Abstract
Verrucous hyperplasia (VH) is a rare exophytic oral mucosal lesion which can transform into verrucous carcinoma (VC), its malignant but clinically similar counterpart. These entities can be distinguished by the lack of invasive growth in VH cases; as such, it is essential to include a margin with adequate depth when performing a biopsy of the epithelium of the lesion. We report an 80-year-old male patient who presented to the Bapuji Dental College & Hospital, Davangere, Karanataka, India, in 2011 with a warty whitish-pink growth on the inside of his cheek. The patient was treated with wide surgical excision of the lesion and a diagnosis of VH was made based on histopathological features. There was no evidence of recurrence at a five-year follow-up. This report highlights the histological variations, pathogenesis and differential diagnosis of VH.
Keywords: Hyperplasia, Verrucous Carcinoma, Squamous Cell Carcinoma, Histology, diagnosis, Case Report, India
Verrucous hyperplasia (vh) is a premalignant exophytic oral mucosal lesion with a predominantly verrucous or papillary surface; this lesion can subsequently transform into verrucous carcinoma (VC), a well-established warty variant of squamous cell carcinoma (SCC).1 Among 324 Taiwanese patients, Hsue et al. found the malignant potential of VH to be 3.1% over an average of 54.6 months.2 As VH and VC may present with similar clinical features, these entities need to be distinguished histologically. In 1980, Shear et al. first differentiated VH from VC based on the absence of endophytic growth in the former entity, wherein the verrucous and hyperplastic epithelium was completely superficial to the adjacent normal epithelium.3 Therefore, it is crucial that biopsies of verrucous lesions include a lesional margin with adequate depth.
Case Report
An 80-year-old male patient presented to the Outpatient Department of the Bapuji Dental College & Hospital, Davangere, Karanataka, India, in 2011 with a growth on the inside of his cheek. Six months prior to presentation, the patient had noticed a pea-sized growth in his mouth which had continued to grow over time. He did not report any pain associated with the growth unless he accidentally bit it whilst chewing food. Although the patient had not smoked or chewed tobacco for the previous 7–8 years, he reported a prior 40-year history of chewing tobacco 3–4 times per day and smoking 2–4 bidis (i.e. thin Indian cigarettes made of unprocessed tobacco wrapped in leaves) per day.
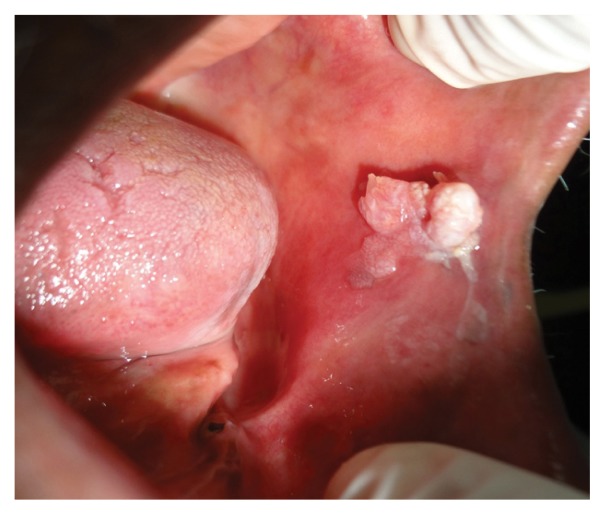
On clinical examination, a whitish-pink sessile oral mass of approximately 1.5 × 1.5 cm was observed with a warty/pebbly superficial surface and clearly defined margins [Figure 1]. It was firm in consistency and non-tender upon palpation. There was no evidence of discharge and no ulcerations were observed on the surface of the lesion, nor in the surrounding mucosa which appeared normal. An extraoral examination revealed an enlarged submandibular lymph node, which was mobile and non-tender upon palpation.
Figure 1.
Intraoral photograph showing a whitish-pink sessile exophytic lesion on the buccal mucosa of an 80-year-old male patient.
