Abstract
OBJECTIVE
To examine whether gender differences may have affected treatment response to S-adenosyl methionine (SAMe) in a recent failed randomized clinical trial (RCT) for adults with major depressive disorder.
METHODS
Data from a two-site, 12-week, double-blind RCT (n=189) assessing the efficacy of SAMe versus placebo and a comparator selective serotonin reuptake inhibitor (escitalopram) were subjected to post-hoc analyses to evaluate effects of patient gender on treatment response.
RESULTS
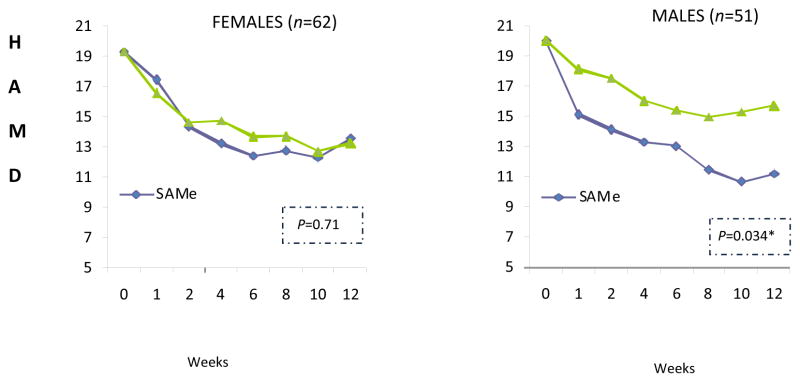
When assessing the efficacy outcomes within each gender separately, SAMe was superior to placebo among males (n=51), but not among females (n=62). Males showed a significant reduction of depression severity from baseline to study endpoint on the 17-item Hamilton Depression Rating Scale (4.3 point difference; p=0.034; d=0.95), while females did not show significant change. This finding emerged despite equivalence on baseline measures of depression severity between the gender groups.
CONCLUSION
Results of this secondary data analysis suggest that gender might impact the antidepressant efficacy of SAMe, with greater therapeutic effect found in males. The underlying mechanism is still relatively unknown. Further work is needed to replicate this observation in independent samples.
Keywords: S-Adenosyl Methionine, SAMe, Antidepressant, Gender, Depression, RCT, One-Carbon Cycle
INTRODUCTION
S-adenosyl methionine (SAMe) is a sulphur-containing compound that is a critical neurochemical component of the one-carbon cycle, involved in the methylation of neurotransmitters responsible for mood regulation (1). SAMe may alleviate depressed mood via enhanced methylation of catecholamines, increased serotonin turnover, reuptake inhibition of norepinephrine, enhanced dopaminergic activity, and increased phosphatidylcholine conversion (2, 3). SAMe has been proposed as a potential treatment for various medical conditions, particularly major depressive disorder (MDD). Double-blind randomized clinical trials (RCTs) have generally supported efficacy of oral and parenteral SAMe as comparable to tricyclic antidepressants, with fewer side effects (4). Recent work supports SAMe augmentation in partial and non-responders to selective serotonin reuptake inhibitors (SSRIs) and serotonin/norepinephrine reuptake inhibitors (SNRIs) (5).
We recently reported the first double-blind RCT comparing the efficacy of SAMe versus placebo and a standard SSRI (escitalopram) in treating MDD (6). The 12-week two-site study was essentially negative, with no significant differences in clinical response occurring between the three treatment groups. Given the considerable body of evidence supporting the antidepressant efficacy of SAMe, we carried out various post-hoc analyses examining possible effects of site, gender, and other patient characteristics. The first of these investigations, a sub-analysis of data from site 1 only, which recruited the majority of participants, revealed a significant effect in favor of SAMe, with a moderate-to-large effect size versus placebo (d=0.74) and remission rates significantly higher for SAMe (34%) than for escitalopram (23%) or placebo (6%) (7). Because the only significant difference in patient characteristics between the two sites was gender (59% males in site 1 and 31% males in site 2; X2 p<0.0001), we explored whether this might explain the difference in outcome between the two sites. This report describes those findings.
