Abstract
Background:
The plasma-derived, pasteurized, nanofiltered C1-inhibitor concentrate (pnfC1-INH) is approved in the United States as an intravenous (IV) on-demand treatment for hereditary angioedema (HAE) attacks, and, in Europe, as on demand and short-term prophylaxis.
Objective:
This analysis evaluated Berinert Patient Registry data regarding IV pnfC1-INH used as long-term prophylaxis (LTP).
Methods:
The international registry (2010–2014) collected prospective and retrospective usage, dosing, and safety data on individuals who used pnfC1-INH for any reason.
Results:
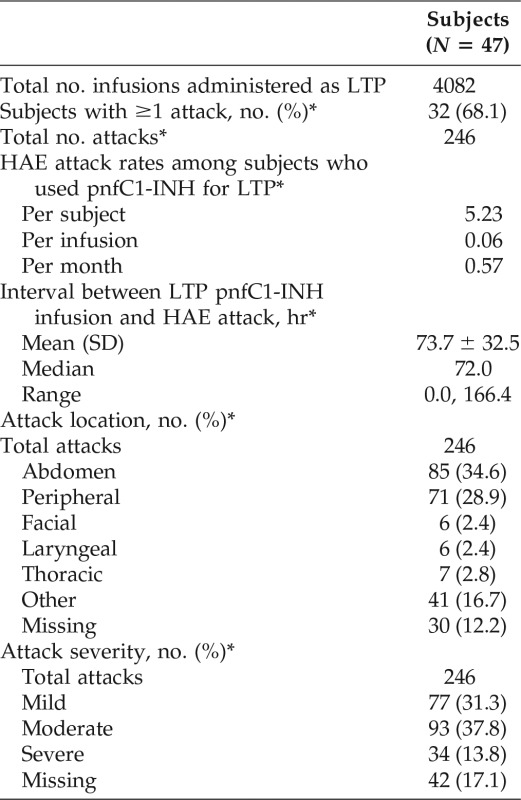
The registry included data on 47 subjects (80.9% female subjects; mean age, 44.8 years), which reflected 4082 infusions categorized as LTP and a total of 430.2 months of LTP administration. The median absolute dose of pnfC1-INH given for LTP was 1000 IU (range, 500–3000 IU), with a median time interval between infusion and a subsequent pnfC1-INH–treated attack of 72.0 hours (range, 0.0–166.4 hours). Fifteen subjects (31.9%) had no pnfC1-INH–treated HAE attacks within 7 days after pnfC1-INH infusion for LTP; 32 subjects (68.1%) experienced 246 attacks, with rates of 0.06 attacks per infusion and 0.57 attacks per month. A total of 81 adverse events were reported in 16 subjects (34.0%) (0.02 events per infusion; 0.19 events per month); only 3 adverse events were considered related to pnfC1-INH (noncardiac chest pain, postinfusion headache, deep vein thrombosis in a subject with an IV port).
Conclusion:
In this international registry, IV pnf-C1-INH given as LTP for HAE was safe and efficacious, with a low rate of attacks that required pnfC1-INH treatment, particularly within the first several days after LTP administration.
