Long-term effects of dexamethasone therapy on host survival, cellular and humoral immunity, viral spread, and corneal pathology following HSV-1 infection.
Keywords: immunosuppression, inflammation, innervation, nerve regeneration
Abstract
Herpes simplex virus type 1 (HSV-1) is a leading cause of neurotrophic keratitis (NTK). NTK is characterized by decreased corneal sensation from damage to the corneal sensory fibers. We have reported on the regression of corneal nerves and their function during acute HSV-1 infection. That nerve loss is followed by an aberrant process of nerve regeneration during the latent phase of infection that lacks functional recovery. We recently showed the elicited immune response in the infected cornea, and not viral replication itself, is part of the mechanism responsible for the nerve degeneration process after infection. Specifically, we showed infected corneas topically treated with dexamethasone (DEX) significantly retained both structure and sensitivity of the corneal nerve network in comparison to mice treated with control eye drops, consistent with decreased levels of proinflammatory cytokines and reduced influx of macrophages and CD8+ T cells into the cornea. This study was undertaken to analyze the long-term effect of such a localized, immunosuppressive paradigm (DEX drops on the cornea surface during the first 8 d of HSV-1 infection) on the immune system and on corneal pathology. We found the profound immunosuppressive effect of DEX on lymphoid tissue was sustained in surviving mice for up to 30 d postinfection (p.i.). DEX treatment had prolonged effects, preserving corneal innervation and its function and blunting neovascularization, as analyzed at 30 d p.i. Our data support previously reported observations of an association between the persistent presence of inflammatory components in the latently infected cornea and structural and functional nerve defects in NTK.
Introduction
The cornea, which receives the densest innervation of the body, can be severely affected by the development of peripheral neuropathies [1]. Corneal fibers, mainly sensory in origin and derived from the trigeminal nerve, respond to thermal, mechanical, and chemical stimuli by releasing factors that are crucial to the maintenance and homeostasis of the ocular surface [1, 2]. Impairment of the trigeminal corneal innervation and its crucial functions causes NTK, a degenerative disease associated with corneal epithelial breakdown, impairment of healing, and development of ulceration, melting, and perforation [3]. The hallmark of NTK is decreased or absent corneal sensation, common to all stages of its diagnosis [3, 4]. Herpetic viral infections of the cornea, such as HSV-1, are thought to be a major cause for the development of NTK [5–7]. Shortly after replicating at the initial site of infection, HSV-1 uses retrograde axonal transport to reach the TG, where it establishes latency within sensory neurons [8]. Repeated cycles of reactivation can lead to HSK, an extensively studied immunopathologic process [9–12]. HSK is characterized by scarring, opacities, and neovascularization of the cornea [10, 13, 14] and is associated with impairment of corneal nerve sensation [15–17]. We previously reported that HSV-1 infection of the mouse cornea caused nerve regression during the acute phase of infection, followed by an abnormal process of regeneration characterized by stromal hyperinnervation and extensive loss of fine, subbasal nerve bundles and corneal sensitivity [18]. Another group suggested that CD4+ T cells promoted the long-term persistence of corneal nerve defects at time points p.i. consistent with latent HSV-1 infection [17]. A follow-up study reported such structural and functional defects involved abnormal corneal reinnervation by sympathetic fibers derived from the superior cervical ganglia [19].
Using a model of topical delivery of the anti-inflammatory drug DEX [20, 21] during the first 8 d p.i., we recently showed the mechanism of nerve regression is dependent on the elicited immune response to infection, rather than on viral replication in the cornea tissue [22]. The present study was undertaken to examine the long-term consequences of DEX treatment applied locally during the first 8 d of HSV-1 infection on host survival, viral replication, corneal pathology (innervation and vascularization), and the immune system. As anticipated, we found topical delivery of DEX on infected corneas significantly increased mouse mortality between 9–14 d p.i. The decreased survival in infected mice treated with DEX correlated with a reduction in serum neutralization titers and increased viral content in TG and BS tissues by 9 d p.i. compared with VEH-treated mice. However, in surviving, infected mice, DEX-elicited immunosuppression was maintained out to at least 30 d p.i., with a reduction in myeloid and T cells in the lymphoid tissues (MLN and spleen), cornea, TG, and BS, in comparison to VEH-treated animals. However, there was no significant difference in the establishment of latency between DEX- and VEH-treated, surviving mice. By comparison, short-term, local DEX treatment preserved corneal nerve function and avascularity at 30 d p.i. Our study is the first, to our knowledge, to show local DEX treatment has a sustained effect on the immune system but does not significantly influence the establishment of viral latency. The data suggest humoral immunity, through the production of neutralizing Abs, is critical for host survival during T cell suppression.
MATERIALS AND METHODS
Animals
All animal procedures were approved by the University of Oklahoma Health Sciences Center and the Dean McGee Eye Institute Institutional Animal Care and Use committees and were performed in adherence to the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. C57BL/6 mice were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). Mice were 6–8 wk old at the time experiments were performed. Before tissue harvesting, mice were deeply anesthetized with ketamine/xylazine and euthanized by cardiac perfusion with PBS.
Virus and in vivo infection
Anesthetized mice were infected by scarification of the corneal surface, followed by the application of 3.0 μl of PBS containing the HSV-1 strain McKrae (103 PFUs/eye). Controls were performed as previously described [18].
Dexamethasone treatment
Starting at 2 h p.i. or at 2 d p.i., nonanesthetized mice were held by the scruff of the neck, and topically delivered 20 µl of 0.1% DEX ophthalmic solution (001-000050-00; Bausch + Lomb, Bridgewater, NJ, USA) onto their corneas. Control treatments (VEH) were given by applying lubricant eye drops (Allergan, Dublin, Ireland) onto each cornea [22]. The treatments were applied 4 times/d between 7:00 AM and 7:00 PM, for up to 8 d p.i.
Corneal sensitivity
A Cochet and Bonnet esthesiometer (8630-1490-29; Luneau SAS, Prunay-le-Gillon, France) was used to test for corneal sensitivity. Briefly, nonanesthetized mice were presented a monofilament at different lengths (6.0–0.5 cm) to elicit a blink response [18].
