Supplemental Digital Content is available in the text
Keywords: ambulatory care, hepatitis B virus reactivation, high-risk immunosuppressive drugs, patient safety
Abstract
Hepatitis B virus (HBV) reactivation in the setting of rituximab use is a potentially fatal but preventable safety event. The rate of HBV screening and proportion of patients at risk who receive antiviral prophylaxis in patients initiating rituximab is unknown.
We analyzed electronic health record (EHR) data from 2 health systems, a university center and a safety net health system, including diagnosis grouper codes, problem lists, medications, laboratory results, procedures codes, clinical encounter notes, and scanned documents. We identified all patients who received rituximab between 6/1/2012 and 1/1/2016. We calculated the proportion of rituximab users with inadequate screening for HBV according to the Centers for Disease Control guidelines for detecting latent HBV infection before their first rituximab infusion during the study period. We also assessed the proportion of patients with positive hepatitis B screening tests who were prescribed antiviral prophylaxis. Finally, we characterized safety failures and adverse events.
We included 926 patients from the university and 132 patients from the safety net health system. Sixty-one percent of patients from the university had adequate screening for HBV compared with 90% from the safety net. Among patients at risk for reactivation based on results of HBV testing, 66% and 92% received antiviral prophylaxis at the university and safety net, respectively.
We found wide variations in hepatitis B screening practices among patients receiving rituximab, resulting in unnecessary risks to patients. Interventions should be developed to improve patient safety procedures in this high-risk patient population.
1. Introduction
The availability of electronic health record (EHR) data provides new and important opportunities to examine patient safety by allowing us to identify patients at-risk for safety issues, to understand clinical reasoning process errors, and to investigate outcomes of patients with adverse events. However, this potential to employ EHR data to enhance patient safety has to date been underutilized.[1] In this study, we use the full spectrum of EHR data—including traditional structured fields along with laboratory results and clinical note narrative text—to understand a common patient safety failure, missed laboratory testing before initiation of a high-risk medication. Missed laboratory testing is known to contribute to adverse drug events and lead to significant patient harm.[2,3] We chose to focus on screening and prophylaxis for hepatitis B virus (HBV) reactivation among patients receiving a high-risk immunosuppressant medication, rituximab, because there is evidence to support screening and prophylaxis for HBV before drug initiation; rituximab has clinical applications in many different specialties; and severe harm is possible for patients who do not receive appropriate screening or prophylaxis for HBV.
The manufacturer of rituximab, the FDA, the CDC, the American Society of Clinical Oncology, and others recommend screening and prophylaxis for HBV before initiation of rituximab.[4–11] Recent studies have reported a higher than expected rate of HBV reactivation among immunosuppressed patients, in particular those receiving rituximab.[12–16] There is evidence supporting the use of antiviral prophylaxis among patients known to be at risk for HBV reactivation.[17–20] However, only a few studies have examined practice patterns around HBV screening before rituximab use,[21–23] antiviral prophylaxis among patients at risk for reactivation, or adverse events among those with gaps in care.
In this study, we used data generated from the EHRs of 2 large healthcare systems affiliated with an academic institution, serving almost 1 million patients, to evaluate screening and prophylaxis patterns for HBV among recent users of rituximab, as well as clinical outcomes among these patients.
2. Methods
2.1. Data sources
Data derive from the EHR of 2 health systems. The first is a tertiary care referral university health center with over 750,000 outpatient visits per year that uses an Epic EHR. The catchment area is large, and includes much of northern California. Many patients are referred from the community for tertiary care including bone marrow transplants, solid organ transplants, and care for immune system disorders requiring the use of rituximab. The second is an urban safety-net health system that has almost 600,000 outpatient visits per year and uses eClinicalWorks as its ambulatory EHR. The catchment area is restricted to residents of a single county; the majority of patients receive integrated care within the safety net system, including on-site lab testing.
For both healthcare systems, all EHR data were available for analysis including demographics, diagnosis grouper codes, problem lists, medications, laboratory studies, procedures, clinical encounter notes, and scanned documents. Because we were interested in determining the feasibility of automated extraction of patient safety errors, variables were initially extracted electronically via back-end access to the EHR data warehouses, using structured fields and text string searches of clinical notes. Following the automated data extraction, a chart review by 2 physicians (GS and JY) was performed to ensure the validity of all extracted variables. In addition, all charts were reviewed to confirm the following variables: clinical indication for rituximab other than lymphoma; absence of any documented HBV screening; positive HBV screening results.
