Abstract
Background:
Allergic rhinitis (AR) is a common disorder. The diagnosis is based on the concordance between allergy sensitization and history. Serum allergen specific immunoglobulin E (sIgE) assessment allows characterization of the relevant sensitizing allergens. Presently, Allergic Rhinitis and its Impact on Asthma (ARIA) classification subdivides AR based on symptoms severity and duration. However, the relationship between sIgE levels and symptom severity is still a matter of debate.
Objective:
Therefore, this study aimed at relating sIgE levels with symptom severity assessed by ARIA classification in a group of patients with AR.
Methods:
We enrolled 217 patients with AR (123 women; median age, 39.5 years). The sIgE levels (expressed in kUA/L) to house-dust mite were detected by the fluorescence enzyme immunoassay in peripheral blood samples. The IgE calibrators were traceable to the second international reference preparation 75/502 of human serum IgE from the World Health Organization. Symptom severity was assessed by ARIA classification.
Results:
We found a significant difference in sIgE levels in patients with mild intermittent versus mild persistent symptoms (p < 0.05), mild intermittent versus moderate-to-severe persistent symptoms (p < 0.001), moderate-to-severe intermittent versus moderate-to-severe persistent symptoms (p < 0.01), and mild persistent versus moderate-to-severe persistent symptoms (p < 0.05).
Conclusion:
Analysis of these findings indicated that the sIgE level to house-dust mite might be a reliable biomarker for symptom severity in patients with AR. This outcome might be clinically relevant, particularly in candidates for immunotherapy.
Keywords: Allergic rhinitis, serum, allergen-specific IgE, ARIA, biomarker
Allergic rhinitis (AR), an inflammatory disease of the nasal membrane, is characterized by symptoms such as sneezing, rhinorrhea, nasal congestion, and nasal itching. AR is often associated with eye symptoms, such as tearing, redness, and itching, and is caused by sensitization to one or more aeroallergens. It is a common disorder worldwide; it may affect up to 40% of the general population. In Italy, its prevalence has steadily increased over the past decades in almost all age classes,1 and currently is estimated ∼25%.2 The diagnosis of AR is based on the demonstration of the production of allergen specific IgE (sIgE) and on the concordance between allergy testing and history, such as the symptom occurrence after inhalation of the sensitizing allergen.
AR was conventionally classified into seasonal AR and perennial AR based on the duration of exposure and symptoms.3 The common allergens for perennial AR include indoor allergens, such as house-dust mite, molds, and animal dander, whereas those allergens for seasonal AR are usually outdoor allergens, such as tree pollen, grass pollen, weed pollen, and molds.4 Some patients sensitized to seasonal allergens have symptoms throughout the year, and some patients sensitized to perennial allergens have symptoms during specific seasons. In addition, many patients are sensitized to both perennial allergens and seasonal allergens simultaneously. The conventional classification has some limitations from a therapeutic standpoint due to its poor association with clinical symptoms. In 2001, the World Health Organization proposed a new Allergic Rhinitis and its Impact on Asthma (ARIA) classification, which classifies AR according to severity and symptom duration.5
Skin-prick test (SPT) and sIgE measurements are the most common methods used to diagnose allergy. Both techniques are widely accepted diagnostic tools. Several researchers investigated the concordance between the level of sIgE and SPT.6–11 SPTs have been used for decades to prove or exclude sensitization to allergens. Also, sIgE assessment is popular and, particularly in patients who are polysensitized, allows defining the relevance of sensitizing allergens more appropriately than SPT in choosing the allergen extract for allergen immunotherapy.12 Previously, two studies reported that serum IgE levels were related to symptom severity in children with seasonal and perennial AR.7,8 Therefore, this study aimed to relate the serum sIgE levels with symptom severity assessed by ARIA criteria in a group of adults with AR.
