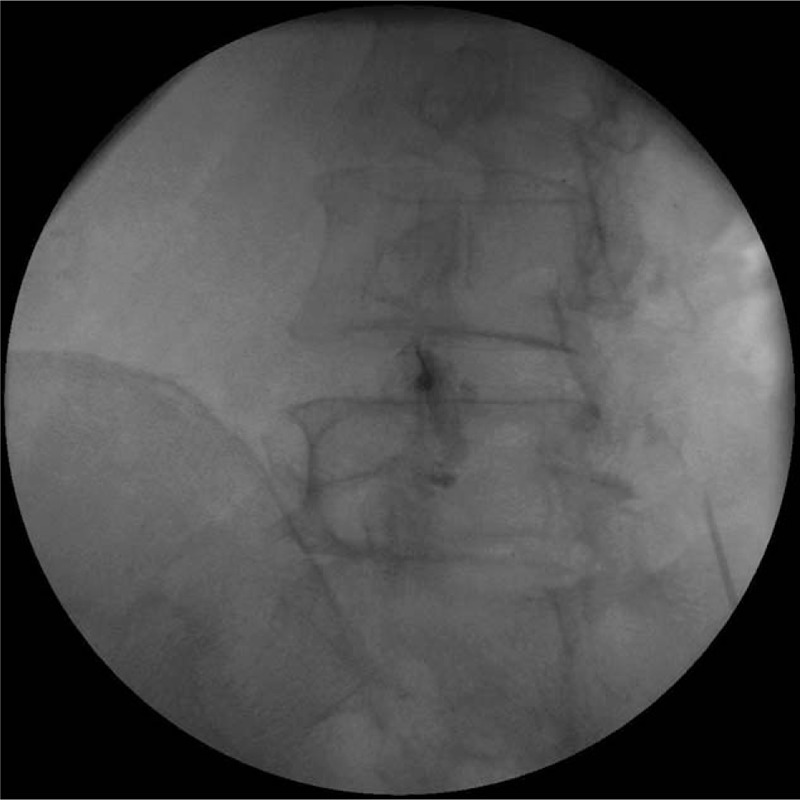
Fluoroscopy-guided intra-articular contrast injection into the left L4-5 facet joint.
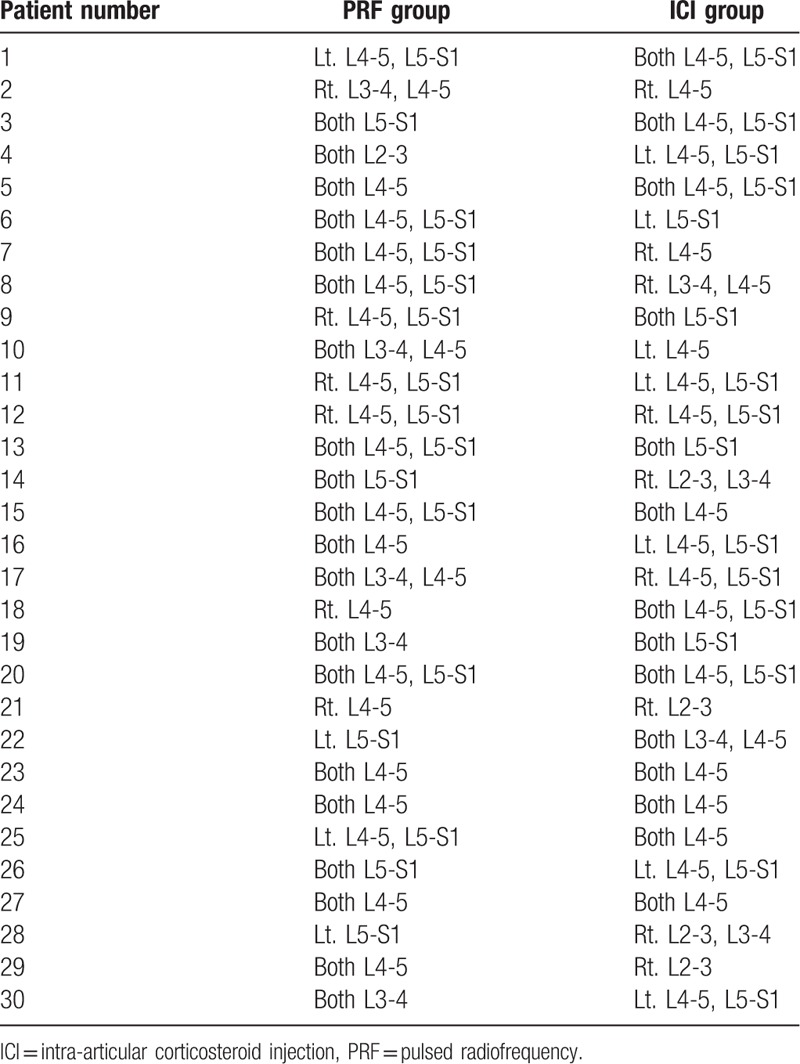
We performed either PRF stimulation or corticosteroid injection in a total of 138 levels of the CFJ (PRF group: 72 levels, ICI group: 66 levels; Table 2). We performed PRF or corticosteroid injection bilaterally in 20 and 14 patients, respectively. We did not perform the procedure unilaterally at more than 2 levels.
Table 2.
The treated facet joint level of each patient.
These IA PRF stimulation and IA LFJ corticosteroid injection procedures were performed by the same physician who had 20 years of training and experience. The physician who performed the procedures was not involved in measuring outcomes.
2.3. Outcome measures
The same investigator performed all pretreatment and follow-up assessments. This investigator was blinded to the grouping of the patients, and did not participate in any treatment. Pain intensity was assessed using a NRS, with values between 0 and 10, with 0 representing “no pain” and 10 representing “the most intense pain imaginable.” The NRS scores were measured before treatment, and 2 weeks, 1, 3, and 6 months after treatment. Successful treatment was defined as more than 50% reduction in the NRS score at 6 months compared with the pretreatment NRS score. To validate the change in pain reduction, NRS scores were evaluated by assessing the difference between the pretreatment and the 6 months after treatment NRS scores (change in NRS [%] = [pretreatment score − score at 6 months after treatment]/pretreatment score × 100).
