Engagement of TLR10 on primary human monocytes has both immediate and long-term suppressive effects on the inflammatory response.
Keywords: regulation, TLR, cytokines
Abstract
TLRs are important pattern-recognition receptors involved in the activation of innate immune responses against foreign pathogens. TLR10 is the only TLR family member without a known ligand, signaling pathway, or clear cellular function. Previous work has shown that TLR10 suppresses proinflammatory cytokine production in response to TLR agonists in a mixed human mononuclear cell population. We report that TLR10 is preferentially expressed on monocytes and suppresses proinflammatory cytokine production resulting from either TLR or CD40 stimulation. TLR10 engagement affects both the MAPK and Akt signaling pathways, leading to changes in the transcriptome of isolated human monocytes. Differentiation of monocytes into dendritic cells in the presence of an αTLR10 mAb reduced the expression of maturation markers and the induction of proinflammatory cytokines, again in response to either TLR or CD40 stimulation. Finally, in coculture experiments, TLR10 differentiated dendritic cells exhibited a decreased capacity to activate T cells as measured by IL-2 and IFN-γ production. These data demonstrate that TLR10 is a novel regulator of innate immune responses and of the differentiation of primary human monocytes into effective dendritic cells.
Introduction
TLRs are a class of pattern-recognition receptors that sense conserved pathogen-associated molecular pattern on a variety of different bacterial, fungal, and viral organisms [1]. TLRs play a significant role in the activation of myeloid cells, comprising monocytes, macrophages, and dendritic cells [2]. These cells represent a group of leukocytes that orchestrate protective immune responses after detection of invading pathogens [3, 4]. Upon activation by pattern-recognition receptors, monocytes residing in the blood secrete proinflammatory cytokines, such as IL-6, TNF-α, and IL-1β, to combat the invading micro-organism. Furthermore, monocytes have the ability to migrate to the site of infection and infiltrate tissues, where they receive signals to differentiate into either macrophages or dendritic cells [5, 6].
Dendritic cells are critically important in the activation of the adaptive immune system. As professional APCs, dendritic cells take up antigen and present it on the MHC complex, a process that is stimulated by TLR activation [7, 8]. Naive T cells interact with the antigen-primed dendritic cell MHC complex via the TCR. The interaction between CD80/86 on dendritic cells and CD28 on T cells provides the costimulation necessary for T cell activation. Cytokines secreted by dendritic cells, such as IL-12, serve to polarize T cells into type 1 effectors [9].
Recognition of the TLR’s cognate ligand initiates dimerization of the receptor and recruitment of MyD88 to the C-terminal TIR domains of the receptor dimers [10–12]. This Myddosome complex further activates downstream signaling proteins that induce gene expression changes through activation of NF-κB and IRF transcription factors [13].
Of the 10 human TLRs, TLRs 1–9 have been extensively studied and have been shown to have critical functions in the activation of myeloid cells. Conversely, TLR10 remains the last human TLR without a known ligand, signaling function, or clear function [14]. TLR10 was first cloned in 2001 and has been shown to be transcriptionally expressed in a variety of different cell types of both the lymphoid and myeloid lineages [6, 15]. We have shown that when overexpressed in a myeloid cell line, proinflammatory cytokine production is suppressed in response to MyD88-dependent and -independent TLR signaling pathways. Furthermore, we generated a transgenic mouse that expresses human TLR10 which also showed suppressed cytokine responses to LPS stimulation [16].
In this study, we expanded upon those initial findings by exploring the effects of an in-house–generated mAb against TLR10 on isolated human monocytes ex vivo. Ab-mediated engagement of TLR10 decreased proinflammatory cytokine production and the phosphorylation status of several signaling pathways activated through either TLR or CD40 stimulation. In addition, engagement of TLR10 affected the differentiation of monocytes into dendritic cells, as evidenced by decreased costimulatory markers, and suppressed cytokine production in response to a stimulus. These TLR10-differentiated dendritic cells had a reduced capacity to activate T cells. The data show that Ab-mediated engagement of TLR10 suppresses both proximal and long-term functions of monocytes that may both advance our understanding of innate immune regulation and have potential medical benefits for treating chronic inflammatory diseases.
MATERIALS AND METHODS
Reagents
Abs for CD33, CD3, CD19, CD83, CD86, CD80, CD40, and HLA-II, as well as all corresponding isotype control Abs were purchased from BD Biosciences (Franklin Lakes, NJ, USA). The αTLR10 mAb (5C2C5) hybridoma was developed in-house using the extracellular domain of TLR10 (aa 20–474) as an antigen. Characterization of our in-house Ab has been published [17]. The 5C2C5 Ab and the MOPC-21 isotype control Ab (BioLegend, San Diego, CA, USA) were directly conjugated to Alexa-647 by using a fluorophore-conjugation kit (Thermo Fisher Scientific, Waltham, MA, USA). The flow buffer was composed of 10% rabbit serum in PBS.
Cell culture
Peripheral blood was obtained from consenting healthy donors under an approved institutional review board protocol. Ficoll-density centrifugation was used to isolate mononuclear cells. For isolated monocytes and T cells, whole blood was mixed with a monocyte or T cell isolation cocktail from StemCell Technologies (Vancouver, BC, Canada) before Ficoll-density centrifugation.
Monocyte-derived dendritic cells were differentiated from isolated monocytes by the addition of 50 ng/ml of GM-CSF and 10 ng/ml of IL-4 (PeproTech, Rocky Hill, NJ) for 4 d, with half of the medium changed after d 2. The TLR10 (5C2C5) or the isotype control (MOPC-21) Ab was added at the same time at a concentration of 10 μg/ml. Cells were plated in a 6-well dish at ∼2 × 106 cells per well. The medium used for all experiments was RPMI 1640 with glutamine supplemented with a 1× concentration of nonessential amino acids (Corning, Corning, NY, USA).
