Abstract
Phytoplankton-bacteria interactions drive the surface ocean sulfur cycle and local climatic processes through the production and exchange of a key compound: dimethylsulfoniopropionate (DMSP). Despite their large-scale implications, these interactions remain unquantified at the cellular-scale. Here we use secondary-ion mass spectrometry to provide the first visualization of DMSP at sub-cellular levels, tracking the fate of a stable sulfur isotope (34S) from its incorporation by microalgae as inorganic sulfate to its biosynthesis and exudation as DMSP, and finally its uptake and degradation by bacteria. Our results identify for the first time the storage locations of DMSP in microalgae, with high enrichments present in vacuoles, cytoplasm and chloroplasts. In addition, we quantify DMSP incorporation at the single-cell level, with DMSP-degrading bacteria containing seven times more 34S than the control strain. This study provides an unprecedented methodology to label, retain, and image small diffusible molecules, which can be transposable to other symbiotic systems.
DOI: http://dx.doi.org/10.7554/eLife.23008.001
Research Organism: phytoplankton-bacteria interaction, sulfur cycle, DMSP, NanoSIMS, stable isotope labelling
eLife digest
Sulfur is an essential element for many organisms and environmental processes. Every year, organisms including microalgae produce more than one billion tons of a sulfur-containing compound called DMSP. Some of this DMSP is released into seawater, where it acts as a key nutrient for microscopic organisms and as a foraging cue to attract fish. DMSP is also the precursor of a gas that helps to form clouds.
Despite DMSP’s potential large-scale effects, it is still not clear what role it plays in the organisms that produce it, or how it is transferred from the microalgae that produce it to the bacteria that use it. It is thought that DMSP could potentially protect the cells from sudden changes in the amount of salt in the seawater (salinity) or from other damage, such as oxidative stress – a build-up of harmful chemicals inside cells.
In a controlled setting using artificial seawater, Raina et al. used high-resolution imaging and chemical analysis to track the journey of DMSP from microalgae to recipient bacteria. The results show that similar to land plants, algae store DMSP in the compartments that regulate cell pressure and photosynthesis. The presence of DMSP in these locations also supports its proposed role in protecting cells from changes in salinity or oxidative damage.
A future step will be to identify the genes involved in producing DMSP in microalgae. This knowledge could be used to create mutants that are either incapable of producing this molecule or that overproduce it. In combination with the high-resolution imaging techniques described here, this will allow researchers to fully understand the role that DMSP plays in these organisms.
Introduction
Interactions between marine phytoplankton and bacteria constitute an important ecological linkage in the oceans (Cole, 1982), controlling chemical cycling and energy transfer to higher trophic levels (Azam and Malfatti, 2007; Falkowski et al., 2008). The cycling of sulfur, an essential element for living organisms, depends on the metabolic interactions between these two Kingdoms (Sievert et al., 2007). A striking example is the production of the sulfur compound dimethylsulfoniopropionate (DMSP) by phytoplankton and its degradation by marine bacteria (and phytoplankton themselves) into the climate-active gas dimethylsulfide (DMS) (Alcolombri et al., 2015; Ayers and Gras, 1991; Howard et al., 2006; Todd et al., 2007). The subsequent release of DMS into the atmosphere contributes 90% of biogenic sulfur emissions and initiates the formation and growth of aerosols, thereby enhancing cloud formation and sunlight scattering (Ayers and Gras, 1991). This highlights how chemical interactions occurring between marine microorganisms across micrometre-scales can ultimately have large-scale impacts on climate (Sievert et al., 2007; Simó, 2001). However, direct measurements of these metabolic interactions, critical to the global sulfur cycling, have not previously been possible at the scale where they occur, the sub-cellular level.
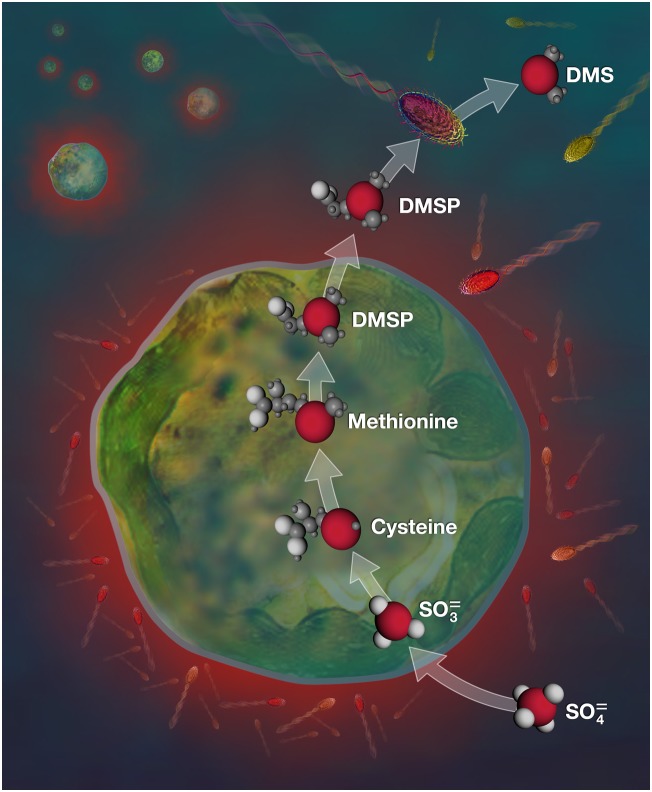
In the surface ocean, the largest quantities of sulfur are present as dissolved sulfate, which constitutes the main sulfur source for phytoplankton (Sievert et al., 2007; Stefels, 2000). Most of the sulfur derived from sulfate uptake is converted by these organisms into sulfur-based amino acids, and a fraction is ultimately used to synthesise DMSP (Stefels, 2000) (Figure 1). Globally, more than a billion tons of DMSP are produced every year, which has been estimated to represent up to 10% of the amount of carbon fixed by phytoplankton (Archer et al., 2001; Simó et al., 2002). However, despite the central role played by DMSP in the marine sulfur cycle, a mechanistic understanding of the biochemistry at the heart of DMSP cycling is currently lacking. Previous studies in higher plants provided strong evidence that DMSP biosynthesis starts in the cytosol and ends in the chloroplast (Trossat et al., 1996, 1998). However, DMSP biosynthesis occur through a different route in phytoplankton (Stefels, 2000), and we still do not know: (1) where this compound is produced and stored in phytoplankton cells; (2) what are its functions; and (3) how efficiently it is transferred from phytoplankton producers to bacterial degraders.
Figure 1. DMSP biosynthetic pathway targeted in this study.
Sulfate (SO42-) taken up from seawater by Symbiodinium is converted to sulfite (SO32-), sulfur-based amino acids and finally DMSP. Some DMSP molecules are then exuded from Symbiodinium cells and can be degraded by a variety of marine bacteria (sulfur atoms (S) and bacterial cells that have taken up sulfur are in red). The biosynthetic pathway presented here is simplified, for more details see Stefels (Stefels, 2000).
DOI: http://dx.doi.org/10.7554/eLife.23008.003
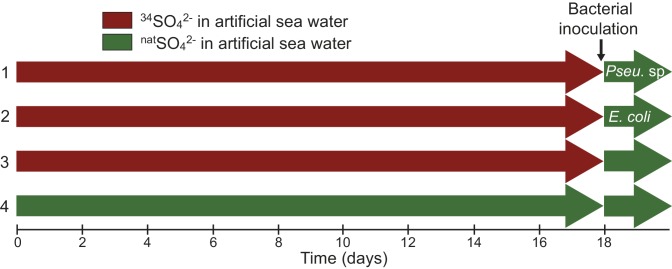
Figure 1—figure supplement 1. Sampling design showing the four different culture treatments.
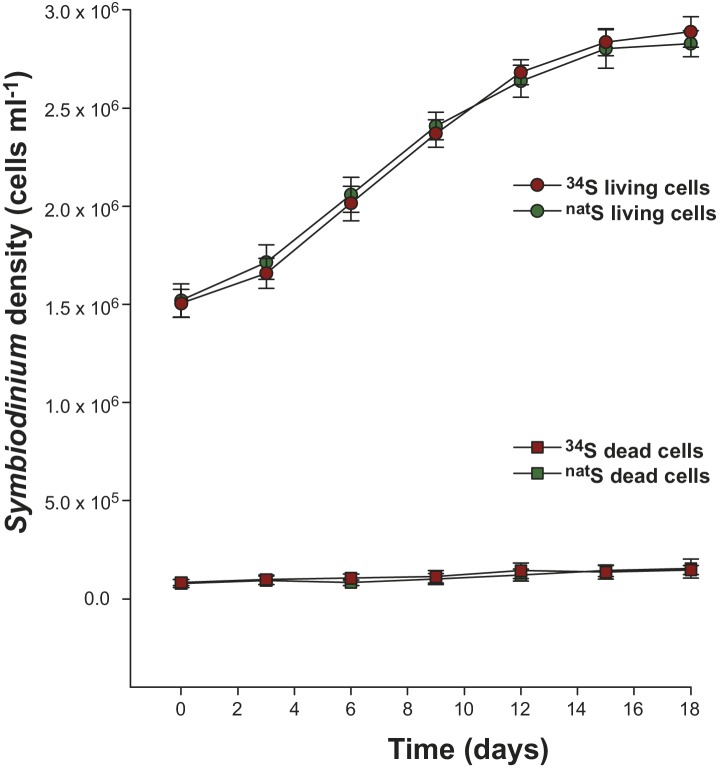
Figure 1—figure supplement 2. Growth kinetics of Symbiodinium cells (strain C1; mean ± SE; n = 8) incubated at 27°C in artificial seawater containing either 34SO42- (red) or natSO42- (green) as the sole sulfur source.
