Abstract
Purpose of Review
Neutropenia lasting for at least for 3 months and not attributable to drugs or a specific genetic, infectious, inflammatory, autoimmune or malignant cause is called chronic idiopathic neutropenia. (CIN) CIN and autoimmune neutropenia (AIN) are very similar and overlapping conditions. The clinical consequences depend upon the severity of neutropenia, but it is not considered a premalignant condition.
Recent findings
Long-term observational studies in children indicate that the disease often lasts for 3 to 5 years in children, then spontaneously remits, but it rarely remits in adult cases. The value of anti-neutrophil antibody testing in both children and adults is uncertain. Most recent data suggest that CIN and AIN are immune mediated diseases, but there are no new clinical or genetic tests to aid in diagnosis. Treatment with granulocyte colony stimulating factor (G-CSF) is effective to increase blood neutrophils in almost all cases; this treatment is reserved, however for patients with both neutropenia and evidence of recurrent fevers, inflammatory symptoms and infections. There is little or no evidence to indicate that G-CSF treatment predisposes to myeloid malignancies in this population.
Summary
It is important to recognize CIN and AIN, the most common causes of chronic neutropenia in both children and adults. If the neutropenia is not severe, i.e. > 0.5 × 109/L, most patients can be observed and not treated prophylactically with antibiotics or a growth factor. When neutropenia is severe treatment with G-CSF is often beneficial.
Keywords: neutropenia, idiopathic neutropenia, autoimmune neutropenia, anti-neutrophil antibodies, granulocyte colony stimulating factor (G-CSF)
Introduction
Neutropenia is usually defined as an absolute neutrophil count (ANC) in the blood that is less than 1.5 × 109/L. [1] In some ethnic groups, specifically persons of African heritage and some Middle Eastern groups, the level that should be regarded as normal is lower, i.e., 0.8 to 1.0 × 109/L. [2, 3] Neutropenia is deemed “severe” and “chronic” when the counts are < 0.5 × 109/L on at least 3 occasions in a 3 month period. It is called “idiopathic” when it cannot be attributed to a drug and there is no definable genetic, infectious, inflammatory, autoimmune or malignant cause. [4, 5] Thus chronic idiopathic neutropenia (CIN) is a diagnosis made by exclusion of other causes. There is also overlap of patients with the diagnosis of CIN and “autoimmune neutropenia (AIN),” because it is difficult to accurately detect circulating antibodies directed toward antigens present on the surface of neutrophils, and clinical interpretation of the anti-neutrophil antibody test result is also difficult. [6, 7] This report summarizes the recent literature on CIN and AIN and presents observations from the Severe Chronic Neutropenia International Registry (SCNIR) on these conditions. [8] Several reviews and commentaries on the diagnosis and treatment of chronic neutropenia in children and adults have been published recently. [9–14]
Epidemiology
CIN and AIN are rare conditions, but they are a common cause for selective neutropenia, i.e., diseases with neutropenia as their primary hematological abnormality, in both children and adults. For this reason these conditions are also referred to as “primary” or “isolated” neutropenia. [15] In a US study of the prevalence of neutropenia, 4.5% of black participants, 0.84% of white participants and 0.44% of Mexican Americans had ANC of 1.5 × 109/L. [16]. In Denmark, a recent study using a national health services data base for 370,000 individuals found that approximately 1% of this population had neutrophil counts less than 1.5 × 109/L. [17] In this study, most commonly recognized causes of neutropenia were viral infections (including HIV) and hematological malignancies. The prevalence of chronic neutropenia was 0.12% and only 2 cases of probable CIN (approximately 5 million) were identified. In an earlier study in Crete, the prevalence of neutropenia (ANC> 0.6–1.7 × 109/L) was 1.4% and no cases of severe neutropenia were observed in 778 adults. [18] For Washington state, USA, the estimated adult prevalence of severe chronic neutropenia is about 5 cases per million. (Dale, unpublished data) In children, acute neutropenia with viral infections is commonly observed and chronic autoimmune neutropenia is also a relatively common entity. The duration of childhood AIN is quite variable, but often remits. Previous reports indicate that it usually lasts until about age 3–5 years with an average duration of 17 months. [19–22] In adults CIN and AIN usually do not remit, but counts remain relatively stable for many years in most patients. [4, 5, 15]
In adults there is a striking female predominance of CIN and AIN with female to male ratio estimated to be as high as 8:1. [4] By contrast, in children the female to male ratio is lower. [4, 5, 15, 22] and there are other features which indicate that idiopathic and autoimmune neutropenia in children and adults are distinct entities. (See below) [19–22] Figure 1 illustrates the age at the time of diagnosis and gender for 501 patients with CIN or AIN enrolled in the Severe Chronic Neutropenia International Registry in North America (hereafter referred to as the SCNIR). [8] This histogram shows an early childhood peak in onset and then a long tail, with female predominance. Based on the shape of this curve, we divided data summaries in this report to patients diagnosed before and after age 10 years. However, there is little difference if the values are divided at age 18, the conventional age break for pediatric and adult populations.