Flow cytometry
Cells (∼1 × 106) were blocked on ice in flow buffer for 30 min before the addition of the different cellular markers. After they were washed, the cells were fixed in 4% paraformaldehyde for 15 min, washed, and resuspended in flow buffer. All flow data were collected on a FACSCanto running FACSDiva software (BD Biosciences). The change in median fluorescence intensity was calculated by subtracting the geometric median fluorescence intensity of the isotype control-treated cells from that of the αTLR10 Ab-treated cells. Prism (GraphPad Software, San Diego, CA, USA) was used to generate the graphs and perform the unpaired t tests to determine statistical significance.
Cellular stimulations
Mononuclear cells, monocytes, or dendritic cells were plated at 2 × 104 cells/well in a 96-well plate. Either the TLR10 mAb (5C2C5) or the isotype control Ab (MOPC-21) was added 10 min before stimulation at 10 μg/ml. LPS 0111:B4 (InvivoGen, San Diego, CA, USA) was added at 10 ng/ml, TL8-506 (InvivoGen) at 50 ng/ml, and αCD40 (R&D Systems, Minneapolis, MN, USA) at 100 ng/ml. After 24 h, cell-free supernatants were collected and stored at −20°C.
For T cell:dendritic cell cocultures, dendritic cells were plated at 4 × 104 dendritic cells/well in a 24-well plate. T cells were added at ∼8−105 T cells per well with a final volume of 300 μl. LPS was added as stated earlier and the superantigen TSST-1 (IBT BioServices, Gaithersburg, MD, USA) was added at 500 pg/ml. After 72 h, cell-free supernatants were collected and stored at −20°C.
Supernatants were analyzed for IL-6, TNFα, IL-1β, IL-12, IL-2, and IFN-γ (Thermo Fisher Scientific) according to the manufacturer’s instructions. Intradonor triplicates were completed for each donor. When appropriate, relative activation was calculated by taking the average triplicate value from TLR10-treated cells divided by the average triplicate value of isotype control-treated cells. GraphPad Prism was used to generate the graphs and perform the unpaired t tests to determine statistical significance.
Western blot analysis
Isolated monocytes were stimulated as stated earlier, and lysates were generated from PBS-washed cells by using the Blue Loading Buffer Pack (Cell Signaling Technologies, Danvers, MA, USA) with 1× HALT Protease and Phosphatase Inhibitor Cocktail (Thermo Fisher Scientific). Gels were run on precast gels (Bio-Rad, Hercules, CA, USA) and transferred to a nitrocellulose membrane. Blots were blocked in a 10% milk-TBST solution for 30 min before overnight incubation with the primary Ab in a 10% BSA-TBST solution. Blots were then washed and blotted with a secondary Ab for 30 min. SuperSignal West Femto (Thermo Fisher Scientific) was used for visualization. ImageLab software (Bio-Rad) was used to generate images and for densitometry measurements. Prism (GraphPad) software was used to generate the bar graphs and perform the unpaired t tests to determine statistical significance.
RNA sequencing
Isolated monocytes were stimulated as stated earlier and RNA was collected after 2 h using an RNeasy Mini Kit (Qiagen, Valencia, CA, USA). DNA digestion was performed on-column and checked by PCR. RNA integrity was assessed by a 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA) to be an RNA integrity number ≥8.8. RNA-seq libraries were prepared using Illumina’s TruSeq Stranded mRNAseq Sample Prep Kit (Illumina, Inc., San Diego, CA, USA). The libraries were quantified by qPCR and sequenced on 2 lanes for 101 cycles from one end of the fragments on a HiSeq2500 with the HiSeq Rapid SBS sequencing kit version 2. Each sample had an average of 25 million reads from 4 different donors. Fastq files were generated and demultiplexed with the bcl2fastq v2.17.1.14 conversion software (Illumina). Analysis was performed by High Performance Biologic Computing Center at the University of Illinois Urbana-Champaign.
Statistics
All data were analyzed using Student’s t test, unless otherwise indicated. Data shown as relative activation were calculated by averaging intradonor triplicate experiments for TLR10- and isotype-treated cells and then dividing the average value from TLR10-treated cells by the average value from isotype-treated cells. Each graph represents the mean of a specified number of replicates, with error bars denoting sem.
RESULTS
Ab-mediated engagement of TLR10 suppresses monocyte cytokine production
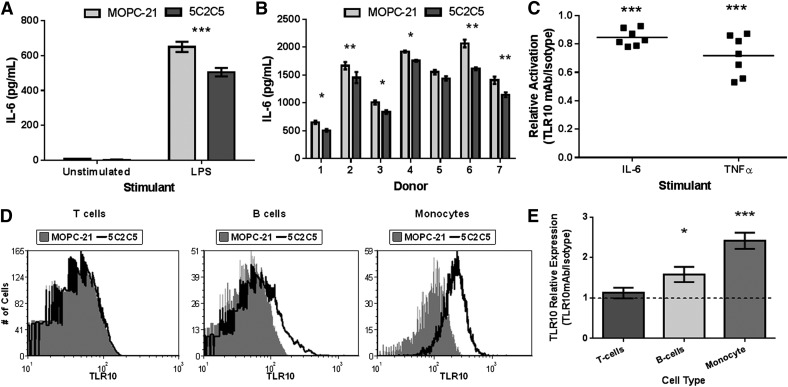
To determine the effect of Ab mediated engagement of TLR10, we first stimulated a population of mononuclear cells with LPS in the presence of either an anti-TLR10 Ab or an isotype control Ab. We observed decreased production of IL-6 in αTLR10-treated cells, compared with isotype control cells after 24 h of stimulation (Fig. 1A). The suppression was consistent across a panel of 7 different donors with αTLR10-treated cells producing on average ∼80% of the IL-6 and TNFα compared to isotype-treated cells (Fig. 1B, C).
