Abstract
Objectives
Policing is a conflict-limiting mechanism observed in many primate species. It is thought to require a skewed distribution of social power for some individuals to have sufficiently high social power to stop others’ fights, yet social power has not been examined in most species with policing behavior. We examined networks of subordination signals as a source of social power that permits policing behavior in rhesus macaques.
Materials and Methods
For each of seven captive groups of rhesus macaques, we (a) examined the structure of subordination signal networks and used GLMs to examine the relationship between (b) pairwise dominance certainty and subordination network pathways and (c) policing frequency and social power (group-level convergence in subordination signaling pathways).
Results
Networks of subordination signals had perfect linear transitivity, and pairs connected by both direct and indirect pathways of signals had more certain dominance relationships than pairs with no such network connection. Social power calculated using both direct and indirect network pathways showed a heavy-tailed distribution and positively predicted conflict policing.
Conclusions
Our results empirically substantiate that subordination signaling is associated with greater dominance relationship certainty and further show that pairs who signal rarely (or not at all) may use information from others’ signaling interactions to infer or reaffirm the relative certainty of their own relationships. We argue that the network of formal dominance relationships is central to societal stability because it is important for relationship stability and also supports the additional stabilizing mechanism of policing.
Keywords: formal signals of dominance, SBT, social power, social stability, subordination networks
Life in a complex social group is a hallmark of primate evolution evident in most primate species (Hinde, 1976). Group living has advantages (e.g., cooperative defense of resources) and disadvantages (e.g., within group competition for resources), some of which increase with group size (Wrangham, 1980; van Schaik and van Hooff, 1983; Sterck et al., 1997; Isbell and Pruetz, 1998; Isbell et al., 1998; Chapman and Chapman, 2000; Vitone et al., 2004). While members of larger groups are at an advantage for monopolizing resources from those in smaller groups, increased competition in larger social groups can increase conflict among group members. Thus, evolved strategies for limiting conflict and reinforcing social relationships among individuals, such as dominance (Preuschoft and Van Schaik, 2000b), post-conflict reconciliation (de Waal, 2000), and conflict intervention by third parties (Flack et al., 2005b; von Rohr et al., 2012; Beisner and McCowan, 2013), allow larger group sizes to persist. For conflict intervention, one mechanism thought to underlie a third party’s ability to limit others’ conflict is “social power” because its distribution can be highly skewed, meaning that social power tends to be concentrated on a few individuals (Flack and de Waal, 2004; Flack and Krakauer, 2006). Yet the distribution of social power and its connection to policing behavior have only been examined in a single captive group of pigtailed macaques (Flack et al., 2005b). In this one study, Flack and colleagues suggest that greater social power arises when an individual receives subordination signals (i.e., formalized communication of subordinate role) from many different signalers; they further suggest that this type of social power appears critical for effective policing within social groups (Flack et al., 2005a; Flack and Krakauer, 2006). To further understand this important but understudied relationship between social power and policing, we examined, using a computational social network approach, a formal subordination signal —the peaceful silent bared teeth (pSBT) display (de Waal and Luttrell, 1985; Beisner and McCowan, 2014)— in rhesus macaques (Macaca mulatta) as a specific behavioral metric of social power and as a predictor of conflict intervention behavior. We provide a summary of the literature to date followed by the specific questions we sought to answer in this study.
Formal signals of dominance or subordination
Any two individuals in a social group can have interactions that are contrary to their dominance relationship. For example, a dominant individual may “win” most, but not all, conflicts with a subordinate, yet still have an established dominance relationship with that subordinate (de Waal, 1986). Formal signals of dominance or subordination, however, are consistently unidirectional communications (typically in the form of gestures, facial movements or vocalizations) within a pair of animals, regardless of the motivational state or the presence of third parties or resources (de Waal, 1986; Preuschoft and van Schaik, 2000a; Setchell and Wickings, 2005). These signals are thus thought to communicate the overall state of a pair’s dominance relationship (i.e., who is subordinate or dominant) and are more reliable indicators of dominance than other behaviors.
The silent-bared-teeth display (SBT; also known as “grimace”) is broadly used by many primate species to communicate a non-threatening disposition (van Hooff, 1962; van Hooff, 1967; van Hooff, 1972). Among some macaque species, it is more specifically a subordination signal (de Waal and Luttrell, 1985; Preuschoft, 1992; Chaffin et al., 1995; Preuschoft, 1995; Preuschoft, 2004; Flack and de Waal, 2007; Beisner and McCowan, 2014). An individual gives a subordination signal once it has learned that it is likely to lose future fights with the opponent, either from direct experience or observational learning and may give multiple signals over time (Flack and Krakauer, 2006). The unidirectional pattern and, in some species non aggressive context, of a subordination signal is thought to communicate to the receiver that the signaler accepts or commits to its subordinate role in the relationship, rather than transient submission in response to a specific event (Flack and de Waal, 2007). Thus, for a given time period, we expect that high dominance certainty, evident from consistency in the direction of aggressive interactions or network paths, is coincident with the use of subordination signals in non-aggressive contexts. Here we use a social network technique called Percolation and Conductance (Fujii et al., 2014) to quantify dominance relationship certainty as the probability that one animal will win against another based upon consistency in the direction of paths in the aggression network. We expect that pairs with subordination signals will have more certain dominance relationships than pairs without signals.
Using social networks to understand social power
Social power is “the degree of consensus about an individual’s capacity to use force” and is quantified as the diversity of individuals that signal subordination to a given receiver (p. E87 Flack and Krakauer, 2006). In pairwise relationships power typically stems from asymmetry in competitive ability or resource holding potential and is largely indistinguishable from the pairwise dominance relationship (Lewis, 2002). Social power, on the other hand, is distinct from dominance rank relationships and is an emergent property of the society because it involves consensus across multiple group members (Flack and Krakauer, 2006). Network analysis is well-suited for quantifying such higher-order phenomena.
Social network analysis is a methodological approach linking individuals (called nodes) that interact. Links between nodes create pathways that can be used to measure the flow of a behavior (or other phenomenon) between group members. The presence/absence (unweighted network) or frequency (weighted network) of behavioral interactions are assembled into a matrix of all group members. For example, A threatens B is represented as the pathway A→B in an aggression network, and if B also threatens A, the relationship is represented as a bidirectional pathway A↔B. Pairs in the network graph that do not directly interact may nevertheless have an indirect pathway connecting them if they have interacted with the same third party (e.g., A→B→C is an indirect pathway linking A and C). This ability to mathematically represent both direct and indirect social connections makes social network analysis ideal for the study of higher order properties of a system (Lusseau, 2003; Pasquaretta et al., 2014).
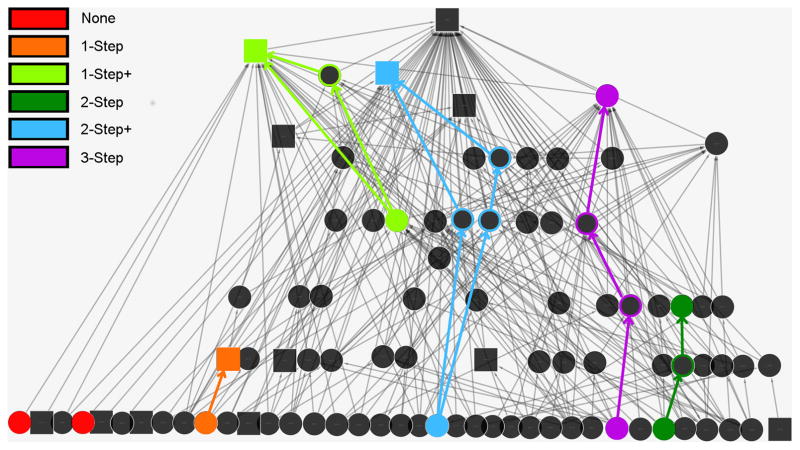
Group-level convergence in subordination signaling has been calculated with only directly received subordination signals to quantify social power. Individuals with the greatest social power have the highest diversity of signal senders (i.e., many individuals signal subordination to the same receiver) and those with no social power do not receive any signals (Flack et al., 2005a; Flack and Krakauer, 2006). However, the unidirectionality of subordination signals within pairs suggests that assembling these signals into a network may produce indirect pathways of signals (i.e., that pass through many nodes) from which both dominance and social power information might be represented. For example, if animal B signals to A on one occasion and animal C signals to B on another, these two subordination signals can be used to generate a network pathway of signals from C→B→A (Figure 1 of a silent-bared-teeth signal network)—a mathematical representation of a potential path through which information may be transmitted in the network. We examine whether all direct and indirect pathways in the network flow in the same direction to test for linear transitivity and the potential utility of these pathways for measuring social power. If perfect linear transitivity exists in the network, there must be no circular triples, quadruples, or other multiples (e.g., A signals to B, B signals to C, C signals to D, and D signals to A) in the subordination signaling network
Figure 1.