2.2. Study population
We defined a cohort of rituximab users from each site. Eligible patients had at least 1 encounter (inpatient or outpatient) after June 2012, the date on which the EHR was implemented (“go-live” date; N = 740,430 at university and 214,458 at safety net). We identified an index date as the date of the first completed rituximab infusion after go-live (see Appendix 1). Patients were included in the study if they had at least 2 inpatient or face-to-face ambulatory encounters during the 12 months before the index date and at least one encounter 30 days after the index date. Because some patients may have received rituximab before “go-live,” this cohort contained both incident and prevalent users.
2.3. Outcomes
We examined 3 outcomes. First, we calculated the proportion of rituximab users who received adequate screening for HBV. Adequate screening was defined according to the drug manufacturer's recommendations for detecting latent HBV infection,[4] that is, as having had a HBV surface antigen (HBsAg) and a HBV core antibody (anti-HBc) test result documented at any time before the index date. If laboratory results were not found in structured fields, we searched for results within the text of clinical notes or in scanned documents with results from outside facilities.
Second, among the patients at risk for HBV reactivation, we calculated the proportion of patients prescribed antiviral prophylaxis by 30 days after the index date. We stratified patients according to their risk for reactivation—patients with “chronic HBV infection” (HBsAg-positive, HBcAb (IgG)-positive, HBsAb-negative, and variable HBV DNA), who have a higher risk for reactivation, versus patients with “resolved/cleared HBV infection” (HBsAg negative, HBcAb (IgG) positive, variable HBsAb, and HBV DNA negative), who have an elevated but lower risk for reactivation.[24] Drugs that qualified for antiviral prophylaxis included lamivudine, entecavir, interferon alfa-2B, adefovir, telbicudine, and tenfovir.
Finally, we confirmed all potential HBV reactivation events via a chart review among patients who received rituximab and had a positive HBsAg, HBcAb, or HBV DNA at any time.
2.4. Covariates
Demographic information including age, sex, and race was extracted from the EHR. Number of outpatient visits in the 180 days before the index date was computed as a measure of healthcare utilization. To identify comorbidities, we extracted diagnoses from encounters, problem lists, invoices, claims, hospital accounts, hospital admissions, and surgical cases at any time leading up to the index date. A modified Charlson score was calculated according to the Deyo protocol.[25] Because of limitations in EHR access, complete assessment of comorbid conditions was not possible in the safety net health system. Ordering clinic was defined by the specialty of the ordering supervising physician for rituximab. Drug administration setting could be inpatient or outpatient. Primary diagnosis requiring rituximab was extracted from the index rituximab infusion order or from the corresponding clinical notes. We assessed oral glucocorticoid use from 30 days before to 30 days after the index date—patients were defined as users if they had a prescription for >14 days during this period. The same window was used to assess use of additional immunosuppressive agents, including antineoplastic or disease-modifying agents (see Appendix 2 for list of included drugs). Because receipt of intravenous immunoglobulin (IVIG) can cause false-positive antibody titers for up to 3 months, we assessed its use in the 90 days leading up to the index date among patients with positive anti-HBc.[26]
2.5. Medical record review of patient safety events
We performed a chart review to identify reasons why patients at known risk for HBV reactivation did not receive antiviral prophylaxis. Two physicians reviewed the entire EHR for these patients (N = 32). Reasons for patient safety failures were coded and compared; any discrepancies were resolved by consensus.
2.6. Sensitivity analyses
We conducted additional analyses on a sample restricted to patients with an index date at least 1 year after go-live. This approach eliminated prevalent users from the sample, who might plausibly have less documentation of screening tests; and mitigated the possible effects of a new EHR reducing complete documentation and ordering.[27]
2.7. Statistical analysis
Because of inherent differences between the study samples from the university vs. safety net health system, analyses were kept separate. We used Chi-squared or t tests to compare baseline characteristics between patients who received adequate HBV testing and those who did not. We used relative risk regression models to identify independent predictors of inadequate testing.[28]Variables included in the models were decided a priori and based on previously reported predictors of risk for HBV that may have influenced providers’ decisions to test.[29,30] All variables included in the models were tested for noncolinearity.
The study was approved by the Committee on Human Research at the University of California, San Francisco.
