Summary
BACKGROUND
Plexiform neurofibromas (PN) are slow growing chemoradiotherapy resistant tumours arising in patients with neurofibromatosis type I (NF1). Currently there are no viable therapeutic options for patients whose life-threatening plexiform neurofibromas cannot be surgically removed due to proximity to vital body structures. Based on identification of molecular targets in genetic mouse models of human NF1 tumours, we hypothesized that the oral kinase inhibitor, imatinib mesylate, may be effective in targeted treatment of these chemoradiotherapy-refractory tumours.
METHODS
An open-label pilot Phase II clinical trial was designed to test whether treatment with imatinib mesylate can decrease volume burden of clinically significant plexiform neurofibromas in NF1 patients. The entry criteria require patients only to have NF1 and a clinically significant plexiform neurofibroma with the specified age limitations (age 3–65). Patients were treated with daily oral imatinib at 440 mg/m2/day for children and 800 mg/day for adults divided twice daily for 6 months. The primary endpoint measure of significant response was a 20% or more reduction in plexiform size by sequential volumetric MRI imaging. Clinical data was analyzed on an intent to treat basis, however to determine the activity of imatinib on NF1-related plexiform tumours, patients able to take imatinib for 6 months were evaluated for their response. Secondary outcomes included evaluation of safety of imatinib mesylate in this group of patients. The trial is registered at http://clinicaltrials.gov/; study number 0512-25. The trial currently is closed to enrollment, however there is a single patient that continues to respond and remains on study.
FINDINGS
On an intent to treat basis, 6 out of 36 patients or 17% (95% CI: 6 – 33%) experienced objective response to imatinib mesylate. In the evaluable study population of patients (n=23) who received drug for at least six months, six patients (26%; 95% CI: 10 – 48%) experienced ≥ 20% decrease in volume of one or more plexiform tumours and 30% of study patients had symptomatic improvement. We noted significant inter-patient and intra-patient heterogeneity of plexiform neurofibroma response. Toxicity of drug was comparable to previous reports in patients with chronic myelogenous leukemia. The most common adverse events were reversible skin rash (5 patients) and edema with weight gain (6 patients). More serious adverse events included reversible grade 3 neutropenia (2 patients) and grade 4 transaminitis (one patient).
INTERPRETATION
Imatinib mesylate caused disease regression in 26% of evaluable patients with clinically significant plexiform neurofibromas due to neurofibromatosis type 1. These results warrant confirmation in a larger multi-institutional clinical trial aimed at this patient population. These findings provide the first demonstration of radiographic volumetric tumour reduction in response to medical therapy in patients with NF1 plexiform neurofibromas using imatinib mesylate based on studies in a pre-clinical genetic mouse model. These translational studies form the framework whereby other agents may be tested/compared to imatinib in the pre-clinical model and moved into the clinic advancing development of more effective therapies for NF1-related plexiform neurofibromas.
INTRODUCTION
Neurofibromatosis type 1 (NF1) is the most common human genetic cancer predisposition syndrome, causing significant morbidity and mortality in approximately one in 3000 individuals1,2. It results from autosomal dominant mutations in the NF1 tumour suppressor gene that encodes a Ras GTPase named neurofibromin3. Deficiency of neurofibromin leads to hyperactivation of the Ras signaling cascade and other signal transduction networks4,5. In approximately 40% of NF1 patients6, the aberrations in these cellular signaling networks culminate in development of tumours known as plexiform neurofibromas (PN). Plexiform neurofibromas occur as multiple primary tumours, each with their own growth characteristics. They arise from loss of heterozygosity in individual Schwann cells in virtually any anatomic location where Schwann cells reside. These locally invasive tumours can be painful, disfiguring, and life-threatening when localized near vital structures such as upper airway or major nerves and blood vessels7,8. Due to their slow-growing nature, plexiform neurofibromas are highly refractory to radiotherapy and chemotherapy, and surgery is often extremely challenging due to localization of these tumours7. Given the lack of viable treatment options, there is an urgent medical need for novel therapeutic approaches to allow successful management of plexiform neurofibromas.
In recent studies, we have demonstrated that administration of the kinase inhibitor, imatinib mesylate, reduces tumour size of plexiform neurofibromas in a preclinical mouse model of NF1 that fully recapitulates the development of plexiform neurofibromas observed in NF1 patients9,10. Mechanistically, this effect is attributed at least in part to targeting cellular phospho-signaling cascades in the tumour microenvironment8,11. Based on these observations, we administered imatinib mesylate (350 mg/m2/d) to a single NF1 patient with life-threatening airway compression by a plexiform neurofibroma, and achieved dramatic (>50%) reduction in tumour size within three months of therapy resulting in significant symptomatic relief8. To build on these observations and determine whether imatinib could decrease volume of individual plexiform neurofibromas in other NF1 patients, we performed a pilot phase II open-label trial of this oral small-molecule kinase inhibitor in NF1 patients with clinically significant plexiform neurofibromas.
