Abstract
Genome wide association study (GWAS) data have linked the G6PC2 gene to variations in fasting blood glucose (FBG). G6PC2 encodes an islet-specific glucose-6-phosphatase catalytic subunit that forms a substrate cycle with the beta cell glucose sensor glucokinase. This cycle modulates the glucose sensitivity of insulin secretion and hence FBG. GWAS data have not linked G6PC2 to variations in body weight but we previously reported that female C57BL/6J G6pc2 knockout (KO) mice were lighter than wild-type littermates on both a chow and high fat diet. The purpose of this study was to compare the effects of G6pc2 deletion on FBG and body weight in both chow fed and high fat fed mice on two other genetic backgrounds. FBG was reduced in G6pc2 KO mice largely independently of gender, genetic background or diet. In contrast, the effect of G6pc2 deletion on body weight was markedly influenced by these variables. Deletion of G6pc2 conferred a marked protection against diet-induced obesity in male mixed genetic background mice whereas in 129SvEv mice deletion of G6pc2 had no effect on body weight. G6pc2 deletion also reduced plasma cholesterol levels in a manner dependent on gender, genetic background and diet. An association between G6PC2 and plasma cholesterol was also observed in humans through electronic health record-derived phenotype analyses. These observations suggest that the action of G6PC2 on FBG is largely independent of the influences of environment, modifier genes or epigenetic events whereas the action of G6PC2 on body weight and cholesterol are influenced by unknown variables.
Introduction
The glucose-6-phosphatase (G6Pase) enzyme system is located in the endoplasmic reticulum and catalyzes the hydrolysis of glucose-6-phosphate (G6P) to glucose and inorganic phosphate (Hutton and O’Brien 2009; O’Brien 2013). In addition to a catalytic subunit, which can be one of three isoforms, G6PC1, G6PC2 or G6PC3, the G6Pase system is composed of a glucose transporter and a G6P/Pi transporter, encoded by the SLC37A4 gene (Hutton and O’Brien 2009; O’Brien 2013). G6PC2 is thought to be expressed exclusively in pancreatic islet beta cells (Hutton and O’Brien 2009; O’Brien 2013). Experiments comparing wild type (WT) and G6pc2 knockout (KO) mouse islets suggest that G6pc2 opposes the action of the beta cell glucose sensor, glucokinase, which catalyzes the formation of G6P from glucose (Iynedjian 2009; Matschinsky 2005). In isolated G6pc2 KO islets G6Pase activity (Pound, et al. 2013) and glucose cycling (Wall, et al. 2015) are abolished. This results in leftward shift in the dose response curve for glucose-stimulated insulin secretion (GSIS) (Pound et al. 2013). Under fasting conditions, where insulin levels are the same in WT and G6pc2 KO mice, this shift results in reduced fasting blood glucose (FBG) in KO mice (Boortz, et al. 2016a; Pound et al. 2013; Wang, et al. 2007). In contrast, under stimulatory conditions using a sub-maximal concentration of glucose this shift results in increased GSIS from G6pc2 KO relative to WT mouse islets (Pound et al. 2013). As predicted from a parallel shift in the dose response curve for GSIS, under stimulatory conditions using a high concentration of glucose, this shift results no difference in GSIS between G6pc2 KO and WT as assessed in either isolated islets in situ (Pound et al. 2013) or mice in vivo using hyperglycemic clamps (Wang et al. 2007).
Consistent with these mouse studies, genome wide association studies (GWAS) have linked the rs560887 single nucleotide polymorphism (SNP) in the G6PC2 gene to variations in FBG (Bouatia-Naji, et al. 2008; Chen, et al. 2008). Molecular studies have shown that the rs560887-G allele represents a gain of function that is associated with increased G6PC2 RNA splicing which is predicted to lead to increased full length G6PC2 protein expression and elevated glucose cycling (Baerenwald, et al. 2013). Since GWAS data show that the rs560887-G allele is associated with elevated FBG (Bouatia-Naji et al. 2008; Chen et al. 2008), the combination of these splicing (Baerenwald et al. 2013) and G6pc2 KO mouse (Pound et al. 2013; Wang et al. 2007) studies suggest that rs560887 is a potentially causative variant. The association between G6PC2 and FBG has been confirmed in multiple GWAS and in different populations (Bouatia-Naji, et al. 2009; Dupuis, et al. 2010; Hu, et al. 2009; Hu, et al. 2010; Prokopenko, et al. 2008; Reiling, et al. 2009; Tam, et al. 2010; Wang, et al. 2013).
Numerous GWAS have also examined the genes that are associated with variations in body weight, fat mass and fat distribution and have shown that greater than 160 loci are linked to these parameters (Lu, et al. 2016). While G6PC2 was not one of the loci identified (Lu et al. 2016) we previously observed that female C57BL/6J G6pc2 KO mice were lighter than wild-type (WT) littermates on both a chow fed and high fat fed diet (Pound et al. 2013). This observation prompted us to examine whether genetic background influences the effect of G6pc2 deletion on body weight and the response to diet-induced obesity (DIO). The results show that the effect of G6pc2 deletion on FBG is largely independent of gender, genetic background and diet whereas the effect of G6pc2 deletion on body weight is highly dependent on these variables. We also found that deletion of G6pc2 reduced plasma cholesterol levels in a manner dependent on gender, genetic background and diet. These observations suggest that the action of G6PC2 on FBG is largely unaffected by the influences of environment, gender, modifier genes or epigenetic events whereas the action of G6PC2 on body weight and cholesterol are influenced by unknown variables.
Materials and Methods
Animal Care
The Vanderbilt University Medical Center Animal Care and Use Committee approved all protocols used. Mice were maintained on either a standard rodent chow diet (calorie contributions: 28% protein, 12% fat, 60% carbohydrate (14% disaccharides); LabDiet 5001; PMI Nutrition International) or a high-fat diet (calorie contributions: 15% protein, 59% fat, 26% carbohydrate (42% disaccharides); Mouse Diet F3282; BioServ). High-fat feeding studies were initiated at 8 weeks of age and mice were maintained on the diet for 8–14 weeks as indicated. Food and water were provided ad libitum.
Generation of G6pc2 Knockout (KO) Mice
Previous studies have described the generation of G6pc2 KO mice on a mixed 129/SvEv × C57BL/6J (Wang et al. 2007), C57BL/6J (Pound et al. 2013) and 129SvEv (Boortz et al. 2016a) genetic background. The targeting vector used to generate the KO allele replaced exons 1–3 of the G6pc2 gene with a LacZ/Neo cassette leaving exons 4 and 5 intact (Wang et al. 2007). Exon 1 contains the translation initiation methionine (Ebert, et al. 1999). As such, the design of the targeting vector completely abolishes G6pc2 expression (Wang et al. 2007). All the mice examined in these studies were littermates generated by interbreeding of heterozygous (HET) mice.
Intraperitoneal Glucose Tolerance Tests
Intraperitoneal glucose tolerance tests (IPGTTs) were performed on ~22 week old male mice as previously described (Pound, et al. 2012).
Phenotypic Analysis of Fasted G6pc2 KO Mice
Mice were fasted for 5 hours and then weighed. After an additional hour of fasting, mice were anesthetized using isoflurane and blood samples were isolated from the retro-orbital venous plexus. Glucose concentrations were measured in whole blood using a glucose monitor (Accu-Check Advantage; Roche, Indianapolis, USA). EDTA (5
 l; 0.5 M) was then added to blood samples prior to isolation of plasma by centrifugation. Insulin samples were assayed using RIA (Morgan and Lazarow 1963) by the Vanderbilt Hormone Assay and Analytical Services Core. Cholesterol was assayed using a cholesterol reagent kit (Raichem, San Diego, CA, USA), while triacylglycerol was assayed using a serum triacylglycerol determination kit (Sigma, St Louis, MO, USA). Body composition was assessed using an mq10 NMR analyzer (Bruker Optics).
l; 0.5 M) was then added to blood samples prior to isolation of plasma by centrifugation. Insulin samples were assayed using RIA (Morgan and Lazarow 1963) by the Vanderbilt Hormone Assay and Analytical Services Core. Cholesterol was assayed using a cholesterol reagent kit (Raichem, San Diego, CA, USA), while triacylglycerol was assayed using a serum triacylglycerol determination kit (Sigma, St Louis, MO, USA). Body composition was assessed using an mq10 NMR analyzer (Bruker Optics).