Flow cytometry
Corneas were harvested and digested in 2.0 mg/ml type 1 collagenase in normal media at 37°C, with mechanical dissociation (a pair of corneas/sample) [22]. Spleen, MLN, TG, and BS tissues were processed as previously described [23]. The resulting cell suspension from each tissue was filtered and labeled for flow cytometric analysis of myeloid and T cell populations Samples were analyzed using a MacsQuant flow cytometer and MacsQuantify software (Miltenyi Biotec, Bergisch Gladbach, Germany), as previously described [22]. Briefly, the phenotype of myeloid populations was based on a gating strategy that selected cells in the scatter range that were CD45+ and, subsequently, F4/80+GR1− (macrophages), F4/80+GR1+ (inflammatory monocytes), and F4/80−GR1+ neutrophils (PMNs). After using scatter properties to select lymphocytes, positive selection for CD45+ cells, and subsequent positive selection for CD3+ cells, all T cells were then analyzed for CD4 (CD4+T) and CD8 (CD8+T) expression. The Abs included were anti-mouse CD45: eFluor450 (clone 30-F11), anti-mouse Ly-6G (Gr1): PE (clone RB6-8C5), anti-mouse CD3e: FITC (145-2C11), anti-mouse CD4: APC (clone GK1.5), anti-mouse CD8a: PE (clone 53-6.7) (all from eBioscience, San Diego, CA, USA), and anti-mouse F4/80: APC (clone Cl:A3-1) (Bio-Rad Laboratories, Hercules, CA, USA).
Immunochemistry and imaging
For immunostaining of cornea flat mounts, eyes were removed, and an incision was made posterior to the limbus to dissect the corneas including a margin of sclera. The corneas were fixed, permeated, and incubated overnight sequentially with blocking, primary Ab and secondary Ab solutions, as previously described [18]. The primary Abs included were anti–β-III tubulin (1:1000; 18207; Abcam, Cambridge, MA, USA) (β-III tubulin), anti-CD31 (1:100; MAB1398Z; EMD Millipore, Billerica, MA), and anti-LYVE-1 (1:100; 14-0443-82; eBioscience). Incisions were made in each cornea to obtain a flower-shaped whole mount (4 quadrants) before mounting in 50% glycerol. Imaging of cornea samples was performed on an Olympus FluoView confocal laser scanning microscope (FV1200, FV10-ASW 4.2; Olympus, Tokyo, Japan). Microscope and software settings were identical for all samples within the experiments. The size of the Z stack generated for cornea flat mounts was 12 slices thick (4.77 µm step size) at ×10 magnification. To quantify changes in corneal innervation and vasculature, the MetaMorph offline software (version 7.7.0.0; Molecular Devices, Sunnyvale, CA, USA) was used to calculate the percentage threshold area positive for β-III tubulin, CD31, and LYVE-1 staining on acquired confocal images. This threshold area is defined as the percentage of β-III tubulin+, CD31+, or LYVE1+ pixels, divided by the total number of pixels in the entire image. For each cornea, a representative image from each quadrant was used for analysis (4 images/sample where the visual field included the peripheral limbus toward the center of the cornea proper).
Viral plaque assay
Corneas, TGs, and BSs were homogenized with a tissue miser, clarified by centrifugation, and then, serially diluted onto a confluent lawn of Vero cells in media containing 10% FBS and antibiotic/antimycotic reagents (15240062; Antibiotic-Antimycotic 100X, Life Technologies; Thermo Fisher Scientific, Waltham, MA, USA; 1× final concentration: 100 U/ml penicillin, 100 µg/ml streptomycin, and 0.25 µg/ml amphotericin B). Plaques were visualized and enumerated with the aid of an inverted microscope 24–48 h later and quantified as mean log PFUs/tissue, as previously described [23].
Real-time quantitative PCR
Total RNA from TG was extracted with TRIzol reagent (Thermo Fisher Scientific) and converted to cDNA (iScript reverse transcription supermix; Bio-Rad Laboratories). The transcript levels of HSV-1 LAT, gB, TK, ICP-27, and Eef1e1 (housekeeping gene; IDT Integrated DNA Technologies, Coralville, IA, USA) were measured using iQSYBR Green PCR supermix and a CFX96 real-time PCR detection system (Bio-Rad Laboratories). Primer sequences to LAT, gB, ICP27, and Eef1e1 were as follows: LAT forward (5′-CCC TCG TCT CCT GTG ATT CT-3); LAT reverse (5′-GGG AGA CAG AAA CAG GAA CAT-3′); gB forward (5′-TCT GCA CCA TGA CCA AGT G-3′); gB reverse (5′-TGG TGA AGG TGG TGG ATA TG-3′); TK forward (5′-ATA CCG ACG ATC TGC GAC CT-3′); TK reverse (5′-TTA TTG CCG TCA TAG CGC GG -3′); ICP27 forward (5′-GCA TCC TTC GTG TTT GTC AT-3′); ICP27 reverse (5′-ACC AAG GGT CGC GTA GTC-3′); Eef1e1 forward (5′- GCA GAA GAA AAG GCA ATG GT-3′); and Eef1e1 reverse (5′-AGG CCG TAGT ACA GCA GGA T-3′) [18, 23]. Relative quantities of gene expression were calculated by the comparative cycle threshold-value method, and the results were expressed as the fold change in expression for each transcript [18, 22].
Virus neutralization assay
Blood was collected from the facial vein of infected mice treated with VEH or DEX at 9, 12, and 30 d p.i., using a 4-mm, sterile Goldenrod animal lancet (MEDIpoint, Inc., Mineola, NY, USA). Blood samples were collected in Eppendorf microcentrifuge tubes, stored at 4°C for 12 h, and centrifuged for 10 min at 4°C. Serum in each sample was separated, aliquoted, and stored at −20°C until used for the virus neutralization assay. Briefly, serum samples were diluted 1:25 with complete media in microtiter plates and serially diluted 2-fold. Next, guinea pig complement (Rockland Immunochemicals, Limerick, PA, USA) diluted 1:20 in complete medium containing 2.85 × 105 PFUs/ml HSV-1 McKrae was added to each well. The plate was gently mixed and incubated for 2 h (37°C, 5% CO2). Confluent Vero cells were subsequently exposed to each virus–serum dilution for 1 h, decanted, and incubated for 24 h in complete medium. Neutralization titers are reported as the reciprocal serum dilution at which a 50% reduction in cytopathic effect was observed [24].