pSBT network of group 8 arranged hierarchically to show the upward flow of all pSBT links in the network
Being connected by an indirect pathway in the subordination signaling network may represent something about a pair’s dominance relationship that would advocate its use in calculating social power. In large groups, many pairs of animals do not regularly interact. Pairs that interact infrequently may gain more frequent access to dominance-subordinance information by observing group members’ interactions. Thus, animal A, who gives subordination signals to animal B, may learn from observation that B gives subordination signals to another animal C, and thereby infers A and C’s dominance relationship.
Considerable evidence indicates that nonhuman primates have the cognitive ability for such observational learning and inference. Many social vertebrates, including primates, rats, birds and fish, can use known relationships to deduce unknown relationships (known as transitive inference) such as deducing A>C from A>B and B>C (McGonigle and Chalmers, 1977; Davis, 1992; Bond et al., 2003; Grosenick et al., 2007). The costs and benefits of larger groups may be selective forces on relative neocortex size and morphology in primates (e.g. Shultz and Dunbar, 2010; David-Barrett and Dunbar, 2013). Therefore, among species that tend to have larger group sizes, individuals are likely better able to track and manage both direct and indirect social relationships (e.g. Shultz and Dunbar, 2007; Huguet et al., 2014).
We hypothesize that an indirect network connection represents a dominance relationship given that subjects are capable of transitive inference. Nonhuman primates may learn, or reaffirm, a formal subordinate or dominant position to an animal with whom it has no recent interactions, if they both have a subordination signaling interaction with the same third party. We therefore expect individuals with access to (indirect) transitive subordination signal information about their relationships to have greater dominance certainty (measured from aggressive interactions) than pairs without such information. In network terminology, we expect that the presence of an indirect pathway is associated with greater pairwise dominance certainty than the absence of that indirect pathway, all else being equal (e.g., both pairs lack direct signals, but one pair has an indirect pathway of signals; both pairs have direct signals, but one pair also has an indirect pathway of signals, etc.).
Conflict intervention
The term ‘policing’ in the animal behavior literature refers to control of group conflict (Ratnieks, 1988) such as intervention to stop intragroup fights in primates (Flack et al., 2005a; von Rohr et al., 2012). In many primate species, it is a robustness mechanism for the maintenance of social stability (Zucker, 1987; Sicotte, 1995; Flack et al., 2005b; McCowan et al., 2011; von Rohr et al., 2012). Recent examinations of the costs and benefits of conflict intervention apply the term policing only when the primary benefit to the intervener appears to be reduced intragroup conflict and improved social stability, as opposed to more selfish benefits from preferentially targeting same-sex competitors or supporting potential mates (Flack et al., 2005a; von Rohr et al., 2012; Beisner and McCowan, 2013). We use the same operational definition of policing found in Beisner and McCowan (2013), which includes both impartial interventions (intervener treats all combatants the same) and support of non-kin subordinates (intervener aggresses the dominant combatant which benefits a subordinate unrelated to the intervener).
Social power underlies conflict policing
Effective policers must have sufficiently greater social power (distinct from dominance rank, as discussed above) than others to stop their fights and this is particularly true for conflicts involving multiple members or individuals with high dominance rank. This suggests that the distribution of social power across group members constrains policing as an effective conflict control mechanism (Flack and de Waal, 2004). However, only one study demonstrates this connection: Flack et al. (2005b) show that in captive pigtail macaque individuals with the greatest social power police intragroup conflict most frequently and with minimal cost. However, there appear to be no other empirical examples of social power distributions in the primate literature.
Despite the rarity of work investigating the distribution of social power that may underlie policing in primates, numerous studies document the existence of conflict policing in other primate species (Bernstein and Sharpe, 1966; Kummer, 1967; Lindburg, 1971; Kurland, 1977; Zucker, 1987; Sicotte, 1995; Vervaecke et al., 2000; von Rohr et al., 2012; Beisner and McCowan, 2013). The cost of policing appears to be low for individuals with highest dominance rank (e.g. Bernstein and Sharpe, 1966; Kaplan, 1978; Beisner and McCowan, 2013). Yet the distribution of social power has not been examined in these species, so it is unknown whether policing in primate societies, where it exists, is accompanied by skewed distributions of social power or is explained by some other mechanism.
Here we examine the social power structure in rhesus macaques—a species with documented policing behavior—and the relationship between subordination signals, social power, and policing behavior in seven captive groups. We evaluate (Q1) whether pairs with direct subordination signals have greater dominance relationship certainty than nonsignaling pairs. We further examine (Q2) whether subordination signal networks show transitive linear structure with all direct and indirect network pathways going in the same direction (i.e., no cyclic pathways such as A→B→C→A) and if so, (Q3) whether pairs linked via indirect pathways of subordination signals have greater dominance certainty than pairs with no indirect connection. Finally, (Q4) we investigate whether indirect pathways of subordination signals are collectively a useful metric of social power; we do so by determining the distribution of social power with and without these indirect pathways and examining whether the most powerful individuals police conflicts more often than those with little or no social power.
MATERIALS AND METHODS
Data collection
We examined subordination signals and conflict policing in seven groups of rhesus macaques at the California National Primate Research Center (CNPRC) between June 2008 and December 2009 (Table 1). Each group was housed in a half-acre outdoor enclosure of similar design, dimensions (30 × 60 m), and physical enrichment (10 A-frame houses and multiple perches, suspended barrels and swings). Macaques were fed a standard monkey chow diet twice per day and water was available ad libitum from multiple water spigots. All research reported here complied with all institutional regulations and laws of the United States government and the American Association of Physical Anthropologists Code of Ethics. This research was approved by the University of California, Davis Institutional Animal Care and Use Committee.
Table 1.
Study group characteristics
| Group | Observation hours | Mean group size | Subjects 3+ yrs | Group formation |
|---|---|---|---|---|
| 1 | 182.1 | 176.5 | 92 | 2003 |
| 5 | 251.8 | 137.1 | 85 | 1986 |
| 8 | 231.8 | 160.1 | 96 | 1991 |
| 10 | 178.1 | 164.4 | 76 | 1976 |
| 14 | 226.3 | 108.3 | 57 | 2003 |
| 16 | 163.9 | 150.3 | 74 | 2004 |
| 18 | 175.5 | 197.9 | 97 | 2001 |
Each group was observed for six hours on four days per week for one week per month during each group’s study period. A team of two observers used an event sampling design to record all occurrences of conflict and dominance interactions among adults (at least 3 years of age) group members as ordered series of pairwise interactions including: displacements, silent bared teeth displays (SBTs), and aggression and submission organized in levels of severity outlined in Beisner and McCowan (2013). Individual animals were identified by natural markings, tattoos, and a dye-marking system implemented by the CNPRC. Inter-observer reliabilities for both behavior and animal identification had mean of 91% agreement and a standard deviation of 3%; range: 86–94%; kappa =0.65, p<0.0001 across three observers.