Figure 1. Onset of Neutropenia.
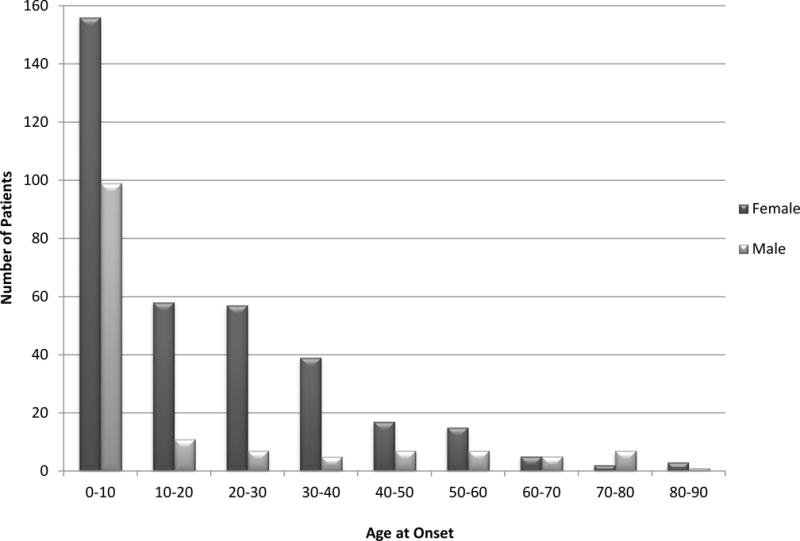
The figure shows the number of male and female patients with the diagnosis of chronic idiopathic or chronic autoimmune, by age groups, enrolled in the Severe Chronic Neutropenia International Registry (SCNIR).
Diagnosis
The diagnosis of CIN or AIN is often suggested by a routine blood count revealing a low ANC. Diagnosis depends on the physical exam and more extensive laboratory testing to exclude other diseases. In young children congenital anomalies suggest a genetic cause for the neutropenia, and in both children and adults generalized enlargement of lymph nodes, hepatomegaly or splenomegaly are unusual findings in CIN or AIN. The examination of the joints is usually unremarkable in CIN or AIN, and the finding of arthritis with or without deformity suggests another diagnosis such as concomitant osteoarthritis or an autoimmune disease, e.g., rheumatoid arthritis (RA including Felty syndrome), systemic lupus erythematosus (SLE), Sjögrens syndrome (SS). Patients with these diseases very rarely present solely with neutropenia, and severe neutropenia is rarely seen as a feature of these diseases except in Felty syndrome. The diagnosis of autoimmune disorders is supported by testing for antinuclear antibodies (ANA) with a reflexive panel of secondary antibody tests. These tests help to secure a diagnosis of one of the autoimmune disorders mentioned above, but some patients with CIN and AIN also have positive tests for autoantibodies (see below).
In patients with CIN and AIN the total white blood cell count (WBC) is usually reduced but the absolute counts for monocytes, eosinophils and basophils are normal. [4, 5, 15] In the absence of active infection or inflammation, the hematocrit/hemoglobin and platelet count are also usually normal or only moderately reduced. Lymphocyte numbers (the absolute lymphocyte count or ALC) may be slightly reduced but are usually within the normal range. [15] The circumstances of the blood cell counts are also important, because fever, inflammation or infection are often associated with a temporary increase in the ANC and decrease in the absolute lymphocyte and monocyte counts (ALC).
It is also important to note that patients with cytopenias involving 2 or more cell lines, e.g., neutropenia and thrombocytopenia or neutropenia and anemia should be investigated for the possibility of idiopathic thrombocytopenic purpura (ITP), autoimmune hemolytic anemia or clonal or malignant hematological disorder. The diagnosis of idiopathic or autoimmune neutropenia should not be applied to patients with these conditions or to patients with drug-induced neutropenia, chronic neutropenia after cancer chemotherapy or neutropenia caused by prolonged immunosuppressive or disease modifying agents. [23] This is important because the prognosis for these conditions differs significantly from CIN and AIN. At present there is no clear evidence that patients with CIN or AIN progress to develop other autoimmune disorders, but there may be yet undiscovered relationships.