METHODS
STUDY PATIENTS
Patients were recruited from the Indiana University School of Medicine Neurofibromatosis Clinic (CH, LW) and the Neurofibromatosis Clinic at UT Southwestern Medical Center/Children’s Medical Center – Dallas (DB) or self-referral. Patient entry criteria included: (1) age of 3–65 years, (2) clinical diagnosis of neurofibromatosis type 1 and (3) presence of clinically significant plexiform neurofibromas defined as (a) potentially life-threatening tumours, (b) tumours impinging on vital structures, or (c) tumours that significantly impair patients’ quality of life from a subjective standpoint due to pain or other symptoms (eg, dyspnea, urinary dysfunction, weakness etc depending on location of individual plexiform tumours). Furthermore, patients must have had at least one plexiform neurofibroma that could be measured by MRI (at least 10 mm in largest dimension) in order to permit objective measurements of tumour response to treatment. Eligible patients must have had a life expectancy of more than two months, Karnofsky12 or Lansky13 performance score of ≥80%, and adequate end-organ function (defined as total bilirubin < 1·5 x upper normal limit (UNL), SGOT and SGPT < 2·5 x UNL, creatinine < 1·5 x ULN, absolute neutrophil count > 1·5 × 109/L, and platelets > 100 × 109/L). Female patients of childbearing potential must have had a negative pregnancy test within 7 days prior to study enrollment, and men and women had to agree to use a barrier birth control method while on study and for three months following discontinuation of study drug.
Key exclusion criteria were: (1) exposure to chemotherapy or any other investigational agents within 28 days prior to enrollment on study; (2) history of another malignancy within 5 years; (3) known brain metastases; (4) New York Heart Association Criteria for class III or IV heart failure14; (5) other uncontrolled medical disease; (6) pregnancy or breast-feeding; (7) HIV infection; (8) history of radiation to >=25% of bone marrow space; (9) history of a major surgery within 2 weeks prior to study entry, and (10) significant concern for medical non-compliance.
Major criteria for discontinuation of study drug included (1) evidence of clinically and radiologically progressive disease, (2) patient’s/parent’s request, and (3) adverse effects requiring removal from study.
MEASUREMENT OF TUMOUR SIZE
At recruitment, patients were imaged with total body MRI using short Tau inversion recovery (STIR) technique, which suppresses fat signal and accentuates the water signal. Neurofibromas typically demonstrate hyperintense signal on T2 weighted imaging. With fat suppression, STIR is able to easily differentiate neurofibromas from surrounding tissue without the addition of intravenous gadolinium. All imaging was performed on a 1·5 Tesla clinical MRI (Magnetom Avanto, Siemens Medical Solutions, Malvern, PA) in the coronal and axial planes at 4 mm gapless slice thickness. Criteria for selection of plexiforms to measure included: 1) tumours likely contributing to clinical problems, 2) images distinct enough for accurate measure, 3) tumours large enough (≥ 10 mm) with a minimum of 3 MRI slices for accurate volumetric determination. Volumes of up to five plexiform neurofibromas per patient were measured using a manual volumetric technique15, 16. Areas of each tumour were measured on sequential MRI sections by manually outlining the tumour, and the sum of area was multiplied by the MRI slice thickness to calculate the tumour volume as described before15, 16. Individual plexiform neurofibromas were measured using the same technique across sequential scans, matching anatomical features of the tumour and surrounding structures from scan to scan. Consistent with previous clinical trials in NF tumours17–19,23, response was defined as a sustained ≥ 20% reduction in tumour volume from baseline, progression was defined as ≥ 20% increase in tumour volume, and tumours that showed < 20% reduction and < 20% increase in volume were categorized as stable. Given that to date there has been no effective therapy for plexiform neurofibromas, and taking into account the presence of multiple individual tumours, our primary goal was to determine if any individual plexiform neurofibromas could respond to imatinib. Inherent in that endpoint is the recognition that with individual tumour growth qualities and molecular evolution, some plexiform neurofibromas may respond while other may remain stable or continue to grow.
STUDY DESIGN AND PROCEDURES
Upon confirmation of eligibility and study recruitment, patients were imaged with MRI STIR imaging to measure baseline tumour size (Figure 1b). Plasma specimens for future baseline tumour biomarker measurements were obtained in addition to routine laboratory studies described above. Imatinib mesylate (provided by Novartis) was administered at the dose of 800 mg/day by mouth divided twice daily in adults and 440 mg/m2/day by mouth in children divided twice daily (dosing at the MTD per recommendations by study drug manufacturer). Patients returned to clinic for follow-up visits including review of symptoms, physical examination, complete blood count and serum chemistries (weekly x 2; every two weeks X 1; monthly x 1; every two months x 2, and then every three months thereafter - or as clinically indicated). Follow-up MRI tumour measurements were performed after two months of treatment, then at six months, one year and yearly thereafter. Plasma samples for follow-up correlative tumour marker studies were obtained at study enrollment and six months after beginning the study drug and stored for future analysis. Treatment with imatinib mesylate was continued for 6 months with an option to continue as long as the patient showed benefit from the study drug and there were no safety concerns. Daily diaries were kept by patients/parents to monitor compliance and toxicities. Given the variation of symptoms in patients and lack of a validated quality of life tool for neurofibromatosis, no quantitative assessment was performed beyond the patient’s subjective impression.