Keywords: Berinert, hereditary angioedema, long-term prophylaxis, plasma-derived C1-inhibitor, registry, safety, efficacy, thromboembolic events, self-administration, dosing
Hereditary angioedema (HAE) is a rare autosomal dominant disease,1–3 with several subtypes, depending on the underlying pathology. The most common variants, HAE with deficient C1-inhibitor (C1-INH) (C1-INH-HAE type 1) and HAE with dysfunctional C1-INH (C1-INH-HAE type 2), result from mutations in the gene SERPING1, which encodes the C1-INH protein.4 A third and less common type of HAE, referred to as HAE with normal C1-INH, may also be familial, with some cases associated with mutations in the F12 gene.5,6
Classic symptoms of HAE include episodic, nonpruritic, localized, nonpitting, spontaneous, and painful subcutaneous edema of the skin and mucosal tissues that primarily affects the face, intestinal tract, extremities, genitals, and upper airway.2,3 Laryngeal attacks can be life-threatening,7,8 and mortality rates are estimated at 30% in patients who are not properly diagnosed or treated.2,3 Individuals with HAE, particularly those who experience frequent and severe attacks, report diminished quality of life,9 a significant negative impact on daily activities (e.g., diminished school or work productivity, absenteeism, impaired work performance, lost leisure time, and activities)9,10 as well as higher rates of anxiety and depression compared with the general population.9–11 HAE attacks can lead to missed school or work attendance and patients also report impaired productivity.9,10
Long-term prophylaxis (LTP) can be an option for some patients based on factors such as attack severity and frequency, and quality-of-life issues.1,12,13 Long-term prophylaxis with intravenous (IV) C1-INH concentrate reduces HAE attack frequency14–17 and improves quality of life in patients with C1-INH-HAE.17 The plasma-derived C1-INH concentrate Berinert (pnfC1-INH) (CSL Behring, King of Prussia, PA) has been available in a pasteurized, nanofiltered formulation since 2010, with a pasteurized predecessor version first marketed in 1985 in the European Union, where it is approved for on-demand treatment and short-term prophylaxis in patients of all ages. In the United States, pnfC1-INH has been available since 2009 and is approved for the treatment of abdominal, facial, or laryngeal attacks in patients of all ages. HAE treatment guidelines recommend IV C1-INH as an option for LTP,4,18,19 although Berinert is not specifically approved for this use. Another IV plasma-derived, pasteurized, nanofiltered C1-INH concentrate (Cinryze; Shire, Boston, MA) is approved for routine prophylaxis in the United States20 as well as on-demand treatment and prophylaxis in the European Union for adolescents and adults. The Berinert Patient Registry (hereafter, “Registry”) collected observational data on pnfC1-INH use in the United States and Europe,21,22 and included a sizable number of patients who used pnfC1-INH as LTP. This report describes usage patterns, safety findings, and HAE attack data in these patients.
METHODS
Study Design
This multicenter, observational, patient registry (NCT01108848) collected data between 2010 and 2014 at 30 U.S. and 7 European sites (Germany, 5; Denmark, 1; Switzerland, 1). Data on usage of C1-INH products other than Berinert were not included. The study was conducted in accordance with local regulatory requirements for noninterventional studies, and patient details and registry data were kept confidential. The study protocol and master informed consent form were reviewed and approved by relevant institutional review boards and independent ethics committees. In compliance with International Conference on Harmonization guidelines, all the subjects provided signed informed consent that was institutional review board approved for collection of treatment data.
Data Collection and Analysis
The Registry collected data regarding patient demographics, reason for pnfC1-INH use, pnfC1-INH dose, anatomic location and severity of HAE attacks, and adverse events (AE), including potential thromboembolic events (TEE) and suspected viral transmission. Data on HAE attacks were recorded only for attacks that were treated with pnfC1-INH and all mentions in this article of attacks after pnfC1-INH LTP infusion should be interpreted as pnfC1-INH–treated attacks, even if not stated explicitly. Both retrospective (infusion occurred before Registry enrollment) and prospective (infusion occurred after enrollment) data on the use of pnfC1-INH were obtained.
For each infusion, investigators were requested to indicate a reason for administration (HAE attack treatment, prophylaxis, or other). The specification of prophylaxis as LTP was based on data analysis rules that defined LTP infusions as those that were investigator designated as prophylaxis and were administered 7 or fewer days apart. In addition, pnfC1-INH infusions that were administered for attack treatment in between LTP infusions were also categorized as LTP infusions for the analyses presented in this article. This convention captured consecutively administered infusions such that they comprised a single LTP dosing interval. Data were analyzed on pnfC1-INH–treated attacks that occurred within 7 days after infusions designated as LTP.
Investigators rated the severity of each AE as mild, moderate, or severe. Serious AEs (SAE) were defined as those that resulted in death, a life-threatening reaction that required hospitalization or events that caused persistent or significant physical disability. Signs and symptoms of HAE attacks were not considered AEs for the purpose of this analysis, with the exception of HAE attacks that also met SAE criteria; these were dually reported as both SAEs and HAE attacks. Any suspected TEEs were to be investigated further with a separate questionnaire designed to obtain additional information. Investigators used their clinical judgment and local standards of clinical care to determine if subjects should undergo monitoring for suspected viral transmissions.