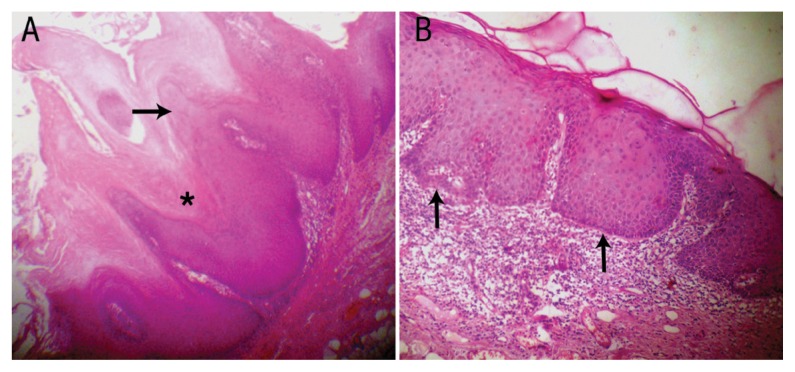
The lesion was treated with wide surgical excision. Histopathological examination of a biopsy specimen revealed a hyperplastic stratified squamous epithelium arranged in the form of exophytic papillary projections, with underlying fibrovascular connective tissue [Figure 2A]. The epithelium exhibited hyper-parakeratinisation with a few koilocytes seen in the superficial layers. The rete ridges had a broad ‘elephant’s foot’ shape and were at the same level as that of the adjoining normal epithelium [Figure 2B]. Some of the cells in the basal layer of the epithelium exhibited dysplastic features. In addition, the underlying connective tissue revealed dense chronic inflammatory cell infiltrates, chiefly concentrated in the juxta-epithelial areas. As a result of these features, a final diagnosis of VH was made. The patient was subsequently followed-up periodically over the next five years with no sign of recurrence [Figure 3].
Figure 2.
Photomicrographs of haematoxylin and eosin stains at x100 magnification showing (A) papillary projections (arrow) with keratin plugging (asterisk) in the clefts and (B) broad ‘elephant’s foot’ rete ridges (arrows) at the same level as that of the adjacent normal epithelium.
Figure 3.
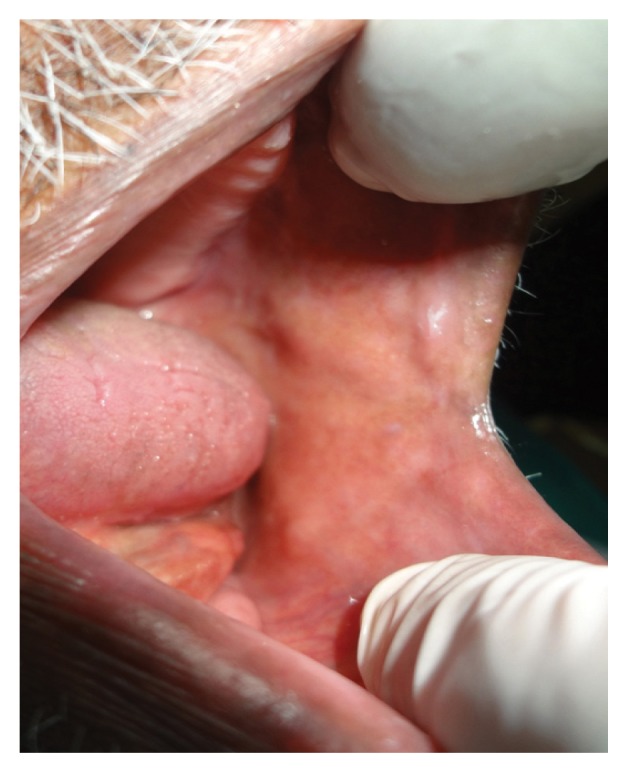
Intraoral photograph of the buccal mucosa of an 85-year-old male patient showing no evidence of recurrence of verrucous hyperplasia five years after wide surgical excision of the lesion.