Analysis of Gene Expression in Mouse Pancreas
Pancreatic gene expression was analyzed as previously described (Boortz et al. 2016a). The following mouse primer pairs were used for the analysis of gene expression:
| G6pc2 Forward | 5′-CCCTGATGGTGGTGGCTCTA-3′ |
| G6pc2 Reverse | 5′-GTCTGTGGGTGGAGCAGGAC-3′ |
| Ins2 Forward | 5′-CACCCAGGCTTTTGTCAAGC-3′ |
| Ins2 Reverse | 5′-CCAGTGCCAAGGTCTGAAGG-3′ |
| Mouse Ppia Forward | 5′-GGCCGATGACGAGCCC-3′ |
| Mouse Ppia Reverse | 5′-TGTCTTTGGAACTTTGTCTGCAA-3′ |
Electronic Health Record (EHR)-Based Phenotyping of Human Research Subjects
EHR-based phenotyping was conducted using data on human subjects in the Vanderbilt University Medical Center (VUMC) BioVU DNA databank. Genotyping data in BioVU is linked to the Synthetic Derivative (SD), a de-identified version of the VUMC EHR repository. Detailed descriptions of program operations, ethical considerations, and continuing oversight and patient engagement have been published (Pulley, et al. 2010; Roden, et al. 2008). For these studies we used a previously genotyped cohort of 29,722 European descendants from VUMC with longitudinal medical care. Genotyping was performed on the Illumina Human Exome BeadChip platform. For this study, we specifically analyzed the intronic G6PC2 SNP rs560887. Lipid measurements utilized routine clinical laboratory testing values present in the EHR.
Statistical Analyses
Other than IPGTTs, data were analyzed using a Student’s t-test: two sample assuming equal variance. The level of significance was as indicated (two-sided Student’s t-test). IPGTT data were analyzed using a two-way ANOVA assuming normal distribution and equal variance. A post hoc analysis was performed using the Bonferroni correction for multiple comparisons. The level of significance was as indicated.
To analyze genetic associations with lipids in BioVU, we used the median value for each individual. The associations between the genotypes and the aggregated laboratory values (as continuous variables) were performed on R with linear model, adjusted for age, sex, and body mass index (BMI). We report beta values, 95% confidence intervals (CI), and p values. P < 0.05 was considered to be significant. All tests assumed a two-tailed distribution.
Results
Analysis of the Effect of G6pc2 Deletion on Body Weight and Composition in Chow and High Fat Fed 129SvEv Mice
We have previously shown that 16 week old chow fed female, but not male, C57BL/6J G6pc2 knockout (KO) mice are slightly lighter than wild type (WT) littermates and have reduced body fat (Pound et al. 2013). These differences were also observed following 12 weeks of high fat feeding in female, but not male, C57BL/6J G6pc2 KO mice (Pound et al. 2013). In this study we repeated these analyses with G6pc2 KO mice on a 129SvEv or mixed genetic background.
In 16 week old chow fed 129SvEv G6pc2 mice no differences in weight or body fat were observed between female WT or KO mice (Table 1). However, male chow fed 129SvEv G6pc2 KO mice were slightly lighter than WT littermates and female chow fed 129SvEv G6pc2 KO mice had slightly increased muscle mass (Table 1).
Table 1. NMR Analysis of Chow Fed 129SvEv G6pc2 KO Mouse Body Composition.
Body composition of 6 hour fasted, 16 week old animals was assessed using a mq10 NMR analyzer. Results are means ± S.E.M. obtained from the number of animals indicated in parentheses. WT=wild type; KO=knockout.
| Gender & Genotype | Body Weight (g) | Fat (g) | Muscle (g) | Free Fluid (g) | Fat (%) | Muscle (%) | Free Fluid (%) |
|---|---|---|---|---|---|---|---|
| Female WT | 22.23 ± 0.26 (15) | 2.76 ± 0.26 (11) | 13.94 ± 0.27 (11) | 0.58 ± 0.05 (11) | 13.04 ± 1.15 (11) | 66.32 ± 0.94 (11) | 2.78 ± 0.26 (11) |
| Female KO | 22.11 ± 0.54 (14) | 3.09 ± 0.46 (9) | 14.69 ± 0.25 (9), * | 0.60 ± 0.03 (9) | 13.48 ± 1.62 (9) | 66.05 ± 1.34 (9) | 2.71 ± 0.15 (9) |
| Male WT | 28.47 ± 0.34 (16) | 2.02 ± 0.35 (11) | 19.23 ± 0.37 (11) | 0.82 ± 0.10 (11) | 7.40 ± 1.29 (11) | 69.90 ± 0.91 (11) | 2.96 ± 0.25 (11) |
| Male KO | 26.93 ± 0.54 (12), * | 1.51 ± 0.25 (9) | 18.21 ± 0.38 (9) | 0.75 ± 0.12 (9) | 6.03 ± 1.00 (9) | 72.45 ± 0.96 (9) | 3.01 ± 0.51 (9) |
- Weight: *p < 0.009 male WT vs KO
- Muscle: *p < 0.04 female WT vs KO
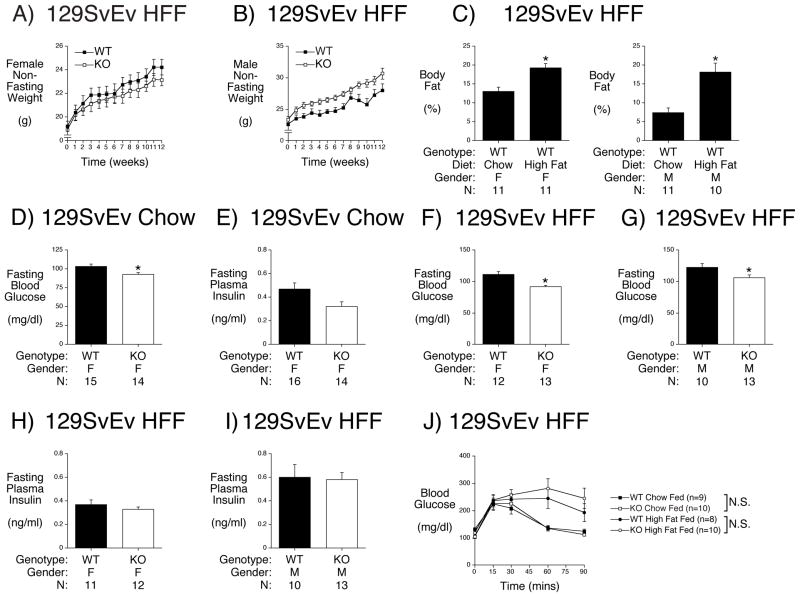
High-fat feeding is a standard nutritional challenge in the field of obesity and diabetes research that induces insulin resistance and is considered to model human disease (Young and Kirkland 2007). High fat feeding of 129SvEv mice was started at 8 weeks of age and continued for 12 weeks. In contrast to C57BL/6J mice that markedly increase their body weight in response to high fat feeding (Pound et al. 2013; Surwit, et al. 1988; Winzell and Ahren 2004) almost no difference in body weight was observed between 16 week old chow fed female and male 129SvEv mice (Table 1) versus 20 week old high fat fed female and male 129SvEv mice (Table 2). Weekly measurements of body weight in non-fasted high fat fed mice during the 12 weeks of high fat feeding showed no evidence for a biphasic change in weight, that would have been suggestive of a toxic effect of prolonged high fat feeding, in either female (Fig. 1A) or male (Fig. 1B) WT and KO mice. These data are consistent with previous studies that have observed that 129SvEv mice are resistant to DIO (Almind and Kahn 2004).
Table 2. NMR Analysis of High Fat Fed 129SvEv G6pc2 KO Mouse Body Composition.