Peaceful SBTs (pSBT) are subordination signals in rhesus macaque society where a subordinate bares its teeth to a dominant, without screaming or other vocalizations, either spontaneously or in response to the dominant’s approach (Beisner and McCowan, 2014). The ‘peaceful’ context was operationally defined by absence of overt or subtle threat such as hard stare or body posture suggestive of threatening behavior (Beisner and McCowan, 2014). Though our previous work identified two different types of pSBT (pSBT-stay and pSBT-leave), these signals differ only in the frequency of grooming between the signaler and receiver, not aggression (Beisner and McCowan, 2014). Both signals were combined for this study.
Interventions involved a third-party entering an on-going fight. Based on previous work reviewed in the introduction, policing interventions were operationally defined as either impartial policing (policer shows no preferential behavior toward any conflict participant, e.g., approach or threaten both participants) or support policing (directing aggression at the dominant conflict participant, thereby helping an unrelated subordinate) (Beisner and McCowan, 2013). Kinship relationships were also defined in order to accurately categorize interventions as support policing that benefits a non-kin subordinate. Two individuals were defined as kin if they were from the same matriline. Males in these captive groups could not disperse, so natal males could also have maternal kin in the group. Each group also included 1–5 unrelated adult males that were added to the groups at the time of initial group formation. We counted the total number of policing interventions performed by each individual across the study period for analysis. Since impartial policing and support policing might have different relationships to social power, these two types of policing were tabulated and analyzed separately.
Social network analyses and metrics
Aggression networks and dominance certainty
Dominance probabilities based upon a standard win/loss matrix suffer from lack of data in much the same way as traditional dominance rank matrices, because many pairs are never observed to interact, and those that have do so infrequently. To address this, we calculated dominance certainty (Table 2) using a network approach called Percolation and Conductance (Fushing et al., 2011; Fujii et al., 2014), whereby multiple indirect dominance pathways in the network (e.g. A→B, B→C generates A→B→C) were used to fill in missing data in the win/loss matrix (e.g., we infer from A→B→C that A is likely dominant to C). Dominance probabilities ranged from certain (consistent direction across all transitive dominance pathways between A and B) to ambiguous (inconsistent direction; some transitive dominance pathways go from A to B whereas other directed pathways go from B to A). Dominance ranks (see Table 2) were also determined from these dyadic dominance probabilities by re-ordering the dominance probability matrix to minimize values greater than 0.5 in the lower triangle (Fushing et al., 2011).
Table 2.
Definitions of metrics
| Measure | Description | Data Source | Analysis | Unit of Analysis |
|---|---|---|---|---|
| Dominance Certainty | Degree of consistency in direction of transitive dominance pathways, reflects the probability animal A will win in a fight against animal B | aggression network pathways | Percolation & Conductance (Fujii et al., 2014) | Dyad |
| Dominance Rank | Linear ordering of individuals in a group from most dominant to least dominant | aggression network pathways | Minimize cost in matrix lower triangle (Fushing et al, 2011) | Individual |
| Linear Transitivity | Degree to which direct and indirect pathways in a network flow the same direction | global property of network structure | Proportion of transitive quadruples, triples, etc. in directed network | Network |
| pSBT Path-type | Categorization of type of connection between two individuals in a network by path length | Network pathways between two nodes | Count of steps in pathways connecting a dyad | Dyad |
| Direct Social Power | The extent to which signals of subordination by multiple individuals converge on the same dominant; the diversity of individuals that signal subordination to a given dominant | direct pSBTs | Shannon information index (Flack & Krakauer, 2006) | Individual |
| Cumulative Social Power | The extent to which pathways in a subordination network converge on the same dominant; diversity of individuals whose subordination signaling pathways lead to a given dominant | pSBT network pathways | Shannon information index | Individual |
Subordination signaling networks and social power
Across the study period, the total number of pSBTs per group ranged from 155 to 420 (rate per individual per hour ranged from 0.010 to 0.022). All signals were used to construct a directed weighted network for each study group. To evaluate whether all direct and indirect pathways in the network flowed in the same direction (show linear transitivity) (Q2), we used Percolation and Conductance to identify and compare directionality of all pathways of different lengths in the network, including 1-step paths (A→B), 2-step paths (A→B→C), 3-step paths (A→B→C→D), and 4-step paths (A→B→C→D→E).
We placed each pair into one of seven mutually exclusive categories according to their types of pathways: (a) 1-step path: pairs with only direct pSBT signal(s); (b) 1-step plus: pairs with a direct pSBT plus one or more indirect paths; (c) 2-step path: pairs with only 2-step indirect path(s) connecting them; (d) 2-step plus: pairs with a 2-step indirect path plus one or more longer indirect paths; (e) 3-step path: pairs with only 3-step indirect path(s) connecting them; (f) 3-step plus: pairs with a 3-step indirect path plus one or more longer indirect paths; (g) 4-step path: pairs with only 4-step indirect path(s) connecting them.
To evaluate whether indirect pathways of subordination signals may be useful for understanding social power, we quantified signaling convergence in two ways: (1) using only direct signals (1-step paths) received, referred to here as direct social power, and (2) using direct and indirect pathways of signals ‘received’, referred to here as cumulative social power (see Table 2). We plotted empirical distributions of social power to evaluate the degree and direction of skew in its distribution. This was done for both direct social power and cumulative social power across all study groups.
Statistical analyses
To examine whether pairs with direct pSBTs had greater dominance certainty than non-signaling pairs (Q1), we fit a generalized linear regression model (McCullagh and Nelder, 1989) to the pairwise dominance certainty values across all possible pairs. Pairs were placed in one of eight categories: no signaling pathway, 1-step path, 1-step plus, 2-step path, 2-step plus, 3-step path, 3-step plus, and 4-step path (see categories defined above). Each group was analyzed separately, and sample size ranged from N=2211 pairs to N=6105 pairs.
To examine whether the presence of an indirect pathway was associated with greater dominance certainty than the absence of that indirect pathway, all else being equal (e.g., no pathway vs. 2-step path; 1-step path pairs versus 1-step plus path) (Q3), we fit a second generalized linear regression model to pairwise dominance certainty across all pairs. For this analysis, pairs were placed in one of the seven categories described above (e.g., 1-step path, 1-step plus, 2-step path, etc.). Groups were analyzed separately and pairs with no network pathways of pSBTs were omitted (sample size range: N=381 pairs to N=1907 pairs). Pairwise dominance certainty values were transformed between 0 (complete certainty of dominance) and 0.5 (complete ambiguity), which fit an inverse Gaussian family distribution. The absolute value of dominance rank difference was included in each model to account for its influence on dyadic dominance certainty.
Finally, to examine whether variance in direct social power and/or cumulative social power predicted policing effort (Q4), we fit a Poisson GLM to the count of impartial policing attempts per individual and the count of support policing. Each group was analyzed separately and sample size ranged from N=57 to N=97 adults (mean = 83.5 adults/group). Observation hours per subject were used as an exposure variable in the analysis of each group to account for temporary absence from the group due to veterinary care.