METHODS
The full methodology of the parent trial is detailed in Mischoulon et al.(6) In brief, consenting outpatients with MDD were recruited from 4/13/05 to 12/22/09. Inclusion criteria consisted of: a diagnosis of MDD by the Structured Clinical Interview for DSM-IV; age 18–80 years old; and a score of 25 or greater on the Inventory of Depressive Symptomatology – Clinician-Rated. The HAM-D-17 was administered concurrently and used as the main outcome measure. Exclusion criteria consisted of: comorbid psychiatric and medical disorders, or use of various medications; current use of other psychotropic drugs; previous moderate use of SAMe or escitalopram; ongoing psychotherapy; multiple treatment failures; and pregnancy or being of child-bearing potential and not using a medically accepted means of contraception.
Participants who met criteria for MDD and had a baseline HAM-D-17 score of ≥14 were analyzed as intent-to-treat using last observation carried forward (LOCF), in which all patients randomized to any of the three treatment arms had their data included in the analysis. We analyzed each gender group to determine whether there were any treatment-based differences. The primary efficacy measure was the difference in change in HAM-D-17 score across the three treatment groups from baseline to week 12 endpoint. Analysis of covariance (ANCOVA), using baseline HAM-D-17 score as the covariate, was conducted to assess differences between Group X Time from baseline to study endpoint. Response rates (HAM-D-17 reduction of ≥ 50%) were also calculated using a Chi-Square analysis.
All analyses were carried out using SPSS version 20.0 software, with statistical significance set at alpha = 0.05.
RESULTS
Baseline HAMD-17 levels were not significantly different between gender groups. While the Group X Time interaction between all three treatments (covarying for baseline HAM-D depression level) was not significant for either gender (male: F2,74 1.41; p=0.16; female F=2,88 0.77; p= 0.71), significant results were revealed for males when directly comparing SAMe to placebo. A significant reduction of depression was seen in males between treatments from baseline to study endpoint; males treated with SAMe had a decline of 8.9 points on the HAM-D compared to 4.6 points for placebo (4.3 point difference, F1,48 4.77; p=0.034; Figure 1). This represents a large effect size, d=0.95. Conversely, females taking placebo had a 6.0-point reduction compared to a 5.4-point reduction for SAMe (n.s). Comparisons of changes in HAM-D-17 score for SAM versus escitalopram and for escitalopram versus placebo in each gender group did not yield any significant findings (data not shown). Gender differences likewise did not impact response rates to treatment (data not shown).
Figure 1.
Gender and response to SAMe versus placebo
Group X Time ANCOVAs were conducted in males and in females. HAMD= Hamilton Depression Rating Scale 17. SAMe= S-adenosyl methionine
DISCUSSION
The present findings suggest that SAMe may be more effective for males than females in reducing depression. This could help explain the negative results from the parent study. While results between sites differed, we saw no obvious reason for this. Both sites employed identical methodologies and staff were highly trained. Further, sample demographics were comparable (Boston and Providence). Other variables such as age, also revealed no significant impact of differential response (data not shown). While these initial analyses suggested that between-site effects might have contributed to the failure to detect treatment differences, the present analysis suggests that gender-based response differences could have impacted outcome in the overall sample.
There are precedents for gender differences in pharmacological antidepressant effects. For example, in a 12-week RCT(8) involving 235 male and 400 female outpatients randomized to sertraline or imipramine, females were significantly more likely to respond to sertraline than to imipramine, and men were significantly more likely to respond to imipramine than to sertraline. Previous studies have also suggested possible gender-based effects for SAMe. Koch et al (9) examined activity-related effects on plasma epinephrine, norepinephrine, dopamine and renin in 16 adults (males and females aged 22–34 years) and 9 athletic adolescent boys (aged 16–17-years). Epinephrine, norepinephrine, and renin activity increased exponentially with exercise, and the effect was greatest in the adolescent boys. Because the assay required conversion of epinephrine, norepinephrine, and dopamine into methyl-derivates in the presence of catechol-O-methyltransferase and SAMe, the findings suggest a possible link between SAMe and gender-specific effects (although we cannot rule out age differences influencing this effect).
Inoue-Choi et al. (10) conducted a cross-sectional analysis of associations between plasma concentrations of one-carbon cycle metabolites and plasma SAMe concentrations in healthy Chinese subjects. Plasma betaine and folate were positively associated with plasma SAMe in men only. Men carrying the variant methionine adenosyltransferase (MAT1A) genotype and having low plasma methionine had significantly lower plasma SAMe concentrations than men carrying the wild type genotype. Again, these findings further suggest the potential for gender-related effects of SAMe.