Body composition of 6 hour fasted, 20 week old animals following 12 weeks of high fat feeding was assessed using a mq10 NMR analyzer. Results are means ± S.E.M. obtained from the number of animals indicated in parentheses. WT=wild type; KO=knockout.
| Gender & Genotype | Body Weight (g) | Fat (g) | Muscle (g) | Free Fluid (g) | Fat (%) | Muscle (%) | Free Fluid (%) |
|---|---|---|---|---|---|---|---|
| Female WT | 23.06 ± 0.69 (11) | 4.46 ± 0.34 (11) | 14.80 ± 0.32 (11) | 0.58 ± 0.06 (11) | 19.26 ± 1.23 (11) | 64.46 ± 144 (11) | 2.48 ± 0.23 (11) |
| Female KO | 22.65 ± 0.58 (11) | 4.30 ± 0.32 (11) | 14.37 ± 0.37 (11) | 0.50 ± 0.04 (11) | 18.88 ± 1.11 (11) | 63.55 ± 1.02 (11) | 2.17 ± 0.15 (11), * |
| Male WT | 26.80 ± 0.93 (10) | 5 ± 0.78 (10) | 16.72 ± 0.50 (10) | 0.55 ± 0.07 (10) | 18.18 ± 2.34 (10) | 62.68 ± 1.63 (10) | 2.02 ± 0.19 (10) |
| Male KO | 30.20 ± 0.82 (13), * | 6.98 ± 0.65 (13) | 17.51 ± 0.35 (13) | 0.60 ± 0.03 (13) | 22.67 ± 1.75 (13) | 58.35 ± 1.58 (13) | 2.00 ± 0.09 (13) |
- Weight: *p < 0.0124 WT vs KO
Figure 1. Effect of G6pc2 Deletion on Body Weight, Composition and Metabolic Parameters in High Fat Fed 129SvEv Mice.
Panels A & B: Starting at 8 weeks of age, female (Panel A) and male (Panel B) mice were fed a high-fat diet with non-fasting body weights measured weekly. Results are the mean ± S.E.M. of data from the following number of animals: female WT n=11; male WT n=11.
Panel C: Body composition was assessed in chow fed 129SvEv mice at 16 weeks of age and in high fat fed 129SvEv mice at 20 weeks of age following 12 weeks of high fat feeding. Mice were fasted for 5 hours and then weighed. One hour later body fat was determined by NMR. Results are the mean ± S.E.M. of data with the genotype, gender and number of animals indicated. WT=wild type; F=female; M= male. *p < 4.61E-10 high fat fed vs chow fed females; *p < 3.94E-05 high fat fed vs chow fed males.
Panels D & E: At 17 weeks of age chow fed mice were fasted for 5 hours and then weighed. One hour later mice were anesthetized and blood isolated. Blood glucose (Panel D) and plasma insulin (Panel E) were determined as described in Methods. Results are the mean ± S.E.M. of data with the genotype, gender and number of animals indicated. WT=wild type; KO=knockout; F=female. *p < 0.014 WT versus KO (Panel D).
Panels F – I: Metabolic parameters were assessed in high fat fed 129SvEv mice at 21 weeks of age following 13 weeks of high fat feeding. Mice were fasted for 5 hours and then weighed. One hour later mice were anesthetized and blood isolated. Blood glucose (Panels F & G) and plasma insulin (Panels H & I) were determined as described in Methods. Results are the mean ± S.E.M. of data with the genotype, gender and number of animals indicated. WT=wild type; KO=knockout; F=female; M= male. *p < 0.001 female WT versus KO (Panel F); *p < 0.037 male WT versus KO (Panel G).
Panel J: Glucose tolerance was assessed in chow fed 129SvEv at ~22 weeks of age and in high fat fed 129SvEv mice at 22 weeks of age following 13 weeks of high fat feeding. IPGTTs using 2.0 g/kg glucose were performed on 6 hr fasted, conscious, chow or high fat fed wild type (WT) and G6pc2 knockout (KO) male mice as described in Methods. The results show the mean glucose concentrations ± S.E.M.
Despite the lack of weight gain, high fat fed female and male 129SvEv mice showed a marked increase in body fat (%) relative to chow fed mice (Fig. 1C). This was associated with a reduction in body muscle (%) in both high fat fed female and male 129SvEv mice (Table 2) relative to chow fed female and male mice (Table 1) (p<0.05).
In 20 week old high fat fed 129SvEv G6pc2 mice no differences in body fat were observed between female or male WT versus KO mice (Table 2). However, male high fat fed 129SvEv G6pc2 KO mice were slightly heavier than WT littermates and free fluid was reduced in female high fat fed 129SvEv G6pc2 KO mice (Table 2).
Analysis of the Effect of G6pc2 Deletion on Fasting Blood Glucose (FBG) and Fasting Plasma Insulin (FPI) in High Fat Fed 129SvEv Mice
We have previously shown that male 129SvEv G6pc2 KO mice have reduced FBG but no change in FPI relative to WT littermates (Boortz et al. 2016a). When this analysis was repeated using female 129SvEv chow fed mice this same reduction in FBG was observed in G6pc2 KO mice (Fig. 1D) with no change in FPI (Fig. 1E).
We next analyzed the effect of high fat feeding on FBG and FPI in 129SvEv mice. Despite 13 weeks of high fat feeding a comparison between female (Fig. 1D) and male (Boortz et al. 2016a) chow fed with female (Fig. 1F) and male (Fig. 1G) high fat fed 129SvEv WT mice revealed surprisingly no increase in FBG. Similarly, a comparison between female (Fig. 1E) and male (Boortz et al. 2016a) chow fed with female (Fig. 1H) and male (Fig. 1I) high fat fed 129SvEv WT mice revealed surprisingly no increase in FPI.
After 13 weeks of high fat feeding a reduction in FBG was observed in both female (Fig. 1F) and male (Fig. 1G) G6pc2 KO relative to WT mice with no differences in FPI in either female (Fig. 1H) or male (Fig. 1I) mice relative to WT.
Analysis of the Effect of High Fat Feeding on Glucose Tolerance in 129SvEv WT and G6pc2 KO Mice
We have previously shown that deletion of G6pc2 does not effect glucose tolerance in chow fed C57BL/6J (Pound et al. 2013) and 129SvEv (Boortz et al. 2016a) mice, consistent with human GWAS data showing no association between G6PC2 SNPs and variations in glucose tolerance (Heni, et al. 2010; Ingelsson, et al. 2010; Li, et al. 2009; Rose, et al. 2009). Although high fat feeding did not result in weight gain in male 129SvEv mice (Table 2) relative to chow fed mice (Table 1), intraperitoneal glucose tolerance tests (IPGTTs) revealed a clear impairment in glucose tolerance in both WT (p<0.0002) and G6pc2 KO (p<0.0001) high fat fed 129SvEv mice relative to chow fed mice (Fig. 1J), suggesting the presence of either insulin resistance and/or impaired GSIS in high fat fed 129SvEv mice. However, even in high fat fed mice, deletion of G6pc2 did not affect glucose tolerance (Fig. 1H).
Analysis of the Effect of G6pc2 Deletion on Body Weight, FBG and FPI in High Fat Fed Mixed Genetic Background Mice
A comparison of data derived from studies on C57BL/6J (Pound et al. 2013) and 129SvEv (Fig. 1; Tables 1 & 2) mice suggest that the effect of G6pc2 deletion on body weight varies with gender and genetic background. We therefore repeated these high fat feeding analyses in mice with a mixed C57BL/6J X 129SvEv genetic background. We have previously shown that FBG is reduced in both female and male mixed C57BL/6J X 129SvEv genetic background G6pc2 KO mice relative to WT with no differences in body weight or FPI (Wang et al. 2007). After starting high fat feeding at 8 weeks of age and continuing for 8 weeks we observed no differences in body weight between female mixed genetic background WT and KO mice (Fig. 2A). In contrast, male mixed genetic background G6pc2 KO mice exhibited a striking protection against DIO (Fig. 2B).
Figure 2. Effect of G6pc2 Deletion on Body Weight and Metabolic Parameters in High Fat fed Mixed Background Mice.
Metabolic parameters were assessed in high fat fed mixed genetic background mice at 16 weeks of age following 8 weeks of high fat feeding. Mice were fasted for 5 hours and then weighed (Panels A & B). One hour later mice were anesthetized and blood isolated. Blood glucose (Panels C & D) and plasma insulin (Panels E & F) were determined as described in Methods. Results are the mean ± S.E.M. of data with the genotype, gender and number of animals indicated. WT=wild type; KO=knockout; F=female; M= male.
*p < 8.32E-05 WT versus KO (Panel B); *p < 3.45E-05 WT versus KO (Panel D); *p < 6.84E-05 WT versus KO (Panel F).