We used the dinoflagellate Symbiodinium, a taxon that includes some of the most prodigious DMSP producers on the planet (Caruana and Malin, 2014; Saltzman and Cooper, 1989). Symbiodinium cells can be free-living in the water column, but are primarily known for the endosymbiotic associations they form with tropical cnidarians that fuel the extremely high productivity of coral reef ecosystems (Dubinsky, 1990). Populations of reef-building corals are major DMSP production hotspots (Broadbent et al., 2002; Raina et al., 2013) and their contribution to the marine sulfur cycle is disproportionately large given their relatively restricted distributions (Raina et al., 2013; Fischer and Jones, 2012). In this ecosystem, DMSP constitutes an important source of carbon and sulfur for the diverse and highly abundant bacterial communities harboured by corals (Raina et al., 2010). Here we tracked and quantified the incorporation of a stable isotope of sulfur into Symbiodinium and its subsequent transfer to associated bacteria. To provide the first sub-cellular imaging and quantification of DMSP, we used a unique suite of analytical techniques, taking advantage of: (i) the spatial resolution afforded by nano-scale secondary ion mass spectrometry (NanoSIMS), (ii) the molecular characterization enabled by Time-of-Fight secondary ion mass spectrometry (ToF-SIMS), and (iii) the precise quantification allowed by nuclear magnetic resonance (NMR) and liquid chromatography-mass spectrometry (LC-MS).
Results and discussion
We used the rare isotope 34S as a tracer to follow the exchange of sulfur between marine micro-organisms at the single-cell level. Symbiodinium cells were incubated for 18 days in artificial seawater containing 34S-labelled sulfate as the sole sulfur source (34S-ASW; Figure 1—figure supplement 1). We relied exclusively on the Symbiodinium cellular machinery to biosynthesise and exude 34S-labelled DMSP following incubation with the 34S-sulfate precursor. To prevent direct uptake of 34S-sulfate by bacteria, all Symbiodinium cultures were rinsed thoroughly and re-inoculated into ASW containing sulfate in natural isotopic abundance (natS-ASW) before addition of bacterial cells. Two different bacterial strains were added to the rinsed cultures and co-incubated for six hours: (i) Pseudovibrio sp. P12, a DMSP-degrading bacterium isolated from healthy corals (Raina et al., 2016), selected because of its worldwide distribution in coastal waters (Shieh et al., 2004) and its abundance in benthic invertebrate communities (Bondarev et al., 2013); and (ii) a control, Escherichia coli W (ATCC 9637), a widely studied and fully sequenced strain, able to grow in seawater and not capable of degrading DMSP. To precisely localise bacterial cells, both strains were pre-grown in a medium enriched in the rare stable isotope 15N (in amino-acids and ammonium form). The cellular incorporation of the stable isotope tracers (34S and 15N) was identified by an increase in the sulfur (34S/32S) and/or nitrogen (15N/14N) ratio above their natural abundance values (0.043 and 0.0037, respectively).
Symbiodinium cell numbers doubled during the incubation period in the medium containing 34S-labelled sulfate, reaching approximately 2.9 million cells ml−1 after 18 days (Figure 1—figure supplement 2). LC-MS analyses carried out at the end of the experiment on extracted Symbiodinium cells confirmed that all cultures initially incubated with 34S-sulfate were highly enriched in 34S-DMSP, which represented up to 46% of the DMSP molecules present in samples analysed (Figure 2, Figure 2—source data 1). This result confirms that sulfur atoms used by dinoflagellates to synthesise DMSP can originate from the uptake of inorganic sulfate derived from seawater (Stefels, 2000). In addition to 34S-DMSP, unexpectedly high levels of 32S-DMSP (ranging from 54% to 66% of total DMSP) were recorded in Symbiodinium cultures (Figure 2—source data 1). The presence of these high levels of 32S-DMSP can be explained by a combination of two factors: (i) Symbiodinium cells density only doubled during the incubation phase in 34S-ASW, retaining a large fraction of the natural pool of 32S initially present in the starting culture prior to the incubation; (ii) new 32S-DMSP might have been synthesised during the six hours immediately preceding sampling, when Symbiodinium cells were incubated in natS-ASW medium. Although high concentrations of DMSP were present in the methanolic Symbiodinium cells extract (Figure 2—source data 1), sulfur containing amino acids (methionine and cysteine) were not detected by LC-MS or 1H NMR.
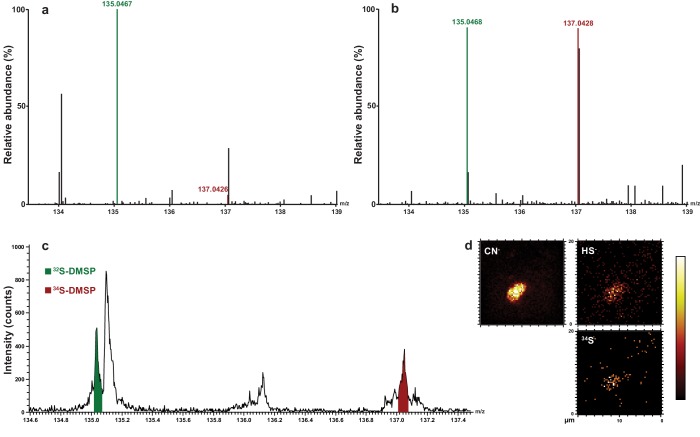
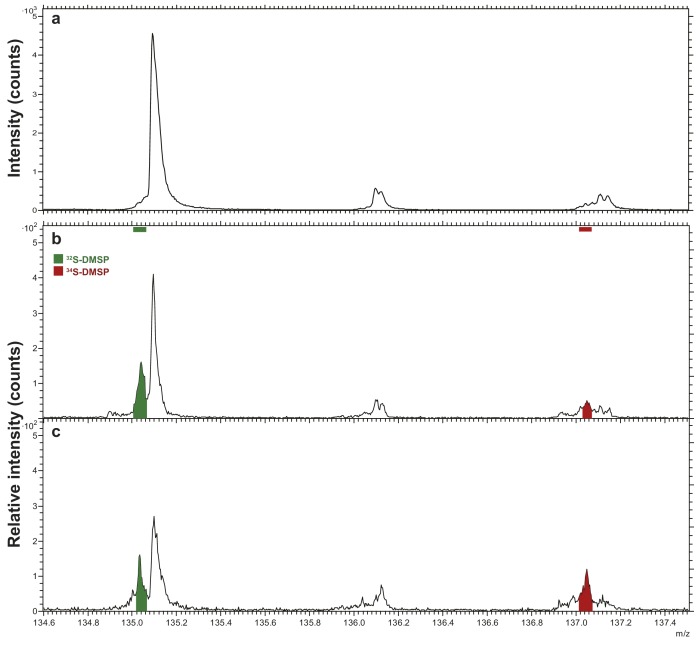
Figure 2. Representative HPLC-MS spectra showing the presence and relative abundance of 32S-DMSP (green peak) and 34S-DMSP (red peak) in methanol extracts derived from Symbiodinium culture (particulate fraction).
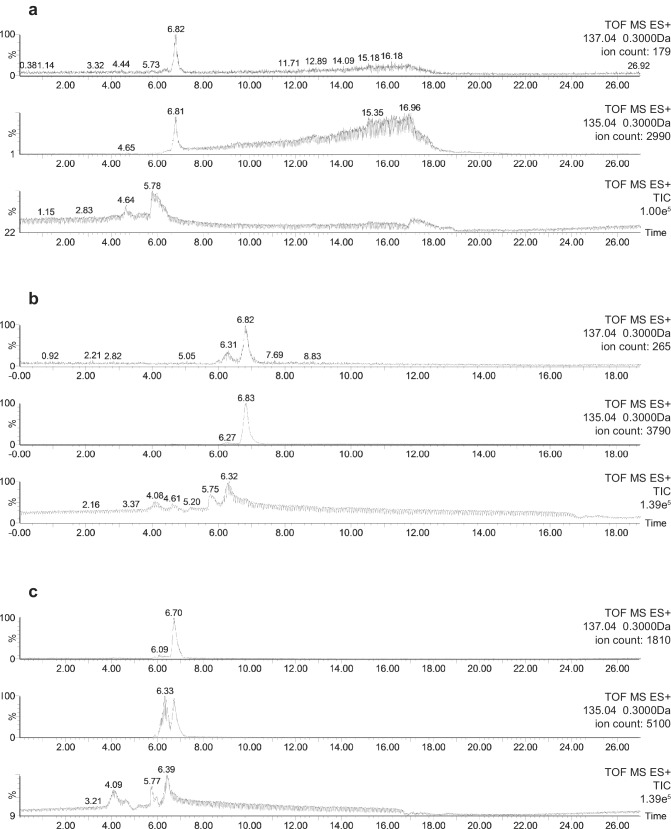
(a) incubated with natS (treatment 4, see Figure 1—figure supplement 1); (b) incubated with 34S (treatment 3, see Figure 1—figure supplement 1). For more detailed spectra, see Figure 2—figure supplement 2; for absolute DMSP abundance, see Figure 2—source data 1. (c) Positive-ion ToF-SIMS spectrum of Symbiodinium incubated with 34S (treatment 3, see Figure 1—figure supplement 1) after resin embedding (34S-DMSP represented 46% of total DMSP counts). For comparison between treatment and control spectra, see Figure 2—figure supplement 1; (d) Negative-ion ToF-SIMS images showing the distribution of CN-, HS- and 34S- species over a Symbiodinium cell (treatment 3, see Figure 1—figure supplement 1) enriched in 34S. Field of view is 20 × 20 μm2 (lateral resolution is ~300 nm).
DOI: http://dx.doi.org/10.7554/eLife.23008.007
Figure 2—figure supplement 1. Representative positive-ion spectra of (a) Araldite 502 resin, and Symbiodinium (b) incubated with natS (treatment 4) and (c) incubated with 34S (treatment 3) after resin embedding.