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5.0 software (GraphPad Software, La Jolla, CA, USA). The data are expressed as the means ± sem for each group. The unpaired t test comparison was performed to assess the significant differences between 2 groups. For multiple comparisons, 1-way ANOVA was performed followed by the Bonferroni post hoc test. For comparison of survival between 2 experimental groups, the calculated survival proportions at the different time points under both treatments were plotted in Kaplan-Meier survival curves for comparison by the log-rank (Mantel-Cox) test (P < 0.05).
RESULTS
Topical application of dexamethasone during the acute phase of HSV-1 infection has a profound, systemic, immunosuppressive effect
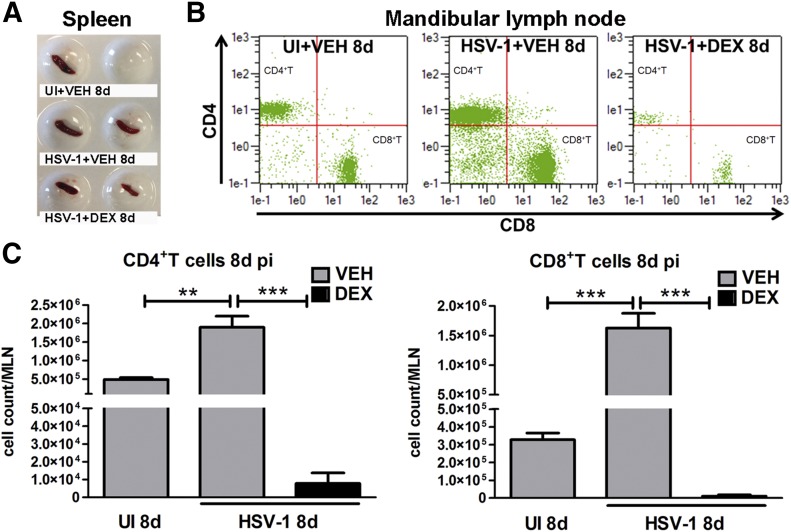
HSV-1 infection of the cornea is characterized by a response from resident cells, including infected epithelial cells, which release chemokines that recruit leukocytes in 2 successive waves consisting of PMNs, macrophages, inflammatory monocytes, and NK cells, followed by cells composed primarily of CD4+ and CD8+ T cells [10, 25]. Previously, we reported HSV-1 causes regression of sensory fibers, innervating the cornea, followed by abnormal nerve regeneration through an immune system–mediated mechanism [18, 22]. The pathologic process elicited by the immune system could be blocked by repeated topical applications of DEX, a clinically prescribed, anti-inflammatory reagent [21, 26], onto the HSV-1–infected corneas. To determine whether the local immunosuppression observed in the cornea was found distal to the site of application, we phenotypically evaluated the immune profile in organized lymphoid organs, including the spleen and MLN. The results show a massive loss in the total cellularity and T cell populations in the spleen and MLN, respectively, by 8 d p.i. (Fig. 1). The reported healthier appearance of immunosuppressed mice correlated with a phenotype of preservation of nerves and avascularity of the cornea proper in comparison to the infected group treated with VEH tear drops by 8 d p.i. [22]. Because, in addition to our observations, steroid treatment has been shown to reactivate latent infection in a variety of experimental models [27–30], and in clinical settings, topical corticosteroid therapy is recommended only in combination with antiviral therapy to reduce viral reactivation [31, 32], we queried whether the action of DEX in our model was long term.
Figure 1. Effect of topical DEX treatment on lymphoid tissues during acute HSV-1 infection.
Mouse corneas were infected with 103 PFU HSV-1 or left UI as controls. Starting at 2 h p.i., mice were topically treated with DEX or VEH onto their corneas for 8 d p.i. before tissue collection. (A) Display of spleens harvested from UI and infected mice treated with VEH or DEX at 8 d p.i. (B and C) MLN tissue was harvested from mice, and MLN single-cell suspensions were stained with specific mAbs to phenotypically identify CD4+ and CD8+ T cells. (A) Representative flow cytometry plots from MLN from UI (top), and infected mice treated with VEH (middle) or DEX (bottom) for 8 d p.i. indicate CD4+ (top left) and CD8+T (bottom right) cell populations. (C) Data summarize the phenotypic leukocyte count means ± sem in MLN tissue, n = 4/UI group and n = 6/infected group, from 2 independent experiments. For the indicated comparisons, **P < 0.01 and ***P < 0.001 by ANOVA, followed by Bonferroni multiple-comparison test.
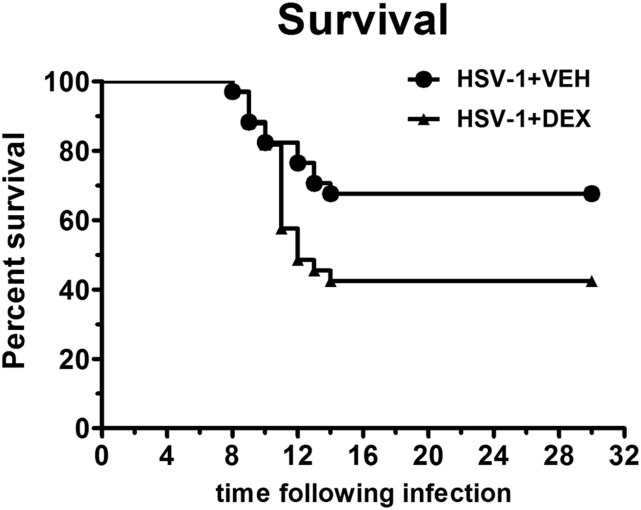
Topical dexamethasone treatment during acute HSV-1 infection decreases survival and has long-term effects on the host immune system
To determine the consequences of local DEX exposure on the immune system in virus-infected mice, C57BL6 mice were ocularly infected, and starting at 2 h p.i., mice were topically treated with 0.1% ophthalmic solution (DEX) or lubricant eye drops as control (VEH) onto their corneas 4 times/d over 8 d p.i. Regardless of treatment, HSV-1–infected mice succumbed to the infection starting at 8 d p.i. (Fig. 2). Death and survival events were recorded daily, plotted in survival curves, and analyzed for a period of 30 d p.i. Even though a greater number of mice succumbed in the DEX-treated group, there were still a significant number of mice that survived to 30 d p.i. (Fig. 2). Most surprising was a profound systemic, immunosuppressive effect in MLN and spleen tissues (Fig. 3A and B) with reduced myeloid (macrophages and inflammatory monocytes) and T cells, compared with VEH-treated mice. These results are consistent with a reduced myeloid (macrophages and inflammatory monocytes) and T cell influx in the cornea (Fig. 3C). Because, after local replication within the cornea, HSV-1 spreads to the nervous system by retrograde transport, we analyzed the presence of leukocytes in the TG and BS under both treatments at 30 d p.i. (Fig. 3D and E). We found no difference in the CD4+ or CD8+ T cells residing in the TG comparing VEH- to DEX-treated animals (Fig. 3D). In contrast, the BS of infected mice treated with VEH had significantly higher CD4+T cell counts relative to UI and DEX-treated mice. Although CD8+ T cells were significantly elevated in the BS of infected mice treated with VEH compared with UI animals, there was no significant reduction in CD8+ T cell counts compared with the DEX-treated mice (Fig. 3E).
