Abstract
Objective
To measure antiphospholipid antibody (aPL) variance during pregnancy; to determine if variation affects outcomes.
Methods
We used data from PROMISSE, a multicenter prospective study of pregnant women with aPL and/or SLE. APL was present if any of the following was positive: anticardiolipin (aCL), anti-β2glycoprotein I (aβ2GPI) titers ≥40 GPL or MPL units, and/or lupus anticoagulant (LAC). APL were measured every trimester and post-partum. Adverse pregnancy outcomes (APOs) were defined as: fetal/neonatal death, preterm delivery <36 weeks due to preeclampsia or placental insufficiency; or growth restriction.
Results
One hundred and fifty-two aPL-positive patients were studied: 57% with clinical APS and 36% with SLE. aPL IgG levels were significantly lower during 2nd and 3rd trimesters compared to screening, but IgG aCL and aβ2GPI remained high-positive through pregnancy in 93% and 85% of patients, respectively. APL IgM titers were negative in the majority of patients and fell modestly during pregnancy. LAC frequency also decreased, but 75% remained positive through the 2nd trimester. Only 4% of patients with aPL at baseline did not have aPL at either 2nd or 3rd trimester. Changes in aPL levels or aPL status were not associated with APOs. LAC was the only aPL associated with APOs.
Conclusion
APL levels decreased marginally during pregnancy, and changes were not associated with pregnancy outcome. Our findings suggest that measurement of aPL early is sufficient to assess risk. Repeat aPL testing through pregnancy is unnecessary.
Antiphospholipid antibodies (aPL), which include anticardiolipin (aCL), anti-β2 glycoprotein I antibodies (aβ2GPI) and lupus anticoagulant (LAC), are a heterogeneous group of antibodies associated with thrombosis, stillbirth, intrauterine growth restriction, preeclampsia and premature birth in patients with antiphospholipid syndrome (APS) (1). Data from animals and human placentas provide strong evidence of a direct pathologic effect of aPL that is believed to be responsible for obstetrical morbidity (2). The mechanisms of these effects may vary with aPL profile, isotype and titer (3). Changes in maternal aPL levels during pregnancy may be associated with different pregnancy outcomes. Currently, the value of repeated testing during pregnancy is unclear. It is unknown whether results from aPL testing in the first trimester are sufficient to predict risk for pregnancy complications, and physicians frequently repeat these tests through pregnancy, adding to the cost of care.
The objective of this study was to evaluate changes in aCL, aβ2GPI and LAC through pregnancy. A second objective was to determine whether aPL variation was associated with pregnancy outcomes.
PATIENTS AND METHODS
Study population
The PROMISSE Study (Predictors of pRegnancy Outcome: bioMarkers In antiphospholipid antibody Syndrome and Systemic lupus Erythematosus) is a prospective multicenter observational study of pregnancies in women with systemic lupus erythematosus (SLE), SLE and aPL or aPL alone, that enrolled patients from September 2003 to August 2014. This report includes 152 aPL-positive patients and 349 SLE patients who were aPL-negative at screening. We have previously reported on characteristics and adverse pregnancy outcomes (APOs) on a subset of PROMISSE patients enrolled from September 2003 to March 2011 (4); Forty-four new aPL positive patients recruited after March 2011 have not been previously reported.
Consecutive pregnant women, age 18 to 45 years, with singleton intra-uterine pregnancy, were enrolled before 18 weeks gestation. Definitions of disease and inclusion and exclusion criteria are described elsewhere (5) and below.
Data collection and follow-up
At screening [T1 (less than 18 weeks gestation)], a medical history and physical examination were performed and blood samples obtained. Patients were followed monthly during the course of pregnancy, and all medical and obstetrical major events were reported as they occurred. Blood tests for aPL were repeated during the second [T2 (20–23 weeks gestation)] and third trimesters [T3 (32–35 weeks gestation)] and at 3 months post-partum.
aPL assays
LAC, aCL and aβ2GPI assays were performed at study core laboratories as previously described (4). The definition of aPL positivity for PROMISSE was a modification of revised Sapporo criteria (6,7) and included: i) presence of aCL and/or aβ2GPI titers ≥40 GPL or MPL units and/or positive LAC [dilute Russel’s viper venom time (dRVVT), dilute prothrombin time (dPT), or activated partial thrombolastin time (aPTT)] and ii) persistence of aPL-positivity in a second assay at least 6 weeks apart (with at least one of the two determination during pregnancy). The PROMISSE study was ongoing when the Sapporo criteria were revised in 2006. We use Sydney criteria in this paper, maintaining, however, the 6-week criterion between APL tests to allow enrollment early in pregnancy (7). In addition, although Sapporo criteria do not include aβ2GPI antibodies, we were able to test this in all patients (6,7).