Figure 2—figure supplement 2. Representative HPLC-MS spectra showing the presence and relative abundance of 32S-DMSP (mass 135.04) and 34S-DMSP (mass 137.04) in methanol extracts: (a) DMSP standard containing natural abundance of 34S-DMSP; (b) Symbiodinium cells incubated with natS (treatment 4); (c) Symbiodinium cells incubated with 34S (treatment 3).
Up to 10% of the carbon fixed by photosynthetic algae is used for the production of DMSP (Sievert et al., 2007; Archer et al., 2001; Simó et al., 2002), which represents a major energy investment for these organisms and strongly suggests that this compound plays a central function in algal cells. To understand more precisely the functional role of DMSP, we used two SIMS approaches to infer its location within cells. To effectively prevent the loss of DMSP from the cells, the entire sampling procedure leading to SIMS analyses had to be water-free, with all steps performed under strict anhydrous conditions. For this, we used cryopreservation techniques followed by freeze substitution in an acrolein-ether mixture. This method has routinely been used to successfully preserve cellular ions and compounds in a variety of systems (Altus and Canny, 1985; Ashford et al., 1999; Kaiser et al., 2015; Marshall et al., 2007; Mostaert et al., 1996), with the acrolein stabilizing and preserving cellular proteins, nucleic and fatty acids through cross linking, while the low temperature, anhydrous conditions ensure preservation and retention of diffusible ions and water-soluble molecules (such as DMSP). The inclusion of acrolein ensures excellent cell structural preservation at a low temperature, which is required for high resolution NanoSIMS analyses (Kaiser et al., 2015; Marshall, 1980).
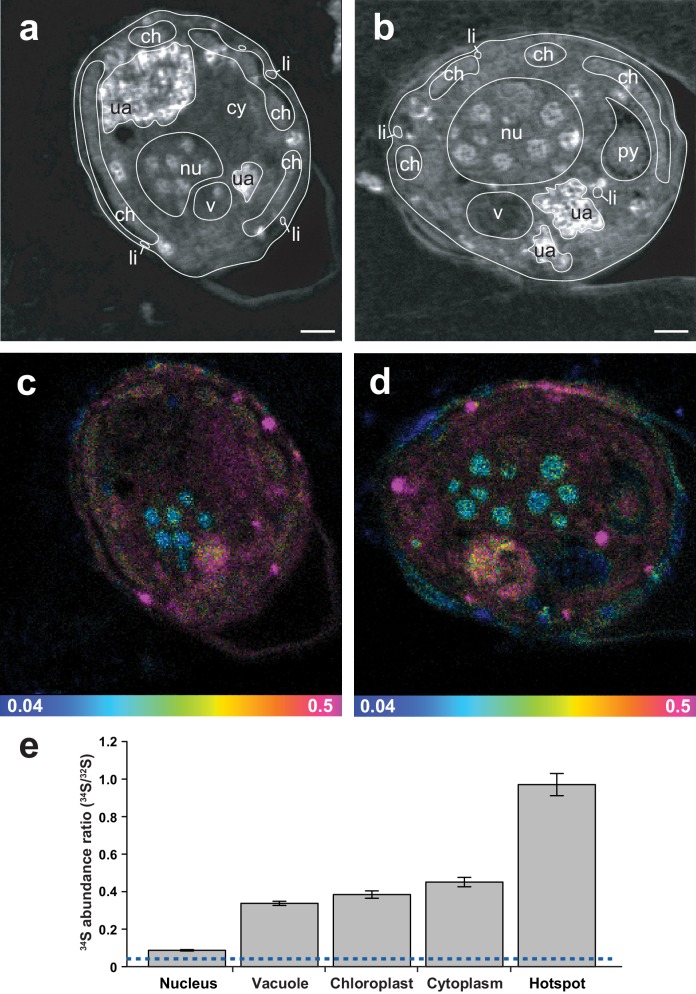
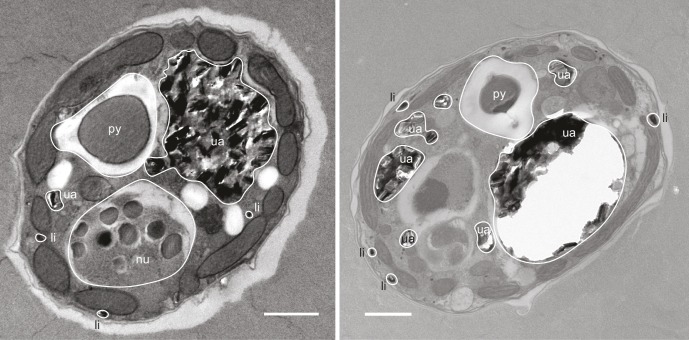
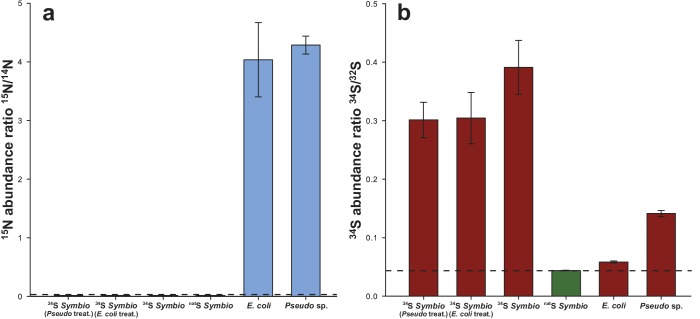
ToF-SIMS revealed that 34S-DMSP was present and abundant in the preserved cells following resin embedding, with a ratio of 34S-DMSP/32S-DMSP matching the bulk analyses carried out with LC-MS prior to embedding (Figure 2c–d, Figure 2—figure supplement 2). NanoSIMS analysis revealed that Symbiodinium exposed to 34S-labelled sulfate were nine times more enriched in 34S than the cells in the control (34S/32S ratio in 34S-ASW treatments: 0.391 ± 0.046, compared to natS-ASW controls 0.044 ± 0.001 [Figure 4—figure supplement 1]). Furthermore, substantial spatial variability in 34S enrichment was detected within Symbiodinium cells. Relatively low level of enrichments were detected in the nucleus (34S/32S: 0.087 ± 0.004) which might correspond to the presence of 34S-labelled amino-acids in the histone-like proteins that condense Symbiodinium DNA into chromosomes (Shoguchi et al., 2013) (Figure 3). Much higher enrichment levels were detected in vacuoles (34S/32S: 0.337 ± 0.011), chloroplasts (34S/32S: 0.384 ± 0.020) and cytoplasm (34S/32S: 0.451 ± 0.025); which means that the enrichment in these cellular structures was 7.7, 8.8 and 10.3 times over the natural abundance levels (Figure 3). However, the largest 34S enrichment was observed in small hotspots often observed near the Symbiodinium cell periphery (34S/32S: 0.971 ± 0.059; Figure 3), reaching more than 22 times the natural abundance level. Based on their small size and their high 34S enrichment, these hotpots are likely storage droplets containing sulfolipids, a group of sulfur compounds known to accumulate in Symbiodinium (Garrett et al., 2013; Yuyama et al., 2016). Lipid droplets of similar sizes and locations can be observed in these cells using electron microscopy (Figure 3—figure supplement 1). We were not able to detect methionine or cysteine using LC-MS or ToF-SIMS, which suggest that the intracellular concentration of these sulfur based amino-acids was relatively low. In contrast, DMSP is known to be by far the most abundant organic sulfur compound present in dinoflagellate cells (Matrai and Keller, 1994), representing more than 50% of the total organic sulfur in these organisms (Matrai and Keller, 1994). DMSP was the only organic sulfur compound we were able to detect in the Symbiodinium cells (through LC-MS, 1H NMR and ToF-SIMS), suggesting that most of the remaining 34S signal measured in Symbiodinium cells with NanoSIMS is highly likely originating from DMSP.
Figure 3. Representative NanoSIMS ion images of Symbiodinium cells showing the sub-cellular distribution of 34S.
(a and b) 12C14N/12C2 mass images showing cellular structures. (c and d) 34S/32S ratio images of the same cells, shown as Hue Saturation Intensity (HSI) images where the colour scale indicates the value of the 34S/32S ratio, with natural abundance in blue, changing to pink with increasing 34S levels. (e) Isotope ratio of 34S/32S in different cellular regions (nucleus n = 10; vacuole n = 3; chloroplast n = 35; cytoplasm n = 12; hotspot n = 20; error bar: SE; source data available: Figure 3—source data 1). The dashed blue line represents the natural 34S abundance recorded in the control samples. nu: nucleus; ch: chloroplast; py: pyrenoid; ua: uric acid storage; v: vacuole; cy: cytoplasm; li: sulfolipids. Scale bars: 1 µm.
DOI: http://dx.doi.org/10.7554/eLife.23008.011
Figure 3—figure supplement 1. Representative electron micrographs of Symbiodinium cells after OsO4 staining showing the position and size of intracellular lipid droplets.
DMSP is an effective scavenger of reactive oxygen species (ROS), particularly hydroxyl radicals (•OH) (Sunda et al., 2002). The in vivo half-life of •OH is 10−9 seconds (Sies, 1993), which implies that these highly reactive molecules can damage lipids, nucleic acids, amino-acids or carbohydrates present in their direct vicinity. To be an effective antioxidant, a molecule needs not only to be able to scavenge ROS, but also to be located close to their source. Although the capacity of DMSP to detoxify ROS is established (Sunda et al., 2002), it has not been previously possible to ascertain its specific cellular function because its location is still unknown. If some DMSP is located in the cytoplasm, as suggested by our NanoSIMS data, it will be ideally localised to act as an osmolyte (Kiene et al., 1996). Furthermore, the presence of strong 34S signals in and around chloroplasts, where ROS are formed, support its role as an antioxidant (Sunda et al., 2002).