RESULTS
Subordination signals and dominance relationship certainty
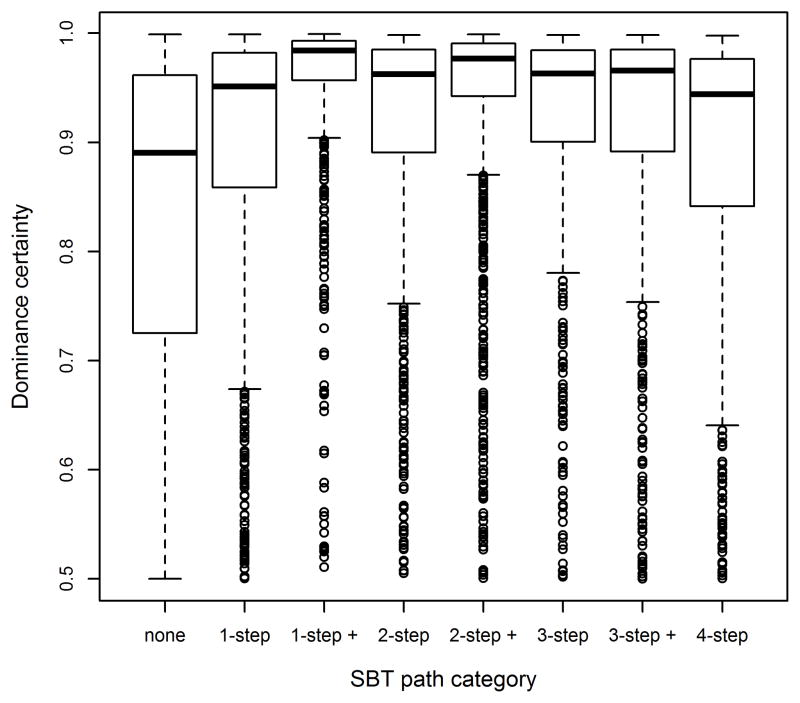
Pairs with only a direct pSBT (i.e., 1-step path) had significantly more certain dominance relationships than pairs with no pathway of connection. In fact, pairs with any length of pSBT pathway between them (direct or indirect) had significantly more certain dominance relationships than pairs with no signaling pathway (Fig. 2). Compared to pairs with no pathway of pSBTs, pairs with a 1-step path (p< 0.01), a 1-step path plus a longer signaling pathway (p< 0.01), a 2-step path (p< 0.01), a 2-step path plus a longer pathway, and a 3-step path (p<0.05) all had greater dominance certainty. Dyads with a 2-step path plus a longer pathway and those with only a 3-step path showed a trend for greater dominance certainty than those with no pathways of pSBTs (0.05 < p < 0.16).
Figure 2.
Dyadic dominance certainty plotted for all dyads with different lengths and combinations of pSBT signaling pathways. Boxes represent the interquartile range and the black bar is the median dominance certainty.
Linear transitivity in subordination signaling networks
The pSBT network of each study group showed perfect linear transitivity—there were no circular pathways of any length (see Fig. 1). Groups did vary, however, in the relative frequencies of each type of pathway (Table S1). Although each group had a similar number of pairs with only a 1-step path (range: 100–174, mean=140.6), they showed greater variance than other types of pairs such as those with a 1-step path plus an indirect pathway (range: 28–164, mean=79). The pSBT networks for all study groups are presented in figures S1–S6.
Indirect pathways of subordination signals and dominance certainty
To examine whether an indirect pathway of signals is associated with greater dominance certainty (and may be representative of a dominance relationship that is relevant to social power), we analyzed only pairs with at least one pSBT pathway (direct or indirect) between them. Amongst pairs with only a single type of pSBT pathway between them (i.e., either a 1-step, or a 2-step, or a 3-step path), there were no significant differences in dyadic dominance certainty—this pattern was true for all groups except Group 16 (See Table 3 and Fig. 2). In other words, direct signals and indirect pathways of signals were associated with equal levels of dyadic dominance certainty. Finally, pairs with multiple types of pSBT pathways between them (i.e.1-step path plus and 2-step path plus) showed either the same or greater dominance certainty than pairs with only a direct pSBT path. Notably, 3-step + and 4-step pathways showed a variable relationship to dominance certainty.
Table 3.
Model coefficients for analyses of dyadic dominance certainty by pSBT path type for each study group. Each path length category is compared to a direct 1-step path.
| Variable | Group 1 | Group 5 | Group 8 | Group 10 | Group 14 | Group 16 | Group 18 |
|---|---|---|---|---|---|---|---|
| Rank diff | 0.11** | 0.13** | 0.12** | 0.036** | 0.072** | 0.059** | 0.11** |
| 1-step + | 0.029** | ns | 0.020** | 0.040** | ns | 0.041** | ns |
| 2-step | ns | ns | ns | ns | ns | 0.031* | ns |
| 2-step + | 0.025* | 0.015* | ns | 0.037* | ns | 0.035* | ns |
| 3-step | ns | ns | ns | ns | ns | 0.031* | ns |
| 3-step + | 0.031* | −0.016* | 0.013* | 0.036* | ns | 0.039** | ns |
| 4-step | ns | −0.016* | ns | ns | ns | 0.037* | ns |
p-value <0.05
p-value <0.01
Non-significant p-values: ns
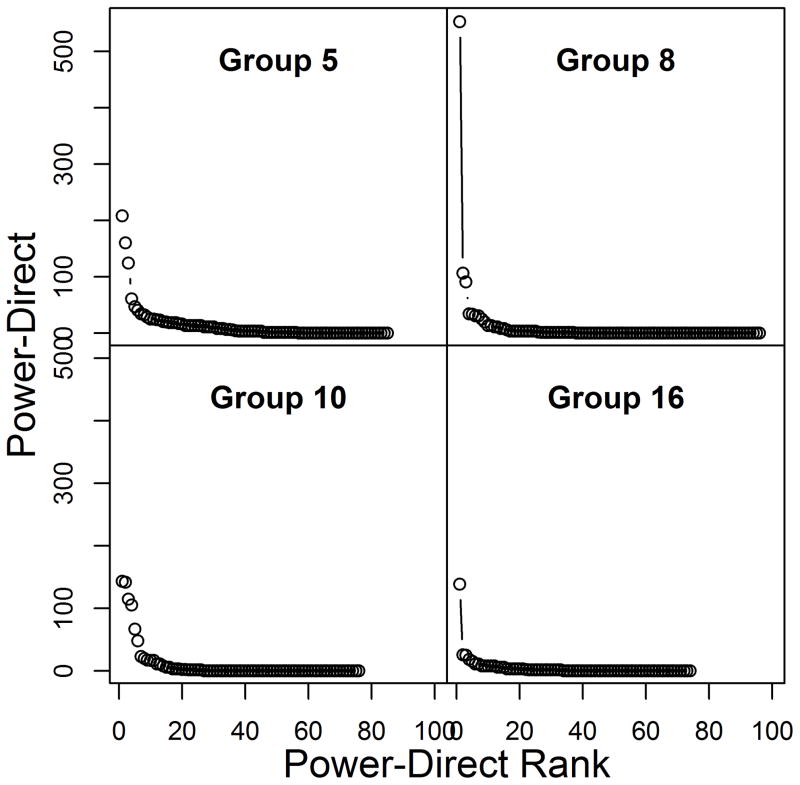
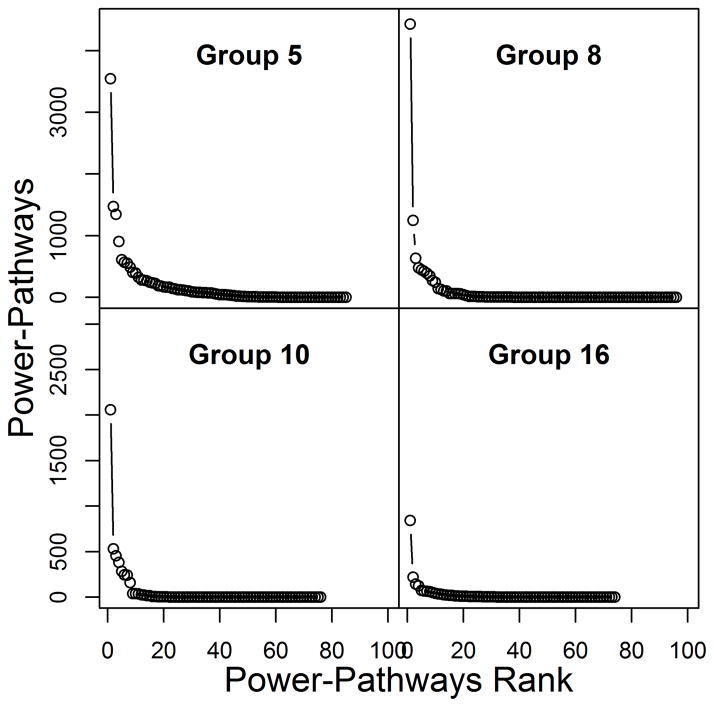
Distribution of social power
The distribution of social power in all study groups showed a heavy-tailed left-skewed distribution, with most individuals accumulating little or no social power (i.e., received no direct pSBT signals and were not on the receiving end of indirect pathways of signals) and a small number of individuals holding far greater social power (Figs. 3–4). The distributions of cumulative social power were more skewed than for direct social power because adding signaling pathways primarily amplified the social power of the individuals with relatively high direct social power (see Figs. S7–S8 for remaining study groups social power distributions).