RESULTS
Subjects
A total of 343 subjects were enrolled in the Registry, 318 of whom received at least one IV dose of pnfC1-INH. Of these, 47 subjects used pnfC1-INH for LTP. The majority of subjects who used pnfC1-INH for LTP were female and white, with a mean age of 44.8 years (range, 13–79 years) (Table 1).
Table 1.
Baseline characteristics of subjects who used pnfC1-INH for long-term prophylaxis
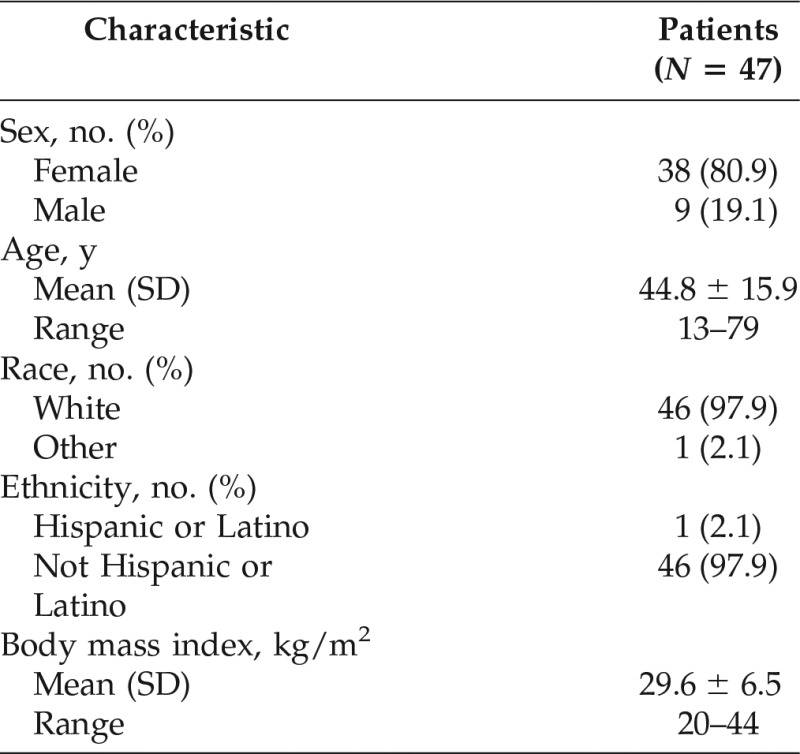
pnfC1-INH = Pasteurized, nanofiltered C1-inhibitor concentrate (Berinert); SD = standard deviation.
pnfC1-INH Dosing and Infusions
A total of 4082 pnfC1-INH infusions were categorized as LTP in 47 subjects. The total duration of pnfC1-INH LTP administration for all the subjects was 430.2 months, with a mean duration of LTP dosing interval per subject of 9.2 months. The median dose of pnfC1-INH given for LTP infusion was 13.77 IU/kg (range, 4.0–32.6 IU/kg), and the median absolute dose per infusion was 1000 IU (range, 500-3000 IU). Forty-two subjects administered pnfC1-INH for LTP at least once outside of a health care setting (95.3% [2807/2944] of infusions for which the setting was known).
Attacks that Occurred During Long-Term Prophylactic Therapy
Among the 47 subjects who used pnfC1-INH for LTP, 15 subjects (31.9%) had no reported HAE attacks (treated with pnfC1-INH) within 7 days after infusion of pnfC1-INH for LTP. The remaining 32 subjects (68.1%) experienced 246 attacks that occurred within 7 days after infusion of pnfC1-INH for LTP (Table 2), for attack rates of 0.06 per infusion and 0.57 per month. The median time interval between LTP infusion and a subsequent attack was 72.0 hours (range, 0.0–166.4 hours). The most common body locations of HAE attacks that occurred within 7 days of an LTP dose of pnfC1-INH (and treated with pnfC1-INH) were abdominal and peripheral; facial and laryngeal attacks were rare (Table 2). The majority of attacks (170/246 [69.1%]) were considered mild or moderate in severity, with 34 (13.8%) classified as severe (intensity not recorded for 42 attacks). The maximum attack intensity by subject was mild in 3 subjects (9.4%), moderate in 13 subjects (40.6%), and severe in 15 subjects (46.9%).