2.4. Statistical analysis
Data were analyzed using the Statistical Package for Social Science (SPSS, v. 22.0, IBM Corporation, Armonk, NY). Demographic data and successful pain relief rate were compared between the 2 groups using the Mann–Whitney U test and chi-square test. The changes in NRS scores in each PRF and ICI group were evaluated using repeated-measure 1-factor analysis. Repeated-measure 2-factor analysis was used to compare changes between groups over time. Multiple comparisons were obtained after a contrast under Bonferroni correction. The level of statistical significance was set at P < 0.05.
3. Results
All patients completed the study. No adverse events were observed in the PRF group. A minor adverse event was observed in 1 patient in the ICI group; the patient had hyperglycemia (blood glucose level of more than 300 mg/dL). There were no significant differences in the demographic data between groups (Table 1, P > 0.05).
In the PRF group, the mean NRS decreased after treatment. The pretreatment NRS was 4.9 ± 0.8. At 2 weeks, the mean NRS was 2.3 ± 1.4; at 1 month, 2.5 ± 1.4; at 3 months, 2.5 ± 1.3; and at 6 months, 2.7 ± 1.5 (Fig. 1). In the ICI group, the mean NRS decreased from 5.0 ± 0.8 pretreatment to 1.4 ± 0.8 at 2 weeks, 1.8 ± 1.2 at 1 month, 2.9 ± 1.4 at 3 months, and 3.2 at 6 months (Fig. 2).
Figure 2.