3. Results
Nine hundred twenty-six patients from the university and 132 from the safety net health system received rituximab during the study period. About half of the patients at each site were women. Pediatric patients were seen exclusively at the university. University patients were mostly white; safety-net patients were diverse with 30% Asian, 19% Hispanic, and 18% African-American patients. Both sites had oncologists as the most common prescribers of rituximab for a primary diagnosis of lymphoma or other malignancies. Additional characteristics of the patients are shown in Table 1.
Table 1.
Patient characteristics N (%), by site.
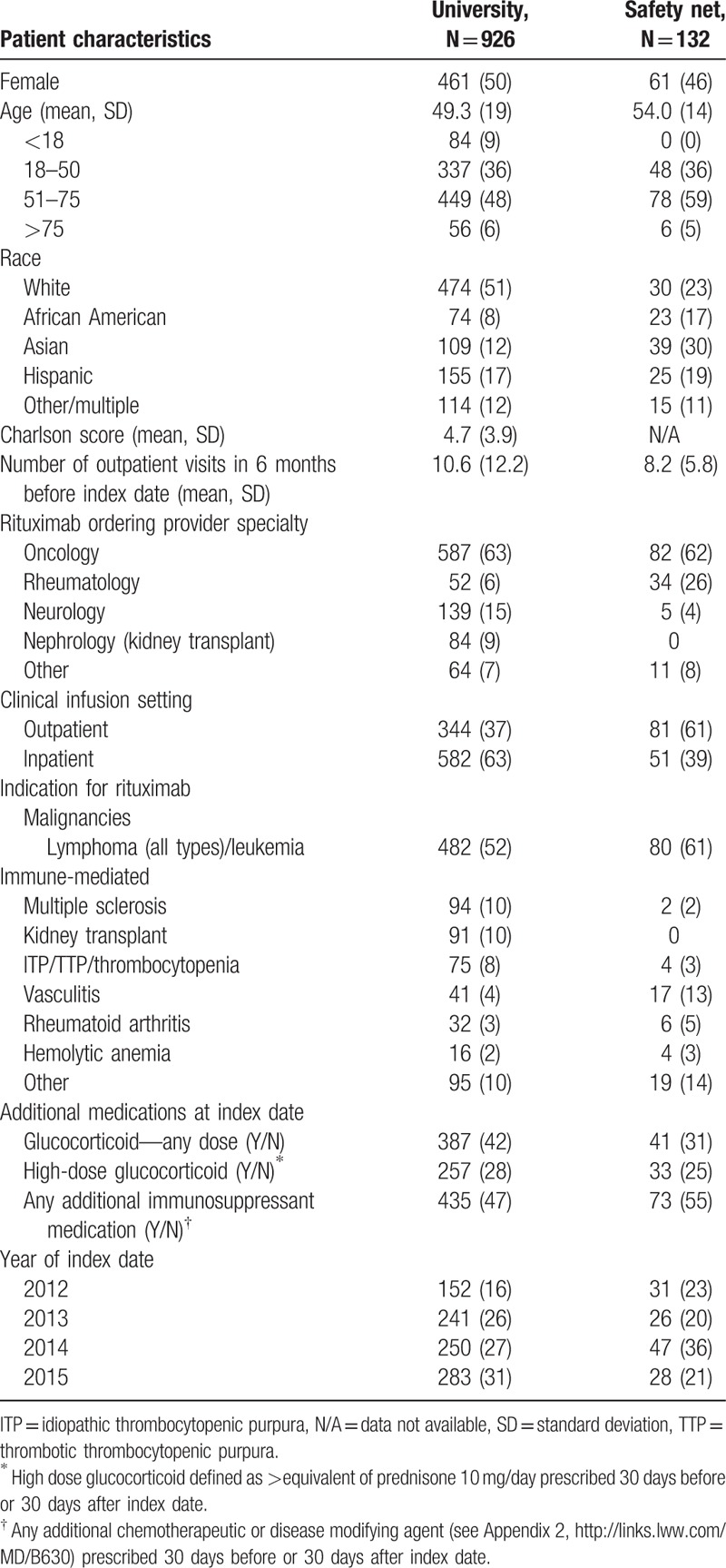
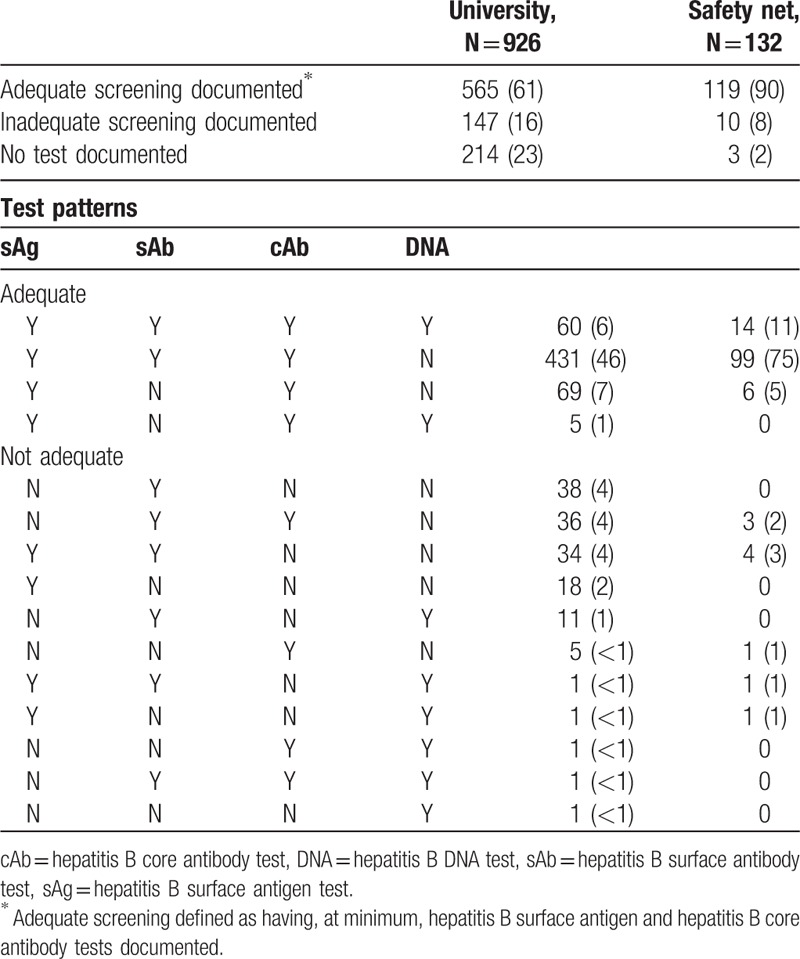
At the university, 565 (61%) patients had adequate HBV screening before the index date and 214 (23%) had no HBV test documented, compared to 119 (90%) and 3 (2%), respectively, in the safety net system. Specific patterns of HBV tests are listed in Table 2. In a sensitivity analysis which excluded patients with an index date the first year of after go-live, results were similar: 19% of 646 patients at the university, and 3% of 117 patients in the safety net system did not have any HBV test documented.
Table 2.
Hepatitis B testing patterns among patients receiving rituximab N (%), by site.
We examined correlates of inadequate screening at the university, where there was sufficient power to perform such analysis. In the bivariate analysis, we found that non-Asian race, lower Charlson score, fewer outpatient visits, lack