Figure 1.
The design of pilot phase II trial of imatinib mesylate in neurofibromatosis patients with plexiform neurofibromas.
SAFETY AND STUDY OVERSIGHT
The protocol was approved by the institutional review boards at Indiana University School of Medicine and the University of Texas Southwestern-Medical School-Dallas, and an IRB approved written informed consent was obtained from all patients. Adverse events were graded according to the National Cancer Institute (NCI) Common Terminology Criteria v3·0 (CTCAE http://ctep.info.nih.gov)). Imatinib mesylate dose modifications were permitted for grade 3/4 adverse effects and for grade two skin rash deemed to be due to study drug. The Principal investigator was required to notify Institutional Review Board, FDA and study drug manufacturer about occurrence of serious adverse effects within three working days.
STUDY OUTCOME STATISTICAL ANALYSIS
The primary outcome was the objective tumour response rate to imatinib mesylate evidenced by volumetric tumour measurements of MRI images. Confidence intervals (95%) were calculated for the response rate to imatinib. The pre-specified secondary outcomes were to assess safety and tolerability of imatinib mesylate in NF1 patients with plexiform neurofibromas. The statistical software is R language, version 2.14.1.
ROLE OF THE FUNDING SOURCE
The study is registered with clinicaltrials.gov; study ID number 0512-25. Novartis Pharmaceuticals provided imatinib mesylate study drug. The study sponsor, Novartis Pharmaceuticals, had no role in the study design, in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication. Kent A. Robertson, M.D., had full access to all the data in the study and had final responsibility for the decision to submit this manuscript for publication.
RESULTS
PATIENTS
Between June 1, 2006 and March 30, 2009, 36 patients were enrolled on the study (Table 1), three patients from UT Southwestern and 33 from Indiana University. Of the 36 patients enrolled, the median age was 13 years, with a median age of 12 years in the 23 evaluable patients. There were 19 males and 17 females enrolled. A total of 23 patients (64%) completed six months of study drug and were evaluable. The remaining 13 patients withdrew from the study prematurely, which precluded the evaluation of the biological impact of the study drug as established by the criteria at the beginning of the study (Table 2). Specifically, nine patients elected to discontinue the study drug prior to 6 months because of minor problems with taking study drug or drug side effects largely a result of dosing at the MTD. Two patients discontinued study drug due to their local physician’s concern for tumour progression that could not be objectively verified due to CT/MRI scan incompatibility. Finally, two patients discontinued study drug because one underwent tumour resection and the other had plexiforms too small to be measured volumetrically. Of the evaluable pediatric patients (age 3–9yr) there were 6 male, 3 female with a median age of 7 yr. Of the evaluable adolescent patients (age 10–18yr) there were 5 male, 3 female with a median age of 13 yr. Of the evaluable adult patients (age ≥19yr) there were 4 male, 2 female with a median age of 26 yr. The study group included patients within the age range of three to 52 years; the majority of patients (n=17) were children and adolescents. The localization of plexiform neurofibromas varied among the patients, with almost half of the tumours localized in the head and neck region (Table 3). Currently, one of the study patients is still receiving imatinib mesylate.
Table 1.
Patient Characteristics
| Characteristic | Enrolled Patients (36) | Evaluable Patients (23) | |
|---|---|---|---|
| Age Range in Years (median) male, female | Total | 36 Pts age 3–52 (13) 19 male, 17 female |
23 Pts age 3–33 (12) 15 male, 8 female |
| Pediatric group (3–9yr) | 13 Pts age 3–9 (4) 9 male, 4 female |
9 Pts age 3–9 (7) 6 male, 3 female |
|
| Adolescent group (10–18yr) | 12 Pts age 10–17 (13) 6 male, 6 female |
8 Pts age 11–17 (13) 5 male, 3 female |
|
| Adult group (≥19 yr) | 11 Pts age 19–52 (28) 4 male, 7 female |
6 pts age 19–33 (26) 4 male, 2 female |
|
| Number Plexiforms | Total (Median per patient) | 107 (3) | 69 (3) |
| Plexiform Site | Head/Neck | 15 | 11 |
| Abd/Pelvis | 5 | 5 | |
| Extremity | 1 | 1 | |
| Paraspinal | 4 | 2 | |
| Generalized | 11 | 4 |
Table 2.
Reasons for discontinuing imatinib and not being included as evaluable patients for 13 patients enrolled on the study
| Age | Reason to Discontinue Drug | Duration |
|---|---|---|
| 4 yo male | Pt refused to take drug, parent decision | 3 months |
| 3 yo male | Initial MRI and follow-up CT not comparable, off per primary MD | 2 months |
| 5 yo male | MRI’s not comparable for volumetric determination; PI decision | 6 months |
| 3 yo female | Pt refused to take drug, parent decision | 1 month |
| 14 yo female | Resection of plexiforms; parent decision | 2 months |
| 13 yo female | Non-compliance; parent decision | 5 months |
| 10 yo female | Edema, felt to be drug related-parent decision | 2 months |
| 13 yo male | Unable to take drug consistently due to extensive GI plexiforms; MD/parent decision | *12 months |
| 52 yo female | Minor anorexia, weight loss, Pt decision | 3 months |
| 37 yo female | Plexiforms too small for volumetric anslysis, off per PI | 12 months |
| 28 yo female | Grade 3–4 drug related edema, seizure, off per MD | 4 months |
| 21 yo female | Grade 4 hepatic toxicity, MD decision | 5 months |
| 48 yo female | Drug related edema, weight gain, GI toxicity, Pt decision | 4 months |
Could only take an occasional dose and then come off drug for weeks at a time. Parents wished to continue to try dosing but without success.