Adverse Pregnancy Outcomes
APOs were reported through pregnancy and included one or more of the following: fetal death after 12 weeks of pregnancy, neonatal death before hospital discharge due to complications of prematurity, pre-term delivery before 36 weeks of pregnancy due to gestational hypertension, preeclampsia, or placental insufficiency and small for gestational-age (SGA) neonate (birthweight < 5th percentile) (4,5).
Statistical analysis
Median titers and median changes in titers during pregnancy were compared to those at screening using the Wilcoxon-Paired test. Variation in aPL positivity during pregnancy was analyzed using the Mac Nemar test for paired-samples, and univariate analysis of factors associated with APOs was done with the Chi-square, Fisher’s exact tests for categorical variables and Mann-Whitney test for continuous variables. Statistical testing was done at the two-tailed α level of 0.05. Data were analyzed using SPSS software package 22.
RESULTS
Characteristics of the study population
Baseline characteristics of all aPL-positive patients are summarized in Table 1. Eighty-seven (57%) patients met criteria for APS, of whom 37 (43%) had history of thrombosis and 72 (83%) of obstetrical complications. At screening, aCL IgG was the most frequently positive aPL, followed by LAC. IgG isotypes of aβ2GPI and aCL were more common than IgM isotypes. Fifty-four patients (36%) met ACR criteria for SLE (8). The majority of patients were treated with antithrombotic therapy at screening: 103 (68%) with heparin and 110 (72%) with aspirin, 43 (28%) were treated with hydroxychloroquine, and immunosuppressive therapy was infrequent. Sixteen patients received prednisone (mean dose 4.3 mg per day, range 1 mg – 10 mg per day) and doses were stable during the pregnancy.
Table 1.
Baseline characteristics of the study population
| Characteristics* | Total population n=152 |
APOs n=46 |
No APOs n=106 |
p-value † |
|---|---|---|---|---|
| Age (years) § | 31.9 (4.6) | 30.6 (5.02) | 32.5 (4.38) | 0.02 |
|
| ||||
| Ethnicity | ||||
| Hispanic | 13 (9%) | 6 (13%) | 7 (7%) | 0.22 |
| Non-Hispanic | 139 (91%) | 40 (87%) | 99 (93%) | |
|
| ||||
| Race | ||||
| White | 128 (90%) | 37 (88%) | 91 (90%) | 0.76 |
| Non-White | 15 (10%) | 5 (12%) | 10 (10%) | |
|
| ||||
| BMI | ||||
| < 25 | 78 (55%) | 15 (35%) | 63 (64%) | 0.01 |
| 25–30 | 34 (24%) | 13 (30%) | 21 (21%) | 0.01 |
| > 30 | 30 (21%) | 15 (35%) | 15 (15%) | 0.001 |
|
| ||||
| SLE | 54 (36%) | 19 (41%) | 35 (33%) | 0.33 |
|
| ||||
| APS | 87 (57%) | 35 (76%) | 52 (49%) | 0.002 |
| History of thrombosis | 37 (24%) | 22 (48%) | 15 (14%) | 0.0001 |
| History of obstetrical manifestations | 72 (47%) | 25 (54%) | 48 (45%) | 0.23 |
|
| ||||
| Treatment during pregnancy | ||||
| Hydroxychloroquine | 43 (28%) | 13 (28%) | 30 (28%) | 0.99 |
| Corticosteroids | 16 (11%) | 3 (7%) | 13 (12%) | 0.39 |
| Aspirin | 110 (72%) | 26 (57%) | 84 (79%) | 0.004 |
| Heparin | 103 (68%) | 35 (76%) | 68 (64%) | 0.12 |
| Azathioprine | 8 (5%) | 4 (9%) | 4 (4%) | 0.24 |
| IVIG once per months | 6 (4%) | 2 (4%) | 4 (4%) | 1.0 |
|
| ||||
| aPL positive at screening # | ||||
| LAC | 81 (55%) | 36 (80%) | 45 (45%) | 0.0001 |
| aCL IgG | 90 (60%) | 30 (67%) | 60 (58%) | 0.30 |
| aCL IgM | 29 (19%) | 5 (11%) | 24 (23%) | 0.09 |
| aβ2GPI IgG | 59 (40%) | 20 (45%) | 39 (38%) | 0.36 |
| aβ2GPI IgM | 26 (18%) | 8 (18%) | 18 (17%) | 0.90 |
Characteristics are expressed as number (% of available data) unless otherwise noted. Proportion of missing data is <10% for all variables.