of glucocorticoid use, lack of IVIG use, earlier index year, outpatient start, and nonnephrology (kidney transplant) ordering clinics were all significantly associated with lower probability of adequate screening. Multivariate regression showed that the strongest predictor of inadequate screening was department of the ordering provider (see Appendix 3). Other independent predictors of inadequate screening included lower Charlson scores, fewer outpatient visits, not receiving glucocorticoids; and index dates before 2015. Patients prescribed rituximab by nephrology (kidney transplant) providers were most likely to have received adequate screening, even after adjusting for other factors.
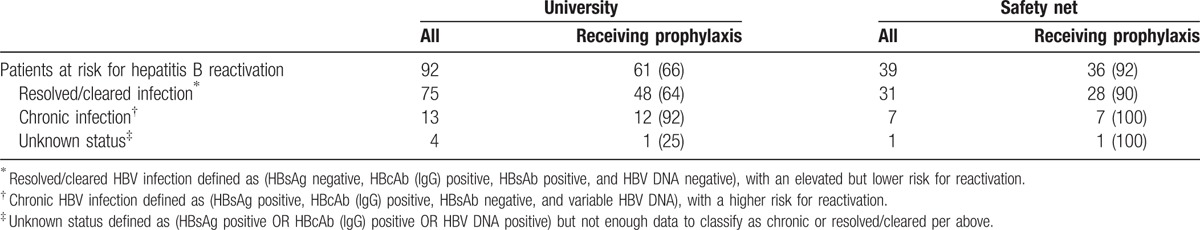
Table 3 describes the proportion of patients known to be at risk for HBV reactivation who were prescribed antiviral prophylaxis by 30 days after the rituximab index date. At the university, 92 patients were confirmed to be at risk for HBV reactivation; 61 (66%) received prescriptions for antiviral prophylaxis. In the safety net, 39 patients were at risk; 36 of these (92%) received prophylaxis. In both systems, patients with chronic infection, who are at highest risk for reactivation, were most likely to receive prophylaxis (92% and 100% at the university and safety net, respectively). The most common antiviral drugs used for HBV prophylaxis were entecavir, lamivudine, and tenofivir (accounting for 66%, 14%, and 5% respectively at the university and 11%, 64%, and 25% respectively at the safety net hospital).