Figure 3.
Distribution of direct social power for four of the seven study groups
Figure 4.
Distribution of cumulative social power for four of the seven study groups
Conflict policing relative to social power
Both direct social power and cumulative social power were positively associated with impartial policing frequency. Three of the seven groups had two best-fit models (model 1 included cumulative social power; model 2 included direct social power) because both calculations of social power were equally good predictors of impartial policing, i.e., individuals with greater social power performed more impartial interventions (see Table 4). The four remaining groups each had one best fit model. In Groups 1 and 14, individuals with higher cumulative social power performed more impartial interventions whereas in Group 5, individuals with higher direct social power performed more impartial interventions. In Group 10, social power was not in the best-fit model, and dominance rank better predicted impartial policing.
Table 4.
Model coefficients for analyses of impartial policing per individual (coefficients are from the best-fit model for each study group)
| Variable | Group 1 | Group 5 | Group 8a | Group 10 | Group 14 | Group 16a | Group 18a |
|---|---|---|---|---|---|---|---|
| Intercept | −4.89 | −5.76 | −6.23 | −3.54 | −4.07 | −6.647 | −6.10 |
| Sex (male) | 1.18** | ns | 2.15** | 2.74** | ns | -- | 1.648 |
| Dominance Rank | −0.05** | −0.032** | −0.04* | −0.14** | −0.13** | ns | −0.045** |
| Cumulative social power | 0.0025* | -- | 0.0003* | -- | 0.0005* | 0.003** | 0.004** |
| Direct social power | -- | 0.011** | -- | -- | -- | -- | -- |
| AIC (dAIC) | 146.7 (2.5) | 107.8 (6.1) | 85.4 (0.6) | 44.8 (7.2) | 98.3 (2.0) | 55.4 (0.3) | 87.3 (1.1) |
Two best-fit models were found
p-value <0.05
p-value <0.01
Non-significant: ns
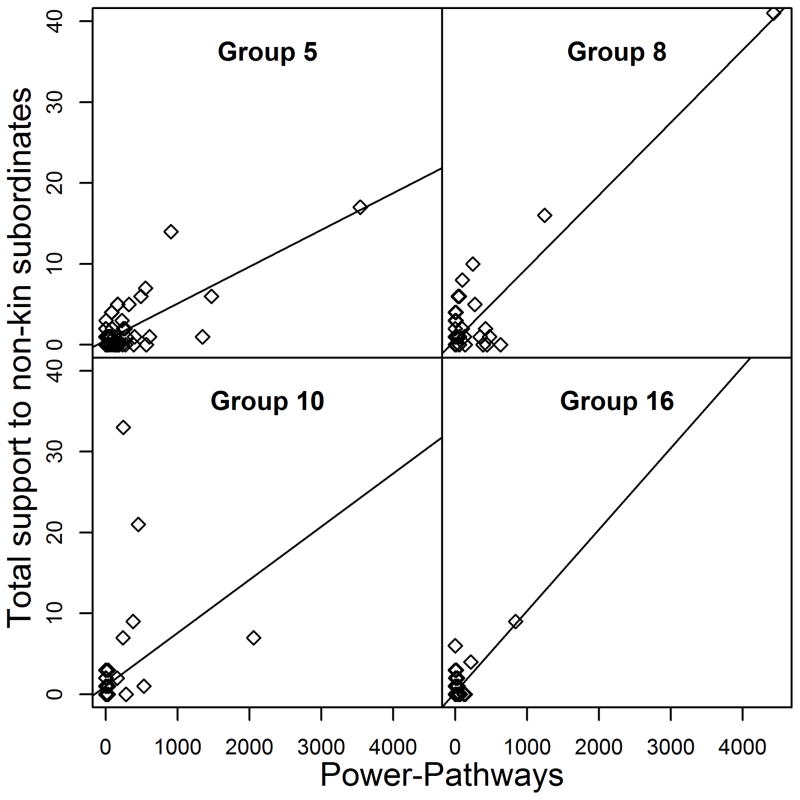
Both direct social power and cumulative social power were also positively associated with support policing frequency per individual (Fig. 5). Similar to impartial policing, three of the seven groups had two best-fit models (model 1 included cumulative social power; model 2 included direct social power) indicating that individuals with greater social power (regardless of how social power was calculated) provided more frequent support to subordinate non-kin (see Table 5). The four remaining groups each had one best fit model. In Groups 1 and 5, individuals with greater cumulative social power supported non-kin subordinates more frequently than those receiving few pSBT signals/pathways. However, in Groups 10 and 14, social power was not in the best-fit model, and dominance rank was a better predictor of policing support to non-kin subordinates.
Figure 5.
Frequency of support given to non-kin subordinates (i.e., policing) by cumulative power for four of the seven study groups
Table 5.
Model coefficients for analyses of support policing (coefficients reported are from the best-fit model for each study group)
| Variable | Group 1 | Group 5 | Group 8a | Group 10 | Group 14 | Group 16a | Group 18a |
|---|---|---|---|---|---|---|---|
| Intercept | −4.06 | −4.70 | −4.42 | −3.17 | −3.84 | −5.29 | −5.28 |
| Sex (male) | 0.88** | 1.11** | 1.43** | 2.50** | 0.84** | 1.20** | 0.92** |
| Dominance Rank | −0.040** | −0.029** | −0.034** | −0.06** | −0.061** | −0.032** | −0.02** |
| Cumulative social power | 0.002** | 0.0003** | 0.0003** | -- | -- | 0.0015* | 0.0025** |
| Direct social power | -- | -- | -- | -- | -- | -- | -- |
| AIC (dAIC) | 267.7 (3.6) | 244.4 (5.1) | 277.8 (1.0) | 175.9 (10.7) | 161.2 (3.0) | 125.8 (0.5) | 250.6 (0.3) |
Two best-fit models were found
p-value <0.05
p-value <0.01
Non-significant: ns
DISCUSSION
Conflict policing is a mechanism to manage group conflict observed in a number of primate species. Effective policers must have sufficiently greater social power than others to stop their fights. Although conflict policing behavior has been identified and studied in many species (though not always referred to as policing), empirical studies are lacking that examine individual attributes, such as social power, that underlie an individual’s ability and/or tendency to police group fights (Flack et al., 2005b). Furthermore, the distribution of social power, and the behavior from which it should be calculated, has not been characterized for most species. We therefore examined the structure of subordination signaling networks as a probable behavioral metric of social power and the relationship between social power and conflict policing in captive rhesus macaques. Our results showed that (a) subordination networks had perfect linear transitivity and (b) direct and indirect pathways of subordination signals were associated with greater dominance certainty. This finding demonstrates that pathways within subordination signaling networks represent formal and settled dominance relationships and are thus ideal for quantifying social power. Furthermore, both direct and cumulative social power were positively associated with conflict policing, substantiating the inclusion of both direct and indirect subordination signaling pathways in calculations of social power. These calculations help identify the underlying social structure that permits policing behavior. Below we discuss these results in detail.