Figure 2. Effect of topical dexamethasone treatment during the first 8 d of HSV-1 infection on mouse survival.
Mouse corneas were infected with 103 PFU HSV-1. Starting at 2 h p.i., mice were topically treated with DEX or VEH onto their corneas for 8 d. Mouse death and survival events were recorded until tissue collection at 30 d p.i. The calculated survival proportions at the different time points under both treatments were fitted in survival curves for comparison. By the log-rank (Mantel-Cox) test, both survival curves were found significantly different (P < 0.05). Analysis began with n = 33 mice for the HSV-1+ VEH group and n = 34 mice for HSV-1+ DEX group, from 7 independent experiments).
Figure 3. Long-term effect of topical dexamethasone treatment on the constituency of immune cell populations.
Mouse corneas were infected with 103 PFU HSV-1 or left UI as controls. Starting at 2 h p.i., mice were topically treated with DEX or VEH onto their corneas for 8 d p.i. before tissue collection at 30 d p.i. Single-cell suspensions were obtained from MLN, spleen, cornea, TG, and BS tissues and were stained with specific mAbs to phenotypically identify the leukocyte populations. Representative flow cytometry plots and bar graphs summarizing the phenotypic leukocyte count means ± sem are shown for MLN (A), spleen (B), cornea (C), TG (D), and BS (E) tissues of mice treated with VEH or DEX. Cell populations in the F4/80 vs. GR1 plots indicate macrophages, inflammatory monocytes, and PMNs. Cell populations inside the CD4 vs. CD8 plots indicate CD4+ (top left) and CD8+ (bottom right) T cells. UI groups: n = 4–5. Infected groups: n = 8–10 for MLN, n = 6–8 for spleen, n = 6–12 for cornea, n = 6–12 for TG, and n = 11–12 for BS, from 4–5 independent experiments. (A, B, D, and E) *P < 0.05, **P < 0.01, and ***P < 0.001 for indicated comparisons by ANOVA, followed by Bonferroni multiple-comparison test. (C) *P < 0.05 by unpaired t test comparison.
Effect of topical dexamethasone treatment on viral spread
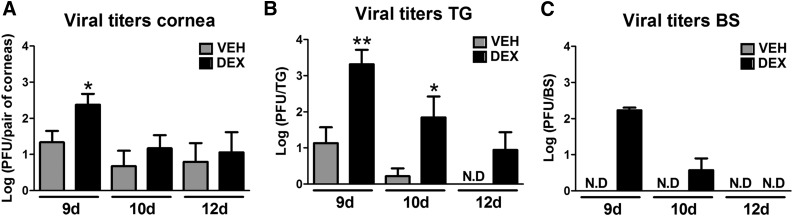
We previously showed DEX significantly decreased the infectious virus recovered from cornea compared with VEH-treated group at 2 d p.i. However, at 4 and 8 d p.i., no differences in the viral load were appreciated between groups [22]. Because we found a profound effect of DEX treatment on the immune system past the resolution of the virus infection (i.e., 30 d p.i.), as well as a partial reduction in cumulative survival in comparison to VEH-treated animals, we measured infectious virus in cornea, TG, and BS at times beyond 8 d p.i. We found DEX-treated mice had significantly more infectious virus in all tissues evaluated at 9 d p.i., consistent with the immunosuppressive state of the mice (Fig. 4A-C). The effect of increased viral spread by DEX was maintained at 10 d p.i., as observed in TG and BS tissues (Fig. 4B and C) but was no longer observed by 12 d p.i. (Fig. 4A-C).
Figure 4. Consequences of dexamethasone treatment during the first 8 d of HSV-1 infection on viral titers.
Mouse corneas were infected with 103 PFU HSV-1. Starting at 2 h p.i., mice were topically treated with DEX or VEH onto their corneas for 8 d before tissue harvest and processing for plaque assay. Bars show means ± sem of viral titer in cornea (A), TG (B), and BS (C) at 9, 10, and 12 d p.i. N.D, viral plaques not detected by the assay. (n = 6 per HSV-1+ VEH group; and n = 3–4 per HSV-1+ DEX group for cornea and TG at 9 d p.i., n = 3–4 per group for BS at 9 and 12 d p.i., and n = 6–8 per group for cornea, TG, and BS at 10 d p.i., from 2–3 independent experiments. *P < 0.05, **P < 0.01 by unpaired t test comparison.
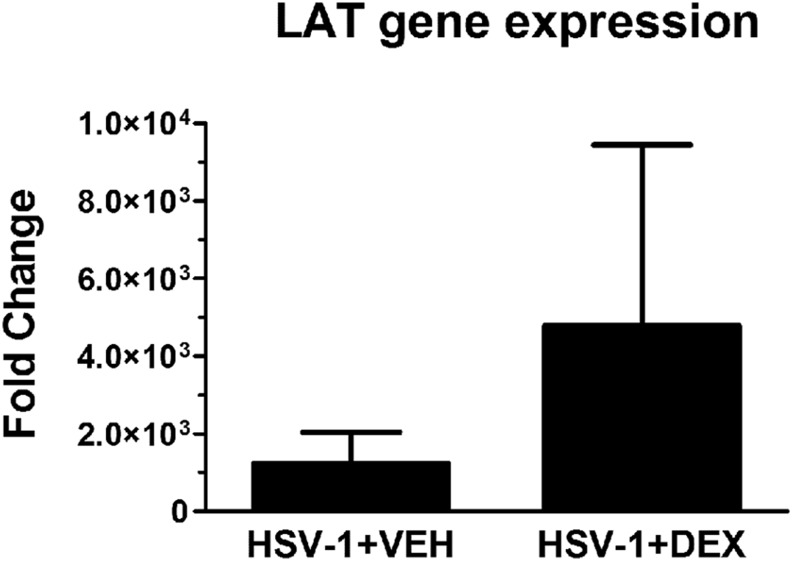
Effect of dexamethasone treatment on the expression of HSV-1 lytic and latent infection-associated transcripts
Steroid treatment has been shown to reactivate latent infection in a variety of experimental models [27–30]. To test whether DEX treatment during acute HSV-1 infection modified viral latency, the transcript levels of lytic genes, including gB, TK, and ICP-27, and the latent transcript LAT were analyzed in TG samples from VEH- or DEX-treated mice at 30 d p.i. No lytic gene expression was detected (data not shown), whereas LAT gene expression was detected, but not significantly different between treated groups of mice, at 30 d p.i. (Fig. 5).