Table 3.
Antiviral prophylaxis for patients receiving rituximab and at risk for reactivation of hepatitis B, N (%).
Through chart review, we explored reasons why patients with a positive HBsAg or anti-HBc were not provided prophylaxis. Out of 34 patients (31 from the university, and 3 from the safety net), in 11 (32%) a positive HBV result was not acknowledged by the provider; in 7 (21%), a positive result was acknowledged but misinterpreted; in 2 (6%) the provider intended to start the antiviral but this was not done; in 11 (32%) the positive HBV test was assumed to be a false positive related to IVIG use, but never rechecked; in 1 (3%) the test was later confirmed to be negative; and in 2 (6%) the reasons were unknown.
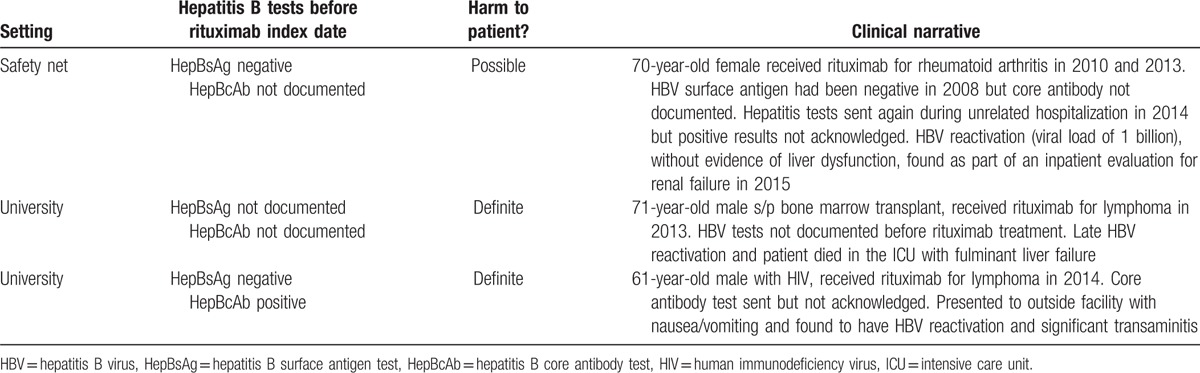
Finally, Table 4 contains clinical vignettes describing the 3 rituximab users in our sample who experienced reactivation of HBV at any time during the study period.
Table 4.
Clinical vignettes of patients with reactivation of hepatitis B following rituximab administration.
4. Discussion
In this study, we found that patient safety errors were relatively common for patients receiving rituximab. A significant proportion of patients were exposed to unnecessary risks (based on documented positive HBV test results but no receipt of antiviral prophylaxis), or had no HBV tests documented in the EHR and were thus potentially at risk for HBV reactivation. Rituximab is just one example of the growing number of high-risk specialty medications, including immunosuppressants, anticoagulants, and others, where missed testing can result in serious or even fatal adverse events. Our findings from the university health system are consistent with prior reports of suboptimal hepatitis B screening among immunosuppressed patients. Reports of HBV screening in rituximab-receiving patients range from 4% to 69%.[21–23,29] Studies looking at a broader range of immunosuppressants reported HBV screening rates of 6% to 62%, with wide variations according to ordering clinic.[30–34] As described in other settings, we also found patients seen by nephrologists and kidney transplant patients to have the highest probability of adequate screening.[30]
We found that patients in the safety net health system were more likely to have documentation of adequate screening for HBV before rituximab compared to patients at the university, despite the fact that many of the same physicians practice in both health systems evaluated in this study. This suggests that health-system factors clearly play a pivotal role in patient safety processes and outcomes. This difference may reflect the fact that office visits, hospital stays, and laboratory tests are all part of an integrated safety net system. Moreover, there appear to be a greater proportion of patients in the safety net with latent HBV infection, which could lead to increased provider awareness of this issue, or other systematic approaches to screening, such as use of preadministration safety checklists (in use at the safety net's infusion center, but not at the university's during the study period). Because care at the university (a tertiary care center) can be fragmented and EHRs may not be interoperable, providers at the university may assume screening laboratory studies were done at an outside hospital while providers referring from the community may assume these tests were performed at the university.[35] These findings suggest that improvements in workflows and EHR systems need to occur and be studied in tandem.[36]
This study sheds light on the feasibility of automatically extracting actionable patient safety information from EHRs. Although automatic extraction of data within the EHR holds great promise in this area, understanding safety errors and resulting adverse events is a complex task that requires detailed clinical and operational knowledge. In the case of rituximab and HBV testing, automated data extraction without chart review would have misrepresented a significant portion of the actual care provided; similar issues have been reported in other studies of automatically extracted quality measures.[37,38] As payers’ reliance on such automation increases, accurate reporting may need to rely on strategies such as collection of critical data elements in structured fields, or the development of natural language processing methods for extraction of unstructured data from clinical notes.