Chi-Square or Fisher exact or Mann Whitney tests compared patients with APOs to patients without APOs.
Age is expressed as mean (Standard Deviation).
APL positivity for each test was defined as: lupus anticoagulant : RVVT, dilute TTI or PTT LA with confirmation; aCL: IgG ≥40 GPL units; IgM ≥40 MPL units ; and anti-β2GPI: IgG ≥40 GPL units; IgM ≥40 MPL units. To be considered postive, each test met these criteria at least twice between 6 weeks and 5 years apart one of which must be in a core lab during the PROMISSE pregnancy (4).
Abbreviations: APOs: adverse pregnancy outcomes; BMI: body mass index; SLE: systemic lupus erythematosus; APS: antiphospholipid syndrome; aPL: antiphospholipid antibodies; IVIG: intravenous immunoglobulin therapy; LAC: lupus anticoagulant; aCL: anticardiolipin antibodies; aβ2GPI: anti-β2 Glycoprotein I antibodies; T1: first trimester of pregnancy; IU: international unit.
Variation in aPL levels through pregnancy
aCL and aβ2GPI decreased throughout pregnancy, but the magnitude of change was small, and IgG titers remained in the high-positive range (≥ 40 GPL units) (Figures 1A, 1B). By three months post-partum, aCL and aβ2GPI IgG titers increased to baseline levels (Figure 1A, 1B). The number of patients positive for IgM was smaller than that for IgG in both aCL and aβ2GPI assays, and trends in levels over pregnancy were similar (Figures 1 A and B).
Figure 1. Measurement of aPL tests through pregnancy according to aPL positivity at baseline.
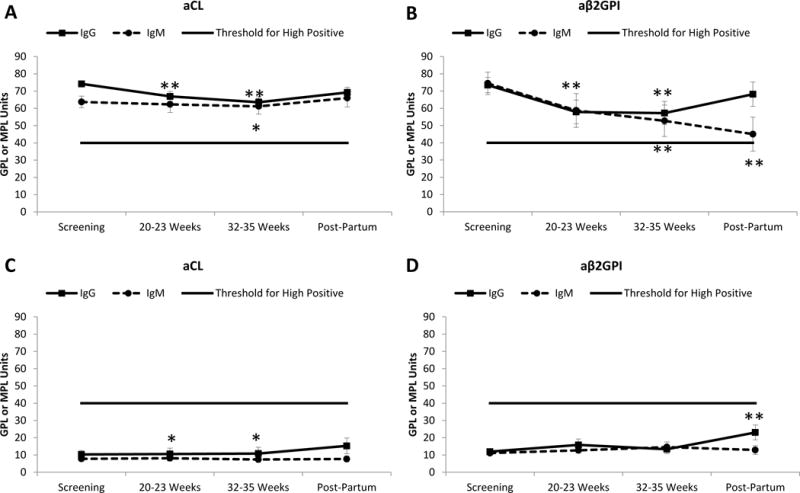
Patients are classified according to positivity for each aPL test at baseline.
A. Variation in aCL titers, among aCL-positive patients at screening (n=90 for IgG and n=29 for IgM). B. Variation in aβ2GPI titers, among aβ2GPI-positive patients at screening (n=59 for IgG and n=26 for IgM). C. Variation in aCL titers, among aCL-negative patients at screening (n=59 for IgG and n=120 for IgM). D. Variation in aβ2GPI titers, among aβ2GPI-negative patients at screening (n=89 for IgG and n=122 for IgM).
aPL positivity was defined for each test. aCL: IgG ≥40 GPL units; IgM ≥40 MPL units; and anti-β2GPI: IgG ≥40 GPL units; IgM ≥40 MPL units. To be considered positive, each test met these criteria at least twice between 6 weeks and 5 years apart, with one determination during the PROMISSE pregnancy at a core lab (4). Negative aCL or aβ2GPI titers were defined as <40 GPL or MPL units.