Table 3.
Plexiform neurofibroma response by age, location, and plexiform size.
| Tumor Response According to Age | ||||
|---|---|---|---|---|
| Age | No. PN | No. (%) Responsive1 | No. (%) Stable2 | No. (%) Progressive3 |
| 3 – 9 yr | 23 | 3 (13%) | 10 (43%) | 10 (43%) |
| 10 – 18 yr | 23 | 2 (9%) | 13 (56%) | 8 (35%) |
| ≥ 19 yr | 23 | 3 (13%) | 13 (56%) | 7 (30%) |
| Total → | 69 | 8 (12%) | 36 (52%) | 25 (36%) |
| Tumor Response According to Location | ||||
|---|---|---|---|---|
| Location | No. PN | No. (%) Responsive1 | No. (%) Stable2 | No. (%)Progressive3 |
| H & N | 33 | 7 (21%) | 15 (45%) | 11 (33%) |
| Trunk | 29 | 1 (3%) | 17 (59%) | 11 (38%) |
| Extremities | 7 | 0 (0%) | 4 (57%) | 3 (43%) |
| Total → | 69 | 8 (12%) | 36 (52%) | 25 (36%) |
| Tumor Response According to Tumor Size | ||||
|---|---|---|---|---|
| Size (cm3) | No. PN | No. (%) Responsive1 | No. (%) Stable2 | No. (%)Progressive3 |
| 1 – 5 | 28 | 4 (14%) | 14 (50%) | 10 (36%) |
| 5 – 10 | 15 | 3 (20%) | 9 (60%) | 3 (20%) |
| 10–20 | 10 | 1 (10%) | 8 (80%) | 1 (10%) |
| > 20 | 16 | 0 (0%) | 5 (31%) | 11 (69%) |
defined as ≥ 20% decrease in volume
defined as < 20% decrease or increase in volume
defined as ≥ 20% increase in volume
EFFICACY
On an intent to treat basis, 6 out of 36 patients or 17% (95% CI: 6 – 33%) experienced objective response to imatinib mesylate. In the evaluable study population of patients who received drug for at least six months, six patients (26%; 95% CI: 10 – 48%) experienced ≥ 20% decrease in volume of one or more plexiform tumours (Figure 2a). In these 23 evaluable patients, sixty nine plexiforms were evaluated, with an average of 3 plexiforms per patient. To adjust for clustering that might occur within individual patients, an analysis was performed for correlated binary outcome. The difference was not significant between adult and pediatric patients (p= 0.89; 95% CI for the odds ratio of response for pediatric patients vs adults is (0.19, 6.5). When individual tumours in the evaluable patients were considered, 12% (8/69) were reduced in volume by 20 – 38% upon treatment with imatinib, (95% CI: 5 – 21%, Figure 2b). The median time to the first measurable response in pediatric patients on this study was 4 months and in adults it was 8 months. However, with the small numbers of patients treated on this trial, this trend does not achieve significance (p-value is 0.83 based on a log-rank test for correlated data). Furthermore, there was heterogeneity in response among different tumours within individual patients. Nineteen evaluable patients had multiple measurable tumours with 12 of these patients (63%) exhibiting a heterogeneous response (a mix of responsive, stable, and/or progressive plexiforms), which likely results from the biology of plexiform neurofibromas arising as genetically distinct primary tumours. When the data is analyzed with respect to age, tumour location, and size (Table 3), there are no statistically significant differences. With respect to age, the power of this analysis would only detect a large effect. However, there are some interesting trends: 1) larger tumours tend to be less responsive, which may reflect drug delivery difficulties into massive solid tumours, and 2) head and neck tumours appear to be more responsive than plexiforms localized in other body parts across age groups. Seven evaluable study patients (30%) reported subjective improvement in disease symptoms, including improved dyspnea noted at ENT evaluation and resolution of snoring/disruptive sleep pattern, improved bladder control as evidenced by loss of need for self-catheterization, and decreased pain and improved sensory or motor symptoms in two patients. Specifically, one patient with cervical cord involvement experienced a decrease in pain/tingling of the hands with improved grip strength, and another patient with lumbar cord plexiforms experienced improved leg strength, which allowed him to walk unassisted.