What can be done to increase HBV testing rates for patients receiving rituximab? First, provider knowledge around adequate testing for HBV in the setting of immunosuppression has been reported to be poor, suggesting a role for educational initiatives.[39–43] Health IT solutions can also be helpful in missed tests and results management.[44] Systems solutions could include the use of clinical decision support at the time the rituximab is ordered or administered.[45] However, the complexity of clinical reasoning and differing clinical workflows across clinics make automated extraction of data for use in clinical decision support or rapid cycle quality improvement challenging. For example, we observed a meaningful number of patients who received IVIG around the time of rituximab infusion, which resulted in false-positive anti-HBc tests. A naïve decision support tool would alert these providers for not prescribing antiviral prophylaxis, when they were in fact taking a prudent approach to spare patients the use of unnecessary medications. Our assessment of factors associated with adequate HBV screening suggests that patients with a larger number of medical issues (higher Charlson scores, receiving glucocorticoids) are less likely to receive adequate screening. This is consistent with some prior literature suggesting that more complex patients receive lower quality care.[46]
This study has several limitations. Because we report on 2 healthcare systems affiliated with an academic institution, our results may not be generalizable to other healthcare systems or populations. The relatively high rates of positive HBV tests in these populations may make providers more likely to screen for hepatitis B and more comfortable in prescribing antiviral prophylaxis compared to providers at other institutions. We did not examine patients who received care at these institutions but who were administered rituximab elsewhere, and cannot rule out that these patients may have had differential risk for inadequate HBV screening. Nevertheless, to our knowledge, this is the largest and most diverse cohort of rituximab users reported in the literature.
In conclusion, in this case study of HBV screening in the setting of rituximab use we demonstrated wide practice variation, unnecessary patient safety risks, and patient safety failures resulting in harm. Standardization of pre-prescribing workflows and use of order sets, checklists, and clinical decision support are increasingly possible with EHRs, and more investment in this area is justified.
Supplementary Material
Footnotes
Abbreviations: anti-HBc = HBV core antibody, CDC = Centers for Disease Control, FDA = Federal Drug Administration, HBsAg = HBV surface antigen, HBV = hepatitis B virus, HER = electronic health record, IVIG = intravenous immunoglobulin.