A healthy population study (11) (n=581) investigating the relationship between fasting concentrations of plasma one-carbon cycle biomarkers (e.g., homocysteine, SAMe, and S-adenosylhomocysteine (SAH)) revealed that, compared to females, males had a significantly lower SAMe:SAH ratio, suggesting that SAMe supplementation could have greater impact on men. A 6-week double-blind RCT of SAMe augmentation for SSRI/SNRI- non-responders revealed that males treated with adjunctive SAMe demonstrated significantly lower sexual arousal dysfunction at study endpoint compared to the adjunctive placebo group,(12) perhaps due to SAMe being a regulator of cystathionine beta-synthase, resulting in nitric oxide-mediated vasodilation of penile tissues (12). A neuroprotective effect via SAMe on dopaminergic neurons might also enhance dopamine’s role in enhancing sexual functioning in men. Despite the data supporting a more specific effect of SAMe in males, an 8-week double-blind RCT with 18 participants with chronic schizophrenia given either SAMe 800 mg or placebo found improvement of depressive symptoms only in females (13).
While these studies are suggestive, they do not provide a clear line of evidence for the potential mechanism underlying a gender difference of SAMe in depression. A few studies have also reported gender-based differences in one-carbon cycle activity in rats and mice. Factors linked to gender effects include the N-acetyltransferase (NAT) enzyme, which plays a role in catabolism of folate, a vitamin on which SAMe synthesis depends. (14) In another study, glycine N-methyltransferase (GNMT), an enzyme that regulates SAMe and can impact methylation and folate metabolism, was significantly increased in the liver of male rats after retinoid administration and SAMe-dependent creatinine synthase was significantly reduced; these effects were less pronounced in female rats.(15) The enzyme S-adenosylmethionine synthetase (AdoMet synthetase) is responsible for the synthesis of SAMe, and is less active in male than female rats (16).
Collectively, these studies suggest that gender-related effects involving SAMe may be due to variances in the one-carbon cycle pathways, perhaps in part secondary to hormonal regulation, but current data do not provide clear support for a single unified theory. The breadth of findings, however, suggests the need for further research in this area.
In summary, our results suggest a gender-based difference in response to SAMe in subjects with MDD, and there are precedents in human and animal studies to suggest mechanisms that might account for this differential. Our investigation is limited by the post-hoc nature of the analysis, and requires prospective studies to replicate the findings. Re-analyses of other published SAMe studies in MDD would also be informative. While we cannot yet say whether a true gender difference in clinical response to SAMe exists, such an effect would represent a novel and important finding that could have implications for the application of this natural product in the treatment of mood disorders.
Acknowledgments
Funding Source:
The study was supported by the NIH and the National Center for Complementary and Alternative Medicine (NCCAM), R01 grant R01AT001638, SAMe tosylate and matching placebo were supplied by Pharmavite LLC, California
Footnotes
Clinicaltrials.gov identifier: NCT00101452
Competing Interests
No direct competing interests identified
Author’s Contributions – JS and DM drafted the initial version of this manuscript. All authors contributed intellectual content to the manuscript and read and approved the final manuscript.