Values represent mean ± SEM. *p<0.05 **p<0.01 compared to screening using Wilcoxon-Paired test.
Abbreviations: aCL: Anticardiolipin antibodies; aβ2GPI: anti-β2 Glycoprotein I antibodies
Despite decreases in average aCL and aβ2GPI titers, few patients with high titers at screening (T1) became negative by Sapporo criteria (<40 GPL or MPL units): none at T2 and only one at T3. Of the 90 patients positive for aCL IgG, only 7% had titers fall to <40GPL units at T2. Table 2 shows the small number of patients who converted from positive to negative, or from negative to positive in any aPL test during pregnancy. Of 152 patients classified as aPL-positive at baseline by one or more of the aPL assays, only 6 (4%) were aPL-negative at either T2 and T3.
Table 2.
Changes in aPL positivity through pregnancy*
| aCL-positive | aβ2GPI-positive | LAC-positive n=79 |
|||
|---|---|---|---|---|---|
| Positive at baseline | IgG n=90 |
IgM n=29 |
IgG n=59 |
IgM n=26 |
|
|
| |||||
| Became negative at T2** Became negative at T3*** |
6 4 |
7 0 |
14 7 |
9 2 |
15 11 |
|
| |||||
| aCL-negative | aβ2GPI-negative |
LAC negative n=65 |
|||
| Negative at baseline |
IgG n=59 |
IgM n=120 |
IgG n=89 |
IgM n=122 |
|
|
| |||||
| Became positive at T2** Became positive at T3*** |
6 0 |
2 3 |
6 1 |
5 2 |
12 1 |
aPL-positivity is defined according to Sapporo criteria: aCL and/or aβ2GPI titers (≥40 GPL or MPL units) and/or positive LAC (RVVT, dPT, aPTT). Negative aCL or aβ2GPI titers were defined as <40 GPL or MPL units.
8 patients had APOs and their pregnancies ended before T2
26 had APOs and their pregnancies ended before T3.
Abbreviations: aCL: Anticardiolipin antibodies; aβ2GPI: anti-β2 Glycoprotein I antibodies; LAC: Lupus anticoagulant; T2: 2nd trimester of pregnancy; T3: 3rd trimester of pregnancy.
Because PROMISSE patients were classified as aPL-positive if one or more of the 3 aPL tests was positive, we had the opportunity to assess levels of aPL at T2 and T3 in patients in whom levels were in the low or moderate range at baseline. When aCL or aβ2GPI levels were <40 GPL at screening, they remained relatively stable during pregnancy (Figure 1C and 1D), and few patients (<10%) classified as being negative for a specific aPL test at baseline met criteria for positivity at T2. Furthermore, none of the 349 SLE patients enrolled in PROMISSE who were classified as aPL-negative at screening met criteria for aPL-positivity at T2 or T3.
LAC test results also tended to decrease during the course of pregnancy. Among the 81 patients who were LAC-positive at screening, 74% remained LAC-positive at T2, 50% at T3, and LAC tests returned to baseline levels at 3 months post-partum. Among the 65 patients LAC-negative at baseline, 18% became positive at T2 (Table 2).
aPL changes and risk of APO
Median pregnancy duration (inter-quartile range) for the entire population was 37.3 (5.9) weeks, while in those with APOs median duration was 28.1 (10.5) weeks. Patients with APOs were younger, had a higher BMI, were more likely to have a history of clinical APS, particularly thrombosis, and LAC (Table 1). Forty-six (30%) patients had APOs, 80% of which occurred during T2. Of patients with APOs, 67% had aCL IgG ≥40, 45% had aβ2GPI IgG ≥40, and 80% LAC, while only 11% had aCL IgM ≥40 and 18% had aβ2GPI IgM ≥40.