Table 2.
HAE attacks in subjects who used pnfC1-INH for long-term prophylaxis
HAE = Hereditary angioedema; pnfC1-INH = pasteurized, nanofiltered C1-inhibitor concentrate (Berinert); LTP = long-term prophylaxis; SD = standard deviation.
*Attacks treated with pnfC1-INH within the 7-day interval after LTP administration of pnfC1-INH.
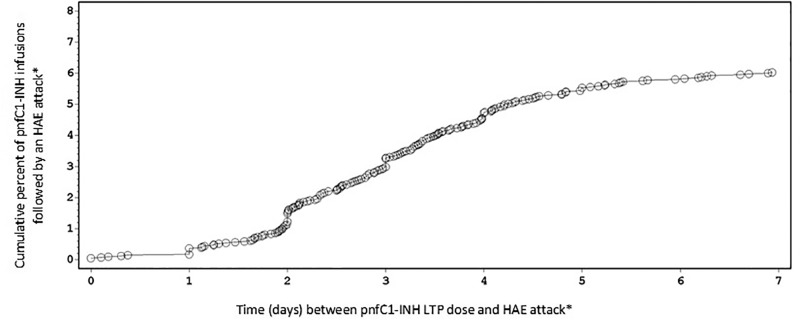
There were 61 pnfC1-INH–treated attacks reported within 2 days after pnfC1-INH infusions that were categorized as LTP, which represented a cumulative percentage of 1% of LTP infusions, followed by an attack (Fig. 1). The cumulative percentage of pnfC1-INH LTP infusions that were followed by a pnfC1-INH–treated HAE attack were 3% at 3 days (n = 133 attacks); 5% at 4 days (n = 193 attacks); and 6% each for the time windows of 5 days (n = 226 attacks), 6 days (n = 237 attacks), and 7 days (n = 246 attacks) after pnfC1-INH LTP infusion (Fig. 1). Analysis of post-LTP infusion attack rates according to weight-based pnfC1-INH dose showed a trend that indicated an inverse dose-dependent relationship (Fig. 2). The highest rate of attacks that occurred within 7 days of infusion (0.08 attacks per infusion) was documented after the subject's most recent pnfC1-INH LTP dose of <15 IU/kg, whereas the lowest attack rate (0.02 attacks per infusion) was observed after preceding pnfC1-INH doses of ≥25 IU/kg. The subjects who received doses of 15 to <20 IU/kg and 20 to <25 IU/kg demonstrated an attack rate of 0.05 attacks per infusion. It should be noted that the highest dose category (≥25 IU/kg) had a small number of subjects (n = 4).
Figure 1.
Cumulative percentage of pnfC1-INH infusions, followed by a hereditary angioedema attack treated with pnfC1-INH that occurred within 7 days of pnfC1-INH infusions designated as long-term prophylaxis. pnfC1-INH = pasteurized, nanofiltered C1-inhibitor concentrate (Berinert). Each circle represents one HAE attack. *HAE attacks treated with pnfC1-INH.
Figure 2.