METHODS
Two-hundred-and-seventeen patients (123 women, 94 men; median age, 39.5 years) were enrolled in the study. Inclusion criteria were (1) documented AR diagnosis, and (2) sensitization to house-dust mite to exclude significant seasonal interference on study evaluation. Blood sampling for assessing sIgE levels was performed in all the subjects. The review board of the Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) San Matteo approved the procedure, and all the patients gave their written informed consent.
Serum levels of sIgE to house-dust mite were detected by the fluorescence enzyme immunoassay (ImmunoCAP; Thermo Fisher Scientific, Milan, Italy) in peripheral blood samples of the patients. The serum was separated from all the blood samples within 4 hours and stored at −20°C until allergen sIgE was evaluated against allergens. The allergen of interest, covalently coupled to ImmunoCAP, reacted with the sIgE in the patient sample. After washing away non-sIgE, enzyme-labeled antibodies against IgE were added to form a complex. After incubation, unbound enzyme–anti-IgE was washed away and the bound complex was then incubated with a developing agent. After stopping the reaction, the fluorescence of the eluate was measured. The higher the response was valued, the more sIgE was present in the specimen. To evaluate the test results, the response for the patient samples was transformed to concentrations with the use of a calibration curve. Quantitative sIgE concentrations were expressed in kU/L according to the traceable calibration to the second international reference preparation 75/502 of human serum IgE from the World Health Organization; sIgE levels were considered positive at >0.35 kU/L. In the present study, the highest sIgE level was considered in each polysensitized patient.
According to the ARIA classification, AR was subdivided based on the duration and/or chronicity of symptoms (intermittent for symptoms <4 days/wk or <4 wk/y or persistent for symptoms >4 days/wk and >4 wk/y), and grading the symptom severity (mild when symptoms do not impair sleep, daily activities, and work and/or school performance; or moderate to severe when symptoms impair sleep, daily activities, and work and/or school performance).5
Statistical Analysis
Statistical analysis was performed by using the statistical software package Medcalc 9 (Frank Schoonjans, Oostende, Belgium). Medians and percentiles (25th and 75th) (interquartile range [IQR]) were used as descriptive statistics. The nonparametric Wilcoxon test was used to compare the samples. The nonparametric Kruskal-Wallis rank test was performed to evaluate the analysis of variance between the patient groups. A p value of <0.05 was considered statistically significant.
RESULTS
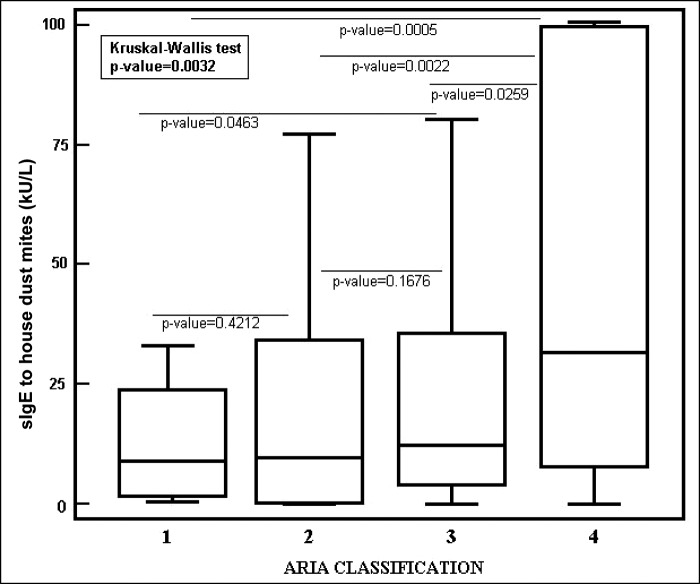
Twenty-five patients had mild intermittent symptoms, 41 had moderate-to-severe intermittent symptoms, 61 had mild persistent symptoms, and 90 moderate-to-severe symptoms. The sIgE levels to aeroallergens in patients with AR significantly (p = 0.0032, Kruskal-Wallis test) changed with regard to ARIA classification, as shown in Fig. 1. In particular, there was a significant difference of sIgE levels to house-dust mite between patients with mild intermittent symptoms (median, 6.91 kU/L; IQR, 1.46–24.28 kU/L) and those with mild persistent symptoms (median, 14.2 kU/L; IQR, 4.74–36.35 kU/L) (p = 0.0463, Wilcoxon test); mild intermittent symptoms and moderate-to-severe persistent symptoms (median, 30.7 kU/L; IQR, 6.69–101 kU/L) (p = 0.0005, Wilcoxon test); between patients with moderate-to-severe intermittent symptoms (median, 11.09 kU/L; IQR, 0.53–37.25 kU/L) and with moderate-to-severe persistent symptoms (p = 0.0022, Wilcoxon test); and patients with mild persistent symptoms and moderate-to-severe persistent symptoms (p = 0.0259, Wilcoxon test).