No reduction in FBG was observed in high fat fed female KO mice relative to WT mice (Fig. 2C) whereas FBG was markedly reduced in high fat fed male KO mice relative to WT mice (Fig. 2D). Similarly, while no difference in FPI was observed between high fat fed female KO mice relative to WT mice (Fig. 2E), FPI was markedly reduced in high fat fed male KO mice relative to WT mice (Fig. 2F).
Comparison of Pancreatic G6pc2 Expression in 129SvEv and C57BL/6J Mice
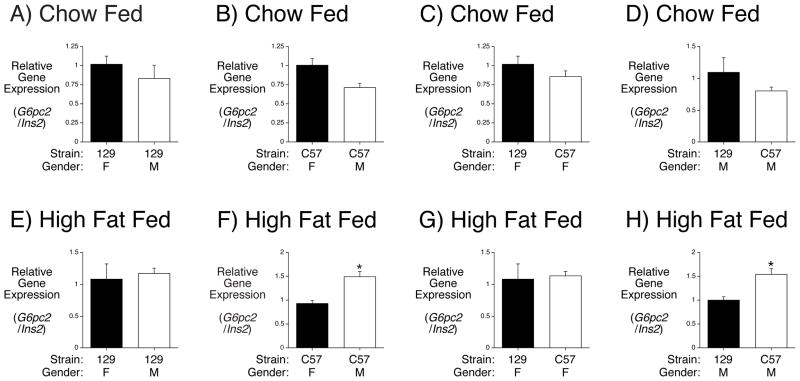
The data derived from studies on C57BL/6J, 129SvEv and mixed genetic background mice reveal that the effect of G6pc2 deletion on body weight varies with gender and genetic background. In addition, FBG is lower in both male chow fed (Boortz et al. 2016a) and high fat fed (Figs. 1F) 129SvEv mice than C57BL/6J mice (Goren, et al. 2004; Mazzaccara, et al. 2008; Pound et al. 2013). While there are likely multiple factors that account for these differences, one potential contributing factor could be variations in G6pc2 gene expression between C57BL/6J and 129SvEv mice. To address this possibility we compared pancreatic G6pc2 and Ins2 gene expression in both mouse strains. There was no difference in the ratio of G6pc2 to Ins2 gene expression between female and male chow fed 129SvEv mice (Fig. 3A) or between female and male chow fed C57BL/6J mice (Fig. 3B). There was also no difference in the ratio of G6pc2 to Ins2 gene expression between chow fed female 129SvEv and C57BL/6J mice (Fig. 3C) or between chow fed male 129SvEv and C57BL/6J mice (Fig. 3D). In contrast, while there was no difference in the ratio of G6pc2 to Ins2 gene expression between female and male high fat fed 129SvEv mice (Fig. 3E) there was a difference between female and male high fat fed C57BL/6J mice (Fig. 3F). Similarly, while there was no difference in the ratio of G6pc2 to Ins2 gene expression between high fat fed female 129SvEv and C57BL/6J mice (Fig. 3G) there was a difference between high fat fed male 129SvEv and C57BL/6J mice (Fig. 3H). These data suggest that G6pc2 expression is induced by high fat feeding relative to Ins2 expression in male C57BL/6J mice, which may contribute to differences versus high fat fed female C57BL/6J mice and male 129SvEv mice.
Figure 3. Comparison of Pancreatic G6pc2 Expression in 129SvEv and C57BL/6J Mice.
Pancreatic RNA was isolated following a 6 hr fast from chow fed (Panels A-D) 129SvEv (129) or C57BL/6J (C57) mice or mice fed a high fat diet for 2 weeks (Panels E-H). G6pc2 and Ins2 expression were quantitated by Real Time PCR. Results show the ratio of G6pc2 to Ins2 expression ± S.E.M. in 3–5 pancreata. *p < 0.05 versus control.
Analysis of the Effect of G6pc2 Deletion on Plasma Cholesterol in 129SvEv, C57BL/6J and Mixed Genetic Background Mice
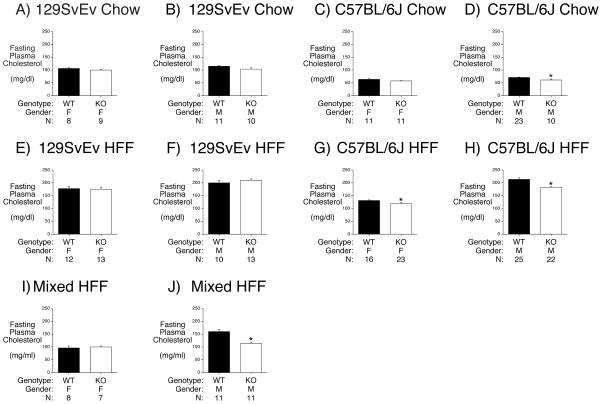
Since multiple plasma lipids change in response to high fat feeding (Eisinger, et al. 2014) we also compared plasma cholesterol levels in G6pc2 KO mice on different genetic backgrounds. We previously observed no change in cholesterol levels in male or female mixed genetic background G6pc2 KO mice relative to WT (Wang et al. 2007). But when we repeated these analyses in chow fed 129SvEv and C57BL/6J mice along with high fat fed 129SvEv, C57BL/6J and mixed genetic background mice we observed that plasma cholesterol levels were reduced in chow fed male C57BL/6J KO mice (Fig. 4D), high fat fed female (Fig. 4G) and male (Fig. 4H) C57BL/6J KO mice, and high fat fed mixed genetic background male KO mice (Fig. 4J).
Figure 4. Effect of G6pc2 Deletion on Plasma Cholesterol in 129SvEv, C57BL6J and Mixed Genetic Background Mice.
Panels A – D: At 17 weeks of age chow fed 129SvEv or C57BL/6J mice were fasted for 5 hours and then weighed. One hour later mice were anesthetized and blood isolated.
Panels E - H: At 21 weeks of age, following 13 weeks of high fat feeding, 129SvEv and C57BL/6J mice were fasted for 5 hours and then weighed. One hour later mice were anesthetized and blood isolated.
Panels I & J: At 16 weeks of age, following 8 weeks of high fat feeding, mixed genetic background mice mice were fasted for 5 hours and then weighed. One hour later mice were anesthetized and blood isolated.
Plasma cholesterol was determined as described in Methods. Results are the mean ± S.E.M. of data with the genotype, gender and number of animals indicated. WT=wild type; KO=knockout; F=female; M= male. *p < 0.0069 WT versus KO (Panel D); *p < 0.02 female WT versus KO (Panel G); *p < 7.01E-06 male WT versus KO (Panel H); *p < 0.001 WT versus KO (Panel J).
Analysis of the Relationship Between G6PC2 SNPs and Metabolic Parameters in Humans using BioVU
Our results in mice demonstrate that the effect of G6pc2 deletion on cholesterol levels varies with gender and genetic background. We next used Vanderbilt’s BioVU DNA databank to determine whether G6PC2 affects these parameters in humans. BioVU individuals with extant genotyping at the intronic G6PC2 SNP rs560887 were screened to identify associations with cholesterol and triglyceride measurements. The rs560887-G allele, which enhances G6PC2 pre-mRNA splicing (Baerenwald et al. 2013), was associated with increased cholesterol (total cholesterol: β = 1.0, p = 0.039; LDL-C: β = 1.1, p = 0.006), but not triglyceride levels (β = 0.90, p = 0.46) or HDL-C (β = −0.07, p = 0.75) (Table 3). We further analyzed the population by sex and found that rs560887-G significantly associated with increased LDL-C in males (p = 0.009) but not in females (p=0.15), although SNP and sex interaction is not significant (p=0.30) (Table 3). Rs560887 did not associate with diabetes status (p=0.37). Thus, as in mice, the impact in humans of modulating G6PC2 expression on plasma lipids is dependent on gender.
Table 3. Association Between G6PC2 SNP rs560887 and Plasma Lipid Measurements Using Electronic Health Record (EHR)-Derived Phenotype Analyses.