The French Chronic Neutropenia Registry recently reported on 108 adult patients, 79% female, with chronic primary neutropenia with ANC less than 1.0 × 109/L. The median age at diagnosis was 28.3 years (range 21.5 to 39.9 years). [15] The median ANC was 0.41, range 0.29–0.65. Thirty-four patients had lymphocytopenia, 8 had monocytopenia and 5 had monocytosis, but monocytopenia and lymphocytopenia were found concomitantly in only 4 patients. More complete information about blood counts for patients with CIN and AIN in the SCNIR is shown in Table 1. In the French report the diagnosis was usually made fortuitously (62%); others had aphthous stomatitis (10%) or infections (23 %) leading to the diagnosis. Overall, recurrent aphthous stomatitis was observed in 45% of the patients. Sixty percent of the patients reported a history of bacterial infections, 15 had at least one episode of severe bacterial infection. Only one fungal infection, an Aspergillus infection, was observed in a patient after treatment with anti-thymocyte globulin, cyclosporine and methylprednisolone. [15] Overall, however, there was a paucity of serious infections in this population, and the patients with the lowest ANCs appeared to be at greatest risk. In the SCNIR, which enrolls patients with lower ANCs, there was a somewhat greater frequency of infections, including more serious infections. (See Table 2.)
Table 1.
*. Complete Blood Counts Before and on G-CSF
| Age at onset of Neutropenia | On/Off G-CSF | Number of patients | WBC median (mean, SEM) | Hematocrit median (mean, SEM) | Hemoglobinmedian (mean, SEM) | Plateletsmedian (mean, SEM) | ANC median (mean, SEM) | ALC median (mean, SEM) | AMC median (mean, SEM) |
|---|---|---|---|---|---|---|---|---|---|
| Under 10 years of age | Before G-CSF | 255 | 5.500 (5.600 +/− 0.17) |
34.1 (34.1 +/− 0.19) |
11.4 (11.4 +/− 0.07) |
333 (343 +/− 6.51) |
0.244 (0.435 +/− 0.05) |
4.053 (4.032 +/− 0.14) |
0.601 (0.663 +/− 0.03) |
| On G-CSF | 183 | 7.000 (7.600 +/− 0.30) |
36.2 (36.4 +/− 0.19) |
12.4 (12.4 +/− 0.06) |
278 (279 +/− 5.62) |
2.400 (3.260 +/− 0.20) |
3.230 (3.190+/− 0.20) |
0.530 (0.560 +/− 0.03) |
|
| Over 10 years of age | Before G-CSF | 246 | 2.600 (2.733 +/− 0.09) |
38.0 (38.0 +/− 0.23) |
12.9 (12.8 +/− 0.08) |
250 (253 +/− 5.04) |
0.407 (0.565 +/− 0.06) |
1.511 (1.531 +/− 0.05) |
0.365 (0.382 +/− 0.02) |
| On G-CSF | 182 | 5.500 (5.800 +/− 0.29) |
38.6 (38.7 +/− 0.29) |
13.0 (13.0 +/− 0.09) |
225 (227 +/− 6.78) |
2.580 (3.400 +/− 0.32) |
1.680 (1.690 +/− 0.07) |
0.400 (0.430 +/− 0.02) |
table contains original data
Table 2.
Patients reporting infections
| Events | Mouth Ulcers | Otitis | Cellulitis | Skin Abscesses | Pneumonia | Sepsis | Peritonitis |
|---|---|---|---|---|---|---|---|
| Before G-CSF | 235 | 213 | 56 | 211 | 84 | 23 | 3 |
| On G-CSF | 82 | 73 | 18 | 44 | 22 | 1 | 0 |
In patients with presumed CIN or AIN, diagnostic testing serves to exclude infectious and malignant diseases as a cause for neutropenia and to confirm the clinical impression of an autoimmune disease mechanism. In the French series bone marrow aspirates were performed in about one third of the patients. [15] Results were normal in 34%; 31% showed late maturation arrest, 15% had granulocytic hypoplasia, 20% had increased cellularity. All cytogenetic analyses were normal. In the French study the ANCs and clinical outcomes could not be directly related to these bone marrow findings.