Funding: This work is supported by AHRQ R01 HS024412, K23 AR063770 (GS), and R01 GM079719 (MS). Drs. Yazdany and Schmajuk are also supported by the Russell/Engleman Medical Research Center for Arthritis. Dr. Sarkar is supported by AHRQ P30HS023558. Dr. Sirota is funded in part by the March of Dimes Prematurity Research Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality or National Institutes of Health.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Jha A, Provonost P. Toward a safer health care system: the critical need to improve measurement. JAMA 2016;315:1831–2. [DOI] [PubMed] [Google Scholar]
- [2].Steinman MA, Handler SM, Gurwitz JH, et al. Beyond the prescription: medication monitoring and adverse drug events in older adults. J Am Geriatr Soc 2011;59:1513–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wahls TL, Cram PM. The frequency of missed test results and associated treatment delays in a highly computerized health system. BMC Fam Pract 2007;8:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].http://www.gene.com/download/pdf/rituxan_prescribing.pdf. Accessed March 1, 2017. [Google Scholar]
- [5].http://www.fda.gov/Drugs/DrugSafety/ucm366406.htm. Accessed March 1, 2017. [Google Scholar]
- [6].Hwang JP, Somerfield MR, Alston-Johnson DE, et al. Hepatitis B virus screening for patients with cancer before therapy: American Society of Clinical Oncology Provisional Clinical Opinion Update. J Clin Oncol 2015;33:2212–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Weinbaum CM, Williams I, Mast EE, et al. Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. MMWR Recomm Rep 2008;57:1–20. [PubMed] [Google Scholar]
- [8].Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology 2009;50:661–2. [DOI] [PubMed] [Google Scholar]
- [9].Liaw YF, Kao JH, Piratvisuth T, et al. Asia-Pacific consensus statement on the management of chronic hepatitis B: a 2012 update. Hepatol Int 2012;6:531–61. [DOI] [PubMed] [Google Scholar]
- [10].European Association for the Study of the Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol 2012;57:167–85. [DOI] [PubMed] [Google Scholar]
- [11].Reddy KR, Beavers KL, Hammond SP, et al. American gastroenterological association institute guideline on the prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology 2015;148:215–9. [DOI] [PubMed] [Google Scholar]
- [12].Dervite I, Hober D, Morel P. Acute hepatitis B in a patient with antibodies to hepatitis B surface antigen who was receiving rituximab. N Engl J Med 2001;344:68–9. [DOI] [PubMed] [Google Scholar]
- [13].Westhoff TH, Jochimsen F, Schmittel A, et al. Fatal hepatitis B virus reactivation by an escape mutant following rituximab therapy. Blood 2003;102:1930. [DOI] [PubMed] [Google Scholar]
- [14].Yang SH, Kuo SH. Reactivation of hepatitis B virus during rituximab treatment of a patient with follicular lymphoma. Ann Hematol 2008;87:325–7. [DOI] [PubMed] [Google Scholar]
- [15].Perrillo RP, Gish R, Falck-Ytter YT. American Gastroenterological Association Institute technical review on prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology 2015;148:221–44. [DOI] [PubMed] [Google Scholar]
- [16].Perrillo RP, Martin P, Lok AS. Preventing hepatitis B reactivation due to immunosuppressive drug treatments. JAMA 2015;313:1617–8. [DOI] [PubMed] [Google Scholar]
- [17].Yeo W, Chan TC, Leung NW, et al. Hepatitis B virus reactivation in lymphoma patients with prior resolved hepatitis B undergoing anticancer therapy with or without rituximab. J Clin Oncol 2009;27:605–11. [DOI] [PubMed] [Google Scholar]
- [18].Huang YH, Hsiao LT, Hong YC, et al. Randomized controlled trial of entecavir prophylaxis for rituximab-associated hepatitis B virus reactivation in patients with lymphoma and resolved hepatitis B. J Clin Oncol 2013;31:2765–72. [DOI] [PubMed] [Google Scholar]
- [19].Hsu C, Tsou HH, Lin SJ, et al. Chemotherapy-induced hepatitis B reactivation in lymphoma patients with resolved HBV infection: a prospective study. Hepatology 2014;59:2092–100. [DOI] [PubMed] [Google Scholar]
- [20].Buti M, Morillas R, Manzano ML, et al. Tenofovir for the prophylaxis of HBV reactivation in anti-HBc-positive patients with hematologic malignancies treated with rituximab: preliminary results of a randomized study (PREBLIN Study). J Hepatol 2014;60suppl:S421–2. [Google Scholar]
- [21].Leonard AN, Love BL, Norris LB, et al. Screening for viral hepatitis prior to rituximab chemotherapy. Ann Hematol 2016;95:27–33. [DOI] [PubMed] [Google Scholar]
- [22].Ramirez J, Duddempudi AT, Sana MM, et al. Screening for hepatitis B in patients with lymphoma. Proc (Bayl Univ Med Cent) 2015;28:438–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hwang JP, Fisch MJ, Zhang H, et al. Low rates of hepatitis B virus screening at the onset of chemotherapy. J Oncol Pract 2012;8:e32–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Paul S, Saxena A, Terrin N, et al. Hepatitis B virus reactivation and prophylaxis during solid tumor chemotherapy: a systematic review and meta-analysis. Ann Intern Med 2016;164:30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613–9. [DOI] [PubMed] [Google Scholar]