Plasma lipid measurements were obtained from routine lipid panels in Vanderbilt University Medical Center’s EHR repository. For each laboratory of each individual, the associations are tested against the median of all lab results for that test. All associations were adjusted for age, sex, and body mass index using linear regression. Cholesterol: total cholesterol; LDL-C: calculated low-density lipoprotein; HDL-C: calculated high-density lipoprotein. The unit for all measurements is mg/dL.
| Lab | Population | N | Beta (G) | 95% CI | P-value | Allele | ||
|---|---|---|---|---|---|---|---|---|
| GG | GA | AA | ||||||
| LDL-C | All | 13087 | 1.15 | 0.33 ~ 1.96 | 0.006 | 102.13 ± 31.43 | 101.58 ± 31.33 | 99.08 ± 31.25 |
| LDL-C | Male | 5863 | 1.60 | 0.4 ~ 2.8 | 0.009 | 97.5 ± 31.23 | 95.77 ± 29.41 | 94.29 ± 32.15 |
| LDL-C | Female | 7224 | 0.82 | −0.29 ~ 1.93 | 0.148 | 105.9 ± 31.09 | 106.33 ± 32.05 | 102.73 ± 30.07 |
| Cholesterol | All | 14349 | 1.00 | 0.05 ~ 1.95 | 0.039 | 183.62 ± 38.55 | 183.25 ± 38.96 | 181.18 ± 40.03 |
| Cholesterol | Male | 6412 | 1.49 | 0.09 ~ 2.87 | 0.037 | 173.36 ± 37.46 | 171. 53 ± 36.79 | 170.63 ± 38.45 |
| Cholesterol | Female | 7937 | 0.68 | −0.59 ~ 1.95 | 0.29 | 191.97 ± 37.39 | 192.81 ± 38.06 | 189.05 ± 39.39 |
| Triglycerides | All | 14213 | 0.90 | −1.48 ~ 3.28 | 0.459 | 149.92 ± 98.13 | 149.41 ± 95.16 | 148.37 ± 99.99 |
| Triglycerides | Male | 6398 | −0.08 | −4.07 ~ 3.9 | 0.967 | 157.72 ± 109.51 | 157.17 ± 105.84 | 157.87 ± 107.95 |
| Triglycerides | Female | 7815 | 1.80 | −1.02 ~ 4.62 | 0.211 | 143.49 ± 87.14 | 143.00 ± 84.82 | 141.09 ± 92.86 |
| HDL-C | All | 13457 | −0.07 | −0.46 ~ 0.33 | 0.746 | 51.42 ± 17.11 | 51.36 ± 17.47 | 51.76 ± 17.56 |
| HDL-C | Male | 6006 | 0.28 | −0.22 ~ 0.78 | 0.269 | 44.00 ± 13.49 | 43.54 ± 12.93 | 43.72 ± 13.33 |
| HDL-C | Female | 7451 | −0.35 | −0.94 ~ 0.24 | 0.245 | 57.43 ± 17.38 | 57.70 ± 18.09 | 57.85 ± 17.93 |
Analysis of the Effect of G6pc2 Deletion on Food Intake
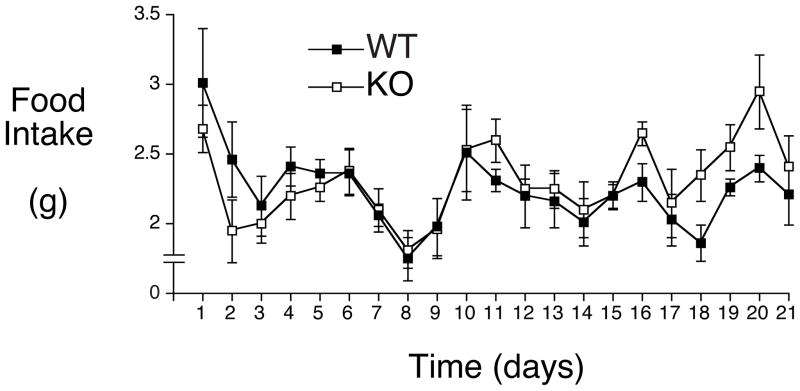
A key question that arises from these studies is how G6PC2, which is thought to be expressed exclusively in pancreatic islet beta cells (Arden, et al. 1999; Martin, et al. 2001), could be affecting body weight. One possible explanation for the link between G6PC2 and body weight is that G6PC2 affects satiety. Thus the leftward shift in the dose response curve for GSIS observed in G6pc2 KO mice (Pound et al. 2013) might result in a faster rise in plasma insulin levels after eating or glucose injection in an IPGTT. Since insulin is a satiety factor (Woods, et al. 2006), this faster rise in insulin could promote a quicker cessation of feeding and ultimately reduced food intake. To address this hypothesis we measured the intake of high fat food in female C57BL/6J WT and G6pc2 KO mice. While female C57BL/6J G6pc2 KO mice are lighter than wild-type (WT) littermates on both a chow fed and high fat fed diet (Pound et al. 2013) no difference in food intake was detected (Fig. 5).
Figure 5. Effect of G6pc2 Deletion on High Fat Food Intake in Female C57BL/6J WT and G6pc2 KO Mice.
Female C57BL/6J WT and G6pc2 KO mice were switched from chow food to high fat food at 8 weeks of age and food intake measured daily for 21 days. Results show the mean food intake ± S.E.M. in 6 WT and 6 G6pc2 KO mice.
Discussion
Our results demonstrate that the effect of G6pc2 deletion in mice on FBG closely parallels human GWAS data in that the effect of G6pc2 deletion on FBG is largely independent of gender and genetic background. We previously showed that, relative to WT mice, FBG is reduced in both female and male chow fed and high fat fed G6pc2 KO on a pure C57BL/6J genetic background (Pound et al. 2013), female and male chow fed G6pc2 KO mice on a mixed genetic background (Wang et al. 2007) and male mice on a 129SvEv genetic background (Boortz et al. 2016a). We show here that FBG is also reduced in 129SvEv chow fed female mice (Fig. 1D) and high fat fed female (Fig. 1F) and male (Fig. 1G) mice. Similarly, FBG is reduced in male high fat fed mixed genetic background G6pc2 KO relative to WT mice (Fig. 2D). FBG was not reduced in female high fat fed mixed genetic background G6pc2 KO mice (Fig. 2C), though the n value in this study was relatively low. These observations are largely consistent with human GWAS data showing an association between G6PC2 and FBG in multiple different populations (Bouatia-Naji et al. 2009; Dupuis et al. 2010; Hu et al. 2009; Hu et al. 2010; Prokopenko et al. 2008; Reiling et al. 2009; Tam et al. 2010; Wang et al. 2013).
With respect to FPI, we previously showed that, relative to WT mice, FPI is unchanged in both female and male chow fed and high fat fed G6pc2 KO mice on a pure C57BL/6J genetic background (Pound et al. 2013), female and male chow fed G6pc2 KO mice on a mixed genetic background (Wang et al. 2007) and male mice on a 129SvEv genetic background (Boortz et al. 2016a). We show here that FBG is also unchanged in 129SvEv chow fed female mice (Fig. 1E) and high fat fed female (Fig. 1H) and male (Fig. 1I) mice. Similarly, FPI is unchanged in female high fat fed mixed genetic background G6pc2 KO mice (Fig. 2E). A reduction in FPI was observed in male high fat fed mixed genetic background G6pc2 KO mice (Fig. 2F) but this is presumably secondary to the marked effect of G6pc2 deletion on body weight in males (Fig. 2B). These observations are consistent with human GWAS data showing no association between G6PC2 and FPI in multiple different populations (Bouatia-Naji et al. 2008; Chen et al. 2008; Mahajan, et al. 2015; Wessel, et al. 2015).