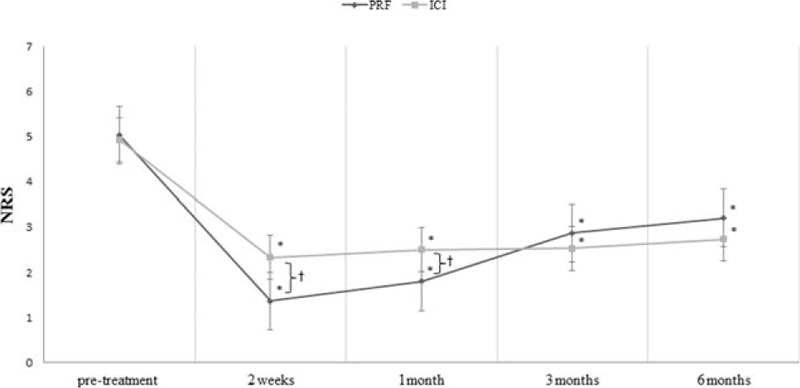
Change in numeric rating scale (NRS). Compared with pretreatment NRS scores, both groups showed a significant decrease in scores at 2 weeks, and 1, 3, and 6 months after each treatment. The changes between groups over time were significantly different. Between groups, at 2 weeks and 1 month after each procedure, the NRS score after intra-articular corticosteroid injection was significantly lower than that after the pulsed radiofrequency (PRF) stimulation. However, at 3 and 6 months after the procedures, the decrements of NRS scores were not significantly different between the 2 groups. ∗P < 0.05: intragroup comparison between posttreatment 1, 3, and 6 months, and pretreatment (repeated-measure 1-factor analysis). †P < 0.05: intergroup comparison in each time point (repeated-measure 2-factor analysis).
Scores on the NRS for each group were significantly different over time (P < 0.001). In both groups, scores at 2 weeks, and 1, 3, and 6 months were significantly decreased when compared with pretreatment scores (P < 0.001). Changes in the NRS scores over time were significantly different between groups (P < 0.001). Two weeks and 1 month after each procedure, we found that the NRS score was significantly lower in the ICI group than in the PRF group (2 weeks: P < 0.001, 1 month: P = 0.011) (Fig. 1). However, at 3 and 6 months after the procedures, the decrements of NRS scores were not significantly different between the 2 groups (3 months: P = 0.497, 3 months: P = 0.315) (Fig. 1).
Six months after treatment, 15 patients (50.0%) in the PRF group reported successful pain relief (pain relief of ≥50%), and 14 patients (46.7%) in the ICI group reported successful pain relief. There was no significant difference in the rates of successful pain relief at 6 months after the procedures (P = 0.796).
4. Discussion
In this study, we evaluated and compared the clinical effect of IA LFJ PRF stimulation and ICI in patients with LFJ pain. Our results show that the severity of pain, which was measured using the NRS, was significantly reduced after each procedure and persisted for the 6-month duration of the study. At 1 month after the procedures, the patients who had corticosteroid injection showed a significantly higher reduction in pain compared with the PRF group. However, at 3 and 6 months after the procedures, the degrees of pain reduction were not significantly different between the 2 groups. Furthermore, in both groups, approximately half of patients reported more than 50% of pain relief at 6 months after each procedure. Summarizing, the short-term pain-relieving effect was superior in the ICI group, but the long-term effect was similar between PRF and ICI.
The mechanisms of how IA PRF reduces joint pain remain unclear. However, we can suggest some possible therapeutic mechanisms of IA PRF. First, PRF stimulation causes damages in the sensory nociceptive axons at a microscopic or subcellular level. These lesions are selectively located in the smaller principal sensory nociceptors (C-fibers and A-delta fibers), but are rarely identified in the larger nonpain-related sensory fibers (A-beta fiber).[22] Therefore, we think IA PRF of the LFJ disrupts the synovial lining nociceptive C-fibers. Additionally, due to insulating properties of bone, the current can be deflected by bony surfaces, and remain inside the joint space without weakening.[28] Accordingly, the residual current in the LFJ appeared to inhibit the excitability of pain-generating afferent nerves, or free nerve endings, which richly innervate the articular capsule. On the contrary, the electrical field induced by a PRF electrode placed in soft tissue rapidly weakens if the distance from the electrode is increased.[28] Finally, the electrical field was reported to reduce the production of proinflammatory or inflammatory cytokines.[26] After the application of PRF into the joint, serum C-reactive protein and cytokines were reduced.[26] In our study, IA PRF stimulation of the LFJ seems to reduce the IA inflammation, leading to reduction of LFJ-origin pain.