LAC at baseline was the only aPL associated with APOs in this expanded population and in our original report (4). In the small number of patients who converted from LAC-negative to LAC-positive at T2 (Table 2), there was no significant increase in the frequency of APOs (3/11 had APOs; all 3 had SLE) compared those who remained LAC-negative (p=0.33). Similarly, changes from negative to positive in aCL or aβ2GPI IgG status was not associated with changes in frequency of APOs, which ranged from 17% to 25% in those who remained negative or converted to positive.
DISCUSSION
Our study is the first description of the evolution of aPL during the course of pregnancy in a prospective, multicenter cohort in which aPL was determined by core labs. Most patients who were positive for a test in first trimester (defined as ≥40 units or LAC) remained in the high-positive range throughout pregnancy. Although we observed modest decreases in all aPL tests through pregnancy, these changes were not associated with changes in pregnancy outcomes. Conversely, among patients negative for a specific aPL test in first trimester, titers did not increase through pregnancy, and the infrequent occurrence of a patient converting from negative to positive was not associated with APOs. In addition, none of the 349 SLE patients enrolled in PROMISSE who were negative for all aPL tests at screening met criteria for aPL later in pregnancy. LAC determination at first trimester and a history of APS were sufficient to identify patients at high risk for APOs. These findings argue that it is not necessary to repeat aPL testing through pregnancy.
Few studies have reported serial aPL determinations through pregnancy. They have significant limitations (9–14): small numbers of patients (7 to 51 patients), not performing all 3 aPL tests, and inclusion of patients with low titer aPLs. Despite these methodological challenges, two studies documented a decrease in aPL through pregnancy (10,11), similar to our results, and one found fluctuations in levels (13). There was no consensus on the clinical relevance of changes in aPL titers. Lynch et al and Donohoe et al found no relationship with APOs (12,13), while others reported favorable pregnancy outcomes associated with falling titers of aPL (10,11,14).
Several potential mechanisms might explain the modest decrease in aPL that we observed. First, dilution by increased intravascular volume could lower the concentration of aPL with no pathologic significance. Indeed, hematocrit normally decreases in pregnancy, reflecting hemodilution, and it did in our patients (results not shown). Second, decreases in aPL levels might result from binding of aPL to placenta, as demonstrated in animal models and in patients; pregnancy complications have been attributed to placental aPL deposition (2,15). However, decreased titer was not associated with APO in our patients. Third, there may be decreased autoantibody production, but we could not assess this possibility. Finally, immunosuppressive drugs, such as corticosteroids, might decrease aPL levels, but only 16 patients received prednisone, and daily doses were 10 mg or less, a dose unlikely to impact antibody titer (16). Our data do not address the effect of higher steroid doses.
The strengths of our study include the large number of patients, prospective data collection and follow-up, and the use of core laboratories. A limitation is that patients were enrolled only at tertiary care centers, which could have selected for a high-risk population compared to the general APS population.
In conclusion, in a large prospective cohort of aPL-positive pregnant women, small decreases in LAC, aCL and aβ2GPI occurred during pregnancy, but changes were not clinically meaningful. Furthermore, negative tests in the first trimester rarely became positive later, suggesting that repeat aPL testing through pregnancy is unnecessary. Only LAC, but not change in LAC status, was associated with APOs (4,5). Thus, risk for poor outcomes in aPL-positive patients can be established based on first trimester LAC. Strategic use of aPL laboratory testing can increase effectiveness of clinical care and minimize the costs associated with repeated tests.
Acknowledgments
ClinicalTrials.gov identifier NCT00198068
SOURCES OF FUNDING
Supported in part by the National Institute of Arthritis and Musculoskeletal and Skin Diseases under award number AR-49772.
Footnotes
CONFLICT(S) OF INTEREST/DISCLOSURE(S)
No conflict of interest to declare.