Figure 1.
The specific immunoglobulin E (sIgE) values (kU/L) to house-dust mite distribution in patients with allergic rhinitis (AR) and with allergy for aeroallergens cause of allergy, evaluated by using the Allergic Rhinitis and its Impact on Asthma (ARIA) classification. Group 1, mild intermittent symptoms; group 2, moderate-to-severe intermittent symptoms; group 3, mild persistent symptoms; and group 4, moderate-to-severe persistent symptoms. Values are represented as medians (black line), quartiles (25th and 75th percentiles, white box), and p values between the groups.
DISCUSSION
The main finding of our analysis was that the presence of an elevated level of sIgE was associated with the severity of AR. The impact of sIgE levels on symptom severity in patients with allergic airway disorders is still a matter of debate. Two pediatric studies were recently performed to directly address this topic. The first study was conducted on 186 children (ages, 2–12 years): 160 with perennial AR and 26 with non-AR as controls.12 On multiple linear regression analysis, the sIgE value was the independent predictor for symptom severity. Therefore, the researchers concluded that serum allergen sIgE may be considered a systemic marker for allergic inflammation and well correlated with the severity of nasal symptoms in children with perennial AR. The second study evaluated 107 children and adolescents with AR due to birch and grass pollen allergy.13 The researchers demonstrated that sIgE levels were associated with symptom severity during the pollen season. More recently, two studies investigated this topic in adult patients with AR.14,15 Both studies demonstrated that the sIgE levels were associated with more-severe allergic symptoms. However, both studies considered the old AR classification. To give a response to the question whether serum sIgE may be related to symptom severity graded on the ARIA criteria, we performed this real-life study.
Our findings were not surprising because patients with moderate and severe persistent asthma shared the presence of elevated serum IgE levels. Previous evidence showed that high levels of IgE are associated with an increased prevalence of asthma, an increased airway hyperresponsiveness,16–19 and an accelerated decrease in lung function.20,21 Our results may be clinically relevant in deciding whether patients are candidates for immunotherapy because high sIgE levels might identify patients with the best indication for such treatment. This would need to be evaluated with a study of the relationship between the sIgE level and symptom improvement with allergen immunotherapy. Moreover, the finding that an elevated level of sIgE was associated with severe symptoms in patients with persistent AR raised the possibility that sIgE could also be considered a prognostic factor of AR severity. This relationship should be investigated in a large longitudinal population study.
In the present study, some limitations should be acknowledged. There may be factors that influence the magnitude of the sIgE value, such as the number and/or density of IgE epitopes on a particular allergen, the affinity and/or avidity of IgE antibodies or the presence of sIgG antibodies. Furthermore, we could not exclude that SPT or a combination of SPT and sIgE may also be reliable biomarkers for symptom severity. In addition, it was discussed that a big trial on allergen immunotherapy included screening patients with positive SPT results but negative sIgE results,22,23 which resulted in a dilution of the observed treatment effect of the investigational product and indicated that, in the setting of a clinical trial, a minimum level of allergen serum sIgE was important in identifying a population sufficiently symptomatic to enable evaluation of treatment benefit. In this regard, it was reported that patients with more-severe symptoms responded better to allergen immunotherapy than patients with milder symptoms.24 Our results were consistent with those of previous studies that demonstrated the high specificity of serum sIgE tests.25,26 In addition, the relevance of biomarkers in AR management is an actual issue, as recently pointed out.27
CONCLUSION
Our findings underlined the importance of sIgE as a feature of rhinitis. Not only because sIgE is a reliable biomarker of symptom severity in patients with persistent AR but also because it might help to assess the prognosis and influence decisions on treatment.