The efficacy of IA LFJ corticosteroid injection remains a subject of discussion. Several studies demonstrated the positive short and long- term effects of IA LFJ corticosteroid injection for managing LFJ pain.[10,13,14] In contrast, Lilius et al[29] reported no differences in outcome between the placebo and ICI groups. Generally, to clinicians, the level of evidence of ICIs is fair or moderate.[15] We adopted strict inclusion criteria in recruiting patients with LFJ origin pain; we included patients who presented with positive findings on both physical examination and diagnostic block. Furthermore, we excluded patients who had disc herniation, lumbar spinal stenosis, or spinal instability on MRI findings. In the current study, IA LFJ corticosteroid injection was shown to be effective during at least 6 months after the injection. The aim of ICI is to bring corticosteroids into the degenerated facet joint based on the belief that there is inflammation. If there is inflammation of the synovium, the synovial lining nociceptive C-fibers are excited, which are responsible for the development of the LFJ pain.[30] The anti-inflammatory properties of corticosteroids block production and release of the inflammatory mediators, and consequently inhibit processes to the inflammation.[31] ICI in our patients with LFJ pain could reduce IA inflammation, which seems to have reduced LFJ pain. However, despite the effectiveness of IA corticosteroids, they have potential adverse effects, including major ones such as suppression of the pituitary-adrenal axis, hyperadrenocorticism, avascular necrosis, osteoporosis, myopathy, and hyperglycemia, to minor ones such as flushing, sweating, and nausea.[16,17] In our study, 1 of the 30 patients who received corticosteroid injection developed hyperglycemia. On the contrary, in the PRF group, no adverse events were reported. Considering the various adverse effects of corticosteroids and devastating results after repeated corticosteroid injections, we think IA PRF stimulation may be a better option in managing LFJ pain than ICI.
As for the usefulness of PRF for managing LFJ pain, several studies have demonstrated the positive efficacy of PRF stimulation on lumbar medial branches.[32–34] However, so far, no study has been conducted on the effect of IA LFJ PRF stimulation. Targeting medial branches of the dorsal ramus is sometimes technically challenging.[35] Compared with PRF stimulation on lumbar medial branches, IA insertion of PRF catheter needle can be technically easier to perform by clinicians. We believe that IA LFJ PRF stimulation would be helpful to reduce the amount of exposing radiation during the procedure and the time taken for the procedure.
In conclusion, we found that both IA PRF stimulation and ICI into the LFJ significantly relieved LFJ pain, and their effects were sustained for at least 6 months after the procedure. The short-term effect was higher after ICI, but the long-term effect was not significantly different between the 2 procedures. Successful pain relief at 6 months was about 50% for patients in both groups. IA PRF can be a useful clinical option for the management of LFJ pain, particularly in patients at risk for development of complications from the use of corticosteroids. Our study involved a small number of subjects; thus, further studies involving larger number of subjects are warranted for a clear elucidation of this topic.
Footnotes
Abbreviations: CRF = conventional radiofrequency, IA = intra-articular, ICI = intra-articular corticosteroid injection, LBP = lower back pain, LFJ = lumbar facet joint, MBB = medial branch block, MRI = magnetic resonance imaging, NRS = numeric rating scale, PRF = pulsed radiofrequency.
The authors report no conflicts of interest.