Figure 5. Effect of topical dexamethasone treatment on LAT gene expression.
Mouse corneas were infected with 103 PFU HSV-1, or left UI as controls. Starting at 2 h p.i., mice were topically treated with DEX or VEH onto their corneas for 8 d p.i. before TG collection at 30 d p.i. TGs were harvested and processed for RNA isolation, followed by cDNA synthesis and real-time quantitative PCR analysis of genes associated with HSV-1 lytic infection (gB, TK, and ICP27) and latent infection (LAT). Although no detection of product was observed for lytic-infection genes in either group (data not shown), the expression of LAT was not significantly different between groups. Results are expressed as fold change LAT transcript expression means ± sem (n = 5–6 replicates for each group from 2 independent experiments) by unpaired t test comparison.
The immunosuppression by topical dexamethasone compromises the humoral immune response to HSV-1
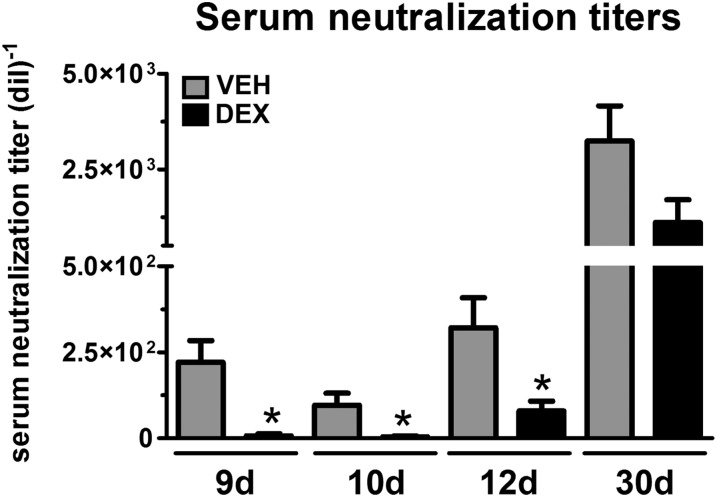
A recent study on the efficacy of a prophylactic HSV-1 vaccine uncovered preexisting humoral immunity as a correlate against HSV-1 ocular disease and latency [24]. Our data showed the significant cellular immunosuppression by topical DEX evidenced in lymphoid tissues during acute infection (Fig. 1) and maintained for 30 d p.i. (Fig. 3) correlated with elevated viral loads in cornea, TG, and BS tissues (Fig. 4). To address whether the compromised immune system and survival of infected mice reflected Ab neutralization titers, serum samples were obtained at 9, 10, 12, and 30 d p.i. and were assessed for HSV-1 neutralization (Fig. 6). Neutralization titers from DEX-treated mice were significantly lower than those of their VEH-treated counterparts at 9, 10, and 12 d p.i.. At 30 d p.i., serum neutralization titers were not significantly different comparing the 2 groups of treated mice (Fig. 6). These results suggest the humoral immune system via serum neutralization titers correlates with long-term survival of the DEX-treated mice.
Figure 6. Effect of topical dexamethasone treatment during the first 8 d of HSV-1 infection on serum neutralization titers.
Blood samples were collected at 9, 10, 12, and 30 d from the facial vein of infected mice treated with VEH and DEX. Serum in each sample was separated and used for the virus neutralization assay. Bar graphs show, for each group, the neutralization titer (reciprocal serum dilution at which a 50% reduction in cytopathic effect was observed) means ± sem. n = 4–6 for 9- and 12-d p.i. groups, n = 6–8 for 10-d p.i. groups (2 independent experiments), and n = 12–14 for 30-d p.i. groups (4 independent experiments). *P < 0.05 by unpaired t test comparison.