Discussion
Clinically, VH presents as a warty or papillary fungating exophytic mucosal mass which can sometimes ulcerate and is predominantly pink in colour with a partly whitish surface.4 The average age at first presentation is between 30–60 years old.4,5 Previous research has indicated the buccal mucosa to be the most common site for VH; this may potentially be correlated with usage of quid (i.e. clumps of chewing tobacco) which is usually placed in this region of the mouth.5,6 In contrast, Shear et al. found that the gingiva and alveolar mucosa were the most common sites among 68 cases of VH.3 Hazarey et al. reported that placement of tobacco-betel-lime quid (i.e. a mixture of slaked lime, chewing tobacco and betel leaf pieces) in the buccal vestibule was the most predominant habit associated with VH growth.6 Wang et al. similarly observed that 91% of 60 patients with VH chewed areca nut quid (i.e. a mixture of areca nut, betel leaf pieces and chewing tobacco).5 Smoking was reported to be the second most common aetiological factor in these two studies.5,6
Shear et al. classified two histological patterns of VH, including the “sharp” variety (comprised of heavily keratinised, long and narrow verrucous processes) and the “blunt” variety (comprised of less heavily keratinised, broader and flatter verrucous processes).3 The former may potentially be referred to as verrucous leukoplakia due to its predominantly white colour resulting from the heavy keratinisation.3 However, no difference in prognosis has been reported according to these patterns. In contrast, Wang et al. classified VH into plaque-type and mass-type lesions and identified significant differences in their malignant transformation rates.5 Plaque-type VH was defined as horizontally-proliferating epithelial hyperplasia resulting in an elevated plaque-like lesion with a verrucous surface, while mass-type VH lesions displayed single or multiple protuberant masses of epithelial hyperplasia, with very little connective tissue at the core and a verrucous surface.5
Histopathologically, all variants of VH exhibit verrucous projections of the hyperplastic epithelium which lie superficially to the adjacent epithelium.3,5 However, there are considerable similarities between VH and VC lesions. The latter is defined as a warty, papillary or fungating exophytic lesion with broad and intact intrusions of rete ridges.3 According to previous research, keratin plugging in the centre of epithelial invaginations is a histological hallmark of VC; however, Slootweg et al. reported that the presence of keratin plugging is not obligatory for a VC diagnosis.4,7 Although dysplasia is rarely seen, mitotic figures are more common.4 The distinction between VH and VC is histological, based on the location of the hyperplastic epithelium in relation to the adjacent normal epithelium; furthermore, the broadened epithelial ridges lie above the adjacent normal epithelium in VH, whereas similar rete ridges display an endophytic growth pattern in VC cases.6 In addition, the verrucous processes in VC often bring a margin of normal epithelium down with them into the underlying connective tissue.3
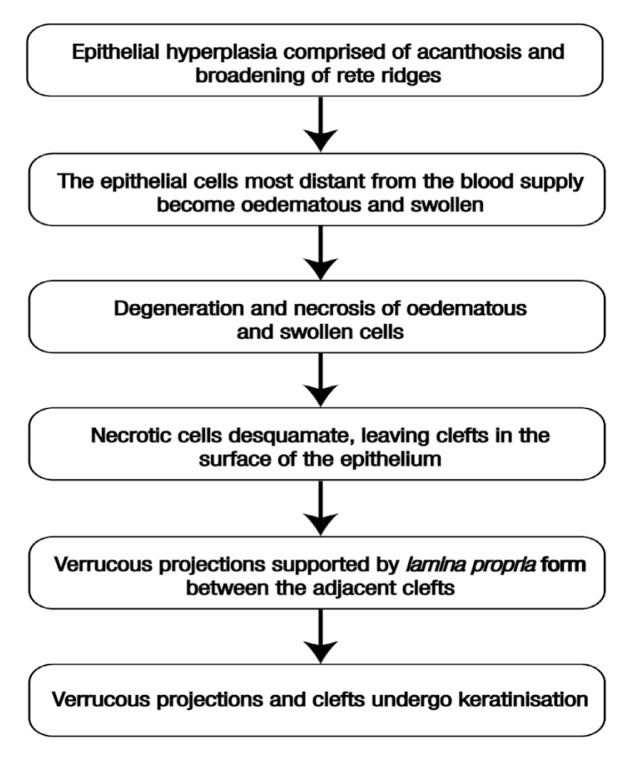
The histogenesis of VH as proposed by Shear et al. is summarised in Figure 4.3 It has been proposed that leukoplakia, if left untreated, may transform into VH or VC over time.3 Moreover, VH can be confused with proliferative verrucous leukoplakia (PVL), a variant of non-homogenous leukoplakia in which the lesions eventually assume an exophytic verrucous appearance.8 While the term VH refers to both clinical and histopathological features, PVL is unequivocally accepted as a clinical term used to define a specific type of non-homogenous leukoplakia with a verrucous surface.4 Histologically, PVL displays clinical foci of hyperkeratosis that progressively spread and become multifocal. Many cases of PVL are extremely resistant to treatment and progress to invasive cancer.8 In cases of papillary squamous cell carcinoma (PSCC), VC can be distinguished by its intact basement membrane which contrasts with the focal or early invasion seen in PSCC; furthermore, the epithelium in PSCC cases is significantly dysplastic when compared with the almost ‘bland’ cytological features of the epithelium of VC lesions.9 The clinicopathological spectrum of verrucous lesions as proposed by Hansen et al. is shown in Table 1.8
Figure 4.
Flowchart depicting the histogenesis of verrucous hyperplasia as proposed by Shear et al.3
Table 1.