Following synthesis by phytoplankton, DMSP constitutes an important carbon and sulfur source for heterotrophic marine bacteria, which can either demethylate the compound and incorporate its sulfur into proteins or cleave it to produce DMS (Curson et al., 2011). At the termination of the experiment, total DMSP concentrations in Symbiodinium cells inoculated with the DMSP-degrading bacterium Pseudovibrio sp. P12 were 31% lower relative to those containing no bacteria or bacteria unable to degrade DMSP (Figure 2—source data 1). As Symbiodinium abundance did not differ between the treatments (Figure 1—figure supplement 2), the lower DMSP concentrations recorded are likely a consequence of the presence of Pseudovibrio cells able to degrade this compound. We sequenced the genome of Pseudovibrio sp. P12, revealing that this bacterium harbours a complete DMSP cleavage pathway, including a DMSP acyl-CoA transferase (encoded by dddD), a DMSP transporter (dddT) and the downstream catabolic enzymes (dddB-C) (Todd et al., 2007; Raina et al., 2016). Further analyses using NMR revealed that this DMSP degradation pathway was functional, enabling this strain to convert high concentrations of DMSP into DMS (Raina et al., 2016). In addition, Pseudovibrio sp. P12 harbours homologues of genes involved in the demethylation pathway (dmdA-B-C-D), though these genes have a relatively low sequence identity (24%, 30%, 43% and 32%, respectively) (Raina et al., 2016) to the genes originally identified in Ruegeria pomeroyi DSS-3 (Reisch et al., 2011).
Bacteria-sized 15N hotspots localised outside Symbiodinium cells in NanoSIMS images were accurately identified as inoculated bacterial cells based on their unique nitrogen isotopic signatures (1151-fold increase on average over natural abundance, n = 79, Figure 4—figure supplement 1). Notably, within the Pseudovibrio treatment, the position of these 15N hotspots correlated exactly with 34S hotspots (Figure 4), which were characterised by a 3.3-fold increase in the 34S/32S ratio over natural abundance (n = 60, Figure 4h). These observations confirmed that Pseudovibrio cells assimilated 34S-labelled Symbiodinium-derived metabolites. A 34% increase was also recorded in the mean 34S/32S ratio of E. coli cells (0.058 ± 0.002; n = 19), which are unable to degrade DMSP (compared to controls: 0.0438, Figure 4h). This enrichment, significantly higher than the expected natural abundance levels (t-Test, n = 19, t = 9.227, *p<0.001), can be explained by: (i) the capacity of E. coli to uptake small quantities of DMSP through betaine transporters to use as an osmoprotectant (Cosquer et al., 1999); (ii) the exudation of small quantities of other sulfur-containing substrates by Symbiodinium, such as methionine, which occur at a ratio of 8.2 ± 2.6 per 1000 amino acid residues in these dinoflagellates (Markell and Trench, 1993). In contrast, the high 34S enrichment recorded in Pseudovibrio cells, together with the significant decrease of particulate DMSP recorded in Pseudovibrio-inoculated treatments (Figure 4i), are likely due to the incorporation and degradation of DMSP. A comparison of 34S uptake between the two bacterial strains further highlights differences in their capacity to metabolise DMSP; Pseudovibrio incorporated seven times more sulfur than E. coli during the six-hours incubation (Pseudovibrio: specific uptake of 6.4 ± 0.3 ng S mg−1 of dry weight, n = 60; E. coli: 0.9 ± 0.1 ng S mg−1 of dry weight, n = 19). However, enzymatic cleavage of 34S-DMSP into volatile 34S-DMS, which diffuses out of Pseudovibrio cells and is therefore not captured by our NanoSIMS measurements, are likely to have caused an underestimation of the amount of sulfur cycled by this bacterium.
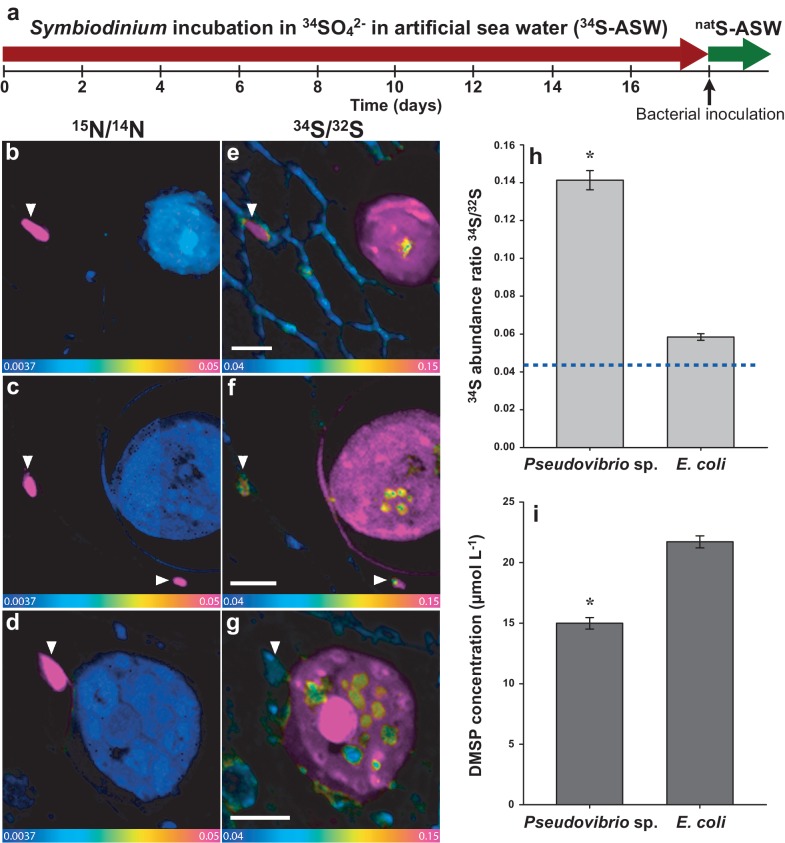
Figure 4. Representative NanoSIMS ion images of Symbiodinium cells exposed to 34S- or natS-artificial seawater (ASW) for 18 days and subsequently inoculated with two different bacterial strains for six hours.
(a) Timeline of the experiment. (b, c and d) 12C15N/12C14N mass images showing the presence of 15N enriched bacterial cells. (e, f and g) 34S/32S ratio image of the same regions. These mass images are shown as HSI images where the colour scale indicates the value of the stable isotope ratios, with natural abundance in blue, changing to pink with increasing 15N or 34S levels. (b, c, e and f) Symbiodinium cultures were inoculated with the DMSP-degrading bacterium Pseudovibrio sp. P12 (treatment 1). (d and g) Symbiodinium cultures were inoculated with Escherichia coli (treatment 2). White arrows indicate bacteria. (h) Isotope ratio of 34S/32S in bacteria, Pseudovibrio cells were significantly more enriched than E. coli (t-Test, n = 60, t = 9.021, *p<0.001, error bars: SE). The dashed blue line represents the natural 34S abundance recorded in the control samples. (i) Total particulate DMSP concentration in Symbiodinium inoculated with Pseudovibrio sp. or E. coli (t-Test, n = 3, t = 9.908, *p<0.001, error bar: SE). Source data available: Figure 4—source data 1. Note: two regions of interest were merged to create Figure 4c due to stage-shifting errors during sequential acquisition of N and S data. Scale bars = 3 µm.
DOI: http://dx.doi.org/10.7554/eLife.23008.014
Figure 4—figure supplement 1. Isotope ratio of (a) 15N/14N and (b) 34S/32S in Symbiodinium and bacteria cells measured by NanoSIMS in the different treatments (values were extracted from the images).
The marine sulfur cycle is a fundamental driver of atmospheric chemistry and climatic processes, yet its global influence is the product of unquantified cellular interactions between microorganisms. Here we used two SIMS approaches to directly visualise the accumulation and subsequent transfer of DMSP between marine microalgae and bacteria with unprecedented sub-cellular resolution. We applied a method that enables the preservation of water-soluble compounds, such as DMSP, in samples. This procedure, applicable to any system, may serve as a template to study the sub-cellular localization and identification of other small and highly diffusible molecules. In addition, similarly to other recent stable isotope approaches (Stefels et al., 2009), our method may be used to quantify the production rate of DMSP at the single cell level. We confirmed that 34S-DMSP was the main organic sulfur compound within the algal cells and we subsequently localised large quantities of the sulfur tracer 34S in algal vacuole, cytoplasm and chloroplasts. This strongly indicates that the relative concentrations of DMSP are higher in these key cellular locations, providing corroborative evidence for its functional role in mitigating both osmotic and oxidative stresses. Taken together, we have demonstrated that it is possible to image and quantify DMSP in phytoplankton and their associated bacteria at the sub-cellular scale. These methods open the way to further studies resolving the role of DMSP in phytoplankton and its contribution to phytoplankton-bacteria interactions.
Materials and methods
Isolation of Symbiodinium and bacteria
Cells of Symbiodinium type C1 (confirmed by sequencing of the ITS1 gene) used in this study were isolated from air-brushed tissues of the coral Acropora tenuis, which had been collected from Magnetic Island, Great Barrier Reef, Australia (latitude 19°10’S; longitude 146°50’E). Cells were sequentially washed three times (5 min at 1600 g) with 0.2 µm filtered seawater. Clean Symbiodinium cells were inoculated into 24 well plates with sterile IMK medium (Wako Chemicals, Richmond, VA, USA) with the antibiotics penicillin (100 μg ml−1), neomycin (100 μg ml−1), streptomycin (100 μg ml−1), nystatin (100 μg ml−1), amphotericin (2.5 μg ml−1), and Germanium dioxide (50 μM)) for 15 days at 27°C, 50 µE and 14:10 light:dark cycle. After this initial incubation, cells from uncontaminated wells were pooled and re-inoculated in new 24-well plates with IMK medium plus antibiotics as above, and incubated for 20 days at the same temperature and lighting conditions. Finally, uncontaminated cells were pooled and inoculated into 25 mL of sterile IMK without antibiotics until the start of the experiment (Santos et al., 2011). Cultures were genotyped by single-strand conformation polymorphism (SSCP) of the ITS1 region (van Oppen et al., 2001).