Figure 1. Ab-mediated engagement of TLR10 suppresses mononuclear cell cytokine production.
(A) Human PBMCs were preincubated with either an αTLR10 Ab (5C2C5) or an isotype-matched control Ab for 10 min before stimulation with LPS for 24 h or were left unstimulated. Cell-free supernatants were collected and Ab-paired ELISA was used to measure the indicated cytokine. Mononuclear cell stimulations were repeated on 7 different donors (B) and plotted as relative activation by dividing TLR10-treated cells by isotype-treated cells for each donor and indicated analyte (C). (D) Representative histograms of PBMCs stained with either an αTLR10 Ab (5C2C5) or an isotype control Ab conjugated to Alexa647 in addition to a cell marker. (E) Data from at least 4 donors were combined by averaging the fold median fluorescence intensity normalized to isotype-treated cells. All error bars represent the sem. *P < 0.05; **P < 0.01, ***P < 0.001.
Mononuclear cells consist of a mixed leukocyte population that could each be affected by TLR10 engagement. To determine which cell types express TLR10, we analyzed 5 different donors by flow cytometry for TLR10 expression. Of the three major constituents of the mixed mononuclear populations, only B cells and monocytes expressed detectable levels of TLR10 (Fig. 1D, E). This confirms our previous finding with a different TLR10 Ab clone (3C10C5) in which we detected TLR10 expression on B cells but not T cells [17].
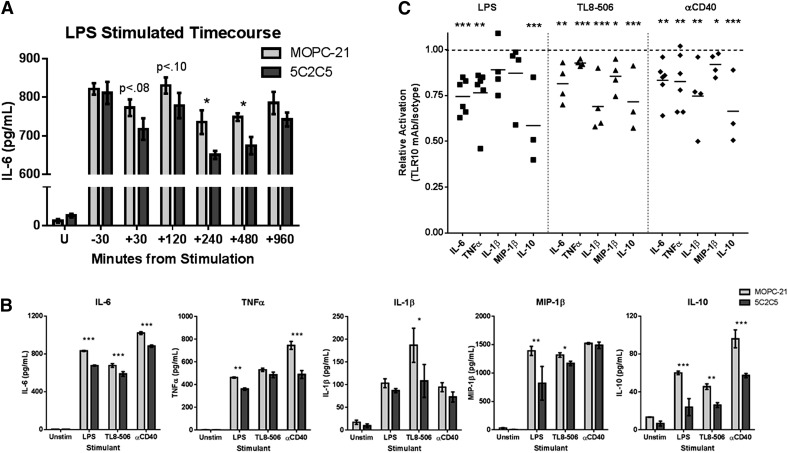
To exclude the possibility that TLR10 engagement on B cells is the mediator of the observed cytokine suppression, we tested isolated monocytes for TLR10-mediated cytokine suppression. Similar to our previous data, isolated monocytes also showed suppressed production of the expanded repertoire of proinflammatory cytokines IL-6, TNFα, IL-1β, and MIP-1β. The anti-inflammatory cytokine IL-10 was also suppressed by TLR10 engagement (Fig. 2). These data suggest that TLR10 engagement directly affects monocyte activation that is not dependent on other leukocytes.
Figure 2. TLR10 suppresses cytokine production from TLR-dependent and -independent stimuli.
Isolated human PMBCs were preincubated with either an αTLR10 (5C2C5) or an isotype control Ab for 10 min before stimulation with the indicated agonist for 24 h. Supernatants were collected and analyzed as described in Fig. 1. (A) Time-course assay where the TLR10 Ab was added at different time intervals before and after LPS stimulation at time 0. (B) Representative stimulation data from 4 to 6 donors. (C) Summary data, where each dot represents a single donor’s TLR10-treated cells after the values are normalized to isotype-treated cells for the indicated analyte and stimulant. *P < 0.05; **P < 0.01; ***P < 0.001.
The TLR10 receptor actively suppresses both the TLR and TNF signaling pathways
To determine whether Ab-engagement before stimulation is essential for suppression, we performed a time-course assay where the αTLR10 Ab was added at different time intervals poststimulation. The αTLR10 Ab suppressed LPS-induced IL-6 production in isolated monocytes up to 8 h after stimulation but not when added 30 min before stimulation (Fig. 2A). These results suggest that the 5C2C5 TLR10 Ab clone is not a blocking Ab but is an activator of the TLR10 receptor.
Previous research has shown that TLR10 can suppress TLR-related signaling but also TLR-independent stimuli in the context of B-cell activation [16, 17]. We wanted to determine whether TLR10 engagement would suppress non-TLR signaling in the context of monocyte activation. We stimulated isolated monocytes with LPS, TL8-506, or αCD40 Abs that represent an extracellular TLR (TLR4) ligand, intracellular TLR (TLR8) ligand, and a stimulator of the TNF pathway (CD40), respectively, We chose CD40 because of its importance in the myeloid lineage with respect to costimulation of T cells and because of our previous work in B cells. Irrespective of the stimulant, TLR10 engagement was equally sufficient to suppress both the pro- and anti-inflammatory cytokines that we tested (Fig. 2B, C).