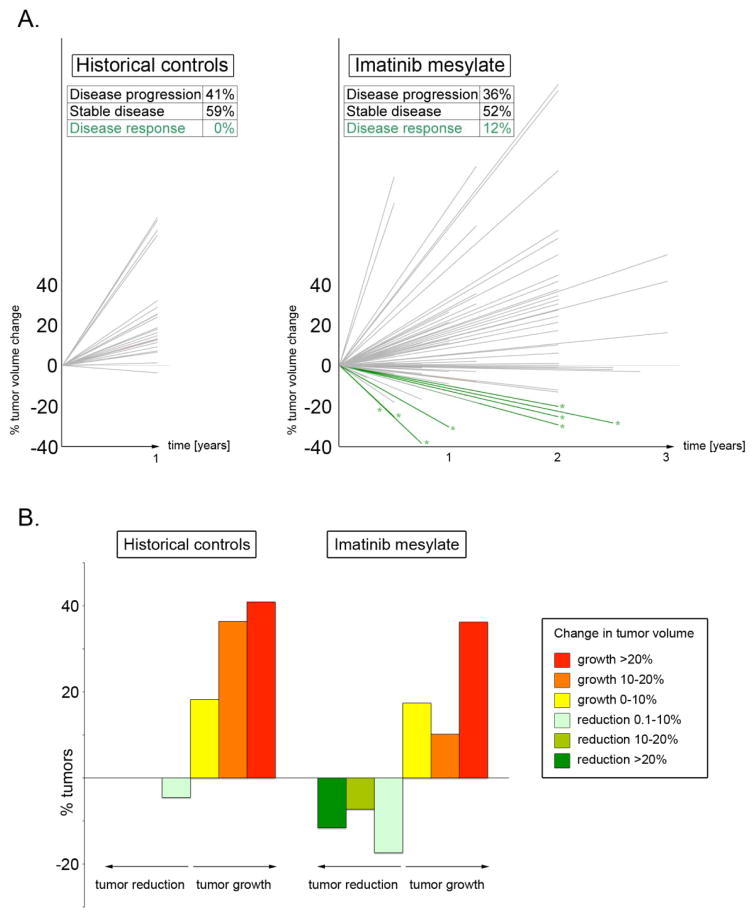
Figure 2.
Treatment with imatinib mesylate changes natural history of plexiform neurofibromas in NF1 patients. 2A, left. Plexiform neurofibroma growth in natural history studies over time in untreated NF1 patients indicates that virtually no tumours shrink over time; instead, they continue to slowly increase in volume (data plotted from reference 17; Table (E)T-3). 2A, right. In striking contrast, a significant fraction of plexiform neurofibromas shrink in response to oral imatinib mesylate. Other plexiform neurofibromas continue to grow in imatinib-treated patients, demonstrating the clinical heterogeneity of these locally invasive tumours. Green lines with asterisks represent tumours that decrease volume over time as evidenced by MRI measurements; grey lines represent growing tumours including those not decreasing by ≥ 20% in volume. 2B. Relative percent of tumours in historical controls (left, ref 17) and imatinib treated patients (right) expressed by percent change in tumour volume.
SAFETY
Nineteen of 36 enrolled patients required dose reductions or interruptions due to treatment-related side effects. The most common adverse effects in the total study population were skin rash and edema (Table 4). Other adverse events included reversible grade 3 neutropenia, weight gain, and grade 4 elevation of hepatic transaminases. There was one death while on study. This death occurred in a patient with a known seizure disorder with an upper respiratory infection, during which the study drug was held. The patient experienced a seizure leading to airway obstruction/aspiration. The death was believed to be unrelated to the study treatment.
Table 4.
Adverse Events
| Grade | Number of Events | Toxicity |
|---|---|---|
| 1 | 8 | Edema(3), nausea(1), depression(1), Abdominal cramping(1), Joint aches(1), neuropathy(1) |
| 2 | 11 | Diarrhea-incontinence(1), hyperbilirubinemia(1), wt gain(1), dyspnea(1), anorexia(1), Rash(1), pain(3), edema(1), ataxia(1) |
| 3 | 10 | Rash(4), seizure(1), neutropenia(2), weight gain(1), motor neuropathy(1), pain(1) |
| 4 | 2 | Elevated transaminases(1), hyperglycemia(1, diabetic patient) |
Panel: Research in context
Systematic review
We searched PubMed for all publications 2000 – 2012, including clinical trials, meta-analysis, and reviews, with the terms “neurofibromatosis type 1”, and “plexiform”. We identified 2 clinical trials of therapies for NF1 plexiform tumours including a phase 1 trial of Tipifarnib19 without objective response, and a phase 1 trial of Pirfenidone18 without objective response using volumetric measures to detect 20% reduction as the response threshold. Finally, a phase 1 trial of pegylated interferon-alpha-2b20 in pediatric NF1 patients with plexiform neurofibromas demonstrated tumour reduction in five out of seventeen patients that were evaluated using volumetric measurements of the tumour. However, compared to the current study, only one individual had a tumour response that was larger than 20%.
Interpretation
Our findings provide the first demonstration of radiographic volumetric tumour reduction in response to medical therapy in patients with NF1 plexiform neurofibromas using imatinib mesylate based on studies in a pre-clinical genetic mouse model.