A coral-associated bacterium, Pseudovibrio sp. P12, was isolated from healthy colonies of the reef-building coral Pocillopora damicornis. This bacterial strain is commonly associated with reef-building corals (Bondarev et al., 2013; Nissimov et al., 2009; Radjasa et al., 2008; Ritchie, 2006; Rypien et al., 2010; Sulistiyani et al., 2010) and capable of metabolizing DMSP as a sole carbon source (Garren et al., 2014). Coral colonies were collected from Davies Reef, Great Barrier Reef, Australia (latitude 18°51’S; longitude 147°41’E) and maintained in aquaria at the Australian Institute of Marine Science (Townsville, Queensland, Australia) prior to strain isolation. A dilution series of coral tissue slurries was inoculated on minimal marine agar plates (1% bacteriological agar; 0.3% casamino acids; 0.4% glucose; in 1 litre of artificial seawater) (Hjelm et al., 2004). After 2 days of incubation at 28°C, single colonies were transferred into Marine Broth (Difco) and grown overnight. Liquid cultures were re-plated on minimal marine agar and the procedure was repeated iteratively until pure cultures were obtained. A laboratory strain of Escherichia coli (E. coli W (ATCC 9637)) was chosen as a control strain based on its ability to grow in the artificial seawater used in this study (see medium composition below), and its lack of DMSP degradation and subsequent sulfur assimilation pathways (unlike many marine or coral bacterial isolates [Raina et al., 2010; Howard et al., 2008]).
Bacterial genomic analysis
High molecular weight DNA from a pure culture of the Pseudovibrio sp. P12 strain was obtained using a miniprep phenol/chloroform based DNA extraction (Ausubel et al., 1987). A paired-end library was prepared using the Illumina Truseq protocol (Illimina, San Diego, CA, USA), with an insert size of 169 bp and a read size of 150 bp. The library was sequenced on an Illumina MiSeq instrument at Monash University (Melbourne, Australia). The genome was assembled with the SPAdes assembler (v2.4.0) (Bankevich et al., 2012) and annotated with the Prokka software (v1.5.2) (Seemann, 2014), providing a draft genome assembly of Pseudovibrio sp. P12. The presence of the genes involved in DMSP metabolism was investigated by searching for homologs of the corresponding genes using reciprocal best BLAST hits.
Synthesis of labelled magnesium sulfate (Mg34SO4)
Magnesium sulfate (Mg34SO4) was synthesised from pure sulfur 34S (purity >99%, Cambridge Isotope, MA) following a two-step reaction:
6HNO3 + 34S → H234SO4 +6NO2 + 2H2O
H234SO4 + MgCO3 → Mg34SO4 +H2O + CO2
Elemental sulfur 34S (0.1069 g) was ground into a fine powder and transferred to a pear-shaped flask. Nitric acid (65%, 4 ml) was added to the flask, heated to 80°C and refluxed for 5 hr. The temperature was subsequently raised to 130°C and refluxed for an additional 24 hr in order to completely oxidise remaining nitric acid. The resulting sulfuric acid (H234SO4) was then converted to Mg34SO4 by the addition of magnesium carbonate (MgCO3) (0.2643 g), giving a yield of 0.3780 g. The solution was subsequently heated to 100°C until all water had completely evaporated. Elemental analysis of the dried crystals was carried out with an electron probe microanalyser (EPMA, Jeol JXA8200), equipped with an energy dispersive spectrometer (EDS), to confirm the synthesis of Mg34SO4.
Symbiodinium growth and experimental conditions
Symbiodinium C1 cells were inoculated into artificial seawater (ASW; starting density: 1.5 × 106 cells ml−1) and incubated at 27°C for 18 days (based on results from a pilot study). LED lights were mounted above the culture, providing an average light intensity of 50 μE over a 14:10 hr light/dark cycle (AI Super Blue LED module 1003, IA, USA). Temperature and light intensities were monitored every 2 min for the duration of the experiment (using a HOBO UA-002-64, 64K temperature/light data logger).
The ASW contained 24.72 g of NaCl, 0.67 g of KCl, 1.36 g of CaCl2·2H2O, 4.66 g of MgCl2·6H2O, 0.18 g of NaHCO3, and 3.8 ml of modified ASP-8A solution (Figure 1—source data 1) in 1 litre of MilliQ water. Magnesium sulfate (MgSO4·7H2O, 6.29 g L−1) was used as the sole sulfur source, with either 34S (99% 34S, hereafter called 34S-ASW) or natural abundance of sulfur (95% 32S, 0.7% 33S, 4.2% 34S; hereafter called natS-ASW). Symbiodinium cells were incubated in 34S-ASW, whereas a batch incubated only in natS-ASW acted as a control. Both growth media were replaced every 5 days in order to actively remove dead and floating cells from the cultures. Symbiodinium cell numbers were monitored every 3 days for both 34S-ASW and natS-ASW treatments, using a light microscope and haemocytometer (depth 0.1 mm; eight replicates were averaged per time point) and cell mortality assessed using a 0.05% (w/v) Evans Blue solution (Morera and Villanueva, 2009).
After 18 days, the medium in both 34S-ASW and natS-ASW Symbiodinium cultures, was decanted and discarded. The Symbiodinium cells were thoroughly rinsed three times with natS-ASW and subsequently resuspended in natS-ASW (5 mins) prior to the addition of bacteria (Figure 1—figure supplement 1). This medium exchange (from 34S-ASW to natS-ASW) was carried out in order to prevent any potential direct bacterial uptake of 34SO42-.
Bacterial growth and inoculation
The two bacterial strains (Pseudovibrio sp. P12 and E. coli W) were grown overnight at 28°C in ASW medium enriched with 15N (in the form of amino-acids and NH4+; Celtone Base Powder; Cambridge Isotope Laboratories, Tewksburry, MA). The bacterial cells were subsequently washed three times in ASW before inoculation. Symbiodinium cells in treatment 1 were subsequently inoculated with the DMSP-degrading bacterium Pseudovibrio sp. P12; treatment 2 with E. coli; treatment 3 acted as a control without bacteria added; and treatment 4, which had no contact with 34S, acted as negative control for sulfur isotope incorporation (Figure 1—figure supplement 1). The two bacterial strains were inoculated at a density of 106 cells ml−1 and all samples were collected six hours after bacterial inoculation (based on results from a pilot study).
Sample preparation for NanoSIMS, electron microscopy and ToF-SIMS
We used high-pressure freezing (Smart et al., 2010), followed by a water-free embedding procedure to effectively prevent the loss of highly soluble compounds such as DMSP from our samples. This method does retain elements in solution (Altus and Canny, 1985; Ashford et al., 1999; Kaiser et al., 2015; Marshall et al., 2007; Mostaert et al., 1996) by effectively replacing the ‘solution’ with resin, without displacing the ions and osmolytes. Symbiodinium cultures pre-incubated with bacteria (20 μl) were dropped onto Thermanox strips (Thermo Fisher Scientific, Waltham, MA, USA, 4 × 18 mm) and then placed in humidified chambers. After 15 min, the cells settled onto the strips and the excess medium was carefully removed with filter paper before being frozen by immersion into liquid nitrogen slush (liquid nitrogen placed under low-vacuum in order to lower its temperature). Samples for structural imaging by electron microscopy (2 µl) were also collected. These were deposited in a gold planchet and high-pressure frozen using an EMPACT2 high-pressure freezer (Leica Microsystems, Wetzlar, Germany). Both sample types were stored in liquid nitrogen until required.
Frozen samples for NanoSIMS were freeze-substituted in anhydrous 10% acrolein in diethyl ether, and warmed progressively to room temperature over three weeks in an EM AFS2 automatic freeze-substitution unit (Leica Microsystems, Wetzlar, Germany) based upon the original method of Marshall (Marshall, 1980), and as described recently in step-by-step detail by Kilburn and Clode (Kilburn and Clode, 2014). The samples were subsequently infiltrated and embedded in anhydrous Araldite 502 resin, after which the Thermanox strip was removed and the sample re-embedded and stored in a desiccator. Although it is possible that not 100% of cellular DMSP may be preserved by this procedure, any losses would affect all samples equally; not impacting the validity of our comparisons between treatments. Furthermore, as 15N was only used as a tag to visualise the bacteria, dilution by processing and resin embedding (Musat et al., 2014) is of no concern here. For 34S analyses, dilution can be expected to be negligible as there is no sulfur contained in processing or resin components. Resin sections (1 µm thick) of embedded Symbiodinium cells were cut dry using a Diatome-Histo diamond knife on an EM UC6 Ultramicrotome (Leica Microsystems, Wetzlar, Germany), mounted on a silicon wafer and coated with 5 nm of gold.
NanoSIMS analysis
The NanoSIMS-50 (Cameca, Gennevilliers, France) at the Centre for Microscopy, Characterisation and Analysis (CMCA) at The University of Western Australia was used for all subsequent analyses. The NanoSIMS-50 allows simultaneous collection and counting of multiple isotopic species, which enables the determination of 15N/14N and 34S/32S ratios. Enrichments of the rare isotopes 34S and 15N were confirmed by an increase in the sulfur (34S/32S) and/or nitrogen (15N/14N) ratio above natural abundance values recorded in controls (equal to 0.0438 and 0.00367, respectively).
NanoSIMS analysis was undertaken by rastering a 2 pA Cs+ beam (~100 nm diameter) across defined 20 μm2 sample areas (256 × 256 pixels). The NanoSIMS-50 was tuned to achieve mass resolution at levels where the isobaric species 12C15N and 13C14N could be separated. The isotope ratio values are represented hereafter using a colour-coded transform (hue saturation intensity (HSI)) showing natural abundance levels in blue, and grading to high enrichment in pink. Images were processed and analysed using Fiji (http://fiji.sc/Fiji) (Schindelin et al., 2012) with the Open-MIMS plug-in (http://nrims.harvard.edu/software). All images were dead-time corrected (Hillion et al., 2008). Quantitative data were extracted from the mass images through manually drawn regions of interest. Ratio data were tested for QSA (quasi-simultaneous arrivals) by applying different beta values from 0.5 to 162. No differences in the data were observed, indicating that the secondary ion count rates were too low to be affected by QSA.