References
- [1].From the Centers for Disease Control and Prevention. Prevalence of disabilities and associated health conditions among adults: United States, 1999. JAMA 2001;285:1571–2. [PubMed] [Google Scholar]
- [2].Hancock MJ, Maher CG, Latimer J, et al. Systematic review of tests to identify the disc, SIJ or facet joint as the source of low back pain. Eur Spine J 2007;16:1539–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Manchikanti L, Pampati V, Fellows B, et al. The inability of the clinical picture to characterize pain from facet joints. Pain Physician 2000;3:158–66. [PubMed] [Google Scholar]
- [4].Manchikanti L, Pampati V, Fellows B, et al. Prevalence of lumbar facet joint pain in chronic low back pain. Pain Physician 1999;2:59–64. [PubMed] [Google Scholar]
- [5].Schwarzer AC, Aprill CN, Derby R, et al. Clinical features of patients with pain stemming from the lumbar zygapophysial joints. Is the lumbar facet syndrome a clinical entity? Spine (Phila Pa 1976) 1994;19:1132–7. [DOI] [PubMed] [Google Scholar]
- [6].Cohen SP, Raja SN. Pathogenesis, diagnosis, and treatment of lumbar zygapophysial (facet) joint pain. Anesthesiology 2007;106:591–614. [DOI] [PubMed] [Google Scholar]
- [7].Taylor JR, Twomey LT. Age changes in lumbar zygapophyseal joints. Observations on structure and function. Spine (Phila Pa 1976) 1986;11:739–45. [DOI] [PubMed] [Google Scholar]
- [8].van Kleef M, Vanelderen P, Cohen SP, et al. 12. Pain originating from the lumbar facet joints. Pain Pract 2010;10:459–69. [DOI] [PubMed] [Google Scholar]
- [9].Amrhein TJ, Joshi AB, Kranz PG. Technique for CT fluoroscopy-guided lumbar medial branch blocks and radiofrequency ablation. AJR Am J Roentgenol 2016;207:631–4. [DOI] [PubMed] [Google Scholar]
- [10].Lakemeier S, Lind M, Schultz W, et al. A comparison of intraarticular lumbar facet joint steroid injections and lumbar facet joint radiofrequency denervation in the treatment of low back pain: a randomized, controlled, double-blind trial. Anesth Analg 2013;117:228–35. [DOI] [PubMed] [Google Scholar]
- [11].Manchikanti L, Manchikanti KN, Manchukonda R, et al. Evaluation of lumbar facet joint nerve blocks in the management of chronic low back pain: preliminary report of a randomized, double-blind controlled trial: clinical trial NCT00355914. Pain Physician 2007;10:425–40. [PubMed] [Google Scholar]
- [12].Manchikanti L, Singh V, Vilims BD, et al. Medial branch neurotomy in management of chronic spinal pain: systematic review of the evidence. Pain Physician 2002;5:405–18. [PubMed] [Google Scholar]
- [13].Ribeiro LH, Furtado RN, Konai MS, et al. Effect of facet joint injection versus systemic steroids in low back pain: a randomized controlled trial. Spine (Phila Pa 1976) 2013;38:1995–2002. [DOI] [PubMed] [Google Scholar]
- [14].Shih C, Lin GY, Yueh KC, et al. Lumbar zygapophyseal joint injections in patients with chronic lower back pain. J Chin Med Assoc 2005;68:59–64. [DOI] [PubMed] [Google Scholar]
- [15].Boswell MV, Colson JD, Spillane WF. Therapeutic facet joint interventions in chronic spinal pain: a systematic review of effectiveness and complications. Pain Physician 2005;8:101–14. [PubMed] [Google Scholar]
- [16].Manchikanti L. Role of neuraxial steroids in interventional pain management. Pain Physician 2002;5:182–99. [PubMed] [Google Scholar]
- [17].Manchikanti L, Boswell MV, Singh V, et al. ASIPP-IPM. Comprehensive evidence-based guidelines for interventional techniques in the management of chronic spinal pain. Pain Physician 2009;12:699–802. [PubMed] [Google Scholar]
- [18].Leon JF, Ortiz JG, Fonseca EO, et al. Radiofrequency neurolysis for lumbar pain using a variation of the original technique. Pain Physician 2016;19:155–61. [PubMed] [Google Scholar]