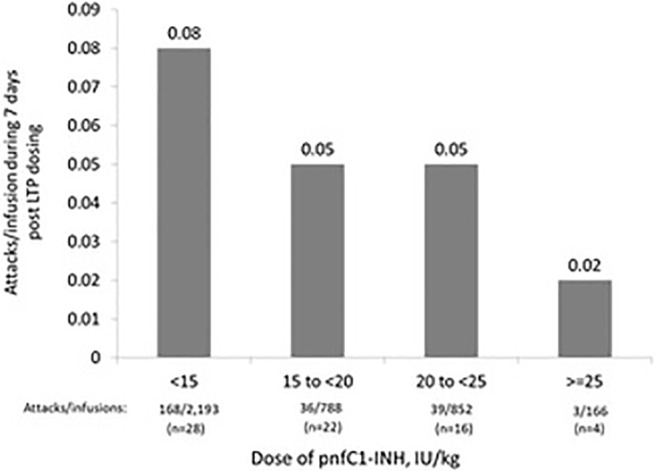
Rate of hereditary angioedema (HAE) attacks treated with pnfC1-INH within 7 days after pnfC1-INH infusions designated as long-term prophylaxis (LTP), categorized by weight-based dose. pnfC1-INH = pasteurized, nanofiltered C1-inhibitor concentrate (Berinert).
Safety of Long-Term Prophylactic Therapy
A total of 81 AEs were reported in 16 subjects (34.0%) who received at least one prospective infusion of pnfC1-INH for LTP. The majority of AEs were mild (n = 21) or moderate (n = 57) in severity. The rates of AEs associated with pnfC1-INH use for LTP were 0.02 per LTP infusion, 1.72 per subject, and 0.19 per subject per month. There was no apparent association between pnfC1-INH dose and the frequency or severity of AEs. The majority of AEs (n = 78 [96.3%]) were not considered to be related to pnfC1-INH. Three AEs were considered to be related to pnfC1-INH: two nonserious (noncardiac chest pain, postinfusion headache) and one serious (deep vein thrombosis).
The one SAE considered related to pnfC1-INH used for LTP was a case of upper-extremity deep vein thrombosis, which also affected the chest, in a 36-year-old woman with HAE and with normal C1-INH who had a subclavian venous access port. The subject's most recent pnfC1-INH doses before the deep vein thrombosis event were 11 days before (500 IU), 7 days before (1000 IU), 3 days before (two doses of 500 IU), and the day of the event (500 IU); she also received a 500 IU dose after the event, on the same day, for treatment of an attack. The port was removed, and the event resolved without sequelae. The subject recovered with no further medical complications and discontinued participation in the Registry. There were no other discontinuations secondary to AEs (regardless of seriousness). The other three SAEs (severe HAE attack, urinary tract infection, and gastrointestinal hemorrhage) reported for Registry subjects who used pnfC1-INH for LTP were not considered related to pnfC1-INH. No AEs were reported that were suggestive of new infection with blood-borne viruses, including human immunodeficiency virus, hepatitis B virus, hepatitis C virus, or parvovirus B19.
DISCUSSION
Berinert (pnfC1-INH and its pasteurized predecessor formulation) has been used for HAE treatment since 1985 in the European Union. The first description of the use of this C1-INH concentrate for LTP was a case report published in 1989.23 Since then, three uncontrolled studies16,24,25 and numerous case reports and/or case series3,26–32 described the clinical use of IV Berinert or its predecessor formulation for LTP, including during pregnancy.3,24,25,27,30 The subgroup of patients in the international patient registry referenced herein has contributed, to date, the largest data set for analysis of pnfC1-INH (Berinert) used for LTP. Although Berinert has not been approved for this indication, all recent treatment guidelines recommend plasma-derived C1-INH as an option for LTP,4,12,13,18,19,33 and the efficacy of another marketed plasma-derived, nanofiltered C1-INH product (Cinryze) for LTP has been confirmed in two placebo-controlled trials,15,34 with further support from several uncontrolled trials.14,15,35
The Registry provided data on 4082 pnfC1-INH infusions characterized as LTP. Approximately one-third of LTP users in the Registry had no HAE attacks reported that required on-demand treatment with pnfC1-INH. It is possible that there were mild attacks that did not require treatment or attacks treated with other interventions that were not captured by the Registry. Within 7 days after LTP administration of pnfC1-INH, HAE attacks that required treatment with pnfC1-INH were reported at rates of 0.57 attacks per month and 0.06 attacks per LTP infusion. Within the first 72 hours after LTP administration of pnfC1-INH, the attack rate was 0.03 per infusion. These results were consistent with those reported with nanofiltered C1-INH (Cinryze). An open-label study that involved 146 patients with HAE who used Cinryze for LTP (fixed dose of 1000 units every 3–7 days) reported a mean breakthrough attack rate of 0.47 attacks per month.34 The percentage of patients with no attacks in the Cinryze study was 34.9%, similar to that seen in the current study (31.9%).