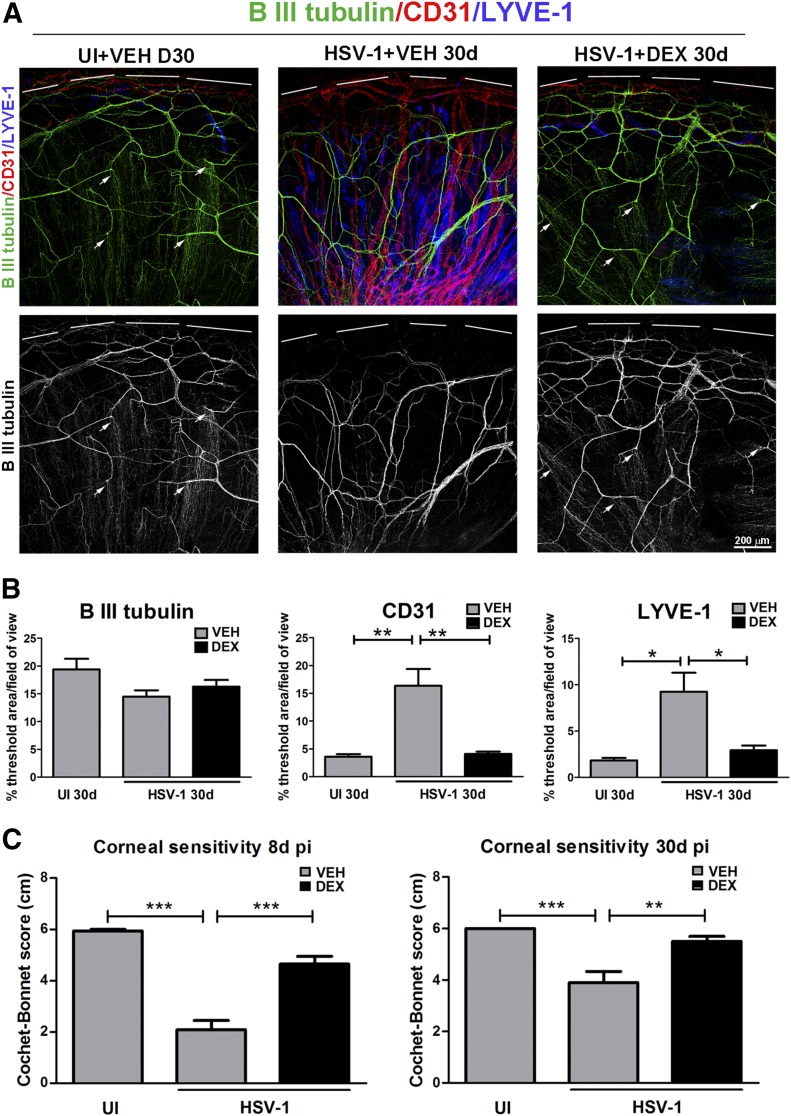
Topical dexamethasone treatment preserves the structure of the corneal innervation and maintains the avascular state of the cornea
The long-term effect of DEX treatment on corneal nerves and blood vessels was evaluated by immunohistochemistry using Abs against β-III tubulin (pan-neuronal marker), and CD31 (endothelial cell marker) and LYVE-1 Abs, respectively. As previously described [22], the UI mice treated with VEH displayed a stromal network formed by thick nerve trunks that ramified into smaller and more-superficial branches as they progressed from the periphery toward the center of the cornea (Fig. 7A and B). A subbasal network composed of thinner, hairpin-like nerves that projected centripetally and presented a roughly parallel orientation from one another terminating in free nerve endings was observed. Consistent with an abnormal phenotype of nerve regeneration secondary to nerve loss [17–19], HSV-1 infection resulted in sustained loss of the fine subbasal nerve bundles, areas of stromal hyperinnervation, and invasion of blood and lymphatic vessels originating from the limbus of the cornea, as observed at 30 d p.i. in the group treated with VEH. DEX treatment applied during the acute viral infection preserved the corneal nerve structure (both stromal and subbasal) and had a sustained effect blunting neovascularization at 30 d p.i. (Fig. 7A and B). We and others have previously reported the effects of HSV-1 inducing corneal denervation [17, 18, 22] in correlation with a significant loss of corneal sensitivity. Such functional impairment was recapitulated in the present study in the infected, VEH-treated group and was significantly prevented by DEX by 8 d p.i. (Fig. 7C). Corneal sensitivity analysis within the surviving mice under both treatments showed DEX treatment maintained corneal nerve function by 30 d p.i., consistent with prolonged structural preservation (Fig. 7C).
Figure 7. Long-term effect of topical dexamethasone treatment during the first 8 d of HSV-1 infection on corneal innervation and vasculature.
Mouse corneas were infected with 103 PFU HSV-1 or left U) as controls. Starting at 2 h p.i., mice were topically treated with DEX or VEH onto their corneas for 8 d p.i. before tissue collection at 30 d p.i. (A) Representative confocal images show corneal nerves (green: β-III tubulin staining) and blood and lymphatic vessels (red: CD31 staining; blue: LYVE-1 staining, top discontinuous white lines depict the limbal margins) at 30 d p.i. (top: merge of β-III tubulin/CD3/LYVE-1 costaining, bottom: grayscale display of β-III tubulin staining only). White arrows depict intact fine bundles of subbasal nerves in the UI group that are preserved in the infected group treated with DEX. (B) Analysis of corneal innervation (left) and vascularization (center and right) expressed as the percentage threshold area positive for β-III tubulin signal per field of view means ± sem, percentage threshold area positive for CD31 signal per field of view means ± sem, and percentage threshold area positive for LYVE-1 signal per field of view means ± sem, respectively (n = 6 for UI and n = 16–17 for infected groups for β-III tubulin, n = 11 for UI and n = 16–23 for uninfected groups for CD31, and n = 9 for UI and n = 11–16 per infected groups for LYVE-1 from 3–4 independent experiments). (C) Bars show Cochet-Bonnet score means ± sem at 8 and 30 d p.i. (n = 16 for UI, n = 40–43 for infected groups at 8 d p.i.; n = 24–28 for infected groups at 30 d p.i. from 5 independent experiments). *P < 0.05, **P < 0.01, and ***P < 0.001 for indicated comparisons by ANOVA, followed by Bonferroni multiple-comparison test.
DISCUSSION
We previously suggested the presence of an elicited immune response to infection, and not local viral replication, was part of the mechanism of corneal nerve degeneration during HSV-1–induced NTK [22]. By using a murine model of HSV-1 infection, we and others have found the significant nerve loss triggered during the acute phase of infection is followed by an aberrant process of corneal reinnervation with sustained deficits at time points corresponding with viral resolution and establishment of latency [17–19]. Work from Yun et al. [17] demonstrated a subset of CD4+ T cells influence the long-term persistence of nerve defects following herpetic infection upon which depletion, nerve structure, and sensitivity can be significantly restored. Our present findings expand on the long-term consequences of topical DEX treatment with emphasis on host survival, cellular and humoral immune responses, viral spread, and corneal pathology, and further support sustained inflammation upon viral resolution as key for the maintenance of the structural and functional nerve deficits common to herpetic NTK.