In contrast to these data showing largely consistent effects of G6pc2 deletion on FBG and FPI regardless of gender, diet and genetic background, the effect of G6pc2 deletion on body weight and body composition is highly dependent on these variables. We previously showed that female, but not male, G6pc2 KO mice on a pure C57BL/6J genetic background had reduced body weight and body fat on both a chow and high fat diet relative to WT mice (Pound et al. 2013). In contrast, we show here that deletion of G6pc2 in female mice on the 129SvEv genetic background has no effect on body weight or body fat on either a chow (Table 1) or high fat (Table 2) diet relative to WT mice. Similarly, deletion of G6pc2 in female mice on a mixed 129SvEv X C57BL/6J genetic background has no effect on body weight on either a chow (Wang et al. 2007) or high fat (Fig. 2A) diet relative to WT mice. In males deletion of G6pc2 on the 129SvEv genetic background was associated with reduced body weight on a chow diet (Table 1) but increased body weight on a high fat diet (Table 2). In contrast, deletion of G6pc2 in male mice on a mixed 129SvEv X C57BL/6J genetic background had no effect on body weight on a chow diet (Wang et al. 2007) whereas this conferred a marked protection against DIO on a high fat diet (Fig. 2B). Overall our results suggest that FBG is a much more tightly regulated variable than body weight. Thus while FBG levels are relatively similar in chow fed C57BL/6J (Pound et al. 2013), 129SvEv (Boortz et al. 2016a) and mixed (Wang et al. 2007) genetic background mice, the increase in body weight and body fat in response to high fat feeding is markedly different in C57BL/6J (Pound et al. 2013; Surwit et al. 1988; Winzell and Ahren 2004) and 129SvEv mice (Fig. 1) (Almind and Kahn 2004). Interestingly, the response to DIO varies remarkably even within inbred mice through poorly understood epigenetic mechanisms (Burcelin, et al. 2002; Koza, et al. 2006; Oey, et al. 2015) though whether such mechanisms and/or environmental factors or modifier genes contribute to the variable effects of G6pc2 deletion on body weight and composition is unknown.
In humans a GWAS performed in a cohort of Mexican Americans linked the G6PC2 rs560887-A allele with a small decrease in BMI and adiposity in this population (Li et al. 2009), however, other GWAS have not associated G6PC2 with variations in body mass index, fat mass or fat distribution (Lu et al. 2016). Similarly, a strong association between G6PC2 and cholesterol was not detected using GWAS (Aulchenko, et al. 2009; Kathiresan, et al. 2009), though a weak association can be detected using BioVU (Table 3). These observations suggest that either the effects of G6pc2 on body mass and cholesterol are quantitatively only easily detected in mice on specific genetic backgrounds or that there is a threshold effect such that G6PC2 will markedly affect these parameters in some human populations but only after a substantial change in expression rather than the subtle changes associated with common SNPs (Baerenwald et al. 2013; Bouatia-Naji, et al. 2010). If correct, the association observed by GWAS between common SNPs in G6PC2 and subclinical atherosclerosis (Rasmussen-Torvik, et al. 2011) is likely secondary to the effect of G6PC2 on FBG rather than a direct effect of G6PC2 on cholesterol metabolism.
A key question that remains to be addressed is how G6PC2, which is thought to be expressed exclusively in pancreatic islet beta cells (Arden et al. 1999; Martin et al. 2001), could be affecting body weight. One possibility, as proposed by Li et al. (Li et al. 2009), is that the differences in body weight they observed in humans are secondary effects due to altered insulin signaling efficacy that arise due to an effect of G6PC2 on the pulsatility of insulin secretion. Another possibility is that G6PC2 expression in other tissues that affect body weight has been overlooked. Indeed while RNA blotting showed no evidence for G6PC2 expression in brain (Martin et al. 2001) and transgenic mouse studies gave inconsistent results (Frigeri, et al. 2004; Wang, et al. 2008), one group has reported G6pc2 expression in the mouse hypothalamus (Goh, et al. 2006), a region critical for the control of body weight (Morton, et al. 2006). However, this expression was only detected using very high template concentrations and PCR cycles (Goh et al. 2006). Moreover, while low levels of expression were detected, it is unlikely to be biologically consequential since expression of the G6pc3 isoform was detected at much higher levels (Goh et al. 2006) and G6pc3 is enzymatically more active than G6pc2 (Petrolonis, et al. 2004; Shieh, et al. 2003). One other potential explanation for the link between G6PC2 and body weight is that G6PC2 affects satiety. Thus the leftward shift in the dose response curve for GSIS observed in G6pc2 KO mice (Pound et al. 2013) might result in a faster rise in plasma insulin levels after eating. Since insulin is a satiety factor (Woods et al. 2006), this faster rise in insulin could promote a quicker cessation of feeding and ultimately reduced food intake. However, an analysis of food intake did not detect a difference between female C57BL/6J WT and G6pc2 KO mice (Fig. 5). Future studies will examine the alternate possibility, specifically whether deletion of G6pc2 affects energy expenditure (Ellacott, et al. 2010). While food intake did not differ between C57BL/6J WT and G6pc2 KO mice, an effect of G6PC2 on the timing of GSIS during glucose tolerance tests could explain the counterintuitive observation that the rs560887-G allele, which confers elevated G6PC2 expression (Baerenwald et al. 2013), is associated with elevated FBG but also higher insulin levels at the 30 minute time point in a glucose tolerance test (Li et al. 2009). We hypothesize that insulin levels may peak earlier in individuals with the rs560887-A allele, which confers lower G6PC2 expression (Baerenwald et al. 2013).
Acknowledgments
We thank Susan Hajizadeh for performing insulin assays and Devin A. Baerenwald for assistance with the food intake study. This research was supported by the following grants: R.O’B., DK92589; O. P. M., DK043748 and DK078188; J.C.D., LM010685. The measurement of plasma insulin by the Vanderbilt Hormone Assay & Analytical Services Core was supported by NIH grant P60 DK20593, to the Vanderbilt Diabetes Research Training Center. The measurement of body composition using the Vanderbilt Mouse Metabolic Phenotyping Center mq10 NMR analyzer was supported by NIH grant DK59637. K. E. S. and L. D. P. were supported by the Vanderbilt Molecular Endocrinology Training Program grant 5T32 DK07563.
R.O’B. is the guarantor of this work, had full access to all the data, and takes full responsibility for the integrity of data and the accuracy of data analysis.
Footnotes
Author Contributions
K. A. B. performed most of the mouse phenotyping studies and manuscript writing.
K. E. S. performed some of the mouse studies.
L. D. P. performed some of the mouse studies.
H. M. performed association studies with EHR data and manuscript editing.
L. B. performed association studies with EHR data.
J. K. O. performed some of the mouse studies.
O. P. M. contributed to the design of experiments and manuscript editing.
J. C. D. contributed to design of EHR studies and manuscript editing.
R. M. O. performed some of the mouse studies and manuscript writing.
None of the authors have a conflict of interest relating to this study.