Subordination signals: dominance certainty and network structure
While the function of SBTs varies across primate species, SBTs given in apparently peaceful contexts are considered subordination signals in pigtail and rhesus macaques (Flack and de Waal, 2007; Beisner and McCowan, 2014). Formal signals of subordination or dominance are thought to decisively communicate who is subordinate versus dominant. Our analysis quantitatively demonstrated that pairs that used subordination signaling had greater dominance certainty (as measured from aggressive interactions) compared to non-signaling pairs. This is consistent with the results of Flack and colleagues (2007) that the peaceful context provides greater assurance about the receiver’s role in the dominance relationship, and more rigorously supports previous claims regarding the function of formal status signals in the literature (Preuschoft and Van Schaik, 2000b; Preuschoft, 2004).
The pSBT network for each study group showed perfect linear transitivity with a clear upward flow of signaling that had no circular pathways To our knowledge, Flack and colleagues’ (2006) description of the pSBT network in a group of pigtailed macaques is the only other empirical example of a subordination signaling network, and they report that its structure is not perfectly transitive. Given that rhesus macaques are more despotic than pigtailed macaques and have a greater degree of asymmetry in their agonistic interactions (Thierry, 2004), perhaps linear transitivity in subordination signaling networks is correlated with a despotic/intolerant social style. However, in order to evaluate this hypothesis, the networks of formal subordination or dominance signals must be characterized from multiple species along a gradient of social tolerance and asymmetry of agonistic interactions. Matrices of subordination signaling interactions are currently lacking in the literature, despite extensive research on SBTs in the genus Macaca.
Indirect pathways
Our analysis also showed that pairs connected by only indirect pathways of signals had greater dominance certainty than pairs with no pathways of subordination signals between them. This suggests that pairs that signal subordination infrequently (or not at all) may still have access to dominance information by observing the signaling behavior of others. Nonhuman primates, like many other vertebrates, are capable of transitive inference (e.g. McGonigle and Chalmers, 1977) and are likely to incorporate third-party information into their understanding of their dominance relationships. Macaques, in particular, are known to use information from third-party social interactions when making social decisions (e.g. ally recruitment Silk, 1999). Further, accumulating evidence suggests that macaques possess neuroanatomical systems that allow them to use information about conspecifics’ actions to guide their own behavior (e.g. mirror neurons, Rizzolatti and Craighero, 2004). Given this cognitive foundation and the transitive structure of the subordination signaling network, we argue that macaques likely infer (or reaffirm) their dominance relationships from assemblages of observed signals that form indirect pathways of signals in the network. We do concede, however, that our data do not allow us to be certain whether macaques actually did infer anything about dominance-subordinance relationships.
Subordination signals were observed infrequently (0.01–0.02 signals/hr/subject), and networks were therefore sparse. This is consistent with previous work showing that affiliation networks of intolerant/despotic macaques are more highly centralized and less dense due to the influence of dominance and nepotism (Sueur et al., 2011; Sueur et al., 2012). This broader pattern of low network density may arise if individuals of low dominance rank tend to avoid direct interaction with those of high dominance rank because the risk of injury is too great. In such a system, transitive inference of dominance relationships (i.e., use of transitive network pathways) would be expected to contribute to the maintenance of these relationships without the cost of direct interaction.
In addition, in four of our seven study groups, we found that pairs with multiple pathways of connection in the pSBT network (e.g. 1-step plus and 2-step plus) had greater dominance certainty than pairs with only a direct signal connection (i.e., 1-step path). For example, among pairs of animals with a direct signal (i.e., pairs whose direct signals were not missed during sampling), those that also had indirect pathways of signals between them had more certain dominance relationships. This suggests that having an indirect pathway of signals adds dominance information beyond a direct signal and that greater dominance certainty is coincident with redundant sources of information on subordination.
Network redundancy
The presence of multiple direct and indirect pathways in rhesus macaque pSBT networks creates redundant information at the level of both the pairwise relationship and the social group. Within pairs, rates of redundant signaling pathways in our rhesus groups ranged from 16 – 63% (mean = 34.9%; percentage of pairs with at least two different signaling pathways between them), and in four of our seven groups, these same pairs had more certain relationships than those connected by a single pSBT pathway. Being connected via redundant network pathways may benefit individuals by creating multiple routes of subordination information transmission (i.e., direct experience or transitive inference), which serves as a buffer against communication error.
Redundancy is a commonly examined feature of complex systems or networks. In scale-free networks, redundancy improves the maintenance of overall connectivity by allowing social systems to survive the failure of a component or node (Albert et al., 2000). Redundancy is also greater in more complex social systems (Anderson and McShea, 2001). Therefore, although redundant network information has been described as “inefficient” and characteristic of primate species with smaller neocortex to body size ratio, such redundancy may be critical for stability in large and dynamic social groups (Pasquaretta et al., 2014). The apparent redundancy found in subordination signaling networks may be a mechanism of group stability in a despotic species with strong hierarchical relationships and large group sizes. The heavy-tailed empirical distributions of social power result from a small number of highly connected and powerful individuals (similar to the ‘hubs’ that are characteristic of scale-free networks). Therefore, redundancy in the pSBT network may buffer the group against the loss of individuals through deaths and emigrations and this in turn allows the group to adjust to the loss of a signaler or receiver or even a change within a single relationship. It maintains the robustness of dominance relationships in much the same way that redundancy in biological systems evolved as function-failure backup mechanisms (Whitacre, 2010). Finally, if pathways in the pSBT network represent group consensus of social power, redundancy in this network may be critical to a power distribution that permits policing.
Power and policing
The two methods of power calculation assigned the highest social power to different individuals in some groups. Individuals at the top of the pSBT network had the highest cumulative social power, but not necessarily the highest direct social power. It is likely more meaningful to receive a signal from another powerful individual than an individual with no social power. Cumulative social power appears to better represent the true group-level convergence in subordination signaling. This is seen in changes in relative power calculations when including these indirect pathways for some individuals such as the drop in the relative social power for a young high-ranked natal male and the increase in relative social power for an alpha female.
Across all of our study groups, individuals with greater social power more frequently policed the fights of others, substantiating previous work by our research group on rhesus macaques (McCowan et al., 2011) and by Flack and colleagues on pigtailed macaques (Flack et al., 2005a; Flack and Krakauer, 2006). Furthermore, we found that cumulative social power was generally the best predictor of policing effort. The few exceptions to this were also informative. First, in Group 5 direct social power better explained impartial policing than cumulative social power. However, the alpha and beta males in group 5 (i.e., those with greatest cumulative social power) did far more support policing (i.e., supporting non-kin subordinates) than impartial interventions. In all other groups, the primary policer(s) used both types of policing equally. Second, in the three groups (8, 16, and 18) in which both direct and cumulative social power were equally good at explaining policing effort, the alpha male was the top policer, performing up to one-third of all policing, and also had the highest power by both calculations. Thus, a large part of the variance in policing in these groups (i.e., the alpha male relative to others) was captured equally well by both power calculations. By contrast, the policing role was shared by multiple individuals in the other groups (2–5 individuals performed 35–45% of all policing interventions), and indirect pathways of signals clarified differences in social power associated with policing effort.
Groups 10 and 14 were notable exceptions to the association between social power and policing. Social power was not part of the best-fit models in the analyses of both types of policing in group 10, nor in the analyses of support policing in group 14. In these analyses, social power had a positive relationship with policing (as in the other study groups), but this effect disappeared once dominance rank was added to the model. Thus dominance rank better explained policing frequency than social power in these instances. Group 10 was also unstable, having low matriline cohesion and relatedness (Beisner et al 2011a), a natal alpha male (Beisner et al., 2011; Jackson et al., 2012), and two recent matrilineal overthrows. Previous work has shown that severe aggression increases when matriline cohesion and relatedness is low and that natal alpha males tend not to police aggression by their high dominance rank female relatives. The most active policers in group 10 were of high dominance rank, but not the highest, nor did they have the highest social power. It appears the policers in that group had limited ability to stop conflicts and provide an adequate stabilizing force in the group. Group 10 appears to be the exception that provides evidence for the general trend, suggesting the relationship between social power and policing may change during unstable periods.