ACKNOWLEDGMENTS
The authors thank Cristina Torre (Pediatric Clinic, IRCCS San Matteo Foundation, Pavia, Italy) and Giorgia Testa (Pediatric Clinic, IRCCS San Matteo Foundation, Pavia, Italy) for outstanding technical support, and Sabrina Nigrisoli (Pediatric Clinic, IRCCS San Matteo Foundation, Pavia, Italy) for data analysis.
Footnotes
No external funding sources reported
The authors have no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Verlato G, Corsico A, Villani S, et al. Is the prevalence of adult asthma and allergic rhinitis still increasing? Results of an Italian study. J Allergy Clin Immunol 111:1232–1238, 2003. [DOI] [PubMed] [Google Scholar]
- 2. Accordini S, Corsico AG, Cerveri I, et al. Diverging trends of chronic bronchitis and smoking habits between 1998 and 2010. Respir Res 14:16, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dykewicz MS, Fineman S. Executive Summary of Joint Task Force Practice Parameters on Diagnosis and Management of Rhinitis. Ann Allergy Asthma Immunol 81:463–468, 1998. [DOI] [PubMed] [Google Scholar]
- 4. Bauchau V, Durham SR. Epidemiological characterization of the intermittent and persistent types of allergic rhinitis. Allergy 60:350–353, 2005. [DOI] [PubMed] [Google Scholar]
- 5. Bousquet J, Khaltaev N, Cruz AA, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 Update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy 63(suppl. 86):8–160, 2008. [DOI] [PubMed] [Google Scholar]
- 6. Brunetto B, Tinghino R, Braschi MC, et al. Characterization and comparison of commercially available extracts for in vivo diagnosis. Allergy 65:184–190, 2010. [DOI] [PubMed] [Google Scholar]
- 7. Sastre J, Landivar ME, Ruiz-Garcia M, et al. How molecular diagnosis can change allergen-specific immunotherapy prescription in a complex pollen area. Allergy 67:709–711, 2012. [DOI] [PubMed] [Google Scholar]
- 8. van der Linden IJ, de Groot MJ, de Jong NC, et al. The diagnostic performance of allergen-molecules in comparison to allergen-extracts. Clin Chem Lab Med 50:129–132, 2011. [DOI] [PubMed] [Google Scholar]
- 9. Patelis A, Gunnbjornsdottir M, Alving K, et al. Allergen extract vs. component sensitization and airway inflammation, responsiveness and new-onset respiratory disease. Clin Exp Allergy 46:730–740, 2016. [DOI] [PubMed] [Google Scholar]
- 10. Silvestri M, Oddera S, Crimi P, Rossi GA. Frequency and specific sensitization to inhalant allergens within nuclear families of children with asthma and/or rhinitis. Ann Allergy Asthma Immunol 79:512–516, 1997. [DOI] [PubMed] [Google Scholar]
- 11. Palao-Ocharan P, Domínguez-Ortega J, Barranco P, et al. Does the profile of sensitization to grass pollen allergens have clinical relevance? J Investig Allergol Clin Immunol 26:188–189, 2016. [DOI] [PubMed] [Google Scholar]
- 12. Ciprandi G, De Amici M, Giunta V, Marseglia GL. Comparison of serum specific IgE and skin prick test in polysensitized patients. Int J Immunopathol Pharmacol 23:1293–1295, 2010. [DOI] [PubMed] [Google Scholar]
- 13. Chen ST, Sun HL, Lu KH, et al. Correlation of immunoglobulin E, eosinophil cationic protein, and eosinophil count with the severity of childhood perennial allergic rhinitis. J Microbiol Immunol Infect 39:212–218, 2006. [PubMed] [Google Scholar]