References
- Almind K, Kahn CR. Genetic determinants of energy expenditure and insulin resistance in diet-induced obesity in mice. Diabetes. 2004;53:3274–3285. doi: 10.2337/diabetes.53.12.3274. [DOI] [PubMed] [Google Scholar]
- Arden SD, Zahn T, Steegers S, Webb S, Bergman B, O’Brien RM, Hutton JC. Molecular cloning of a pancreatic islet-specific glucose-6-phosphatase catalytic subunit-related protein. Diabetes. 1999;48:531–542. doi: 10.2337/diabetes.48.3.531. [DOI] [PubMed] [Google Scholar]
- Aulchenko YS, Ripatti S, Lindqvist I, Boomsma D, Heid IM, Pramstaller PP, Penninx BW, Janssens AC, Wilson JF, Spector T, et al. Loci influencing lipid levels and coronary heart disease risk in 16 European population cohorts. Nat Genet. 2009;41:47–55. doi: 10.1038/ng.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baerenwald DA, Bonnefond A, Bouatia-Naji N, Flemming BP, Umunakwe OC, Oeser JK, Pound LD, Conley NL, Cauchi S, Lobbens S, et al. Multiple functional polymorphisms in the G6PC2 gene contribute to the association with higher fasting plasma glucose levels. Diabetologia. 2013;56:1306–1316. doi: 10.1007/s00125-013-2875-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boortz KA, Syring KE, Dai C, Pound LD, Oeser JK, Jacobson DA, Wang JC, McGuinness OP, Powers AC, O’Brien RM. G6PC2 Modulates Fasting Blood Glucose In Male Mice in Response to Stress. Endocrinology. 2016a;157:3002–3008. doi: 10.1210/en.2016-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boortz KA, Syring KE, Lee RA, Dai C, Oeser JK, McGuinness OP, Wang JC, O’Brien RM. G6PC2 Modulates the Effects of Dexamethasone on Fasting Blood Glucose and Glucose Tolerance. Endocrinology. 2016b doi: 10.1210/en.2016-1678. en20161678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouatia-Naji N, Bonnefond A, Baerenwald DA, Marchand M, Bugliani M, Marchetti P, Pattou F, Printz RL, Flemming BP, Umunakwe OC, et al. Genetic and Functional Assessment of the Role of the rs13431652-A and rs573225-A Alleles in the G6PC2 Promoter that Strongly Associate With Elevated Fasting Glucose Levels. Diabetes. 2010;59:2662–2671. doi: 10.2337/db10-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouatia-Naji N, Bonnefond A, Cavalcanti-Proenca C, Sparso T, Holmkvist J, Marchand M, Delplanque J, Lobbens S, Rocheleau G, Durand E, et al. A variant near MTNR1B is associated with increased fasting plasma glucose levels and type 2 diabetes risk. Nat Genet. 2009;41:89–94. doi: 10.1038/ng.277. [DOI] [PubMed] [Google Scholar]
- Bouatia-Naji N, Rocheleau G, Van Lommel L, Lemaire K, Schuit F, Cavalcanti-Proenca C, Marchand M, Hartikainen AL, Sovio U, De Graeve F, et al. A polymorphism within the G6PC2 gene is associated with fasting plasma glucose levels. Science. 2008;320:1085–1088. doi: 10.1126/science.1156849. [DOI] [PubMed] [Google Scholar]
- Burcelin R, Crivelli V, Dacosta A, Roy-Tirelli A, Thorens B. Heterogeneous metabolic adaptation of C57BL/6J mice to high-fat diet. Am J Physiol Endocrinol Metab. 2002;282:E834–842. doi: 10.1152/ajpendo.00332.2001. [DOI] [PubMed] [Google Scholar]
- Chen WM, Erdos MR, Jackson AU, Saxena R, Sanna S, Silver KD, Timpson NJ, Hansen T, Orru M, Grazia Piras M, et al. Variations in the G6PC2/ABCB11 genomic region are associated with fasting glucose levels. J Clin Invest. 2008;118:2620–2628. doi: 10.1172/JCI34566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuis J, Langenberg C, Prokopenko I, Saxena R, Soranzo N, Jackson AU, Wheeler E, Glazer NL, Bouatia-Naji N, Gloyn AL, et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet. 2010;42:105–116. doi: 10.1038/ng.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert DH, Bischof LJ, Streeper RS, Chapman SC, Svitek CA, Goldman JK, Mathews CE, Leiter EH, Hutton JC, O’Brien RM. Structure and promoter activity of an islet-specific glucose-6-phosphatase catalytic subunit-related gene. Diabetes. 1999;48:543–551. doi: 10.2337/diabetes.48.3.543. [DOI] [PubMed] [Google Scholar]
- Eisinger K, Liebisch G, Schmitz G, Aslanidis C, Krautbauer S, Buechler C. Lipidomic analysis of serum from high fat diet induced obese mice. Int J Mol Sci. 2014;15:2991–3002. doi: 10.3390/ijms15022991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellacott KL, Morton GJ, Woods SC, Tso P, Schwartz MW. Assessment of feeding behavior in laboratory mice. Cell Metab. 2010;12:10–17. doi: 10.1016/j.cmet.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigeri C, Martin CC, Svitek CA, Oeser JK, Hutton JC, Gannon M, O’Brien RM. The Proximal Islet-Specific Glucose-6-Phosphatase Catalytic Subunit Related Protein (IGRP) Autoantigen Promoter is Sufficient to Initiate but not Maintain Transgene Expression in Mouse Islets In Vivo. Diabetes. 2004;53:1754–1764. doi: 10.2337/diabetes.53.7.1754. [DOI] [PubMed] [Google Scholar]
- Goh BH, Khan A, Efendic S, Portwood N. Expression of glucose-6-phosphatase system genes in murine cortex and hypothalamus. Horm Metab Res. 2006;38:1–7. doi: 10.1055/s-2006-924964. [DOI] [PubMed] [Google Scholar]
- Goren HJ, Kulkarni RN, Kahn CR. Glucose homeostasis and tissue transcript content of insulin signaling intermediates in four inbred strains of mice: C57BL/6, C57BLKS/6, DBA/2, and 129X1. Endocrinology. 2004;145:3307–3323. doi: 10.1210/en.2003-1400. [DOI] [PubMed] [Google Scholar]
- Heni M, Ketterer C, t Hart LM, Ranta F, van Haeften TW, Eekhoff EM, Dekker JM, Boomsma DI, Nijpels G, Kramer MH, et al. The Impact of Genetic Variation in the G6PC2 Gene on Insulin Secretion Depends on Glycemia. J Clin Endocrinol Metab. 2010;95:E479–484. doi: 10.1210/jc.2010-0860. [DOI] [PubMed] [Google Scholar]
- Hu C, Zhang R, Wang C, Ma X, Wang C, Fang Q, Bao Y, Xiang K, Jia W. A genetic variant of G6PC2 is associated with type 2 diabetes and fasting plasma glucose level in the Chinese population. Diabetologia. 2009;52:451–456. doi: 10.1007/s00125-008-1241-3. [DOI] [PubMed] [Google Scholar]
- Hu C, Zhang R, Wang C, Yu W, Lu J, Ma X, Wang J, Jiang F, Tang S, Bao Y, et al. Effects of GCK, GCKR, G6PC2 and MTNR1B variants on glucose metabolism and insulin secretion. PLoS One. 2010;5:e11761. doi: 10.1371/journal.pone.0011761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton JC, O’Brien RM. The glucose-6-phosphatase catalytic subunit gene family. J Biol Chem. 2009;284:29241–29245. doi: 10.1074/jbc.R109.025544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingelsson E, Langenberg C, Hivert MF, Prokopenko I, Lyssenko V, Dupuis J, Magi R, Sharp S, Jackson AU, Assimes TL, et al. Detailed physiologic characterization reveals diverse mechanisms for novel genetic Loci regulating glucose and insulin metabolism in humans. Diabetes. 2010;59:1266–1275. doi: 10.2337/db09-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iynedjian PB. Molecular physiology of mammalian glucokinase. Cell Mol Life Sci. 2009;66:27–42. doi: 10.1007/s00018-008-8322-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathiresan S, Willer CJ, Peloso GM, Demissie S, Musunuru K, Schadt EE, Kaplan L, Bennett D, Li Y, Tanaka T, et al. Common variants at 30 loci contribute to polygenic dyslipidemia. Nat Genet. 2009;41:56–65. doi: 10.1038/ng.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koza RA, Nikonova L, Hogan J, Rim JS, Mendoza T, Faulk C, Skaf J, Kozak LP. Changes in gene expression foreshadow diet-induced obesity in genetically identical mice. PLoS Genet. 2006;2:e81. doi: 10.1371/journal.pgen.0020081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Shu YH, Xiang AH, Trigo E, Kuusisto J, Hartiala J, Swift AJ, Kawakubo M, Stringham HM, Bonnycastle LL, et al. Additive Effects of Genetic Variation in Gck and G6pc2 on Insulin Secretion and Fasting Glucose. Diabetes. 2009;58:2946–2953. doi: 10.2337/db09-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Day FR, Gustafsson S, Buchkovich ML, Na J, Bataille V, Cousminer DL, Dastani Z, Drong AW, Esko T, et al. New loci for body fat percentage reveal link between adiposity and cardiometabolic disease risk. Nat Commun. 2016;7:10495. doi: 10.1038/ncomms10495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan A, Sim X, Ng HJ, Manning A, Rivas MA, Highland HM, Locke AE, Grarup N, Im HK, Cingolani P, et al. Identification and Functional Characterization of G6PC2 Coding Variants Influencing Glycemic Traits Define an Effector Transcript at the G6PC2-ABCB11 Locus. PLoS Genet. 2015;11:e1004876. doi: 10.1371/journal.pgen.1004876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CC, Bischof LJ, Bergman B, Hornbuckle LA, Hilliker C, Frigeri C, Wahl D, Svitek CA, Wong R, Goldman JK, et al. Cloning and Characterization of the Human and Rat Islet-Specific Glucose-6-Phosphatase Catalytic Subunit-Related Protein (IGRP) Genes. J Biol Chem. 2001;276:25197–25207. doi: 10.1074/jbc.M101549200. [DOI] [PubMed] [Google Scholar]