Time-of-flight secondary ion mass spectrometry (ToF-SIMS)
During ToF-SIMS analysis the sample surface is sputtered with a focused primary ion beam to produce ionic species (secondary ions) of the atoms, molecules and molecular fragments from the uppermost monolayers of the surface. The secondary ions are extracted into a flight column (time-of-flight analyser) and their masses determined by the exact time at which they arrive at the detector. The data collected can provide: (i) mass spectral information in the form of an accumulated mass spectrum, and (ii) image information in the XY dimensions showing the intensity distribution of the specific secondary ions from the area analysed.
The mass resolution of the ToF-SIMS analysis is determined by the temporal pulse width of the primary ions hitting the sample surface; whereas the spatial resolution is determined by the spot size of the primary ion beam. ToF-SIMS analyses are conducted with the instruments optimised either for high mass resolution or for high spatial resolution, as achieving both short pulses (for mass resolution) and narrow focus (for spatial resolution) simultaneously will greatly reduce the primary ion current density.
In this study, ToF-SIMS analyses were conducted using the TOF.SIMS five instrument (ION-TOF GmbH, Münster, Germany) at the Mark Wainwright Analytical Centre (MWAC), University of New South Wales. The instrument is equipped with a bismuth liquid metal cluster ion gun for analysis and an electron flood gun for charge compensation. Analysis was performed using a 30 keV Bi3+ cluster ion beam on resin sections (1 µm thick) mounted on silicon wafers. The ‘spectrometry’ mode was used to acquire high-mass resolution spectra (m/△m > 4000) and ‘fast-imaging’ mode was used to acquire high spatial resolution images (lateral resolution ~300 nm, m/Δm ~ 200).
In a typical analysis, a positive ion spectrum was acquired over a defined area of 20 × 20 μm2 or 50 × 50 μm2. The area of interest was identified by negative ion images acquired over areas of 20 × 20 μm2 (64 × 64 pixels) to 200 × 200 μm2 (128 × 128 pixels), where maps of CN- (m/z 26), S- (m/z 32), HS- (m/z 33) and 34S- (m/z 34) were generated to locate the position of cells and the presence of sulfur-containing compounds within the sample. Care was taken to ensure the ion dose density was kept below the static SIMS limit (1012–1013 primary ions per cm2) (Lindgren et al., 2014) when acquiring imaging data, e.g. no more than 5–10 scans over areas of 20 × 20 μm2. Keeping the static limit in the imaging mode prevents any significant damage to the sample structure or chemistry (Vickerman and Briggs, 2013), and enables further analyses of the same area in the positive polarity in this case. In the positive spectrum, the molecular ion [M + H]+ peak of both the 32S-and 34S-containing DMSP molecules (C5H1132SO2+ and C5H1134SO2+, respectively) are closely spaced with peaks arise from the resin (Figure 2—figure supplement 2). To maximise signal-to-noise ratio, data acquisition over a relatively small area encompassing the cell was desired, allowing unambiguous identification of the C5H1132SO2+ and C5H1134SO2+ peaks. High mass resolution positive spectra were calibrated using the masses of CH2+, C2H4+, C4H8+ and C6H12+ molecules. Data processing and evaluation were conducted using the SurfaceLab six software package (ION-TOF GmbH, Münster, Germany).
Prior to the analyses of the resin sections, the mass spectrum of dimethyl-β-propiothetin standard (Research Plus Inc., USA) was recorded to provide spectral information of DMSP generated by ToF-SIMS analysis. The molecular ion [M + H]+ peak (C5H11SO2+, m/z 135.05) was observed to be the most intense peak in the spectrum, and was used as the mass peak position when determining the presence of DMSP molecules in the samples. The mass spectrum of a mixture of methionine and cysteine (Sigma-Aldrich, USA) was also acquired to serve as a reference standard. Both methionine and cysteine were not detected or the amounts were below the detection limit of the instrument (ppm range).
Transmission electron microscopy (TEM)
High-pressure frozen samples for structural imaging were freeze-substituted in 1% OsO4 in acetone over two days and similarly infiltrated and embedded as described above. Sections 90 nm thick were cut on water using a diamond knife, collected on copper grids and imaged unstained at 120 kV in a JEOL 2100 TEM (Tokyo, Japan) fitted with a Gatan ORIUS camera (California, USA). Please note: the high solubility of DMSP in water prevented the coupling of NanoSIMS with TEM images (Clode et al., 2009) to identify the location of small organelles such as mitochondria, as ultrathin sections cannot be prepared without exposing the samples to water.
High pressure liquid chromatography-mass spectrometry (HPLC-MS)
After samples were collected for NanoSIMS analysis, all Symbiodinium cultures were centrifuged (3000 g) for 5 min, the medium was discarded and pelleted cells were extracted with 5 mL of HPLC-grade methanol. Crude methanol extracts were then analysed by reverse-phase (RP18) HPLC-MS in triplicate along with pure DMSP and amino acid standards.
A 10 µL aliquot of the methanol extract was diluted with an equal volume of acetonitrile and chromatographed using a Waters Alliance 2695 HPLC system comprising a quaternary pump, autosampler and photodiode array detector (200–400 nm) coupled to a Waters Micromass LCT Premier orthogonal acceleration time-of-flight (oa-TOF) mass spectrometer. Separation was achieved on an Alltima HP HILIC column (250 × 4.6 mm with a particle size of 5 µm) at 27°C and a flow rate of 0.75 ml min-1. The gradient was: acetonitrile (90%):0.1% formic acid (10%) at 0 min; acetonitrile (60%):0.1% formic acid (40%) at 0.4 min; acetonitrile (10%):0.1% formic acid (90%) at 12 min; acetonitrile (90%):0.1% formic acid (10%) at 12.25 min.
TOF-MS accurate mass measurements (scan-range m/z 100–1000 at 4 GHz, resolution = 9500) were acquired using an electrospray ionization (ESI) source in W positive mode with the following operation parameters: capillary voltage: 3000 V; cone voltage: 80V; ion source temperature: 80°C; desolvation temperature: 350°C; cone gas flow: 10 l hr−1; desolvation gas flow: 750 l hr−1; ion energy: 33 V; acceleration voltage: 100 V. MassLynx software (version 4.1, Waters) was used for operating the HPLC-MS, as well as for data acquisition and processing. Leucine Enkephalin was used as the external reference.
Quantitative nuclear magnetic resonance (qNMR)
The MeOH extracts remaining after HPLC-MS analysis was dried using a vacuum-centrifuge and dissolved in a mixture of deuterium oxide (D2O, D 99.8%, 250 μl) and deuterated methanol (CD3OD, D 99.8%, 750 μl) (Cambridge Isotope Laboratories, Andover, MA, USA). A 700 µl aliquot of the particulate-free extract was transferred into a 5 mm Norell 509-UP-7 NMR tube (Norell Inc., Landisville, NJ, USA) and analysed immediately by 1H NMR.
1H NMR spectra were recorded on a Bruker Avance 600 MHz NMR spectrometer with TXI 5 mm probe and quantification performed using the ERETIC method (Tapiolas et al., 2013). This technique generates an internal electronic reference signal, calibrated using stock solutions of DMSP.
Sulfur uptake
Bacterial strains and Symbiodinium were counted (Becton Dickinson LSR II flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA), and pellets were subsequently freeze-dried and weighed in order to determine their total sulfur content (equal to 5390 ng S mg−1). Samples were analysed on a Thermo Scientific FLASH 2000 Series (Thermo Scientific, Waltham, MA, USA). The sulfur uptake per mg of bacterial cells (ρ) was expressed in ng S mg−1 and was calculated by normalizing the 34S-incorporation measured using NanoSIMS to the average sulfur content (% of dry mass) according to the equation of Dugdale and Wilkerson (1986), presented in Pernice et al. (2012).:
ρ = ((Smes - Snat)/(Senr - Snat)) × Scontent ×103
Where:
Smes: 34S/32S measured in labelled samples by NanoSIMS
Snat: natural abundance of 34S/32S measured in unlabelled samples by NanoSIMS
Senr: 34S-enrichment of the Symbiodinium cells measured by NanoSIMS
Scontent: average sulfur content (%) measured by Thermo Scientific FLASH 2000 Series.
The calculated uptake (in nmol S mg−1) was then converted into an estimate uptake rate per day (nmol S l−1 day−1), based on: the bacterial exposure to 34S (6 hr), and the bacterial cell density for a given dry weight (acquired through flow cytometry; equal to 7.12 × 10−7 g for 5 × 105 bacterial cells).
Acknowledgements
The authors would like to thank Sabina Belli for valuable comments on the manuscript. We specially thank John Cliff, Mike House, Sigrid Fraser, Jeremy Shaw, John Murphy, Lyn Kirilak, Brett Baillie, and Sara Bell for their technical assistance. We also thank Janja Ceh and Bernard O’Reilly for their indefectible support. Credit for Figure 1: Glynn Gorick, Jean-Baptiste Raina and Justin Seymour. This work was supported by ANNiMS (Australian Government, Department of Education, Employment and Workplace Relations), the AMMRF Centre for Microscopy, Characterisation and Analysis (UWA) and by Australian Research Council Grant DE160100636 to JBR.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Funding Information
This paper was supported by the following grants:
Australian Research Council DE160100636 to Jean-Baptiste Raina.
ANNiMS to Jean-Baptiste Raina.
AMMRF Centre for Microscopy, Characterisation and Analysis to Jean-Baptiste Raina.
Additional information
Competing interests
The authors declare that no competing interests exist.
Author contributions
J-BR, Conceptualization, Data curation, Formal analysis, Funding acquisition, Methodology, Writing—original draft, Writing—review and editing.