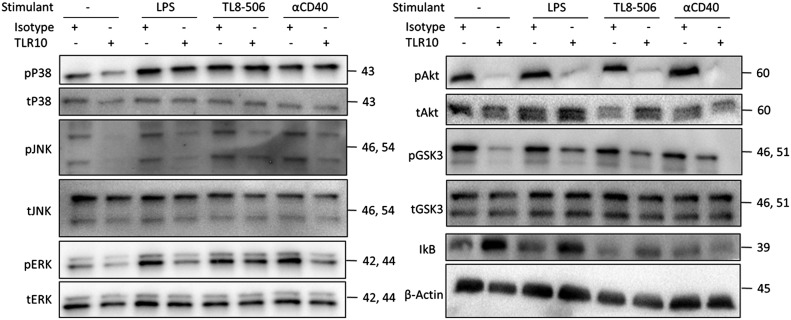
Although there are many differences between the TLR and TNF signaling pathways proximally, there is also considerable cross talk that may allow TLR10 engagement to suppress both pathways. To examine which signaling proteins are affected by TLR10 engagement, we analyzed the phosphorylation status of the NF-κB regulator IκB, several MAPK families, and the main kinase in the PI3K signaling pathway, Akt (PKB). As shown in Fig. 3 and Supplemental Fig. 1, TLR10 engagement suppressed the degradation of IκB, as well as the phosphorylation of both the MAPK and PI3K/Akt signaling pathways 20 min after stimulation. This effect was detected when the cells were stimulated with either a TLR agonist or αCD40 stimulant. Alternatively, these pathways can be activated by integrins on the surface of monocytes, without any additional stimulant. TLR10 engagement also suppressed the residual integrin-mediated activation (mediated by receptor tyrosine kinases) of these pathways from the initial plating of the cells (seen in the unstimulated condition). These data suggest that TLR10 acts as a broad proximal suppressor of inflammatory signaling.
Figure 3. TLR10 suppresses the phosphorylation of the Akt and MAPK signaling pathways.
Isolated human PMBCs were preincubated with either an αTLR10 (5C2C5) or an isotype control Ab for 10 min before stimulation with the indicated agonist for 15 min. Cell lysates were collected and analyzed by immunoblot analysis for each signaling target, as indicated. Data are representative of at least 3 independent experiments.
TLR10 alters the transcriptome of primary monocytes
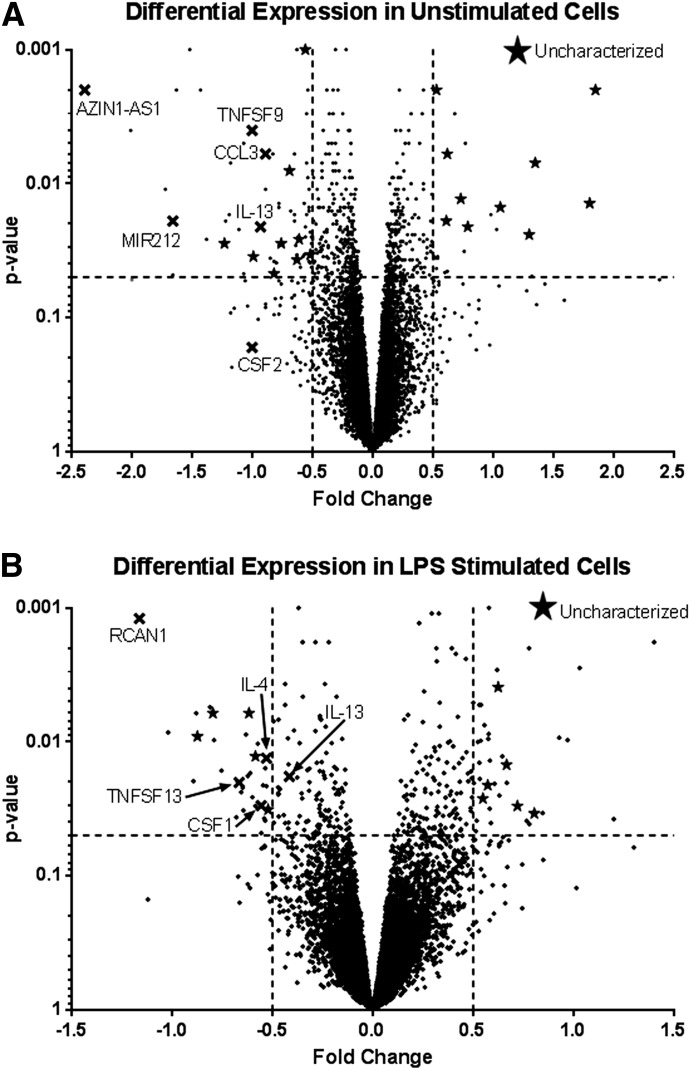
To understand the broad effect of TLR10 engagement on primary monocytes, we evaluated the transcriptome of monocytes in the presence and absence of the αTLR10 Ab, with and without LPS stimulation. RNA from 4 different donors was collected and sequenced. After setting an arbitrary cutoff for significance at P < 0.05 and a fold change >50%, relatively few genes were significantly differentially expressed, either negatively or positively (Fig. 4, Supplemental Table 1). Of these genes, KEGG analysis showed that genes involved in cytokine–cytokine receptor interactions were the largest family of genes negatively regulated in both the unstimulated and LPS-stimulated conditions, which included the genes CSF1 (M-CSF), CSF2 (GM-CSF), IL-4, IL-13, CCL3, and several members of the TNF superfamily. Of the genes that were positively regulated, no functional pathway was identified because of the high number of uncharacterized genes present. To confirm the differential expression, the genes TNFα, CSF1, COX7C, ALOX12, EGF, IL-4, and MGLL, some of which did not necessarily make our RNA-seq significance cutoff but were significant at a level close to 0.05 and were functionally interesting, were validated by qPCR and showed expression patterns that matched those of the RNA-seq data (Supplemental Fig. 2).