Our previous report supported a time-dependent, beneficial effect of immunosuppression on the sensory innervation of the cornea in the absence of antiviral drug intervention [22]. Such protection was consistent with the antiangiogenic properties known to be exerted by this drug in the cornea [20]. The nerve structure and functional preservation by DEX occurred in the context of decreased cell counts of macrophages at all time-points studied and decreased CD8+ T cells at 8 d p.i. In addition, the nerve protection was lost when the DEX therapy was delayed for 2 d, which suggested the innate immune response to infection as the trigger for such neurodegenerative mechanism [22]. Along with the hypothesis that corneal nerve degeneration is immune mediated, the prolonged nerve damage in the VEH-treated group at 30 d p.i., along with the sustained neovascularization with blood and lymphatic vessels of the cornea was consistent with the sustained presence of inflammatory cells in the tissue. The “prolonged” immunosuppression observed in MLN and spleen, well beyond DEX treatment, correlated with decreased infiltrated macrophages and inflammatory monocytes and significant reductions in both CD4+ and CD8+ T cells in the corneas of latently infected mice, compared with the VEH-treated counterparts. Further studies will address the contribution of the innate and adaptive immune responses in the nerve degeneration that coexists with sustained neovascularization after infection of the cornea. An important difference between experimental models of HSV-1 infection and human corneal disease is that, although, in mice, the development of the immunopathology associated to HSK and nerve degeneration is the consequence of a single acute infection (in this case, 1000 PFUs HSV-1/cornea), HSK in humans is thought to be the result of repeated cycles of viral reactivation followed by viral replication. This fundamental difference between both hosts obscures our interpretation as to whether the nerve damage in patients with recurrent HSK is progressive or is caused by the innate immune response defined during primary infection in the face of an already primed, adaptive immune response. Further research, including successful murine models of HSV-1 reactivation, might be of value to address this relevant question.
A previous study investigating CNS pathology after HSV-1 infection reported that, in wild-type mice, initial neurologic signs do not manifest until 7 d p.i., with mortality arising shortly after that [33]. Conversely, mice with a compromised innate immune system were more susceptible to infection, which correlated with development of CNS deficits and 100% death at earlier time points than in wild-type mice [33]. Consistent with an association between immunosuppression, lack of local viral replication control, increased neuroinvasion, and development of CNS pathology, topical DEX treatment of infected corneas resulted in an elevation in viral titers at 9 d p.i. in TG and BS tissues. The viral titer data correlated with lower serum neutralization titers in the DEX-treated group at time points consistent with higher mortality rates (9–12 d). In the DEX-treated mice that survived the acute infection, serum neutralization titers were not significantly different from those of the VEH-treated mice, suggesting that humoral immunity is a critical component of the host immune response to control CNS infection and pathology. Consistent with these results, VEH- and DEX-treated mice that survived infection retained similar levels of latent virus in the TG. As such, we suggest the neutralization capacity, along with innate immune components also reported to be critical in viral resistance in the CNS [34], are necessary for the survival of the DEX-treated, HSV-1–infected mice.
AUTHORSHIP
A.J.C.E. conceived and performed experiments, analyzed data, interpreted results, and generated the figures. M.M.C. performed experiments and collected data. D.J.J.C. conceived experiments and interpreted results. A.J.C.E. and D.J.J.C. wrote the manuscript.
ACKNOWLEDGMENTS
The authors would like to acknowledge the generous support from the U.S. National Institutes of Health (NIH) National Eye Institute (NEI) (Grants R01 EY021238 and NEI core P30 EY021725), an Oklahoma Center for Adult Stem Cell Research (OCASCR) grant through the Oklahoma Tobacco Settlement Endowment Trust, and an unrestricted grant from Research to Prevent Blindness. The authors would like to thank Dr. Derek Royer, Hem Gurung, and Chandra Menendez for the critical discussions during the preparation of this manuscript
Glossary
- BS
brainstem
- DEX
dexamethasone
- Eef1e1
eukaryotic translation elongation factor 1 ε-1
- gB
HSV-1 glycoprotein B
- HSK
herpes stromal keratitis
- ICP-27
HSV-1–infected cell protein 27
- LAT
latency-associated transcript
- MLN
mandibular lymph node
- NTK
neurotrophic keratitis
- PMN
polymorphonuclear cell
- p.i.
after infection
- TG
trigeminal ganglia
- TK
thymidine kinase
- UI
uninfected
- VEH
vehicle
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- 1.Müller L. J., Marfurt C. F., Kruse F., Tervo T. M. (2003) Corneal nerves: structure, contents and function. Exp. Eye Res. 76, 521–542. [DOI] [PubMed] [Google Scholar]
- 2.Beuerman R. W., Schimmelpfennig B. (1980) Sensory denervation of the rabbit cornea affects epithelial properties. Exp. Neurol. 69, 196–201. [DOI] [PubMed] [Google Scholar]
- 3.Bonini S., Rama P., Olzi D., Lambiase A. (2003) Neurotrophic keratitis. Eye (Lond.) 17, 989–995. [DOI] [PubMed] [Google Scholar]
- 4.Sacchetti M., Lambiase A. (2014) Diagnosis and management of neurotrophic keratitis. Clin. Ophthalmol. 8, 571–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cavanagh H. D., Pihlaja D., Thoft R. A., Dohlman C. H. (1976) The pathogenesis and treatment of persistent epithelial defects. Trans. Sect. Ophthalmol. Am. Acad. Ophthalmol. Otolaryngol. 81, 754–769. [PubMed] [Google Scholar]
- 6.Davis E. A., Dohlman C. H. (2001) Neurotrophic keratitis. Int. Ophthalmol. Clin. 41, 1–11. [DOI] [PubMed] [Google Scholar]
- 7.Liesegang T. J. (1985) Corneal complications from herpes zoster ophthalmicus. Ophthalmology 92, 316–324. [DOI] [PubMed] [Google Scholar]
- 8.Miller C. S., Danaher R. J., Jacob R. J. (1998) Molecular aspects of herpes simplex virus I latency, reactivation, and recurrence. Crit. Rev. Oral Biol. Med. 9, 541–562. [DOI] [PubMed] [Google Scholar]