DISCUSSION
This open-label pilot phase II trial revealed the efficacy and safety of high-dose oral imatinib mesylate in the treatment of clinically significant plexiform neurofibromas in children and adults with neurofibromatosis type 1. Over the last several years, our bench-to-bedside approach to analysis of NF1-controlled signaling networks in animal and cell culture models of neurofibromatosis9, 10, 21, 22 culminated in identification of a small-molecule drug8 whose activity against cells within the microenvironment of plexiform neurofibromas in human patients is supported by this trial. This study is to our knowledge the first demonstration in a human cancer that targeting the genetically altered microenvironment results in a reduction in tumour size.
We have observed objective responses to imatinib mesylate in 26% of evaluable patients enrolled on the study. The rationale for presenting the data in terms of evaluable patients is based on our observations that the majority of the patients who came off study did so because of issues related to compliance, and they never took the drug long enough to test the biological impact of the drug. The reasons for poor compliance relate to the biology of the tumour and the initial dosing of drug. Overwhelmingly, plexiform tumours are slow growing, and consequently many patients have been living with them for a long time, usually measured in years. Thus, unlike patients with highly malignant tumours who are tolerant of at least some side effects, patients with plexiform neurofibromas patients have a very low threshold for any drug related discomfort. The initial choice for dosing of drug also contributed to the lack of compliance. Given that there are no known active or effective agents for plexiforms, we determined that initial dosing would be at the previously established MTD (maximal tolerated dose) to see if imatinib was active for any plexiforms. Collectively, these factors set the stage for a high likelihood of having minor and major side effects in a population of patients that would not tolerate side effects. This resulted in refusal to take drug and compliance issues (9 of the 13 patients coming off study). In fact, because taking the study drug was problematic for these patients, we have significantly modified the dosing regimen in the ongoing follow-up trial with improvement in drug tolerability. Of the other four patients, one came off study after what seemed to be an early response, because the parents saw a window of opportunity to resect the tumour. A second individual was excluded from the study because the tumours were too small to measure volumetrically. Furthermore, two patients withdrew early in the course because of referring physicians’ concerns for tumour progression, although these concerns were not confirmed by CT/MRI scans (Table 2). At the individual tumour level, 12 % of tumours shrank by 20–38% in volume. Natural history studies of NF1 patients report that plexiform tumours never regress but rather display variable progressive growth17. In contrast to historical data, we noted a profound response (≥ 20% decrease in tumour volume) to the study drug in a subset of tumours, some of which reduced in volume by almost 40% with a median reduction of 26%. Furthermore, an even larger subset of tumours in evaluable patients had a decrease in tumour volume as compared to historical controls, but less than the 20% threshold (Figure 2b). Importantly, tumour response was associated with substantial subjective improvement of symptoms reported by patients, including some tumours that had a reduction of less than the 20% threshold. In several cases, the observed clinical improvement was truly remarkable, including better airway patency, regained bladder control and improved lower extremity motor symptoms. It is possible that the response of plexiform neurofibromas to imatinib may in part be due to cells expressing the c-kit receptor in the tumour microenvironment as characterized in the pre-clinical model8. To our knowledge, this is the first successful reduction of plexiform neurofibromas using targeted oral chemotherapy.
It is interesting to note that disease response to the study drug varied not only between patients, but also between different tumours in individual patients. Additionally, the median time to the first measurable response in pediatric patients (4 months) tended to be shorter than in adult patients (8 months). This provocative observation may open new inroads to understanding pathobiology of plexiform neurofibromas, and potentially unravel novel therapeutic targets. We are actively pursuing these clinically relevant hypotheses using a systems-biology approach, as the answers may facilitate customized treatment and screening programs in patients with this common genetic disorder.
Our study is not free of limitations, which include relatively small sample size and significant heterogeneity of patient population with respect to age, tumour location and disease extent. This pilot study takes a necessary step towards developing effective therapeutic strategies for NF1-related plexiform neurofibromas by addressing whether individual tumours can respond to targeted therapy. The inclusion criteria were purposefully kept very broad and inclusive to determine if imatinib showed any activity without restriction of entry criteria other than having NF1 and a clinically significant plexiform neurofibroma with the specified age limitations (age 3–65). A large-scale, multi-institutional non-placebo phase III clinical trial is needed to confirm the results of this pilot study with more open eligibility criteria that avoid the limitations of smaller scale phase 2 trials. A placebo controlled Phase III trial would be unethical since this report establishes imatinib activity against a fraction of plexiform neurofibromas and there are no other treatments available. Based on data in this trial we believe a minimum of one year of evaluation on treatment is important to allow patients to demonstrate responsiveness. Finally, many study patients reported subjective improvement of quality of life/clinical symptoms that frequently (but not always) correlate with tumour response as evidenced by sequential MRIs. We are developing questionnaires and other study tools to allow quantification of subjective clinical improvement and address potential placebo effects in future clinical trials in this patient population. There is an ongoing clinical trial (NCT01140360) further defining the activity of imatinib in NF1 patients.
In summary, this pilot phase II study provides evidence that imatinib mesylate may be successfully used in targeted chemotherapy of plexiform neurofibromas in patients with neurofibromatosis type 1. A multi-institutional clinical trial is warranted to confirm these results and establish new standards of care for patients with this common genetic cancer predisposition syndrome.