Figure 4. The monocyte transcriptome is differentially regulated by TLR10 engagement.
Isolated human monocytes from peripheral blood were preincubated with either an αTLR10 (5C2C5) or an isotype control Ab for 10 min before either no or LPS stimulation. RNA was collected after 2 h of stimulation and sequenced. Two pairwise calculations were performed. One that compared isotype and anti-TLR10 treated cells that were not stimulated (A) and the other that compared isotype and anti-TLR10 treated cells stimulated with LPS (B). Dotted line: cutoff values of at least a 50% fold change and P < 0.5. X’s denote genes of interest, and stars denote uncharacterized proteins within the prescribed cutoff values.
Long-term engagement of TLR10 suppresses monocyte differentiation
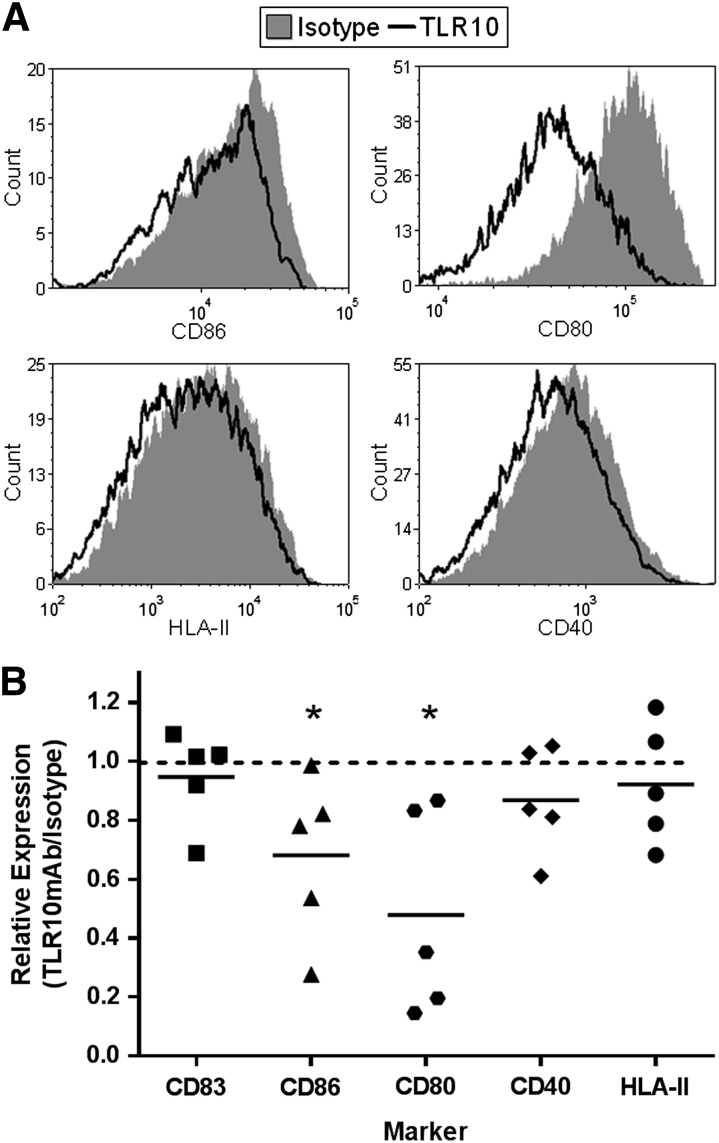
The suppression of the colony stimulating factor genes in the RNA-seq data suggested to us that TLR10 has an effect on monocyte differentiation. To test this hypothesis, monocytes were differentiated for 4 d using CSF2 (GM-CSF) and IL-4 in the presence of either αTLR10 or isotype control Abs and tested for the expression of several dendritic cell maturation markers. Although CD83 expression was not affected, the expression of the B7 complex consisting of CD80/86 was significantly lower in αTLR10-treated cells. The other markers examined, CD40 and HLA-II had increased variability but trended toward lower expression levels, as well (Fig. 5).
Figure 5. TLR10 engagement suppresses dendritic cell maturation marker expression of differentiating monocytes.
Monocytes were differentiated into dendritic cells with GM-CSF/IL-4 for 4 d in the presence of either the TLR10 Ab (5C2C5) or an isotype control Ab. Cells were then collected and stained for the indicated maturation markers. (A) Representative histograms from 5 donors. (B) Summary data where each dot represents single-donor TLR10-treated cells after normalizing the values to isotype-treated cells for the indicated marker. *P < 0.05.
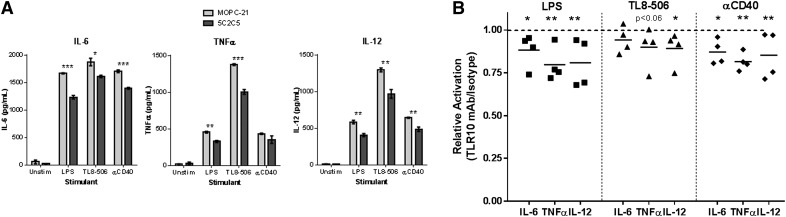
The αTLR10-cultured monocyte-derived immature dendritic cells were next tested for their ability to produce cytokines after TLR or αCD40 stimulation. The cytokines IL-6, TNFα, and IL-12 either trended downward or were significantly suppressed in the presence of the αTLR10 Ab (Fig. 6). In addition, the expression of TLR10 itself decreased upon differentiation into dendritic cells, although the degree of cytokine suppression seen in monocytes compared to dendritic cells did not change (Supplemental Fig. 3). Overall, the data show that monocytes differentiated in the presence of the αTLR10 Ab produced dendritic cells with reduced expression of costimulatory markers and the production of inflammatory cytokines.