- 9.Biswas P. S., Rouse B. T. (2005) Early events in HSV keratitis—setting the stage for a blinding disease. Microbes Infect. 7, 799–810. [DOI] [PubMed] [Google Scholar]
- 10.Carr D. J., Härle P., Gebhardt B. M. (2001) The immune response to ocular herpes simplex virus type 1 infection. Exp. Biol. Med. (Maywood) 226, 353–366. [DOI] [PubMed] [Google Scholar]
- 11.Rowe A. M., St Leger A. J., Jeon S., Dhaliwal D. K., Knickelbein J. E., Hendricks R. L. (2013) Herpes keratitis. Prog. Retin. Eye Res. 32, 88–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stumpf T. H., Shimeld C., Easty D. L., Hill T. J. (2001) Cytokine production in a murine model of recurrent herpetic stromal keratitis. Invest. Ophthalmol. Vis. Sci. 42, 372–378. [PubMed] [Google Scholar]
- 13.Wuest T. R., Carr D. J. (2010) VEGF-A expression by HSV-1-infected cells drives corneal lymphangiogenesis. J. Exp. Med. 207, 101–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng M., Schwarz M. A., Lee S., Kumaraguru U., Rouse B. T. (2001) Control of stromal keratitis by inhibition of neovascularization. Am. J. Pathol. 159, 1021–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallar J., Tervo T. M., Neira W., Holopainen J. M., Lamberg M. E., Miñana F., Acosta M. C., Belmonte C. (2010) Selective changes in human corneal sensation associated with herpes simplex virus keratitis. Invest. Ophthalmol. Vis. Sci. 51, 4516–4522. [DOI] [PubMed] [Google Scholar]
- 16.Hamrah P., Cruzat A., Dastjerdi M. H., Zheng L., Shahatit B. M., Bayhan H. A., Dana R., Pavan-Langston D. (2010) Corneal sensation and subbasal nerve alterations in patients with herpes simplex keratitis: an in vivo confocal microscopy study. Ophthalmology 117, 1930–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yun H., Rowe A. M., Lathrop K. L., Harvey S. A., Hendricks R. L. (2014) Reversible nerve damage and corneal pathology in murine herpes simplex stromal keratitis. J. Virol. 88, 7870–7880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chucair-Elliott A. J., Zheng M., Carr D. J. (2015) Degeneration and regeneration of corneal nerves in response to HSV-1 infection. Invest. Ophthalmol. Vis. Sci. 56, 1097–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yun H., Lathrop K. L., Hendricks R. L. (2016) A central role for sympathetic nerves in herpes stromal keratitis in mice. Invest. Ophthalmol. Vis. Sci. 57, 1749–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mirabelli P., Peebo B. B., Xeroudaki M., Koulikovska M., Lagali N. (2014) Early effects of dexamethasone and anti-VEGF therapy in an inflammatory corneal neovascularization model. Exp. Eye Res. 125, 118–127. [DOI] [PubMed] [Google Scholar]
- 21.Weijtens O., Schoemaker R. C., Romijn F. P., Cohen A. F., Lentjes E. G., van Meurs J. C. (2002) Intraocular penetration and systemic absorption after topical application of dexamethasone disodium phosphate. Ophthalmology 109, 1887–1891. [DOI] [PubMed] [Google Scholar]
- 22.Chucair-Elliott A. J., Jinkins J., Carr M. M., Carr D. J. (2016) IL-6 contributes to corneal nerve degeneration after herpes simplex virus type I infection. Am. J. Pathol. 186, 2665–2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Menendez C. M., Jinkins J. K., Carr D. J. (2016) Resident T cells are unable to control herpes simplex virus-1 activity in the brain ependymal region during latency. J. Immunol. 197, 1262–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Royer D. J., Gurung H. R., Jinkins J. K., Geltz J. J., Wu J. L., Halford W. P., Carr D. J. (2016) A highly efficacious herpes simplex virus 1 vaccine blocks viral pathogenesis and prevents corneal immunopathology via humoral immunity. J. Virol. 90, 5514–5529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaye S., Choudhary A. (2006) Herpes simplex keratitis. Prog. Retin. Eye Res. 25, 355–380. [DOI] [PubMed] [Google Scholar]
- 26.Pinto R. D., Lira R. P., Abe R. Y., Zacchia R. S., Felix J. P., Pereira A. V., Arieta C. E., de Castro R. S., Bonon S. H. (2014) Dexamethasone/povidone eye drops versus artificial tears for treatment of presumed viral conjunctivitis: a randomized clinical trial. Curr. Eye Res. 40, 870–877. [DOI] [PubMed] [Google Scholar]
- 27.Cook S. D., Paveloff M. J., Doucet J. J., Cottingham A. J., Sedarati F., Hill J. M. (1991) Ocular herpes simplex virus reactivation in mice latently infected with latency-associated transcript mutants. Invest. Ophthalmol. Vis. Sci. 32, 1558–1561. [PubMed] [Google Scholar]
- 28.Halford W. P., Gebhardt B. M., Carr D. J. (1996) Mechanisms of herpes simplex virus type 1 reactivation. J. Virol. 70, 5051–5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haruta Y., Rootman D. S., Xie L. X., Kiritoshi A., Hill J. M. (1989) Recurrent HSV-1 corneal lesions in rabbits induced by cyclophosphamide and dexamethasone. Invest. Ophthalmol. Vis. Sci. 30, 371–376. [PubMed] [Google Scholar]
- 30.Shimeld C., Hill T. J., Blyth W. A., Easty D. L. (1990) Reactivation of latent infection and induction of recurrent herpetic eye disease in mice. J. Gen. Virol. 71, 397–404. [DOI] [PubMed] [Google Scholar]
- 31.Barron B. A., Gee L., Hauck W. W., Kurinij N., Dawson C. R., Jones D. B., Wilhelmus K. R., Kaufman H. E., Sugar J., Hyndiuk R. A., Laibson P. R., Stulting R. D., Asbell P. A.; Herpetic Eye Disease Study Group (1994) Herpetic eye disease study: a controlled trial of oral acyclovir for herpes simplex stromal keratitis. Ophthalmology 101, 1871–1882. [DOI] [PubMed] [Google Scholar]
- 32.Wilhelmus K. R., Gee L., Hauck W. W., Kurinij N., Dawson C. R., Jones D. B., Barron B. A., Kaufman H. E., Sugar J., Hyndiuk R. A., , Laibson P. R., Stulting R. D., Asbell P. A.; Herpetic Eye Disease Study Group (1994) Herpetic eye disease study: a controlled trial of topical corticosteroids for herpes simplex stromal keratitis. Ophthalmology 101, 1883–1896; discussion 1895–1896. [DOI] [PubMed] [Google Scholar]
- 33.Conrady C. D., Zheng M., van Rooijen N., Drevets D. A., Royer D., Alleman A., Carr D. J. (2013) Microglia and a functional type I IFN pathway are required to counter HSV-1-driven brain lateral ventricle enlargement and encephalitis. J. Immunol. 190, 2807–2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Royer D. J., Conrady C. D., Carr D. J. (2016) Herpesvirus-associated lymphadenitis distorts fibroblastic reticular cell microarchitecture and attenuates CD8 T cell responses to neurotropic infection in mice lacking the STING-IFNα/β defense pathways. J. Immunol. 197, 2338–2352. [DOI] [PMC free article] [PubMed] [Google Scholar]