PLC, Conceptualization, Formal analysis, Supervision, Validation, Methodology, Writing—original draft, Writing—review and editing.
SC, Formal analysis, Writing—review and editing.
JB, Formal analysis.
MRK, Conceptualization, Formal analysis, Validation.
AR, Formal analysis.
SF, Resources, Formal analysis.
MS, Resources, Investigation.
VB, Resources, Methodology.
PT-H, Investigation, Methodology.
DT, Conceptualization, Investigation.
CMM, Conceptualization, Resources, Methodology.
BG, Methodology.
MP, Formal analysis, Investigation, Methodology.
CEM, Resources, Methodology.
JRS, Resources, Supervision, Writing—original draft.
BLW, Conceptualization, Supervision, Writing—original draft.
DGB, Conceptualization, Supervision, Writing—original draft, Writing—review and editing.
References
- Alcolombri U, Ben-Dor S, Feldmesser E, Levin Y, Tawfik DS, Vardi A. MARINE SULFUR CYCLE. identification of the algal dimethyl sulfide-releasing enzyme: a missing link in the marine sulfur cycle. Science. 2015;348:1466–1469. doi: 10.1126/science.aab1586. [DOI] [PubMed] [Google Scholar]
- Altus D, I, Canny MJ. Loading of assimilates in wheat leaves. II. the path from chloroplast to vein. Plant, Cell & Environment. 1985;8:275–285. doi: 10.1111/1365-3040.ep11604677. [DOI] [Google Scholar]
- Archer SD, Widdicombe CE, Tarran GA, Rees AP, Burkill PH. Production and turnover of particulate dimethylsulphoniopropionate during a coccolithophore bloom in the northern north sea. Aquatic Microbial Ecology. 2001;24:225–241. doi: 10.3354/ame024225. [DOI] [Google Scholar]
- Ashford AE, Vesk PA, Orlovich DA, Markovina AL, Allaway WG. Dispersed polyphosphate in fungal vacuoles in Eucalyptus pilularis/Pisolithus tinctorius ectomycorrhizas. Fungal Genetics and Biology. 1999;28:21–33. doi: 10.1006/fgbi.1999.1140. [DOI] [PubMed] [Google Scholar]
- Ausubel FM, Bent R, Kingston RE. Current Protocols in Molecular Biology. Wiley and Sons; 1987. pp. 2.10–2.12. [Google Scholar]
- Ayers GP, Gras JL. Seasonal relationship between cloud condensation nuclei and aerosol methanesulphonate in marine air. Nature. 1991;353:834–835. doi: 10.1038/353834a0. [DOI] [Google Scholar]
- Azam F, Malfatti F. Microbial structuring of marine ecosystems. Nature Reviews Microbiology. 2007;5:782–791. doi: 10.1038/nrmicro1747. [DOI] [PubMed] [Google Scholar]
- Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, Pyshkin AV, Sirotkin AV, Vyahhi N, Tesler G, Alekseyev MA, Pevzner PA. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. Journal of Computational Biology. 2012;19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank RJ. Cell architecture of the dinoflagellate Symbiodinium sp. inhabiting the hawaiian stony coral Montipora verrucosa. Marine Biology. 1987;94:143–155. doi: 10.1007/BF00392906. [DOI] [Google Scholar]
- Bondarev V, Richter M, Romano S, Piel J, Schwedt A, Schulz-Vogt HN. The genus Pseudovibrio contains metabolically versatile bacteria adapted for symbiosis. Environmental Microbiology. 2013;15:2095–2113. doi: 10.1111/1462-2920.12123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent AD, Jones GB, Jones RJ. DMSP in corals and benthic algae from the great barrier reef. Estuarine, Coastal and Shelf Science. 2002;55:547–555. doi: 10.1006/ecss.2002.1021. [DOI] [Google Scholar]
- Caruana AMN, Malin G. The variability in DMSP content and DMSP lyase activity in marine dinoflagellates. Progress in Oceanography. 2014;120:410–424. doi: 10.1016/j.pocean.2013.10.014. [DOI] [Google Scholar]
- Clode PL, Kilburn MR, Jones DL, Stockdale EA, Cliff JB, Herrmann AM, Murphy DV. In situ mapping of nutrient uptake in the rhizosphere using nanoscale secondary ion mass spectrometry. Plant Physiology. 2009;151:1751–1757. doi: 10.1104/pp.109.141499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole JJ. Interactions between Bacteria and algae in aquatic ecosystems. Annual Review of Ecology and Systematics. 1982;13:291–314. doi: 10.1146/annurev.es.13.110182.001451. [DOI] [Google Scholar]
- Cosquer A, Pichereau V, Pocard JA, Minet J, Cormier M, Bernard T. Nanomolar levels of dimethylsulfoniopropionate, dimethylsulfonioacetate, and glycine betaine are sufficient to confer osmoprotection to Escherichia coli. Applied and Environmental Microbiology. 1999;65:3304–3311. doi: 10.1128/aem.65.8.3304-3311.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curson AR, Todd JD, Sullivan MJ, Johnston AW. Catabolism of dimethylsulphoniopropionate: microorganisms, enzymes and genes. Nature Reviews Microbiology. 2011;9:849–859. doi: 10.1038/nrmicro2653. [DOI] [PubMed] [Google Scholar]
- Dubinsky Z. Coral Reefs. Elsevier; 1990. pp. 75–87. [Google Scholar]
- Dugdale RC, Wilkerson FP. The use of 15 N to measure nitrogen uptake in eutrophic oceans; experimental considerations1,2. Limnology and Oceanography. 1986;31:673–689. doi: 10.4319/lo.1986.31.4.0673. [DOI] [Google Scholar]
- Falkowski PG, Fenchel T, Delong EF. The microbial engines that drive Earth's biogeochemical cycles. Science. 2008;320:1034–1039. doi: 10.1126/science.1153213. [DOI] [PubMed] [Google Scholar]
- Fischer E, Jones G. Atmospheric dimethysulphide production from corals in the great barrier reef and links to solar radiation, climate and coral bleaching. Biogeochemistry. 2012;110:31–46. doi: 10.1007/s10533-012-9719-y. [DOI] [Google Scholar]
- Garren M, Son K, Raina JB, Rusconi R, Menolascina F, Shapiro OH, Tout J, Bourne DG, Seymour JR, Stocker R. A bacterial pathogen uses dimethylsulfoniopropionate as a cue to target heat-stressed corals. The ISME Journal. 2014;8:999–1007. doi: 10.1038/ismej.2013.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett TA, Schmeitzel JL, Klein JA, Hwang JJ, Schwarz JA. Comparative lipid profiling of the cnidarian Aiptasia pallida and its dinoflagellate symbiont. PLoS One. 2013;8:e57975. doi: 10.1371/journal.pone.0057975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillion F, Kilburn MR, Hoppe P, Messenger S, Weber PK. The effect of QSA on S, C, O and si isotopic ratio measurements. Goldschmidt Conference Abstracts, A377.2008. [Google Scholar]
- Hjelm M, Bergh O, Riaza A, Nielsen J, Melchiorsen J, Jensen S, Duncan H, Ahrens P, Birkbeck H, Gram L. Selection and identification of autochthonous potential probiotic bacteria from turbot larvae (Scophthalmus maximus) rearing units. Systematic and Applied Microbiology. 2004;27:360–371. doi: 10.1078/0723-2020-00256. [DOI] [PubMed] [Google Scholar]
- Howard EC, Henriksen JR, Buchan A, Reisch CR, Bürgmann H, Welsh R, Ye W, González JM, Mace K, Joye SB, Kiene RP, Whitman WB, Moran MA. Bacterial taxa that limit sulfur flux from the ocean. Science. 2006;314:649–652. doi: 10.1126/science.1130657. [DOI] [PubMed] [Google Scholar]
- Howard EC, Sun S, Biers EJ, Moran MA. Abundant and diverse bacteria involved in DMSP degradation in marine surface waters. Environmental Microbiology. 2008;10:2397–2410. doi: 10.1111/j.1462-2920.2008.01665.x. [DOI] [PubMed] [Google Scholar]
- Kaiser C, Kilburn MR, Clode PL, Fuchslueger L, Koranda M, Cliff JB, Solaiman ZM, Murphy DV. Exploring the transfer of recent plant photosynthates to soil microbes: mycorrhizal pathway vs direct root exudation. New Phytologist. 2015;205:1537–1551. doi: 10.1111/nph.13138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiene RP, Visscher PT, Keller MD, Kirst GO. Biological and Environmental Chemistry of DMSP and Related Sulfonium Compounds. Plenum Press; 1996. pp. 121–129. [Google Scholar]
- Kilburn MR, Clode PL. Electron Microscopy. Ch. 33. Humana Press; 2014. pp. 733–755. [Google Scholar]
- Lindgren J, Sjövall P, Carney RM, Uvdal P, Gren JA, Dyke G, Schultz BP, Shawkey MD, Barnes KR, Polcyn MJ. Skin pigmentation provides evidence of convergent melanism in extinct marine reptiles. Nature. 2014;506:484–488. doi: 10.1038/nature12899. [DOI] [PubMed] [Google Scholar]
- Markell DA, Trench RK. Macromolecules exuded by symbiotic dinoflagellates in culture: amino acid and sugar composition1. Journal of Phycology. 1993;29:64–68. doi: 10.1111/j.1529-8817.1993.tb00280.x. [DOI] [Google Scholar]
- Marshall AT, Clode PL, Russell R, Prince K, Stern R. Electron and ion microprobe analysis of calcium distribution and transport in coral tissues. Journal of Experimental Biology. 2007;210:2453–2463. doi: 10.1242/jeb.003343. [DOI] [PubMed] [Google Scholar]
- Marshall AT. Freeze-substitution as a preparation technique for biological X-ray microanalysis. Scanning Electron Microscopy. 1980;2:395–408. [PubMed] [Google Scholar]