Figure 6. Dendritic cells differentiated with αTLR10 have a decreased capacity to produce proinflammatory cytokines.
Monocytes were differentiated into dendritic cells with GM-CSF/IL-4 for 4 d in the presence of either the TLR10 Ab (5C2C5) or an isotype control Ab and then stimulated for 24 h with the indicated agonist. Supernatants were collected and analyzed as described in Fig 1. (A) Representative data from 4 different donors. (B) Summary data, where each dot represents single-donor TLR10-treated cells after the values were normalized to isotype-treated cells for the indicated analyte and stimulant. *P < 0.05; **P < 0.01; ***P < 0.001.
αTLR10-differentiated dendritic cells have a reduced capacity to activate T cells
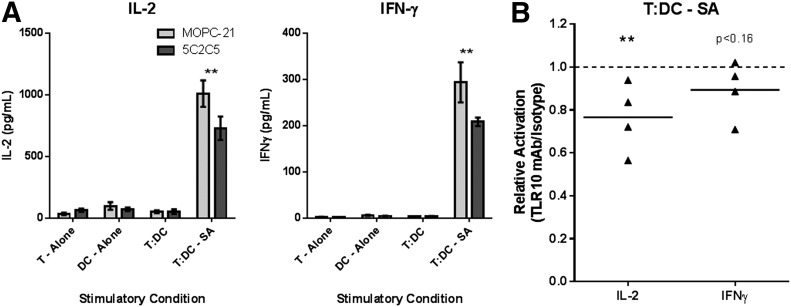
Last, we examined whether the αTLR10-differentiated dendritic cells have reduced functional capacity to stimulate T cells. To this end, we cocultured dendritic cells with autologous T cells in the presence of superantigen and measured the induction of the T cell cytokines IL-2 and IFN-γ. Neither the T cells nor the dendritic cells, alone or in coculture, produced a significant amount of IL-2 and IFN-γ without superantigen present. When treated with the superantigen, both IL-2 and IFN-γ were strongly induced, with neither IL-4 nor -17 being produced under these conditions (data not shown). T cell activation in the presence of the superantigen was significantly suppressed when cocultured with the αTLR10-differentiated dendritic cells (Fig. 7). These data show that TLR10 engagement on differentiating monocytes yields dendritic cells with a reduced functional capacity to activate T cells ex vivo.
Figure 7. TLR10-cultured dendritic cells have a decreased capacity to activate T cells.
Monocytes were differentiated into dendritic cells with GM-CSF/IL-4 for 4 d in the presence of either the TLR10 Ab (5C2C5) or an isotype control Ab. Dendritic cells were cocultured at a 1:20 ratio with autologous T cells and incubated with or without superantigen (SA) for 3 d, as indicated. Supernatants were collected and analyzed as described in Fig. 1. (A) Representative data from 4 donors. (B) Summary data, where each dot represents a single donor’s TLR10-treated cells after normalizing the values to isotype-treated cells for the indicated analyte. **P < 0.01; ***P < 0.001.
DISCUSSION
Our group had shown that when overexpressed, TLR10 suppresses cytokine production in human monocytic cell lines and in a transgenic mouse expressing human TLR10 [16]. This study expands upon our finding that TLR10 functions as a suppressor of MyD88-dependent and -independent responses by using an in-house–generated αTLR10 Ab. One limitation of this study is that we do not possess direct evidence that the 5C2C5 αTLR10 Ab is a blocking or activating Ab, although we have several indirect lines of evidence that suggest the latter. The 2 hypotheses that explain why we see decreased cytokine production upon addition of the αTLR10 Ab is that either the proinflammatory function is blocked or the anti-inflammatory function of TLR10 is activated. For it to be blocked, we would have to assume TLR10 induces a proinflammatory response that no group has been able to detect. Much of the support for the activation of an anti-inflammatory function comes from our publication in which we reported suppression when TLR10 is overexpressed as a homodimer [16]. In addition, our time-course experiment that detected TLR10-mediated suppression up to 8 h after stimulation suggests an active signal propagating from the TLR10 complex after ligation by our Ab. For those reasons, we believe that Ab-mediated engagement of the TLR10 receptor induces homodimerization and activation of a suppressive response.
TLR10 is most homologous to TLR-1 and -6, which are arranged in tandem on the chromosome and are thought to have arisen from several gene-duplication events [18]. Despite this close homology, TLR10 contains several key mutations within its TIR domain and more specifically within the BB loop of the TIR domain that has been shown to be critical in TLR signaling [19, 20]. The crystal structure of TLR10 reveals a TIR domain fold similar to the other TLR family members, with the BB loop exposed and available to interact with potential TIR domain-containing adaptors [21]. The amino acids within the BB loop of human TLR10 are unique among the human TLR family and, more important, are conserved across different mammalian species, suggesting that those mutations are critical for TLR10’s function (unpublished observations).