Conclusion
Group-level convergence in subordination signaling concentrates social power on a small number of conflict policers in the group and yields a remarkable pattern of global linear transitivity of signaling that highlights the importance of indirect signaling pathways and use of third-party dominance information. We argue that subordination signaling networks are critical for communicating formal and settled dominance relationships, thereby upholding social stability in complex and large primate systems. The relationship between dominance certainty and pSBT signaling pathways highlights an important connection between two aspects of rhesus macaque society – aggression and status signaling - and corroborates previous findings by Beisner et al. (2015). This demonstrates an interdependence between group-wide patterns of directionality in dyadic aggression and status signaling as it relates to social instability. In a hierarchical system, the relationship between different networks can degrade if the power structure of the system breaks down (Fushing et al., 2014). In rhesus macaque society, pSBT networks are representative of this power structure, and relate both to dominance certainty, which is important for relationship stability and therefore group stability, and also support the additional stabilizing mechanism of policing. We argue that this network of formal dominance relationships is central to rhesus macaque societal stability, particularly with increasing group size.
Supplementary Material
Acknowledgments
This project was supported by NIH grants #R24 RR024396 (BM) and #PR 51 RR000169 (CNPRC base grant). We thank our observation staff Megan Jackson and Shannon Seil, Jessica Vandeleest and Jian Jin for helpful conversations, the editors, and two anonymous reviewers for their valuable suggestions for improving the manuscript. BM conceived and designed the study and supplied the materials; BB performed the data collection; BB, BM, DH, and KF developed the hypotheses; BB performed the analyses; BB, DH, KF, and BM wrote the paper.
Grant sponsorship: NIH grants #R24 RR024396 (BM) and #PR 51 RR000169 (CNPRC base grant)
LITERATURE CITED
- Albert R, Jeong H, Barabasi AL. Error and attack tolerance of complex networks. Nature. 2000;406:378–381. doi: 10.1038/35019019. [DOI] [PubMed] [Google Scholar]
- Anderson C, McShea DW. Individual versus social complexity, with particular reference to ant colonies. Biological Reviews. 2001;76(2):211–237. doi: 10.1017/s1464793101005656. [DOI] [PubMed] [Google Scholar]
- Beisner BA, Jackson ME, Cameron A, McCowan B. Effects of natal male alliances on aggression and power dynamics in rhesus macaques. Am J Primatol. 2011;73:790–801. doi: 10.1002/ajp.20907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisner BA, Jin J, Fushing H, McCowan B. Detection of social group instability among captive rhesus macaques using joint network modeling. Current Zoology. 2015 doi: 10.1093/czoolo/61.1.70. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisner BA, McCowan B. Policing in nonhuman primates: partial interventions serve a prosocial conflict management function in rhesus macaques. PLoS ONE. 2013;8(10):e77369. doi: 10.1371/journal.pone.0077369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisner BA, McCowan B. Signaling context modulates social function of silent bared teeth displays in rhesus macaques (Macaca mulatta) Am J Primatol. 2014;76:111–121. doi: 10.1002/ajp.22214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein I, Sharpe LG. Social roles in a rhesus monkey group. Behaviour. 1966;26(1):91–104. doi: 10.1163/156853966x00038. [DOI] [PubMed] [Google Scholar]
- Bond AB, Kamil AC, Balda RP. Social complexity and transitive inference in corvids. Anim Behav. 2003;65(3):479–487. [Google Scholar]
- Chaffin CL, Friedlen K, De Waal FBM. Dominance style of Japanese macaques compared with rhesus and stumptail macaques. Am J Primatol. 1995;35(2):103–116. doi: 10.1002/ajp.1350350203. [DOI] [PubMed] [Google Scholar]
- Chapman CA, Chapman LJ. Constraints on group size in red colobus and red-tailed guenons: examining the generality of the ecological constraints model. International Journal of Primatology. 2000;21(4):565–585. [Google Scholar]
- David-Barrett T, Dunbar RIM. Processing power limits social group size: computational evidence for the cognitive costs of sociality. P Roy Soc B-Biol Sci. 2013;280(1765) doi: 10.1098/rspb.2013.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis H. Transitive inference in rats (Rattus norvegicus) J Comp Psychol. 1992;106(4):342–349. doi: 10.1037/0735-7036.106.4.342. [DOI] [PubMed] [Google Scholar]
- de Waal FBM. The integration of dominance and socialbonding in primates. Q Rev Biol. 1986;61:459–479. doi: 10.1086/415144. [DOI] [PubMed] [Google Scholar]
- de Waal FBM. Primates: a natural heritage of conflict resolution. Science. 2000;289(5479):586–590. doi: 10.1126/science.289.5479.586. [DOI] [PubMed] [Google Scholar]
- de Waal FBM, Luttrell LM. The formal hierarchy of rhesus macaques: an investigation of the bared-teeth display. Am J Primatol. 1985;9:73–85. doi: 10.1002/ajp.1350090202. [DOI] [PubMed] [Google Scholar]
- Flack JC, de Waal FBM. Dominance style, social power, and conflict. In: Thierry B, Singh M, Kaumanns W, editors. Macaque Societies: A Model for the Study of Social Organization. Cambridge: Cambridge University Press; 2004. pp. 157–182. [Google Scholar]
- Flack JC, de Waal FBM. Context modulates signal meaning in primate communication. Proceedings of the National Academy of Science. 2007;104:1581–1586. doi: 10.1073/pnas.0603565104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flack JC, de Waal FBM, Krakauer DC. Social structure, robustness, and policing cost in a cognitively sophisticated species. The American Naturalist. 2005a;165:E000. doi: 10.1086/429277. [DOI] [PubMed] [Google Scholar]
- Flack JC, Krakauer DC. Encoding power in communication networks. The American Naturalist. 2006;168:E87–E102. doi: 10.1086/506526. [DOI] [PubMed] [Google Scholar]
- Flack JC, Krakauer DC, de Waal FBM. Robustness mechanisms in primate societies: a perturbation study. Proc R Soc Biol Sci Ser B. 2005b;272:1091–1099. doi: 10.1098/rspb.2004.3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii K, Fushing H, Beisner BA, McCowan B. Technical Report. Dept of Statistics; UC Davis: 2014. Computing power structures in directed biosocial networks: flow percolation and imputed conductance. [Google Scholar]
- Fushing H, Jordà Ò, Beisner B, McCowan B. Computing systemic risk using multiple behavioral and keystone networks: The emergence of a crisis in primate societies and banks. International Journal of Forecasting. 2014;30(0):797–806. doi: 10.1016/j.ijforecast.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fushing H, McAssey M, Beisner BA, McCowan B. Ranking network of a captive rhesus macaque society: a sophisticated corporative kingdom. PLoS ONE. 2011;6(3):e17817. doi: 10.1371/journal.pone.0017817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosenick L, Clement TS, Fernald RD. Fish can infer social rank by observation alone. Nature. 2007;445(7126):429–432. doi: 10.1038/nature05511. [DOI] [PubMed] [Google Scholar]
- Hinde RA. Interactions, relationships and social structure. Man. 1976;11(1):1–17. [Google Scholar]