Disease mechanisms
Most investigations of the mechanisms for CIN and AIN have focused on autoimmunity and aberrant T-cell functions. In children with chronic neutropenia, the diagnosis of AIN versus CIN usually is based on results of measuring circulating anti-neutrophil antibodies. [19, 20] The International Granulocyte Immunology Workshops (IGIW) recommend using both the granulocyte immunofluorescence test (GIFT) and the granulocyte agglutination test (GAT) because of the frequency of false positive results. A recent study of 157 pediatric patients with AIN from the Italian Neutropenia Registry (median age at onset was 0.7 years) reported results of immunological testing and bone marrow examinations in patients with AIN with a primary diagnosis based on clinical presentation and subsequent resolution of neutropenia. [24] The bone marrows in 52 of 54 patients were normal; only 2 showed a moderate decrease in myeloid cellularity. The granulocyte immunofluorescence tests (GIFT) performed in three laboratories showed a sensitivity of 62% with a single assay; repeat assay showed 82% were positive.
Using data from the SCNIR, Boxer et al recently reported that antibody testing is not helpful in predicting outcomes in children with the diagnosis of AIN or CIN. [21] Outcomes were the same for children with either positive or negative tests. Boxer et al. also described a few cases in which children with severe congenital neutropenia were first tested for anti-neutrophil antibodies. When these tests were positive, the diagnosis of AIN was assigned, but when the neutropenia did not remit and the patients developed severe infections, genetic studies revealed that they had severe congenital neutropenia due to mutations in ELANE. Vu et al recently reported a similar case of misdiagnosis. [25] It is important to recognize that misdiagnosis is a potential adverse outcome of antibody testing.
In adults, the value of the GIFT and GAT tests is less certain. The recently reported French series excluded patients with RA, SLE, SS and the LGL syndrome. [15] In this series, anti-neutrophil antibody tests were positive in 35% (23 of 66 patients) and 47% (39 of 83 patients) had positive tests for autoantibodies other than neutrophil antibodies. Results were: ANA positive 24%, % ANCA positive 17%, rheumatoid factor positive 15% and anti-dsDNA positive 14%. Clonal rearrangement of T-cell receptor genes was found in 13%. Kyritsi et al has also recently reported a high frequency of thyroid disorders in patients with chronic neutropenia. [26] In this report 218 consecutive neutropenic patients were prospectively evaluated with thyroid tests and tests to detect clonal T-cell disorders. Among these patients, 95 (44%) had some evidence of thyroid disease.
A series of studies from Crete indicate that neutropenia in adults with CIN is attributable to apoptosis of developing neutrophils in the bone marrow mediated through activated T lymphocytes and excessive production of pro inflammatory, myelosuppressive cytokines. In studies of 54 CIN patients, Koumaki et al. found increased levels of IL-1 beta, tumor necrosis factor-alpha, IL-6 and transforming growth factor-beta 1 and decreased levels of IL-10 in bone marrow culture supernatants compared to 30 healthy volunteers. In subsequent studies transforming growth factor (TGF)-beta-1 levels were particularly high in some patients. [27] Damianaki recently reported investigations in 91 CIN patients demonstrating the presence of small numbers of PNH type clones in these patients using flow cytometry. [28] Cumulatively this work suggests that CIN is an immune-mediated bone marrow failure syndrome that impairs normal production of neutrophils in the bone marrow. [29]
Neutropenia in patients with the LGL syndrome is associated with similar findings and probably has the same basic mechanisms, except there is a recognized population of lymphocytes producing these myelosuppressive cytokines. [30,31]
Genetic testing
Understanding of the genetic mechanisms for severe chronic neutropenia is still unfolding. In the French series sequencing was done for some patients, all with negative results; ELANE (55 patients,) GATA2 (12 patients) and CXCR4 (8 patients). At present it is assumed that AIN and severe congenital neutropenia are distinct and not overlapping entities, but more data is necessary to confirm this hypothesis. A recent study from Taiwan examined the association of granulocyte specific autoantibodies and HLA subtypes.[32] In this study of young children (mean age at onset 9.8 months) 74% had antibodies against HNA-1a. There was a significant association of HLA-DQB1*0503 and positive test for neutrophil directed antibodies (odds ratio, 6.48; p = 0.0002).
Treatment
For many years there was no effective treatment for neutropenia; the mainstays of care were good hygiene, observation for early signs of infection and treatment with antibiotics when infections occur. A randomized, controlled trial of G-CSF for treatment of severe chronic neutropenia, including 42 patients with CIN, established G-CSF as an effective therapy for this condition. [33] In this study marrow aspirate comparisons showed a significant increase in post mitotic marrow neutrophils, the post mitotic to mitotic ratio for myeloid cells and an increase in the myeloid to erythroid ratio. There was a decrease in infection related events and antibiotic use in treated patients. Numerous subsequent studies confirm the effectiveness of G-CSF to increase blood neutrophils in CIN and AIN.