- Matrai PA, Keller MD. Total organic sulfur and dimethylsulfoniopropionate in marine phytoplankton: intracellular variations. Marine Biology. 1994;119:61–68. doi: 10.1007/BF00350107. [DOI] [Google Scholar]
- Morera C, Villanueva MA. Heat treatment and viability assessment by Evans blue in cultured Symbiodinium kawagutii cells. World Journal of Microbiology and Biotechnology. 2009;25:1125–1128. doi: 10.1007/s11274-009-9987-4. [DOI] [Google Scholar]
- Mostaert AS, Orlovich DA, King RJ. Ion compartmentation in the red alga Caloglossa leprieurii in response to salinity changes: freeze-substitution and X-ray microanalysis. New Phytologist. 1996;132:513–519. doi: 10.1111/j.1469-8137.1996.tb01871.x. [DOI] [PubMed] [Google Scholar]
- Musat N, Stryhanyuk H, Bombach P, Adrian L, Audinot JN, Richnow HH. The effect of FISH and CARD-FISH on the isotopic composition of (13)C- and (15)N-labeled Pseudomonas putida cells measured by nanoSIMS. Systematic and Applied Microbiology. 2014;37:267–276. doi: 10.1016/j.syapm.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Nissimov J, Rosenberg E, Munn CB. Antimicrobial properties of resident coral mucus bacteria of Oculina patagonica. FEMS Microbiology Letters. 2009;292:210–215. doi: 10.1111/j.1574-6968.2009.01490.x. [DOI] [PubMed] [Google Scholar]
- Pernice M, Meibom A, Van Den Heuvel A, Kopp C, Domart-Coulon I, Hoegh-Guldberg O, Dove S. A single-cell view of ammonium assimilation in coral-dinoflagellate symbiosis. The ISME Journal. 2012;6:1314–1324. doi: 10.1038/ismej.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radjasa OK, Wiese J, Sabdono A, Imhoff JF. Coral as source of Bacteria with antimicrobial activity. Journal of Coastal Development. 2008;11:121–130. [Google Scholar]
- Raina JB, Dinsdale EA, Willis BL, Bourne DG. Do the organic sulfur compounds DMSP and DMS drive coral microbial associations? Trends in Microbiology. 2010;18:101–108. doi: 10.1016/j.tim.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Raina JB, Tapiolas D, Motti CA, Foret S, Seemann T, Tebben J, Willis BL, Bourne DG. Isolation of an antimicrobial compound produced by Bacteria associated with reef-building corals. PeerJ. 2016;4:e2275. doi: 10.7717/peerj.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raina JB, Tapiolas DM, Forêt S, Lutz A, Abrego D, Ceh J, Seneca FO, Clode PL, Bourne DG, Willis BL, Motti CA. DMSP biosynthesis by an animal and its role in coral thermal stress response. Nature. 2013;502:677–680. doi: 10.1038/nature12677. [DOI] [PubMed] [Google Scholar]
- Reisch CR, Stoudemayer MJ, Varaljay VA, Amster IJ, Moran MA, Whitman WB. Novel pathway for assimilation of dimethylsulphoniopropionate widespread in marine bacteria. Nature. 2011;473:208–211. doi: 10.1038/nature10078. [DOI] [PubMed] [Google Scholar]
- Ritchie KB. Regulation of microbial populations by coral surface mucus and mucus-associated Bacteria. Marine Ecology Progress Series. 2006;322:1–14. doi: 10.3354/meps322001. [DOI] [Google Scholar]
- Rypien KL, Ward JR, Azam F. Antagonistic interactions among coral-associated bacteria. Environmental Microbiology. 2010;12:28–39. doi: 10.1111/j.1462-2920.2009.02027.x. [DOI] [PubMed] [Google Scholar]
- Saltzman ES, Cooper JC. Biogenic Sulphur in the Environment. American Chemical Society; 1989. pp. 167–182. [Google Scholar]
- Santos EO, Alves N, Dias GM, Mazotto AM, Vermelho A, Vora GJ, Wilson B, Beltran VH, Bourne DG, Le Roux F, Thompson FL. Genomic and proteomic analyses of the coral pathogen Vibrio coralliilyticus reveal a diverse virulence repertoire. The ISME Journal. 2011;5:1471–1483. doi: 10.1038/ismej.2011.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nature Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. 2014;30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- Shieh WY, Lin YT, Jean WD. Pseudovibrio denitrificans gen. nov., sp. nov., a marine, facultatively anaerobic, fermentative bacterium capable of denitrification. International Journal of Systematic and Evolutionary Microbiology. 2004;54:2307–2312. doi: 10.1099/ijs.0.63107-0. [DOI] [PubMed] [Google Scholar]
- Shoguchi E, Shinzato C, Kawashima T, Gyoja F, Mungpakdee S, Koyanagi R, Takeuchi T, Hisata K, Tanaka M, Fujiwara M, Hamada M, Seidi A, Fujie M, Usami T, Goto H, Yamasaki S, Arakaki N, Suzuki Y, Sugano S, Toyoda A, Kuroki Y, Fujiyama A, Medina M, Coffroth MA, Bhattacharya D, Satoh N. Draft assembly of the Symbiodinium minutum nuclear genome reveals dinoflagellate gene structure. Current Biology. 2013;23:1399–1408. doi: 10.1016/j.cub.2013.05.062. [DOI] [PubMed] [Google Scholar]
- Sies H. Strategies of antioxidant defense. European Journal of Biochemistry. 1993;215:213–219. doi: 10.1111/j.1432-1033.1993.tb18025.x. [DOI] [PubMed] [Google Scholar]
- Sievert S, Kiene R, Schulz-Vogt H. The sulfur cycle. Oceanography. 2007;20:117–123. doi: 10.5670/oceanog.2007.55. [DOI] [Google Scholar]
- Simó R, Archer SD, Pedrós-Alió C, Gilpin L, Stelfox-Widdicombe CE. Coupled dynamics of dimethylsulfoniopropionate and dimethylsulfide cycling and the microbial food web in surface waters of the North Atlantic. Limnology and Oceanography. 2002;47:53–61. doi: 10.4319/lo.2002.47.1.0053. [DOI] [Google Scholar]
- Simó R. Production of atmospheric sulfur by oceanic plankton: biogeochemical, ecological and evolutionary links. Trends in Ecology & Evolution. 2001;16:287–294. doi: 10.1016/S0169-5347(01)02152-8. [DOI] [PubMed] [Google Scholar]
- Smart KE, Smith JA, Kilburn MR, Martin BG, Hawes C, Grovenor CR. High-resolution elemental localization in vacuolate plant cells by nanoscale secondary ion mass spectrometry. The Plant Journal. 2010;63:870–879. doi: 10.1111/j.1365-313X.2010.04279.x. [DOI] [PubMed] [Google Scholar]
- Stefels J, Dacey JWH, Elzenga JTM. In vivo DMSP-biosynthesis measurements using stable-isotope incorporation and proton-transfer-reaction mass spectrometry (PTR-MS) Limnology and Oceanography: Methods. 2009;7:595–611. doi: 10.4319/lom.2009.7.595. [DOI] [Google Scholar]
- Stefels J. Physiological aspects of the production and conversion of DMSP in marine algae and higher plants. Journal of Sea Research. 2000;43:183–197. doi: 10.1016/S1385-1101(00)00030-7. [DOI] [Google Scholar]
- Sulistiyani S, Nugraheni A, Radjasa OK, Sabdono A, Khoeri MM. Antibacterial activities of bacterial symbionts of soft coral Sinularia sp. against tuberculosis bacteria. Journal of Coastal Development. 2010;14:45–50. [Google Scholar]
- Sunda W, Kieber DJ, Kiene RP, Huntsman S. An antioxidant function for DMSP and DMS in marine algae. Nature. 2002;418:317–320. doi: 10.1038/nature00851. [DOI] [PubMed] [Google Scholar]
- Tapiolas DM, Raina J-B, Lutz A, Willis BL, Motti CA. Direct measurement of dimethylsulfoniopropionate (DMSP) in reef-building corals using quantitative nuclear magnetic resonance (qNMR) spectroscopy. Journal of Experimental Marine Biology and Ecology. 2013;443:85–89. doi: 10.1016/j.jembe.2013.02.037. [DOI] [Google Scholar]
- Todd JD, Rogers R, Li YG, Wexler M, Bond PL, Sun L, Curson AR, Malin G, Steinke M, Johnston AW. Structural and regulatory genes required to make the gas dimethyl sulfide in bacteria. Science. 2007;315:666–669. doi: 10.1126/science.1135370. [DOI] [PubMed] [Google Scholar]
- Trossat C, Nolte KD, Hanson AD. Evidence that the pathway of dimethylsulfoniopropionate biosynthesis begins in the cytosol and ends in the chloroplast. Plant Physiology. 1996;111:965–973. doi: 10.1104/pp.111.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trossat C, Rathinasabapathi B, Weretilnyk EA, Shen TL, Huang ZH, Gage DA, Hanson AD. Salinity promotes accumulation of 3-dimethylsulfoniopropionate and its precursor S-methylmethionine in chloroplasts. Plant Physiology. 1998;116:165–171. doi: 10.1104/pp.116.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oppen MJ, Palstra FP, Piquet AM, Miller DJ. Patterns of coral-dinoflagellate associations in Acropora: significance of local availability and physiology of Symbiodinium strains and host-symbiont selectivity. Proceedings of the Royal Society B: Biological Sciences. 2001;268:1759–1767. doi: 10.1098/rspb.2001.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickerman JC, Briggs D. TOF-SIMS: Materials Analysis by Mass Spectrometry. Ch. 10. IM Publications & SurfaceSpectra; 2013. pp. 271–290. [Google Scholar]
- Yuyama I, Higuchi T, Takei Y. Sulfur utilization of corals is enhanced by endosymbiotic algae. Biology Open. 2016;5:1299–1304. doi: 10.1242/bio.020164. [DOI] [PMC free article] [PubMed] [Google Scholar]