- Huguet P, Barbet I, Belletier C, Monteil JM, Fagot J. Cognitive Control Under Social Influence in Baboons. Journal of Experimental Psychology-General. 2014;143(6):2067–U2367. doi: 10.1037/xge0000026. [DOI] [PubMed] [Google Scholar]
- Isbell LA, Pruetz JD. Differences between vervets (Cercopithecus aethiops) and patas monkeys (Erythrocebus patas) in agonistic interactions between adult females. International Journal of Primatology. 1998;19(5):837–855. [Google Scholar]
- Isbell LA, Pruetz JD, Young TP. Movements of vervets (Cercopithecus aethiops) and patas monkeys (Erythrocebus patas) as estimators of food resource size, density, and distribution. Behavioral Ecology and Sociobiology. 1998;42(2):123–133. [Google Scholar]
- Jackson ME, Hannibal DL, Beisner BA, McCowan B. Matrilineal relatedness influences the efficacy of policing by alpha male rhesus macaques(Macaca mulatta) Am J Primatol. 2012;74(S1):74. [Google Scholar]
- Kaplan JR. Fight interference and altruism in rhesus monkeys. Amer J Phys Anthrop. 1978;49:241–249. [Google Scholar]
- Kummer H. Tripartite relations in Hamadryas baboons. In: Altmann SA, editor. Social Communication Among Primates. Chicago: University of Chicago Press; 1967. [Google Scholar]
- Kurland JA. Kin selection in the Japanese monkey. Basel, New York: S. Karger; 1977. p. 145. [PubMed] [Google Scholar]
- Lewis R. Beyond dominance: The importance of leverage. Q Rev Biol. 2002;77:149–164. doi: 10.1086/343899. [DOI] [PubMed] [Google Scholar]
- Lindburg DG. The rhesus monkey in north India: an ecological and behavioral study. In: Rosenblum LA, editor. Primate Behavior: Developments in Field and Laboratory Research. New York: Academic Press; 1971. pp. 1–106. [Google Scholar]
- Lusseau D. Emergent properties of a dolphin social network. Proc R Soc B. 2003;270:S186–S188. doi: 10.1098/rsbl.2003.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCowan B, Beisner BA, Capitanio JP, Jackson ME, Cameron A, Seil S, Atwill ER, Fushing H. Network stability is a balancing act of personality, power, and conflict dynamics in rhesus macaque societies. PLoS ONE. 2011;6(8):e22350. doi: 10.1371/journal.pone.0022350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullagh P, Nelder JA. Generalized Linear Models. London: Chapman and Hall; 1989. [Google Scholar]
- McGonigle BO, Chalmers M. Are monkeys logical? Nature. 1977;267(5613):694–696. doi: 10.1038/267694a0. [DOI] [PubMed] [Google Scholar]
- Pasquaretta C, Leve M, Claidiere N, van de Waal E, Whiten A, MacIntosh AJJ, Pele M, Bergstrom ML, Borgeaud C, Brosnan SF, et al. Social networks in primates: smart and tolerant species have more efficient networks. Scientific Reports. 2014:4. doi: 10.1038/srep07600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuschoft S. Laughter and smile in barbary macaques (Macaca sylvanus) Ethology. 1992;91(3):220–236. [Google Scholar]
- Preuschoft S. Macaques: and Evolutionary Perspective. Utrecht: Tessel Offset B. V; 1995. ‘Laughter’ and ‘Smiling’. [Google Scholar]
- Preuschoft S. Power and communication. In: Thierry B, Singh M, Kaumanns W, editors. Macaque Societies. Cambridge: Cambridge University Press; 2004. pp. 56–60. [Google Scholar]
- Preuschoft S, van Schaik CP. Dominance and communication: conflict management in various social settings. In: Aureli F, de Waal FBM, editors. Natural Conflict Resolution. Berkeley: University of California Press; 2000a. pp. 77–105. [Google Scholar]
- Preuschoft S, Van Schaik CP. Dominance, social relationships, and conflict management. In: Aureli F, De Waal FBM, editors. Natural Conflict Resolution. Berkeley: California University Press; 2000b. pp. 77–105. [Google Scholar]
- Ratnieks FLW. Reproductive harmony via mutual policing by workers in eusocial Hymenoptera. The American Naturalist. 1988;132:217–236. [Google Scholar]
- Rizzolatti G, Craighero L. The mirror-neuron system. Annu Rev Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Setchell JM, Wickings EJ. Dominance, status signals and coloration in male mandrills (Mandrillus sphinx) Ethology. 2005;111(1):25–50. [Google Scholar]
- Shultz S, Dunbar RIM. The evolution of the social brain: anthropoid primates contrast with other vertebrates. P Roy Soc B-Biol Sci. 2007;274(1624):2429–2436. doi: 10.1098/rspb.2007.0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz S, Dunbar RIM. Species differences in executive function correlate with hippocampus volume and neocortex ratio across nonhuman primates. Journal of Comparative Psychology. 2010;124(3):252–260. doi: 10.1037/a0018894. [DOI] [PubMed] [Google Scholar]
- Sicotte P. Interposition in conflicts between males in bi-male groups of mountain gorillas. Folia Primatol. 1995;65:14–24. doi: 10.1159/000156871. [DOI] [PubMed] [Google Scholar]
- Silk JB. Male bonnet macaques use information about third party rank relationships to recruit allies. Anim Behav. 1999;58:45–51. doi: 10.1006/anbe.1999.1129. [DOI] [PubMed] [Google Scholar]
- Sterck EHM, Watts DP, van Schaik CP. The evolution of female social relationships in nonhuman primates. Behavioral Ecology and Sociobiology. 1997;41(5):291–309. [Google Scholar]
- Sueur C, Deneubourg J-L, Petit O. From social network (centralized vs. decentralized) to collective decision-making (unshared vs. shared consensus) PLoS ONE. 2012;7(2):e32566. doi: 10.1371/journal.pone.0032566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueur C, Petit O, De Marco A, Jacobs AT, Watanabe K, Thierry B. A comparative network analysis of social style in macaques. Anim Behav. 2011;82(4):845–852. [Google Scholar]
- Thierry B. Social epigenesis. In: Thierry B, Singh M, Kaumanns W, editors. Macaque Societies. Cambridge: Cambridge University Press; 2004. pp. 267–290. [Google Scholar]
- van Hooff JARAM. Facial expressions in higher primates. Symposia of the Zoological Society of London. 1962;8:97–125. [Google Scholar]
- van Hooff JARAM. The facial displays of the catarrhine monkeys and apes. In: Morris P, editor. Primate Ethology. Chicago: Aldine Press; 1967. pp. 7–69. [Google Scholar]
- van Hooff JARAM. A comparative approach to the phylogeny of laughter and smiling. In: Hinde RA, editor. Non-Verbal Communication. Cambridge: Cambridge University Press; 1972. pp. 209–241. [Google Scholar]
- van Schaik CP, van Hooff JARAM. On the ultimate causes of primate social systems. Behaviour. 1983;85:91–117. [Google Scholar]
- Vervaecke H, de Vries H, van Elsacker L. Function and distribution of coalitions in captive bonobos (Pan paniscus) Primates. 2000;41:249–265. doi: 10.1007/BF02557595. [DOI] [PubMed] [Google Scholar]
- Vitone ND, Altizer S, Nunn CL. Body size, diet and sociality influence the species richness of parasitic worms in anthropoid primates. Evolutionary Ecology Research. 2004;6:183–199. [Google Scholar]
- von Rohr CR, Koski SE, Burkart JM, Caws C, Fraser ON, Ziltener A, van Schaik CP. Impartial third-party interventions in captive chimpanzees: a reflection of community concern. PLoS ONE. 2012;7(3):e32494. doi: 10.1371/journal.pone.0032494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitacre JM. Degeneracy: a link between evolvability, robustness and complexity in biological systems. Theor Biol Med Model. 2010;7:12. doi: 10.1186/1742-4682-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrangham RW. An ecological model of female-bonded primate groups. Behaviour. 1980;75:267–300. [Google Scholar]
- Zucker EL. Control of intragroup aggression by a captive male orangutan. Zoo Biol. 1987;6:219–223. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.