Acknowledgments
Funding Novartis Pharmaceuticals
Footnotes
CONTRIBUTORS
Kent A. Robertson, M.D. – Oversight of all phases of the trial including design, conduct of the trial, analysis of data, and writing the manuscript.
Grzegorz Nalepa, M.D. - analyzed data, measured tumour volumes, performed statistical analysis, generated figures, and wrote the manuscript
Feng-Chun Yang, M.D. – analyzed tumour volumes data, generated figures, plasma samples for biochemical analysis.
Daniel C. Bowers, M.D. - Served as institutional PI for the study at my institution (includes institutional regulatory issues, administrative supervision, IRB approval), enrolled patients on the clinical trial at my institution and reviewed, edited and approved the writing of the manuscript
Chang Y. Ho, M.D. – tumour measurements, text contribution and manuscript editing
Gary D. Hutchins, Ph.D. – Tumour volumetric analysis
James M. Croop, M.D. – assisted in study design, evaluating data and reviewed manuscript
Terry A. Vik, M.D. – assisted in protocol design and procedural execution of the trial, and data review.
Scott C. Denne, M.D. – assisted in regulatory issues with the IRB, scientific review committee, and data interpretation.
Luis F. Parada, Ph.D. – Protocol design input from the pre-clinical model
Cynthia M. Hingtgen, M.D. – patient enrollment, neurologic expertise in managing NF1 in adults
Larry E. Walsh, M.D. – patient enrollment, history taking and examination, neurologic expertise in managing NF1 in children
Menggang Yu, Ph.D. – Biostatistician
Kamnesh R. Pradhan, M.D. – provided neuro-oncology expertise and protocol design
Mary K. Edwards-Brown, M.D. – MRI interpretation and tumour measurements.
Mervyn D. Cohen, M.D. – interpreted MRI images and participated in manuscript review
James W. Fletcher, M.D. – review and interpretation of imaging data
Jeffrey B. Travers, M.D. – Provided dermatologic management of NF-related and imatinib-related skin findings
Karl W. Staser, Ph.D. – performed cytokine assays, collected and analyzed data, assisted with manuscript preparation
Melissa W. Lee, BS– provided oversight of the clinical research office and regulatory interface with the study sponsor.
Marcie R. Sherman, BS, MS – clinical coordinator, patient enrollment, scheduling
Cynthia J. Davis, RN – research nurse, patient enrollment, scheduling-screening
Lucy C. Miller, RN– provided regulatory interface with IRB submissions.
David A. Ingram, M.D. – Study design based on pre-clinical model, patient referral
D. Wade Clapp, M.D. – Study design conception and development through all phases from pre-clinical work to execution of the clinical trial.
Study design-development- KAR, FCY, CYH, JMC, TAV, LFP, JWF, DAI, DWC
protocol writing-review- KAR, CYH, JMC, TAV, LFP, KRP, MWL,
(IRB, SRC, FDA, Novartis) regulatory approval- KAR, DCB, JMC, TAV, SCD, MWL,
Subject referral, enrollment, follow-up – KAR, DCB, CMH, LEW, KRP, JBT, MWL, MRS,
data collection- KAR, DCB, CYH, CMH, MKEB, JBT, KWS, MWL, MRS,
data-biostatistical analysis- KAR, GN, FCY, JMC, TAV, MY,
image analysis- KAR, GN, CYH, GDH, MKEB, MDC, JWF,
cytokine assays- FCY, KWS
manuscript preparation- KAR, GN, FCY, CYH, JMC, TAV, SCD, CMH, MY, MDC, KWS, DAI, DWC
All authors have seen and approved the final draft.
CONFLICT OF INTEREST
The authors have no financial or personal interests that would inappropriately influence their work on this manuscript.