- Matschinsky FM. Glucokinase, glucose homeostasis, and diabetes mellitus. Curr Diab Rep. 2005;5:171–176. doi: 10.1007/s11892-005-0005-4. [DOI] [PubMed] [Google Scholar]
- Mazzaccara C, Labruna G, Cito G, Scarfo M, De Felice M, Pastore L, Sacchetti L. Age-Related Reference Intervals of the Main Biochemical and Hematological Parameters in C57BL/6J, 129SV/EV and C3H/HeJ Mouse Strains. PLoS One. 2008;3:e3772. doi: 10.1371/journal.pone.0003772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CR, Lazarow AL. Immunoassay of insulin: two antibody system: plasma insulin of normal, subdiabetic, and diabetic rats. Am J Med Sci. 1963;257:415–419. [Google Scholar]
- Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- O’Brien RM. Moving on from GWAS: functional studies on the G6PC2 gene implicated in the regulation of fasting blood glucose. Curr Diab Rep. 2013;13:768–777. doi: 10.1007/s11892-013-0422-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oey H, Isbel L, Hickey P, Ebaid B, Whitelaw E. Genetic and epigenetic variation among inbred mouse littermates: identification of inter-individual differentially methylated regions. Epigenetics Chromatin. 2015;8:54. doi: 10.1186/s13072-015-0047-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrolonis AJ, Yang Q, Tummino PJ, Fish SM, Prack AE, Jain S, Parsons TF, Li P, Dales NA, Ge L, et al. Enzymatic characterization of the pancreatic islet-specific glucose-6-phosphatase-related protein (IGRP) J Biol Chem. 2004;279:13976–13983. doi: 10.1074/jbc.M307756200. [DOI] [PubMed] [Google Scholar]
- Pound LD, Oeser JK, O’Brien TP, Wang Y, Faulman CJ, Dadi PK, Jacobson DA, Hutton JC, McGuinness OP, Shiota M, et al. G6PC2: A Negative Regulator of Basal Glucose-Stimulated Insulin Secretion. Diabetes. 2013;62:1547–1556. doi: 10.2337/db12-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pound LD, Sarkar SA, Ustione A, Dadi PK, Shadoan MK, Lee CE, Walters JA, Shiota M, McGuinness OP, Jacobson DA, et al. The physiological effects of deleting the mouse slc30a8 gene encoding zinc transporter-8 are influenced by gender and genetic background. PLoS One. 2012;7:e40972. doi: 10.1371/journal.pone.0040972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokopenko I, Langenberg C, Florez JC, Saxena R, Soranzo N, Thorleifsson G, Loos RJ, Manning AK, Jackson AU, Aulchenko Y, et al. Variants in MTNR1B influence fasting glucose levels. Nat Genet. 2008;41:77–81. doi: 10.1038/ng.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulley J, Clayton E, Bernard GR, Roden DM, Masys DR. Principles of human subjects protections applied in an opt-out, de-identified biobank. Clin Transl Sci. 2010;3:42–48. doi: 10.1111/j.1752-8062.2010.00175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen-Torvik LJ, Li M, Kao WH, Couper D, Boerwinkle E, Bielinski SJ, Folsom AR, Pankow JS. Association of a fasting glucose genetic risk score with subclinical atherosclerosis: The Atherosclerosis Risk in Communities (ARIC) study. Diabetes. 2011;60:331–335. doi: 10.2337/db10-0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiling E, van ‘t Riet E, Groenewoud MJ, Welschen LM, van Hove EC, Nijpels G, Maassen JA, Dekker JM, t Hart LM. Combined effects of single-nucleotide polymorphisms in GCK, GCKR, G6PC2 and MTNR1B on fasting plasma glucose and type 2 diabetes risk. Diabetologia. 2009;52:1866–1870. doi: 10.1007/s00125-009-1413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roden DM, Pulley JM, Basford MA, Bernard GR, Clayton EW, Balser JR, Masys DR. Development of a large-scale de-identified DNA biobank to enable personalized medicine. Clin Pharmacol Ther. 2008;84:362–369. doi: 10.1038/clpt.2008.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose CS, Grarup N, Krarup NT, Poulsen P, Wegner L, Nielsen T, Banasik K, Faerch K, Andersen G, Albrechtsen A, et al. A variant in the G6PC2/ABCB11 locus is associated with increased fasting plasma glucose, increased basal hepatic glucose production and increased insulin release after oral and intravenous glucose loads. Diabetologia. 2009;52:2122–2129. doi: 10.1007/s00125-009-1463-z. [DOI] [PubMed] [Google Scholar]
- Sadikot RT, Jansen ED, Blackwell TR, Zoia O, Yull F, Christman JW, Blackwell TS. High-dose dexamethasone accentuates nuclear factor-kappa b activation in endotoxin-treated mice. Am J Respir Crit Care Med. 2001;164:873–878. doi: 10.1164/ajrccm.164.5.2008059. [DOI] [PubMed] [Google Scholar]
- Shieh JJ, Pan CJ, Mansfield BC, Chou JY. A glucose-6-phosphate hydrolase, widely expressed outside the liver, can explain age-dependent resolution of hypoglycemia in glycogen storage disease type Ia. J Biol Chem. 2003;278:47098–47103. doi: 10.1074/jbc.M309472200. [DOI] [PubMed] [Google Scholar]
- Surwit RS, Kuhn CM, Cochrane C, McCubbin JA, Feinglos MN. Diet-induced type II diabetes in C57BL/6J mice. Diabetes. 1988;37:1163–1167. doi: 10.2337/diab.37.9.1163. [DOI] [PubMed] [Google Scholar]
- Tam CH, Ho JS, Wang Y, Lee HM, Lam VK, Germer S, Martin M, So WY, Ma RC, Chan JC, et al. Common polymorphisms in MTNR1B, G6PC2 and GCK are associated with increased fasting plasma glucose and impaired beta-cell function in Chinese subjects. PLoS One. 2010;5:e11428. doi: 10.1371/journal.pone.0011428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall ML, Pound LD, Trenary I, O’Brien RM, Young JD. Novel Stable Isotope Analyses Demonstrate Significant Rates of Glucose Cycling In Mouse Pancreatic Islets. Diabetes. 2015;64:2129–2137. doi: 10.2337/db14-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Liu L, Zhao J, Cui G, Chen C, Ding H, Wang DW. Large Scale Meta-Analyses of Fasting Plasma Glucose Raising Variants in GCK, GCKR, MTNR1B and G6PC2 and Their Impacts on Type 2 Diabetes Mellitus Risk. PLoS One. 2013;8:e67665. doi: 10.1371/journal.pone.0067665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Flemming BP, Martin CC, Allen SR, Walters J, Oeser JK, Hutton JC, O’Brien RM. Long-range enhancers are required to maintain expression of the autoantigen islet-specific glucose-6-phosphatase catalytic subunit-related protein in adult mouse islets in vivo. Diabetes. 2008;57:133–141. doi: 10.2337/db07-0092. [DOI] [PubMed] [Google Scholar]
- Wang Y, Martin CC, Oeser JK, Sarkar S, McGuinness OP, Hutton JC, O’Brien RM. Deletion of the Gene Encoding the Islet-Specific Glucose-6-Phosphatase Catalytic Subunit-Related Protein Autoantigen Results in a Mild Metabolic Phenotype. Diabetologia. 2007;50:774–778. doi: 10.1007/s00125-006-0564-1. [DOI] [PubMed] [Google Scholar]
- Wessel J, Chu AY, Willems SM, Wang S, Yaghootkar H, Brody JA, Dauriz M, Hivert MF, Raghavan S, Lipovich L, et al. Low-frequency and rare exome chip variants associate with fasting glucose and type 2 diabetes susceptibility. Nat Commun. 2015;6:5897. doi: 10.1038/ncomms6897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winzell MS, Ahren B. The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes. 2004;53(Suppl 3):S215–219. doi: 10.2337/diabetes.53.suppl_3.s215. [DOI] [PubMed] [Google Scholar]
- Woods SC, Lutz TA, Geary N, Langhans W. Pancreatic signals controlling food intake; insulin, glucagon and amylin. Philos Trans R Soc Lond B Biol Sci. 2006;361:1219–1235. doi: 10.1098/rstb.2006.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young GS, Kirkland JB. Rat models of caloric intake and activity: relationships to animal physiology and human health. Appl Physiol Nutr Metab. 2007;32:161–176. doi: 10.1139/h06-082. [DOI] [PubMed] [Google Scholar]