In the recently reported French study, 50 of 108 were treated with G-CSF, 24 regularly and 26 sporadically. The median G-CSF dose was 4.5 mcg/kg per week or 0.65 mcg/kg/day. Treatment was regarded as clinically effective in 23 of 24 treated regularly with G-CSF and in 20 of 26 treated sporadically. The mean increase in neutrophils was from 0.59 × 109/L to 2.06 × 109/L. Table 3 shows the doses and exposures for G-CSF treatment of patients in the SCNIR, with the doses expressed mcg/kg/day. Almost all patients took G-CSF daily, every other day or three times per week to smooth the exposure and to minimize adverse effects, principally bone pain, headache and aching symptoms; doses were calculated as total dose per week divided by 7.
Table 3.
G-CSF Treatment
| Age at onset of Neutropenia | Number of patients | *G-CSF dose (mg/kg) median (mean, SEM) | G-CSF exposure in years median (mean, SEM) | G-CSF exposure in grams median (mean, SEM) |
|---|---|---|---|---|
| Under 10 years of age | 226 | 1.41 (1.47 +/− 0.15) | 2.0 (4.1 +/− 0.30) | 0.01 (0.11 +/− 0.03) |
| Over 10 years of age | 236 | 1.04 (1.04 +/− 0.24) | 8.0 (9.4 +/− 0.48) | 0.12 (0.28 +/− 0.03) |
If treatment was not given daily, the total weekly dose was divided by seven to arrive at the daily dose.
Pretreatment there was a predictable pattern of infections; most commonly these patients have respiratory infections, periodontal disease and mouth ulcers and skin infections. (See Table 2) Severe infections were rare, the most common probably being pneumonia. With treatment there is subjectively a sense of improved well-being, fewer of the common problems and substantially fewer severe infections. (See Table 2)
The patterns of infections and inflammatory symptoms vary considerably from patient to patient. Many patients with CIN and AIN have mild to moderate neutropenia and few infections, and some patients even with more severe neutropenia do well. It is the consensus of most expert clinicians to be guided by the patient’s history, rather than simply their blood counts in deciding whether or not to treat with G-CSF. Other treatments such as splenectomy, corticosteroids, androgens, and immunosuppressive and immune modulating therapies are not recommended, unless the patient fails to respond to G-CSF. In the SCNIR data base, 48 CIN/AIN patients reported treatment with another agent before G-CSF (corticosteroids 30, gamma globulin 15, methotrexate 5, cyclosporine 3, other agents 3) and almost all these treatments were stopped soon after the patient began G-CSF and not resumed. The French report concluded that “other treatments (mainly methylprednisolone, cyclophosphamide, and methotrexate) were effective in less than half of the patients, and relapses were observed at treatment with all in nearly all patients.” [15] Thus, immunosuppressive therapy should only be proposed for symptomatic patients who have failed G-CSF.
Adverse effects of G-CSF in CIN and AIN
Most patients readily comply with long term treatment with G-CSF, administered daily or several times per week in a dose sufficient to maintain an ANC in the low-normal range. Very few patients find the adverse effects sufficiently severe to warrant discontinuing treatment. As a stimulant to myeloid cell proliferation, there have long been concerns about G-CSF causing myeloid malignancies. Fattizzo et al reported on 76 patients with chronic neutropenia (49 with CIN, 27 with AIN, mediated age 56 years, median neutrophils 1.1 × 109/L). They observed 5 cases of NK cell expansion, 4 cases of hairy cell leukemia, and 3 cases of myelodysplasia over a 5 year period. [34] In the French series, there were no secondary hematological malignancies. Data for 501 CIN and AIN patients reporting to the North American office of the SCNIR and exposed to G-CSF for an average treatment period of 6.3 years or for total patient exposure of approximately 3100 patient-years revealed two patients developed hematological malignancies. In brief the cases were:
Patient #1 was a 21 year old female initially diagnosed with chronic idiopathic neutropenia in 2004 at the age of 18. At the time of enrollment in the SCNIR, her bone marrow showed “left shifted” in the myeloid series and flow cytometry revealed lymphocytosis with T cell predominance. She was treated with G-CSF at 3.5 mcg/kg/day for 3 years until she developed a Staphylococcus aureus infection, pneumonia, sepsis and died. The autopsy revealed a T-cell lymphoproliferative process involving the bone marrow, spleen and lymph nodes.