References
- 1.Williams VC, Lucas J, Babcock MA, Gutmann DH, Korf B, Maria BL. Neurofibromatosis type 1 revisited. Pediatrics. 2009;123:124–33. doi: 10.1542/peds.2007-3204. [DOI] [PubMed] [Google Scholar]
- 2.Wolkenstein P, Rodriguez D, Ferkal S, et al. Impact of neurofibromatosis 1 upon quality of life in childhood: a cross-sectional study of 79 cases. Br J Dermatol. 2009;160:844–8. doi: 10.1111/j.1365-2133.2008.08949.x. [DOI] [PubMed] [Google Scholar]
- 3.Le LQ, Parada LF. Tumor microenvironment and neurofibromatosis type I: connecting the GAPs. Oncogene. 2007;26:4609–16. doi: 10.1038/sj.onc.1210261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Staser K, Yang FC, Clapp DW. Plexiform neurofibroma genesis: questions of Nf1 gene dose and hyperactive mast cells. Curr Opin Hematol. 2010;17:287–93. doi: 10.1097/MOH.0b013e328339511b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Staser K, Yang FC, Clapp DW. Mast cells and the neurofibroma microenvironment. Blood. 2010;116:157–64. doi: 10.1182/blood-2009-09-242875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mautner VF, Asuagbor FA, Dombi E, et al. Assessment of benign tumor burden by whole-body MRI in patients with neurofibromatosis 1. Neuro Oncol. 2008;10:593–8. doi: 10.1215/15228517-2008-011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lantieri L, Meningaud JP, Grimbert P, et al. Repair of the lower and middle parts of the face by composite tissue allotransplantation in a patient with massive plexiform neurofibroma: a 1-year follow-up study. Lancet. 2008;372:639–45. doi: 10.1016/S0140-6736(08)61277-5. [DOI] [PubMed] [Google Scholar]
- 8.Yang FC, Ingram DA, Chen S, et al. Nf1-dependent tumors require a microenvironment containing Nf1+/−- and c-kit-dependent bone marrow. Cell. 2008;135:437–48. doi: 10.1016/j.cell.2008.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vogel KS, Klesse LJ, Velasco-Miguel S, Meyers K, Rushing EJ, Parada LF. Mouse tumor model for neurofibromatosis type 1. Science. 1999;286:2176–9. doi: 10.1126/science.286.5447.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu Y, Ghosh P, Charnay P, Burns DK, Parada LF. Neurofibromas in NF1: Schwann cell origin and role of tumor environment. Science. 2002;296:920–2. doi: 10.1126/science.1068452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lasater EA, Bessler WK, Mead LE, et al. Nf1+/− mice have increased neointima formation via hyperactivation of a Gleevec sensitive molecular pathway. Hum Mol Genet. 2008;17:2336–44. doi: 10.1093/hmg/ddn134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schag CC, Heinrich RL, Ganz PA. Karnofsky performance status revisited: reliability, validity, and guidelines. J Clin Oncol. 1984 Mar;2(3):187–93. doi: 10.1200/JCO.1984.2.3.187. [DOI] [PubMed] [Google Scholar]
- 13.Lansky SB, List MA, Lansky LL, Ritter-Sterr C, Miller DR. The measurement of performance in childhood cancer patients. Cancer. 1987 Oct 1;60(7):1651–6. doi: 10.1002/1097-0142(19871001)60:7<1651::aid-cncr2820600738>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 14.Hurst JW, Morris DC, Alexander RW. The use of the New York Heart Association’s classification of cardiovascular disease as part of the patient’s complete Problem List. Clin Cardiol. 1999 Jun;22(6):385–90. doi: 10.1002/clc.4960220604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poussaint TY, Jaramillo D, Chang Y, Korf B. Interobserver reproducibility of volumetric MR imaging measurements of plexiform neurofibromas. AJR Am J Roentgenol. 2003;180:419–23. doi: 10.2214/ajr.180.2.1800419. [DOI] [PubMed] [Google Scholar]
- 16.Solomon J, Warren K, Dombi E, Patronas N, Widemann B. Automated detection and volume measurement of plexiform neurofibromas in neurofibromatosis 1 using magnetic resonance imaging. Comput Med Imaging Graph. 2004;28:257–65. doi: 10.1016/j.compmedimag.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Dombi E, Solomon J, Gillespie AJ, et al. NF1 plexiform neurofibroma growth rate by volumetric MRI: relationship to age and body weight. Neurology. 2007;68:643–7. doi: 10.1212/01.wnl.0000250332.89420.e6. [DOI] [PubMed] [Google Scholar]
- 18.Babovic-Vuksanovic D, Widemann BC, Dombi E, et al. Phase I trial of pirfenidone in children with neurofibromatosis 1 and plexiform neurofibromas. Pediatr Neurol. 2007;36:293–300. doi: 10.1016/j.pediatrneurol.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 19.Widemann BC, Salzer WL, Arceci RJ, et al. Phase I trial and pharmacokinetic study of the farnesyltransferase inhibitor tipifarnib in children with refractory solid tumors or neurofibromatosis type I and plexiform neurofibromas. J Clin Oncol. 2006;24:507–16. doi: 10.1200/JCO.2005.03.8638. [DOI] [PubMed] [Google Scholar]
- 20.Jakacki RI, Dombi E, Potter DM, Goldman S, Allen JC, Pollack IF, Widemann BC. Phase 1 trial of pegylated-interferon-alpha-2b in young patients with plexiform neurofibromas. Neurology. 76:265–72. doi: 10.1212/WNL.0b013e318207b031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang FC, Chen S, Clegg T, et al. Nf1+/− mast cells induce neurofibroma like phenotypes through secreted TGF-beta signaling. Hum Mol Genet. 2006;15:2421–37. doi: 10.1093/hmg/ddl165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang FC, Ingram DA, Chen S, et al. Neurofibromin-deficient Schwann cells secrete a potent migratory stimulus for Nf1+/− mast cells. J Clin Invest. 2003;112:1851–61. doi: 10.1172/JCI19195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Plotkin SR, Halpin C, McKenna MJ, et al. Erlotinib for Progressive Vestibular Schwannoma in Neurofibromatosis 2 Patients. Otology & Neurotology. 2010;31:1135–1143. doi: 10.1097/MAO.0b013e3181eb328a. [DOI] [PMC free article] [PubMed] [Google Scholar]