Clinicopathological spectrum of verrucous lesions8
| Grade | Entity | Histopathological features |
|---|---|---|
| 0 | None | Normal oral mucosa |
| 2 | Clinical leukoplakia | Hyperkeratosis with or without dysplasia |
| 4 | Verrucous hyperplasia | Leukoplakia with papillary exophytic proliferation of the epithelium |
| 6 | Verrucous carcinoma | Downgrowth of the well-differentiated squamous epithelial blunt rete ridges with intact basement membrane |
| 8 | Papillary carcinoma | Exophytic and invasive growth of the well-differentiated squamous epithelium with keratin formation and minimal dysplasia |
| 10 | SCC | Loss of cohesion of moderately/poorly-differentiated tumour cells with moderate to severe dysplasia and minimal keratin formation |
SCC = squamous cell carcinoma.
The malignant potential of VH is well established. 1–3 Slootweg et al. concluded that VH probably represents a morphological variant of VC after noting an association between VH and SCC in 37% of 27 patients.4 Chen et al. reported high expression of inducible nitric oxide synthase (iNOS) proteins and messenger ribonucleic acid in VH lesions and concluded that an iNOS-dependent mechanism may be involved in the malignant transformation of VH.10,11 Additionally, the higher expression of interleukin-1β and glutathione S-transferase pi isoenzymes and the allelic loss at 19 loci on seven different chromosome arms may also play an important role in the malignant transformation of VH.12–14 Tumour protein p53, epidermal growth factor receptor and human epidermal growth factor receptor 3 expression can also be used to differentiate VH from VC and SCC.15,16 Although Greer Jr et al. found an association between the human papilloma virus and VH, the role of the virus in the malignant transformation of VH has yet to be confirmed.17
In terms of treatment modality, surgery alone is the most common method of management for both VC and VH cases, due to their overlapping clinicopathological features.18,19 However, it is important to ensure wide surgical excision of the lesion with adequate soft tissue margins so as to avoid recurrence. Although sporadic cases of cervical and distant metastasis have been reported, the overall rate of metastasis is insignificant.20 An incorrect histological diagnosis or the presence of occult foci indicating SCC has been proposed to justify the metastatic nature of lesions otherwise characteristic of VC; as such, thorough sampling of the surgical specimen is highly recommended.20,21
Conclusion
Distinction between VC and VH lesions can only be made histologically, by comparing the level of the rete ridges of the epithelium of the lesion with that of the adjacent normal epithelium. In addition, VH cases may also be confused with verrucous leukoplakia. Thus, biopsies of verrucous lesions should include the adjacent normal epithelium in order to ensure correct diagnosis. As VH has the potential for malignant transformation, patients should be treated in a similar manner to those with VC.
References
- 1.Neville BW, Damm DD, Allen CM, Bouquot JE. Oral and Maxillofacial Pathology. 3rd ed. Philadelphia, Pennsylvania, USA: Saunders; 2008. Epithelial pathology; pp. 388–97. [Google Scholar]
- 2.Hsue SS, Wang WC, Chen CH, Lin CC, Chen YK, Lin LM. Malignant transformation in 1458 patients with potentially malignant oral mucosal disorders: A follow-up study based in a Taiwanese hospital. J Oral Pathol Med. 2007;36:25–9. doi: 10.1111/j.1600-0714.2006.00491.x. [DOI] [PubMed] [Google Scholar]
- 3.Shear M, Pindborg JJ. Verrucous hyperplasia of the oral mucosa. Cancer. 1980;46:1855–62. doi: 10.1002/1097-0142(19801015)46:8<1855::AID-CNCR2820460825>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 4.Slootweg PJ, Müller H. Verrucous hyperplasia or verrucous carcinoma: An analysis of 27 patients. J Maxillofac Surg. 1983;11:13–19. doi: 10.1016/S0301-0503(83)80006-X. [DOI] [PubMed] [Google Scholar]
- 5.Wang YP, Chen HM, Kuo RC, Yu CH, Sun A, Liu BY, et al. Oral verrucous hyperplasia: Histologic classification, prognosis, and clinical implications. J Oral Pathol Med. 2009;38:651–6. doi: 10.1111/j.1600-0714.2009.00790.x. [DOI] [PubMed] [Google Scholar]