- [19].Nedelka T, Nedelka J, Schlenker J, et al. Mechano-transduction effect of shockwaves in the treatment of lumbar facet joint pain: comparative effectiveness evaluation of shockwave therapy, steroid injections and radiofrequency medial branch neurotomy. Neuro Endocrinol Lett 2014;35:393–7. [PubMed] [Google Scholar]
- [20].Vatansever D, Tekin I, Tuglu I, et al. A comparison of the neuroablative effects of conventional and pulsed radiofrequency techniques. Clin J Pain 2008;24:717–24. [DOI] [PubMed] [Google Scholar]
- [21].Sluijter ME, Cosman ER, Rittmann WB, et al. The effects of pulsed radiofrequency fields applied to the dorsal root ganglion—A preliminary report. Pain Clin 1998;11:109–17. [Google Scholar]
- [22].Erdine S, Bilir A, Cosman ER, et al. Ultrastructural changes in axons following exposure to pulsed radiofrequency fields. Pain Pract 2009;9:407–17. [DOI] [PubMed] [Google Scholar]
- [23].Hagiwara S, Iwasaka H, Takeshima N, et al. Mechanisms of analgesic action of pulsed radiofrequency on adjuvant-induced pain in the rat: roles of descending adrenergic and serotonergic systems. Eur J Pain 2009;13:249–52. [DOI] [PubMed] [Google Scholar]
- [24].Choi GS, Ahn SH, Cho YW, et al. Long-term effect of pulsed radiofrequency on chronic cervical radicular pain refractory to repeated transforaminal epidural steroid injections. Pain Med 2012;13:368–75. [DOI] [PubMed] [Google Scholar]
- [25].Park CH, Lee YW, Kim YC, et al. Treatment experience of pulsed radiofrequency under ultrasound guided to the trapezius muscle at myofascial pain syndrome -a case report-. Korean J Pain 2012;25:52–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Schianchi PM. A new technique to treat facet joint pain with pulsed radiofrequency. Anesth Pain Med 2015;5:e21061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Schianchi PM, Sluijter ME, Balogh SE. The treatment of joint pain with intra-articular pulsed radiofrequency. Anesth Pain Med 2013;3:250–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sluijter ME, Teixeira A, Serra V, et al. Intra-articular application of pulsed radiofrequency for arthrogenic pain: report of six cases. Pain Pract 2008;8:57–61. [DOI] [PubMed] [Google Scholar]
- [29].Lilius G, Laasonen EM, Myllynen P, et al. Lumbar facet joint syndrome. A randomised clinical trial. J Bone Joint Surg Br 1989;71:681–4. [DOI] [PubMed] [Google Scholar]
- [30].Chen C, Lu Y, Kallakuri S, et al. Distribution of A-delta and C-fiber receptors in the cervical facet joint capsule and their response to stretch. J Bone Joint Surg Am 2006;88:1807–16. [DOI] [PubMed] [Google Scholar]
- [31].Lee DG, Ahn SH, Lee J. Comparative effectiveness of pulsed radiofrequency and transforaminal steroid injection for radicular pain due to disc herniation: a prospective randomized trial. J Korean Med Sci 2016;31:1324–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Colini-Baldeschi G. Evaluation of pulsed radiofrequency denervation in the treatment of chronic facet joint pain: an observational study. Anesth Pain Med 2012;1:168–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lindner R, Sluijter ME, Schleinzer W. Pulsed radiofrequency treatment of the lumbar medial branch for facet pain: a retrospective analysis. Pain Med 2006;7:435–9. [DOI] [PubMed] [Google Scholar]
- [34].Mikeladze G, Espinal R, Finnegan R, et al. Pulsed radiofrequency application in treatment of chronic zygapophyseal joint pain. Spine J 2003;3:360–2. [DOI] [PubMed] [Google Scholar]
- [35].Moussa WM, Khedr W. Percutaneous radiofrequency facet capsule denervation as an alternative target in lumbar facet syndrome. Clin Neurol Neurosurg 2016;150:96–104. [DOI] [PubMed] [Google Scholar]