With TLR10 containing several key amino acid differences in its BB loop and the novel suppressive function of the receptor, it is unlikely that TLR10 mediates this function through one of the classic TLR adaptor proteins, including MyD88, MAL (TIRAP), TRIF, and the TRIF-related adaptor molecule (TRAM). However, there are several other TIR domain-containing proteins that have been discovered more recently and whose functions within the cell are not yet clearly defined [22]. These proteins include the sterile α- and armadillo-motif–containing protein [23], BCAP [24], and B-cell scaffold protein with ankyrin repeats-1 [25]. Both sterile α- and armadillo-motif-containing protein and BCAP have been suggested to have regulatory roles within the cell. BCAP in particular has been shown to connect TLR signaling to the PI3K/Akt signaling pathway that can lead to suppression of inflammatory cytokine release [24].
An alternate hypothesis is that TLR10-mediated activation induces other anti-inflammatory cytokines such as IL-10. Our data suggest that this is not what occurred in isolated monocytes where we measured suppressed levels of IL-10 after stimulation. In addition, the TLR10-mediated suppression was detected in the MAPK and Akt signaling proteins after only 15 min of stimulation, suggesting that suppression occurs before the transcription of proinflammatory or anti-inflammatory cytokines. Last, from our analysis of the transcriptome of isolated monocytes after TLR10 engagement, we show a lack of anti-inflammatory cytokines up-regulated in the conditions we tested. Currently, we do not believe anti-inflammatory cytokine production to be the primary mechanism of TLR10-mediated suppression.
In support of our results, another publication reported that Ab-mediated engagement of TLR10 with a different TLR10 mAb suppressed TLR2-mediated inflammatory responses in mononuclear cells. In that study, TLR10 engagement blocked the inflammatory responses of both primary human cells and a TLR10 transgenic mouse treated with the TLR2 agonist PAM3CSK4 or whole Borrelia burgdorferi [26]. Our study expands on this data to show a much broader suppression profile for TLR10, including other TLRs and CD40 stimulations.
Two other studies have found proinflammatory roles for TLR10 in the context of live H1N1 flu virus or Listeria monocytogenes infections. In these studies the effect of knocking down TLR10 expression on proinflammatory cytokine production by either THP-1 or HT-29 cells was measured. It is interesting to note that, in both studies, TLR10 knockdown cells exhibited a proinflammatory role only when infected with live and not UV-inactivated or heat-killed pathogens [27, 28]. Although in our study we challenged cells with an intracellular TLR8 agonist resulting in suppression, there is perhaps a role for intracellular TLR10 in the recognition of an in vivo pathogen-associated molecular pattern that results in alternative signaling outputs [29].
Overall, this work advances our knowledge of the last orphan human TLR and its potential function in vivo. Our data show that Ab-mediated engagement of TLR10 is capable of suppressing classic proinflammatory signaling pathways induced by several different agonists that result in decreased levels of proinflammatory cytokines. In addition, long-term engagement of TLR10 is sufficient to effect the differentiation of monocytes into dendritic cells by lowering their capacity to active T cells. Although the true biologic function of TLR10 may not be fully understood until the discovery of a natural ligand, the suppressive function of TLR10 described in this report has far-reaching therapeutic implications for a variety of diseases characterized by dysregulated immune activation.
AUTHORSHIP
N.J.H. and R.I.T. designed the study; N.J.H., C.F., and J.G. performed the research; N.J.H. analyzed the data; and N.J.H. and R.I.T. wrote the manuscript.
ACKNOWLEDGMENTS
This work was supported by Grant 1R01-AI097639 from the National Institute of Allergy and Infectious Diseases (NIAID) of the U.S. National Institutes of Health. The authors thank the Functional Genomics, DNA Services, and HPCBio groups at the University of Illinois Urbana-Champaign, Roy J. Carver Biotechnology Center, for their assistance in designing, performing, and analyzing the RNA-sequencing data.
Glossary
- BCAP
B cell adaptor for PI3K
- TIR
toll/interleukin receptor
- TIRAP
TIR domain containing adaptor protein
- TRIF
TIR-domain-containing adaptor-inducing IFN-β
Footnotes
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
Disclosures
The authors declare no conflicts of interest.
REFERENCES
- 1.Kawai T., Akira S. (2010) The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 11, 373–384. [DOI] [PubMed] [Google Scholar]
- 2.Hornung V., Rothenfusser S., Britsch S., Krug A., Jahrsdörfer B., Giese T., Endres S., Hartmann G. (2002) Quantitative expression of toll-like receptor 1-10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J. Immunol. 168, 4531–4537. [DOI] [PubMed] [Google Scholar]
- 3.Geissmann F., Jung S., Littman D. R. (2003) Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19, 71–82. [DOI] [PubMed] [Google Scholar]
- 4.Waschbisch A., Schröder S., Schraudner D., Sammet L., Weksler B., Melms A., Pfeifenbring S., Stadelmann C., Schwab S., Linker R. A. (2016) Pivotal role for CD16+ monocytes in immune surveillance of the central nervous system. J. Immunol. 196, 1558–1567. [DOI] [PubMed] [Google Scholar]