Analysis of the Registry data implied that prophylaxis efficacy was greatest within the first 72 hours after pnfC1-INH administration and an LTP dosing strategy of every 3 to 4 days was appropriate and logical. Analysis of the data also indicated a trend toward greater efficacy of LTP with higher weight-based doses of pnfC1-INH. In a recent study that involved 20 patients with HAE inadequately controlled on a fixed, regular dose of Cinryze 1000 units for LTP, dose escalation up to 2500 units was necessary in 12 of them.14 These findings reinforce the need for individualization of LTP regimens. The Registry data reflected a wide range of pnfC1-INH dosing for LTP, with absolute doses that ranged from 500 to 3500 IU per infusion.
The safety of pnfC1-INH used as LTP in patients with HAE is of particular interest, given the regular, ongoing frequency of infusions, typically one to two times per week, over long periods of time. Thus, the cumulative exposure to pnfC1-INH is typically greater compared with less frequently administered acute HAE therapy. Analysis of the Registry data supported the overall safety of pnfC1-INH when used for LTP, with a very low rate of AEs per LTP infusion (0.2), and no evidence of a dose-response relationship for AEs. Although there have been rare postmarketing reports of systemic allergic reactions and/or anaphylaxis with the use of pnfC1-INH in Europe,36 there were no such reports in this large international registry nor in other clinical trials with pnfC1-INH that indicated that the risk of hypersensitivity seemed to be extremely small.
The observed risk of TEE was low, with one TEE reported among subjects who used pnfC1-INH for LTP over a duration of 430 months for all the subjects and which occurred in a patient with preexisting risk factors for TEE. Similarly, a worldwide survey of HAE-treating physicians, which reflects experience with 856 patients with HAE, revealed a minimal risk of TEE; three of five reported TEEs were associated with the use of indwelling catheters.37 It should be noted that the official product labeling for both Berinert and Cinryze includes a precaution to closely monitor patients with known risk factors for TEEs.
One clear limitation of the Registry data with regard to assessing LTP efficacy is the lack of a control group or comparative historic attack frequency. In addition, because the Registry was designed to gather data on pnfC1-INH use, breakthrough attacks were only captured if they were treated with pnfC1-INH; attacks that went untreated or may have been managed with other on-demand HAE medication were not recorded. However, it is likely that most patients who used pnfC1-INH for prophylaxis would also use it to treat breakthrough attacks. At minimum, analysis of the data provides strong evidence that the incidence of HAE attacks of a severity that warranted pnfC1-INH treatment was very low for several days after LTP infusion and increased gradually over the 7-day postinfusion period. Further, the attack patterns found in the Registry data mimic those reported in other reports.
Investigators and subjects who participated in the Registry adhered to routine practices regarding pnfC1-INH use and dosing. Therefore, the use of IV pnfC1-INH varied according to local standards of care in different countries and between individual prescribers. Although this can be considered a limitation in some regards, it also adds value to the data with regard to understanding usage patterns and outcomes in a real-world setting. Further, although Berinert is not indicated for prophylaxis, “prophylaxis” was offered as one reason for pnfC1-INH use that the investigator could choose to report because such use is consistent with HAE treatment guidelines. However, specification of short-term prophylaxis or LTP was not offered. Therefore, a designation of prophylaxis as LTP was determined according to data analysis rules as described in the Methods section. Also, given the nature of the Registry and its focus on collecting Berinert usage and safety data, concomitant medication data were often incomplete; thus it was possible that some subjects were using other prophylactic medications. Another potential limitation of a registry-based data set was the possibility of self-selection bias, given that participation was voluntary. Therefore, the findings may not be entirely reflective of the HAE population in general. Yet, this study, of 318 subjects with HAE and >4000 pnfC1-INH infusions given for LTP, represents one of the largest data sets for this indication to date. Also, although the Registry population was diverse with respect to geography, age, and sex, it was a racially homogenous group, with only one nonwhite subject.