Patient #2 was a 66 year old female diagnosed with chronic idiopathic neutropenia in 1998 at the age of 50. She started on G-CSF in 2001 at 0.5 mcg/kg/day and was treated for five years with G-CSF in doses ranging from 0.7 to 0.16 mcg/kg/day. G-CSF was discontinued at age 59, because her neutropenia had resolved. Six years later, at age 65, she was diagnosed with AML-M4.
Pregnancy
Three observational studies recently reported outcomes associated with the administration of G-CSF to pregnant women, the majority having CIN. In one study of 107 women reporting on 224 pregnancies treatment with G-CSF (median dose 1.0 mcg/kg per day) in spontaneous terminations or preterm labors, and a suggestion of benefit. [35] Adverse events in the neonates were similar for the two groups. Another study analyzing 38 pregnancies, 21 in women with CIN, also showed no differences in outcomes with or without treatment for the mothers or their newborns. [36] Transient profound neutropenia in infants born to women with CIN or AIN sometimes with severe infectious ramifications were also reported. [35, 36] Clinically this means that blood counts should be done at least a few times after birth in infants of mothers with CIN or AIN. If the neonate is severely neutropenic and there is concern about the risk of severe infections, G-CSF may be helpful in this specific setting. [37] A recently published, large, retrospective study of 348 neonatal intensive care units with 30,705 neutropenic infants, 2142 treated with G-CSF (7%), showed that G-CSF shortened the time for hematological recovery but did not reduce secondary sepsis or death at 14 days compared to untreated infants. [38] Thus the specific cause for neutropenia and other clinical factors should be considered in all cases.
Conclusion
CIN and AIN are the most common diagnosis in children and adults presenting with isolated or primary neutropenia. The diagnosis depends on the history of chronicity of the neutropenia and absence of evidence of other causes such as infectious, inflammatory or malignant diseases. In young children it is estimated that about 1/3 will resolve spontaneously. In adults, remissions are very rare. G-CSF is the only predictably effective treatment, and should be reserved for patients with recurrent fevers and infections.
Key points.
Chronic idiopathic and autoimmune neutropenia are the most common cause for chronic neutropenia in children and adults
Most evidence suggests that these conditions are due to immune mechanisms impairing neutrophil production in the bone marrow
Granulocyte colony-stimulating factor is an effective chronic therapy treatment in doses much lower than those for prevention of chemotherapy induced neutropenia.
Acknowledgments
We acknowledge the assistance of Laurie Steele in the preparation of this manuscript for publication.
Financial support and sponsorship: NIH grant 5R 24AI049393, Severe Chronic Neutropenia International Registry
DCD is a consultant and receives research support from Amgen, a manufacturer of granulocyte colony-stimulating factor (G-CSF)
Footnotes
Conflict of interest:
AAB has no financial conflict of interest
References
Papers of particular interest, published within the annual period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1*.Dale DC, Welte K. Neutropenia and Neutrophilia. In: Kaushansky K, Lichtman MA, Prchal JT, et al., editors. Williams Hematology. 9th. New York, NY: McGraw-Hill; 2016. pp. 991–1004. A current textbook review. [Google Scholar]
- 2.Thobakgale CF, Ndung’u T. Neutrophil counts in persons of African origin. Curr Opin Hematol. 2014;21:50–57. doi: 10.1097/MOH.0000000000000007. [DOI] [PubMed] [Google Scholar]
- 3*.Denic S, Narchi H, Al Mekaini LA, et al. Prevalence of neutropenia in children by nationality. BMC Hematol. 2016;16:15. doi: 10.1186/s12878-016-0054-8. An important resource on neutropenia by country and ethnicity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kyle RA, Linman JW. Chronic idiopathic neutropenia. A newly recognized entity? N Engl J Med. 1968;279:1015–1019. doi: 10.1056/NEJM196811072791902. [DOI] [PubMed] [Google Scholar]