References
- 1.de Jesus GR, Agmon-Levin N, Andrade CA, Andreoli L, Chighizola CB, Porter TF, et al. 14th International Congress on Antiphospholipid Antibodies Task Force report on obstetric antiphospholipid syndrome. Autoimmunity reviews. 2014;13(8):795–813. doi: 10.1016/j.autrev.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Viall CA, Chamley LW. Histopathology in the placentae of women with antiphospholipid antibodies: A systematic review of the literature. Autoimmunity reviews. 2015;14(5):446–71. doi: 10.1016/j.autrev.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 3.Mekinian A, Loire-Berson P, Nicaise-Roland P, Lachassinne E, Stirnemann J, Boffa MC, et al. Outcomes and treatment of obstetrical antiphospholipid syndrome in women with low antiphospholipid antibody levels. Journal of reproductive immunology. 2012;94(2):222–6. doi: 10.1016/j.jri.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Lockshin MD, Kim M, Laskin CA, Guerra M, Branch DW, Merrill J, et al. Prediction of adverse pregnancy outcome by the presence of lupus anticoagulant, but not anticardiolipin antibody, in patients with antiphospholipid antibodies. Arthritis and rheumatism. 2012;64(7):2311–8. doi: 10.1002/art.34402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buyon JP, Kim MY, Guerra MM, Laskin CA, Petri M, Lockshin MD, et al. Predictors of Pregnancy Outcomes in Patients With Lupus: A Cohort Study. Annals of internal medicine. 2015;163(3):153–63. doi: 10.7326/M14-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson WA, Gharavi AE, Koike T, Lockshin MD, Branch DW, Piette JC, et al. International consensus statement on preliminary classification criteria for definite antiphospholipid syndrome: report of an international workshop. Arthritis and rheumatism. 1999;42(7):1309–11. doi: 10.1002/1529-0131(199907)42:7<1309::AID-ANR1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 7.Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) Journal of thrombosis and haemostasis : JTH. 2006;4(2):295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 8.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis and rheumatism. 1997;40(9):1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 9.Sanchez-Guerrero J, Alarcon-Segovia D. Course of antiphospholipid antibodies in patients with primary antiphospholipid syndrome before, during and after pregnancy treated with low dose aspirin. Relationship of antibody levels to outcome in 7 patients. The Journal of rheumatology. 1992;19(7):1083–8. [PubMed] [Google Scholar]
- 10.Topping J, Quenby S, Farquharson R, Malia R, Greaves M. Marked variation in antiphospholipid antibodies during pregnancy: relationships to pregnancy outcome. Human reproduction. 1999;14(1):224–8. doi: 10.1093/humrep/14.1.224. [DOI] [PubMed] [Google Scholar]
- 11.Salazar-Paramo M, Jara LJ, Ramos A, Barile L, Machado G, Garcia-De La Torre I. Longitudinal study of antinuclear and anticardiolipin antibodies in pregnant women with systemic lupus erythematosus and antiphospholipid syndrome. Rheumatology international. 2002;22(4):142–7. doi: 10.1007/s00296-002-0207-x. [DOI] [PubMed] [Google Scholar]
- 12.Lynch AM, Rutledge JH, Stephens JK, Murphy JR, Marlar RA, Davila GH, et al. Longitudinal measurement of anticardiolipin antibodies during normal pregnancy: a prospective study. Lupus. 1995;4(5):365–9. doi: 10.1177/096120339500400506. [DOI] [PubMed] [Google Scholar]
- 13.Donohoe S, Quenby S, Mackie I, Panal G, Farquharson R, Malia R, et al. Fluctuations in levels of antiphospholipid antibodies and increased coagulation activation markers in normal and heparin-treated antiphospholipid syndrome pregnancies. Lupus. 2002;11(1):11–20. doi: 10.1191/0961203302lu132oa. [DOI] [PubMed] [Google Scholar]
- 14.Kwak JY, Barini R, Gilman-Sachs A, Beaman KD, Beer AE. Down-regulation of maternal antiphospholipid antibodies during early pregnancy and pregnancy outcome. American journal of obstetrics and gynecology. 1994;171(1):239–46. doi: 10.1016/0002-9378(94)90476-6. [DOI] [PubMed] [Google Scholar]
- 15.Holers VM, Girardi G, Mo L, Guthridge JM, Molina H, Pierangeli SS, et al. Complement C3 activation is required for antiphospholipid antibody-induced fetal loss. The Journal of experimental medicine. 2002;195(2):211–20. doi: 10.1084/jem.200116116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Out HJ, de Groot PG, Hasselaar P, dan Vliet M, Derksen RH. Fluctuations of anticardiolipin antibody levels in patients with systemic lupus erythematosus: a prospective study. Annals of the rheumatic diseases. 1989;48(12):1023–8. doi: 10.1136/ard.48.12.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]