References
- 1.Williams AL, Girard C, Jui D, Sabina A, Katz DL. S-adenosylmethionine (SAMe) as treatment for depression: a systematic review. Clin Invest Med. 2005;28(3):132–9. [PubMed] [Google Scholar]
- 2.Bottiglieri T, Hyland K. S-adenosylmethionine levels in psychiatric and neurological disorders: a review. Acta Neurol Scand Suppl. 1994;154:19–26. doi: 10.1111/j.1600-0404.1994.tb05405.x. [DOI] [PubMed] [Google Scholar]
- 3.Papakostas GI, Alpert JE, Fava M. S-adenosyl-methionine in depression: a comprehensive review of the literature. Current psychiatry reports. 2003;5(6):460–6. doi: 10.1007/s11920-003-0085-2. [DOI] [PubMed] [Google Scholar]
- 4.Papakostas GI. Evidence for S-adenosyl-L-methionine (SAM-e) for the treatment of major depressive disorder. J Clin Psychiatry. 2009;70(Suppl 5):18–22. doi: 10.4088/JCP.8157su1c.04. [DOI] [PubMed] [Google Scholar]
- 5.Papakostas GI, Mischoulon D, Shyu I, Alpert JE, Fava M. S-adenosyl methionine (SAMe) augmentation of serotonin reuptake inhibitors for antidepressant nonresponders with major depressive disorder: a double-blind, randomized clinical trial. Am J Psychiatry. 2010;167(8):942–8. doi: 10.1176/appi.ajp.2009.09081198. [DOI] [PubMed] [Google Scholar]
- 6.Mischoulon D, Price LH, Carpenter LL, Tyrka AR, Papakostas GI, Baer L, et al. A Double-blind, randomized, placebo-controlled clinical trial of S-adenosyl-L-methionine (SAMe) versus escitalopram in major depressive disorder. J Clin Psychiatry. 2014 doi: 10.4088/JCP.13m08591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarris J, Papakostas G, Vitolo O, Fava M, Mischoulon D. S-Adenosyl Methionine (SAMe) versus Escitalopram and Placebo in Major Depression: Efficacy and Effects of Histamine and Carnitine as Moderators of Response. Journal of Affective Disorders. 2014;164:76–81. doi: 10.1016/j.jad.2014.03.041. [DOI] [PubMed] [Google Scholar]
- 8.Kornstein SG, Schatzberg AF, Thase ME, Yonkers KA, McCullough JP, Keitner GI, et al. Gender differences in treatment response to sertraline versus imipramine in chronic depression. Am J Psychiatry. 2000;157(9):1445–52. doi: 10.1176/appi.ajp.157.9.1445. [DOI] [PubMed] [Google Scholar]
- 9.Koch G, Johansson U, Arvidsson E. Radioenzymatic determination of epinephrine, norepinephrine and dopamine in 0.1 ml plasma samples: plasma catecholamine response to submaximal and near maximal exercise. Journal of clinical chemistry and clinical biochemistry Zeitschrift fur klinische Chemie und klinische Biochemie. 1980;18(6):367–72. doi: 10.1515/cclm.1980.18.6.367. [DOI] [PubMed] [Google Scholar]
- 10.Inoue-Choi M, Nelson HH, Robien K, Arning E, Bottiglieri T, Koh WP, et al. One-carbon metabolism nutrient status and plasma S-adenosylmethionine concentrations in middle-aged and older Chinese in Singapore. International journal of molecular epidemiology and genetics. 2012;3(2):160–73. [PMC free article] [PubMed] [Google Scholar]
- 11.King WD, Ho V, Dodds L, Perkins SL, Casson RI, Massey TE. Relationships among biomarkers of one-carbon metabolism. Molecular biology reports. 2012;39(7):7805–12. doi: 10.1007/s11033-012-1623-y. [DOI] [PubMed] [Google Scholar]
- 12.Dording CM, Mischoulon D, Shyu I, Alpert JE, Papakostas GI. SAMe and sexual functioning. European psychiatry: the journal of the Association of European Psychiatrists. 2012;27(6):451–4. doi: 10.1016/j.eurpsy.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Strous RD, Ritsner MS, Adler S, Ratner Y, Maayan R, Kotler M, et al. Improvement of aggressive behavior and quality of life impairment following S-adenosyl-methionine (SAM-e) augmentation in schizophrenia. Eur Neuropsychopharmacol. 2009;19(1):14–22. doi: 10.1016/j.euroneuro.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 14.Witham KL, Butcher NJ, Sugamori KS, Brenneman D, Grant DM, Minchin RF. 5-methyl-tetrahydrofolate and the S-adenosylmethionine cycle in C57BL/6J mouse tissues: gender differences and effects of arylamine N-acetyltransferase-1 deletion. PLoS One. 2013;8(10):e77923. doi: 10.1371/journal.pone.0077923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McMullen MH, Rowling MJ, Ozias MK, Schalinske KL. Activation and induction of glycine N-methyltransferase by retinoids are tissue- and gender-specific. Archives of biochemistry and biophysics. 2002;401(1):73–80. doi: 10.1016/S0003-9861(02)00030-9. [DOI] [PubMed] [Google Scholar]
- 16.Oscarsson J, Gardmo C, Eden S, Mode A. Pulsatile growth hormone secretion decreases S-adenosylmethionine synthetase in rat liver. American journal of physiology Endocrinology and metabolism. 2001;280(2):E280–6. doi: 10.1152/ajpendo.2001.280.2.E280. [DOI] [PubMed] [Google Scholar]