- 5.Dale DC, Guerry D, Werwerka JR, et al. Chronic neutropenia. Medicine. 1979;58:128–144. doi: 10.1097/00005792-197903000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Farruggia P, Dufour C. Diagnosis and management of primary autoimmune neutropenia in children: insights for clinicians. Ther Adv Hematol. 2015;6:15–24. doi: 10.1177/2040620714556642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akhtari M, Curtis B, Waller EK. Autoimmune neutropenia in adults. Autoimmun Rev. 2009;9:62–66. doi: 10.1016/j.autrev.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Dale DC, Bolyard AA, Schwinzer BG, et al. The Severe Chronic Neutropenia International Registry: 10-year follow-up report. Support Cancer Ther. 2006;3:220–231. doi: 10.3816/SCT.2006.n.020. [DOI] [PubMed] [Google Scholar]
- 9*.Palmblad J, Nilsson CC, Höglund P, Papadaki HA. How we diagnose and treat neutropenia in adults. Expert Rev Hematol. 2016;9:479–487. doi: 10.1586/17474086.2016.1142867. An excellent review focused on adult patients. [DOI] [PubMed] [Google Scholar]
- 10*.Dale DC. How I diagnose and treat neutropenia. (Review) Curr Op Hematol. 2016;23:1–4. doi: 10.1097/MOH.0000000000000208. A recent review describing a staged approach to diagnosis and comments on appropriate uses of G-CSF in the treatment of CIN. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palmblad J, Dufour C, Papadaki HA. How we diagnose neutropenia in the adult and elderly patient. Haematologica. 2014;99:1130–1133. doi: 10.3324/haematol.2014.110288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibson C, Berliner N. How we evaluate and treat neutropenia in adults. Blood. 2014;124:1251–1258. doi: 10.1182/blood-2014-02-482612. [DOI] [PubMed] [Google Scholar]
- 13.Newburger PE, Dale DC. Evaluation and management of patients with isolated neutropenia. Sem Hematol. 2013;50:198–206. doi: 10.1053/j.seminhematol.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walkovich K, Boxer LA. How to approach neutropenia in childhood. Pediatr Rev. 2013;34:173–184. doi: 10.1542/pir.34-4-173. [DOI] [PubMed] [Google Scholar]
- 15**.Sicre de Fontbrune F, Moignet A, Beaupain B, et al. Severe chronic primary neutropenia in adults: report on a series of 108 patients. Blood. 2015;126:1643–1650. doi: 10.1182/blood-2015-03-634493. An excellent, comprehensive review of adult patients with CIN in France. [DOI] [PubMed] [Google Scholar]
- 16.Hsieh MM, Everhart JE, Byrd-Holt DD, et al. Prevalence of neutropenia in the U.S. population: age, sex, smoking status, and ethnic differences. Ann Intern Med. 2007;146:486–492. doi: 10.7326/0003-4819-146-7-200704030-00004. [DOI] [PubMed] [Google Scholar]
- 17**.Andersen CL, Tesfa D, Siersma VD, et al. Prevalence and clinical significance of neutropenia discovered in routine complete blood cell counts: a longitudinal study. J Intern Med. 2016;279:566–575. doi: 10.1111/joim.12467. A very useful population-based study of the prevalence of neutropenia in Denmark. [DOI] [PubMed] [Google Scholar]
- 18.Papadaki HA, Xylouri I, Coulocheri S, et al. Prevalence of chronic idiopathic neutropenia of adults among an apparently healthy population living on the island of Crete. Ann Hematol. 1999;78:293–297. doi: 10.1007/s002770050518. [DOI] [PubMed] [Google Scholar]
- 19.Bruin M, Dassen A, Pajkrt D, et al. Primary autoimmune neutropenia in children: a study of neutrophil antibodies and clinical course. Vox Sang. 2005;88:52–59. doi: 10.1111/j.1423-0410.2005.00585.x. [DOI] [PubMed] [Google Scholar]
- 20.Bux J, Behrens G, Jaeger G, Welte K. Diagnosis and clinical course of autoimmune neutropenia in infancy: analysis of 240 cases. Blood. 1998;91:181–186. [PubMed] [Google Scholar]
- 21*.Boxer LA, Bolyard AA, Marrero TM, et al. Is there a role for anti-neutrophil antibody testing in predicting spontaneous resolution of neutropenia in young children? Blood. 2015;126:2211. (abstract). A recent abstract describing the clinical utility of anti-neutrophil antibody testing in children. [Google Scholar]
- 22.van der Veen JP, Hack CE, Engelfriet CP, et al. Chronic idiopathic and secondary neutropenia: clinical and serological investigations. Br J Haematol. 1986;63:161–171. doi: 10.1111/j.1365-2141.1986.tb07506.x. [DOI] [PubMed] [Google Scholar]
- 23.Yeoh SA, Fox C, Hull R. Neutropenia in the elderly: A rheumatology perspective. Drugs Aging. 2016;33:585–601. doi: 10.1007/s40266-016-0383-0. Review. [DOI] [PubMed] [Google Scholar]