- 6.Hazarey VK, Ganvir SM, Bodhade AS. Verrucous hyperplasia: A clinico-pathological study. J Oral Maxillofac Pathol. 2011;15:187–91. doi: 10.4103/0973-029X.84492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rajendran R, Sivapathasundaram B, editors. Shafer’s Textbook of Oral Pathology. 5th ed. New Delhi, India: Elsevier; 2005. pp. 164–5. [Google Scholar]
- 8.Hansen LS, Olson JA, Silverman S., Jr Proliferative verrucous leukoplakia: A long-term study of thirty patients. Oral Surg Oral Med Oral Pathol. 1985;60:285–98. doi: 10.1016/0030-4220(85)90313-5. [DOI] [PubMed] [Google Scholar]
- 9.Ferlito A, Devaney KO, Rinaldo A, Putzi MJ. Papillary squamous cell carcinoma versus verrucous squamous cell carcinoma of the head and neck. Ann Otol Rhinol Laryngol. 1999;108:318–22. doi: 10.1177/000348949910800318. [DOI] [PubMed] [Google Scholar]
- 10.Chen YK, Hsuen SS, Lin LM. Increased expression of inducible nitric oxide synthase for human oral submucous fibrosis, verrucous hyperplasia, and verrucous carcinoma. Int J Oral Maxillofac Surg. 2002;31:419–22. doi: 10.1054/ijom.2002.0246. [DOI] [PubMed] [Google Scholar]
- 11.Chen YK, Hsuen SS, Lin LM. Expression of inducible nitric oxide synthase in human oral premalignant epithelial lesions. Arch Oral Biol. 2002;47:387–92. doi: 10.1016/S0003-9969(02)00011-0. [DOI] [PubMed] [Google Scholar]
- 12.Tsai CC, Chen CC, Lin CC, Chen CH, Lin TS, Shieh TY. Interleukin-1 beta in oral submucous fibrosis, verrucous hyperplasia and squamous cell carcinoma tissues. Kaohsiung J Med Sci. 1999;15:513–19. [PubMed] [Google Scholar]
- 13.Chen YK, Lin LM. Immunohistochemical demonstration of epithelial glutathione S-transferase isoenzymes in normal, benign, premalignant and malignant human oral mucosa. J Oral Pathol Med. 1995;24:316–21. doi: 10.1111/j.1600-0714.1995.tb01192.x. [DOI] [PubMed] [Google Scholar]
- 14.Poh CF, Zhang L, Lam WL, Zhang X, An D, Chau C, et al. A high frequency of allelic loss in oral verrucous lesions may explain malignant risk. Lab Invest. 2001;81:629–34. doi: 10.1038/labinvest.3780271. [DOI] [PubMed] [Google Scholar]
- 15.Sakurai K, Urade M, Takahashi Y, Kishimoto H, Noguchi K, Yasoshima H, et al. Increased expression of c-erbB-3 protein and proliferating cell nuclear antigen during development of verrucous carcinoma of the oral mucosa. Cancer. 2000;89:2597–605. doi: 10.1002/1097-0142(20001215)89:12<2597::AID-CNCR12>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 16.Wu M, Putti TC, Bhuiya TA. Comparative study in the expression of p53, EGFR, TFG-alpha, and cyclin D1 in verrucous carcinoma, verrucous hyperplasia, and squamous cell carcinoma of head and neck region. Appl Immunohistochem Mol Morphol. 2002;10:351–6. doi: 10.1097/00129039-200212000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Greer RO, Jr, Eversole LR, Crosby LK. Detection of human papillomavirus-genomic DNA in oral epithelial dysplasia, oral smokeless tobacco-associated leukoplakia and epithelial malignancies. J Oral Maxillofac Surg. 1990;48:1201–5. doi: 10.1016/0278-2391(90)90538-D. [DOI] [PubMed] [Google Scholar]
- 18.Kang CJ, Chang JT, Chen TM, Chen IH, Liao CT. Surgical treatment of oral verrucous carcinoma. Chang Gung Med J. 2003;26:807–12. [PubMed] [Google Scholar]
- 19.Mehrotra D, Goel M, Kumar S, Pandey R, Ram H. Oral verrucous lesions: Controversies in diagnosis and management. J Oral Biol Craniofac Res. 2012;2:163–9. doi: 10.1016/j.jobcr.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asproudis I, Gorezis S, Aspiotis M, Tsanou E, Kitsiou E, Merminga E, et al. Orbital metastasis from verrucous carcinoma of the oral cavity: Case report and review of the literature. In Vivo. 2007;21:909–12. [PubMed] [Google Scholar]
- 21.Kallarakkal TG, Ramanathan A, Zain RB. Verrucous papillary lesions: Dilemmas in diagnosis and terminology. Int J Dent. 2013;2013:298249. doi: 10.1155/2013/298249. [DOI] [PMC free article] [PubMed] [Google Scholar]