- [26].Ramsay I, Gorton RL, Patel M, et al. Transmission of hepatitis B core antibody and galactomannan enzyme immunoassay positivity via immunoglobulin products: a comprehensive analysis. Clin Infect Dis 2016;63:57–63. [DOI] [PubMed] [Google Scholar]
- [27].Burke HB, Sessums LL, Hoang A, et al. Electronic health records improve clinical note quality. J Am Med Inform Assoc 2015;22:199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004;159:702–6. [DOI] [PubMed] [Google Scholar]
- [29].Vaughn BP, Doherty GA, Gautam S, et al. Screening for tuberculosis and hepatitis B prior to the initiation of antitumor necrosis therapy. Inflamm Bowel Dis 2012;18:1057–63. [DOI] [PubMed] [Google Scholar]
- [30].Paul S, Shuja A, Tam I, et al. Gastroenterologists have suboptimal hepatitis B virus screening rates in patients receiving immunosuppressive therapy. Dig Dis Sci 2016;61:2236–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lee R, Vu K, Bell CM, et al. Screening for hepatitis B surface antigen before chemotherapy: current practice and opportunities for improvement. Curr Oncol 2010;17:32–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Conway R, Doran MF, O'Shea FD, et al. The impact of hepatitis screening on diagnosis and treatment in rheumatoid arthritis. Clin Rheumatol 2014;33:1823–7. [DOI] [PubMed] [Google Scholar]
- [33].van der Have M, Belderbos TD, Fidder HH, et al. Screening prior to biological therapy in Crohn's disease: adherence to guidelines and prevalence of infections. Results from a multicentre retrospective study. Dig Liver Dis 2014;46:881–6. [DOI] [PubMed] [Google Scholar]
- [34].Visram A, Chan KK, McGee P, et al. Poor recognition of risk factors for hepatitis B by physicians prescribing immunosuppressive therapy: a call for universal rather than risk-based screening. PLoS ONE 2015;10:e0120749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Madden JM, Lakoma MD, Rusinak D, et al. Missing clinical and behavioral health data in a large electronic health record (EHR) system. J Am Med Inform Assoc 2016;23:1143–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Carayon P, Schoofs Hundt A, Karsh BT, et al. Work system design for patient safety: the SEIPS model. Qual Saf Health Care 2006;Suppl 1:i50–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Baker DW, Persell SD, Thompson JA, et al. Automated review of electronic health records to assess quality of care for outpatients with heart failure. Ann Intern Med 2007;146:270–7. [DOI] [PubMed] [Google Scholar]
- [38].Kern LM, Malhotra S, Barrón Y, et al. Accuracy of electronically reported “meaningful use” clinical quality measures: a cross-sectional study. Ann Intern Med 2013;158:77–83. [DOI] [PubMed] [Google Scholar]
- [39].Lee RSM, Bell CM, Singh JM, et al. Hepatitis B screening before chemotherapy: a survey of practitioners’ knowledge, beliefs, and screening practices. J Oncol Pract 2012;8:321–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Turker K, Oksuzoglu B, Balci E, et al. Awareness of hepatitis B virus reactivation among physicians authorized to prescribe chemotherapy. Eur J Intern Med 2013;24:E90–2. [DOI] [PubMed] [Google Scholar]
- [41].Day FL, Link E, Thursky K, et al. Current hepatitis B screening practices and clinical experience of reactivation in patients undergoing chemotherapy for solid tumors: a nationwide survey of medical oncologists. J Oncol Pract 2011;7:141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Tran TT, Rakoski MO, Martin P, et al. Screening for hepatitis B in chemotherapy patients: survey of current oncology practices. Aliment Pharmacol Ther 2010;31:240–6. [DOI] [PubMed] [Google Scholar]
- [43].Khokhar OS, Farhadi A, McGrail L, et al. Oncologists and hepatitis B: a survey to determine current level of awareness and practice of antiviral prophylaxis to prevent reactivation. Chemotherapy 2009;55:69–75. [DOI] [PubMed] [Google Scholar]
- [44].Roy CL, Rothschild JM, Dighe AS, et al. An initiative to improve the management of clinically significant test results in a large health care network. Jt Comm J Qual Patient Saf 2013;39:517–27. [DOI] [PubMed] [Google Scholar]
- [45].Sun WC, Hsu PI, Yu HC, et al. The compliance of doctors with viral hepatitis B screening and antiviral prophylaxis in cancer patients receiving cytotoxic chemotherapy using a hospital-based screening reminder system. PLoS ONE 2015;10:e0116978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Zulman DM, Asch SM, Martins SB, et al. Quality of care for patients with multiple chronic conditions: the role of comorbidity interrelatedness. J Gen Intern Med 2014;29:529–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