- 24**.Farruggia P, Fioredda F, Puccio G, et al. Autoimmune neutropenia of infancy: Data from the Italian neutropenia registry. Am J Hematol. 2015;90:E221–222. doi: 10.1002/ajh.24187. A very helpful study of the utility of antibody testing and outcomes in Italian patients. [DOI] [PubMed] [Google Scholar]
- 25.Vu QV, Wada T, Tran TT, et al. Severe congenital neutropenia caused by the ELANE gene mutation in a Vietnamese boy with misdiagnosis of tuberculosis and autoimmune neutropenia: a case report. BMC Hematol. 2015;15:2. doi: 10.1186/s12878-015-0020-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26*.Kyritsi EM, Yiakoumis X, Pangalis GA, et al. High frequency of thyroid disorders in patients presenting with neutropenia to an outpatient hematology clinic STROBE-compliant article. Medicine (Baltimore) 2015;94:e886. doi: 10.1097/MD.0000000000000886. A comprehensive study of the association of thyroid disease and neutropenia from Greece. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koumaki V, Damianaki A, Ximeri M, et al. Pro-inflammatory bone marrow milieu in patients with chronic idiopathic neutropenia is associated with impaired local production of interleukin-10. Br J Haematol. 2006;135:570–573. doi: 10.1111/j.1365-2141.2006.06345.x. [DOI] [PubMed] [Google Scholar]
- 28*.Damianaki A, Stagakis E, Mavroudi I, et al. Minor populations of paroxysmal nocturnal haemoglobinuria type cells in patients with chronic idiopathic neutropenia. Eur J Haematol. 2016;28 doi: 10.1111/ejh.12766. [Epub ahead of print] A recent study examining the association of paroxysmal nocturnal haemoglobinuria and neutropenia. [DOI] [PubMed] [Google Scholar]
- 29.Stavroulaki E, Kastrinaki MC, Pontikoglou C, et al. Mesenchymal stem cells contribute to the abnormal bone marrow microenvironment in patients with chronic idiopathic neutropenia by overproduction of transforming growth factor-β1. Stem Cells Dev. 2011;20:1309–1318. doi: 10.1089/scd.2010.0425. [DOI] [PubMed] [Google Scholar]
- 30.Pontikoglou C, Kalpadakis C, Papadaki HA. Pathophysiologic mechanisms and management of neutropenia associated with large granular lymphocytic leukemia. Expert Rev Hematol. 2011;4:317–328. doi: 10.1586/ehm.11.26. [DOI] [PubMed] [Google Scholar]
- 31.Prochorec-Sobieszek M. Advances in diagnosis and treatment of large granular lymphocyte syndrome. Curr Opin Hematol. 2011;18:55–62. doi: 10.1097/MOH.0b013e328340dc12. [DOI] [PubMed] [Google Scholar]
- 32.Wang LY, Wang CL, Chu CC, et al. Primary autoimmune neutropenia in children in Taiwan. Transfusion. 2009;49:1003–1006. doi: 10.1111/j.1537-2995.2008.02084.x. [DOI] [PubMed] [Google Scholar]
- 33.Dale DC, Bonilla MA, Davis MW, et al. A randomized controlled phase III trial of recombinant human G-CSF for treatment of severe chronic neutropenia. Blood. 1993;81:2496–2502. [PMC free article] [PubMed] [Google Scholar]
- 34*.Fattizzo B, Zaninoni A, Consonni D, et al. Is chronic neutropenia always a benign disease? Evidences from a 5-year prospective study. Eur J Intern Med. 2015;26:611–615. doi: 10.1016/j.ejim.2015.05.019. A report of a series of Italian adults with chronic neutropenia, several developing secondary hematological disorders malignancies. [DOI] [PubMed] [Google Scholar]
- 35.Boxer LA, Bolyard AA, Kelley ML, et al. Use of granulocyte colony-stimulating factor during pregnancy in women with chronic neutropenia. Obstet Gynecol. 2015;125:197–203. doi: 10.1097/AOG.0000000000000602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeidler C, Grote UA, Nickel A, et al. Outcome and management of pregnancies in severe chronic neutropenia patients by the European Branch of the Severe Chronic Neutropenia International Registry. Haematologica. 2014;99:1395–1402. doi: 10.3324/haematol.2013.099101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maheshwari A. Neutropenia in the newborn. Curr Opin Hematol. 2014;21:43–49. doi: 10.1097/MOH.0000000000000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38*.Lee JA, Sauer B, Tuminski W, et al. Granulocyte colony-stimulating factor in hospitalized infants with neutropenia. Am J Perinatol. 2016 Sep 20; doi: 10.1055/s-0036-1593349. [Epub ahead of print] A comprehensive analysis of the benefits of G-CSF in neonates with neutropenia. [DOI] [PMC free article] [PubMed] [Google Scholar]