- 5.Lauvau G., Loke P., Hohl T. M. (2015) Monocyte-mediated defense against bacteria, fungi, and parasites. Semin. Immunol. 27, 397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiong H., Pamer E. G. (2015) Monocytes and infection: modulator, messenger and effector. Immunobiology 220, 210–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnold-Schrauf C., Berod L., Sparwasser T. (2015) Dendritic cell specific targeting of MyD88 signalling pathways in vivo. Eur. J. Immunol. 45, 32–39. [DOI] [PubMed] [Google Scholar]
- 8.Krawczyk C. M., Holowka T., Sun J., Blagih J., Amiel E., DeBerardinis R. J., Cross J. R., Jung E., Thompson C. B., Jones R. G., Pearce E. J. (2010) Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood 115, 4742–4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Den Haan J. M. M., Arens R., van Zelm M. C. (2014) The activation of the adaptive immune system: cross-talk between antigen-presenting cells, T cells and B cells. Immunol. Lett. 162(2 Pt B), 103–112. [DOI] [PubMed] [Google Scholar]
- 10.Jenkins K. A., Mansell A. (2010) TIR-containing adaptors in toll-like receptor signalling. Cytokine 49, 237–244. [DOI] [PubMed] [Google Scholar]
- 11.Gay N. J., Symmons M. F., Gangloff M., Bryant C. E. (2014) Assembly and localization of toll-like receptor signalling complexes. Nat. Rev. Immunol. 14, 546–558. [DOI] [PubMed] [Google Scholar]
- 12.Kawasaki T., Kawai T. (2014) Toll-like receptor signaling pathways. Front. Immunol. 5, 461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin S.-C., Lo Y.-C., Wu H. (2010) Helical assembly in the MyD88-IRAK4-IRAK2 complex in TLR/IL-1R signalling. Nature 465, 885–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guan Y., Ranoa D. R. E., Jiang S., Mutha S. K., Li X., Baudry J., Tapping R. I. (2010) Human TLRs 10 and 1 share common mechanisms of innate immune sensing but not signaling. J. Immunol. 184, 5094–5103. [DOI] [PubMed] [Google Scholar]
- 15.Chuang T., Ulevitch R. J. (2001) Identification of hTLR10: a novel human toll-like receptor preferentially expressed in immune cells. Biochim. Biophys. Acta 1518, 157–161. [DOI] [PubMed] [Google Scholar]
- 16.Jiang S., Li X., Hess N. J., Guan Y., Tapping R. I. (2016) TLR10 is a negative regulator of both MyD88-dependent and -independent TLR signaling. J. Immunol. 196, 3834–3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hess N. J., Jiang S., Li X., Guan Y., Tapping R. I. (2017) TLR10 is a B-cell intrinsic suppressor of adaptive immune responses. J. Immunol. 198, 699–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mikacenic C., Reiner A. P., Holden T. D., Nickerson D. A., Wurfel M. M. (2013) Variation in the TLR10/TLR1/TLR6 locus is the major genetic determinant of interindividual difference in TLR1/2-mediated responses. Genes Immun. 14, 52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mikami T., Miyashita H., Takatsuka S., Kuroki Y., Matsushima N. (2012) Molecular evolution of vertebrate toll-like receptors: evolutionary rate difference between their leucine-rich repeats and their TIR domains. Gene 503, 235–243. [DOI] [PubMed] [Google Scholar]
- 20.Roach J. C., Glusman G., Rowen L., Kaur A., Purcell M. K., Smith K. D., Hood L. E., Aderem A. (2005) The evolution of vertebrate toll-like receptors. Proc. Natl. Acad. Sci. USA 102, 9577–9582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nyman T., Stenmark P., Flodin S., Johansson I., Hammarström M., Nordlund P. (2008) The crystal structure of the human toll-like receptor 10 cytoplasmic domain reveals a putative signaling dimer. J. Biol. Chem. 283, 11861–11865. [DOI] [PubMed] [Google Scholar]
- 22.Kondo T., Kawai T., Akira S. (2012) Dissecting negative regulation of Toll-like receptor signaling. Trends Immunol. 33, 449–458. [DOI] [PubMed] [Google Scholar]
- 23.Panneerselvam P., Ding J. L. (2015) Beyond TLR signaling: the role of SARM in antiviral immune defense, apoptosis and development. Int. Rev. Immunol. 34, 432–444. [DOI] [PubMed] [Google Scholar]
- 24.Troutman T. D., Hu W., Fulenchek S., Yamazaki T., Kurosaki T., Bazan J. F., Pasare C. (2012) Role for B-cell adapter for PI3K (BCAP) as a signaling adapter linking Toll-like receptors (TLRs) to serine/threonine kinases PI3K/Akt. Proc. Natl. Acad. Sci. USA 109, 273–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu Y.-Y., Kumar R., Iida R., Bagavant H., Alarcón-Riquelme M. E. (2016) BANK1 regulates IgG production in a lupus model by controlling TLR7-dependent STAT1 activation. PLoS One 11, e0156302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oosting M., Cheng S.-C., Bolscher J. M., Vestering-Stenger R., Plantinga T. S., Verschueren I. C., Arts P., Garritsen A., van Eenennaam H., Sturm P., Kullberg B.-J., Hoischen A., Adema G. J., van der Meer J. W. M., Netea M. G., Joosten L. A. B. (2014) Human TLR10 is an anti-inflammatory pattern-recognition receptor. Proc. Natl. Acad. Sci. USA 111, E4478–E4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee S. M. Y., Kok K.-H., Jaume M., Cheung T. K. W., Yip T.-F., Lai J. C. C., Guan Y., Webster R. G., Jin D.-Y., Peiris J. S. M. (2014) Toll-like receptor 10 is involved in induction of innate immune responses to influenza virus infection. Proc. Natl. Acad. Sci. USA 111, 3793–3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Regan T., Nally K., Carmody R., Houston A., Shanahan F., Macsharry J., Brint E. (2013) Identification of TLR10 as a key mediator of the inflammatory response to Listeria monocytogenes in intestinal epithelial cells and macrophages. J. Immunol. 191, 6084–6092. [DOI] [PubMed] [Google Scholar]
- 29.Mourao-Sa D., Roy S., Blander J. M. (2013) Vita-PAMPs: signatures of microbial viability. Adv. Exp. Med. Biol. 785, 1–8. [DOI] [PubMed] [Google Scholar]