- 14. Ciprandi G, Comite P, Ferrero F, et al. Serum allergen-specific IgE, allergic rhinitis severity, and age. Rhinology 54:231–238, 2016. [DOI] [PubMed] [Google Scholar]
- 15. Ciprandi G, Comite P, Ferrero F, et al. Birch allergy and oral allergy syndrome: the practical relevance of serum IgE to Bet v 1. Allergy Asthma Proc 37:43–49, 2016. [DOI] [PubMed] [Google Scholar]
- 16. Rolinck-Werninghaus C, Keil T, Kopp M, et al. Specific IgE serum concentration is associated with symptom severity in children with seasonal allergic rhinitis. Allergy 63:1339–1344, 2008. [DOI] [PubMed] [Google Scholar]
- 17. Burrows B, Martinez FD, Halonen M, et al. Association of asthma with serum IgE levels and skin-test reactivity to allergens. N Engl J Med 320:271–277, 1989. [DOI] [PubMed] [Google Scholar]
- 18. Peat JK, Toelle BG, Dermand J, et al. Serum IgE levels, atopy, and asthma in young adults: Results from a longitudinal cohort study. Allergy 51:804–810, 1996. [PubMed] [Google Scholar]
- 19. Sunyer J, Anto JM, Sabria J, et al. Relationship between serum IgE and airway responsiveness in adults with asthma. J Allergy Clin Immunol 95:699–706, 1995. [DOI] [PubMed] [Google Scholar]
- 20. Shadick NA, Sparrow D, O'Connor GT, et al. Relationship of serum IgE concentration to level and rate of decline of pulmonary function: The Normative Aging Study. Thorax 51:787–792, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Siroux V, Oryszczyn MP, Paty E, et al. Relationships of allergic sensitization, total immunoglobulin E and blood eosinophils to asthma severity in children of the EGEA Study. Clin Exp Allergy 33:746–751, 2003. [DOI] [PubMed] [Google Scholar]
- 22. Cox LS, Didier A, Demoly P, et al. Methodological aspects of a meta-analysis of grass pollen allergen sublingual immunotherapy tablets. J Allergy Clin Immunol 138:314–315.e4, 2016. [DOI] [PubMed] [Google Scholar]
- 23. Didier A, Worm M, Horak F, et al. Sustained 3-year efficacy of pre- and coseasonal 5-grass-pollen sublingual immunotherapy tablets in patients with grass pollen-induced rhinoconjunctivitis. J Allergy Clin Immunol 128:559–566, 2011. [DOI] [PubMed] [Google Scholar]
- 24. Howarth P, Malling HJ, Molimard M, Devillier P. Analysis of allergen immunotherapy studies shows increased clinical efficacy in highly symptomatic patients. Allergy 67:321–327, 2012. [DOI] [PubMed] [Google Scholar]
- 25. Wood RA, Phipatanakul W, Hamilton RG, Eggleston PA. A comparison of skin prick tests, intradermal skin tests, and RASTs in the diagnosis of cat allergy. J Allergy Clin Immunol 103:773–779, 1999. [DOI] [PubMed] [Google Scholar]
- 26. Sharma HP, Wood RA, Bravo AR, Matsui EC. A comparison of skin prick tests, intradermal skin tests, and specific IgE in the diagnosis of mouse allergy. J Allergy Clin Immunol 121:933–939, 2008. [DOI] [PubMed] [Google Scholar]
- 27. Tsybikov NN, Egorova EV, Kuznik BI, et al. Biomarker assessment in chronic rhinitis and chronic rhinosinusitis: Endothelin-1, TARC/CCL17, neopterin, and α-defensins. Allergy Asthma Proc 37:35–42, 2016. [DOI] [PubMed] [Google Scholar]