CONCLUSION
The results of this large international patient registry indicated that IV pnfC1-INH was being used for LTP of HAE attacks in both the United States and Europe with good safety and no observable dose-response for AE occurrence. Such use was associated with a low 7-day post-LTP infusion attack rate. A possible dose-response phenomenon was evident for weight-based dosing regimens, which indicated that higher doses provide better protection. Analysis of these data support a role for the use of IV pnfC1-INH in patients with HAE who are considered candidates for LTP.
ACKNOWLEDGMENTS
Writing and editorial assistance was provided by Carole Alison Chrvala, Ph.D., and Sandra Westra, Pharm.D., Churchill Communications (Maplewood, NJ). This assistance was funded by CSL Behring (Marburg, Germany). The authors retained full control over manuscript content.
Footnotes
This study was funded by CSL Behring LLC
T. Craig has received institutional grants, consulting grants (for participation as a reviewer), and lecture fees from CSL Behring; he has served as a board member for the Hereditary Angioedema Association (HAE-A); he has received consultancy fees from Biocryst; he is employed at Penn State. A. Vegh has received consultancy fees from CSL Behring. J. W. Baker has received grants from Amgen, AstraZeneca, BioCryst, Circassia Limited, CSL Behring, Dyax Corp, Genentech, Inc, GlaxoSmithKline, Maruho Co, Ltd, Mylan Technologies Inc, Novartis Pharmaceuticals Corp, Pearl Therapeutics, Inc, Pfizer Inc, Pharming, Regeneron, Sanofi Aventis, Shire, Teva Pharmaceuticals USA, Tigercat Pharma, Inc, and ViroPharma. J. A. Bernstein has received grants, consulting fees, and travel support from Shire and CSL Behring; he has received grants from Dyax; he has served as a board member on the HAEA medical advisory board; he has served as a paid consultant for GLG. P. Busse has received grants and has served as a paid consultant for CSL Behring; she has served as a paid consultant for and received grants from Viropharma; she has served as a paid consultant for Dyax. M. Magerl has served as a board member and consultant for, and has received payment for expert testimony, manuscript preparation, and speaking from Shire and Viropharma; he has served as a consultant for, provided expert testimony to, and has received payment for speaking and manuscript preparation from CSL Behring; he has received payment for expert testimony and speaking from Sobi. I. Martinez-Saguer has received speaker/consultancy fees and institutional research grant money from CSL Behring, Jerini AG/Shire, Sobi, BioCrystm and ViroPharma. M. A. Riedl has received grants and consultancy fees from Biocryst, CSL Behring, Shire, and Dyax; he has received grants from Pharming and Amgen; he has served as a consultant for Baxalta, Salix, and Arrowhead. W. Lumry has received grants, consulting fees, and travel support fees from Shire, CSL Behring, Dyax, and BioCryst; he has received fees for participation in review from BioCryst; he has served as a board member for the HAE-A; he has received consultancy fees, speaking fees, and grants from Genetech/Roche; he has received consultancy and speaking fees from Meda; he has received grants and speaking fees from Teva; he has received grants from Perigo, BioProduct Laboratory, Mylan, Circassia, and Optinose. D. Williams-Herman was an employee of CSL Behring at time of study conduct. J. Edelman, H. Feuersenger, and M. Rojavin are employees at CSL Behring. R. Machnig is an employee and stockholder at CSL Behring. R. Shapiro has no conflicts of interest to declare pertaining to this article
Presented at the 2015 annual meeting of the American Academy of Allergy Asthma and Immunology, Houston, Texas, February 20–24, 2015
REFERENCES
- 1. Bhardwaj N, Craig TJ. Treatment of hereditary angioedema: A review (CME). Transfusion 54:2989–2996; quiz 2988, 2014. [DOI] [PubMed] [Google Scholar]
- 2. Constantino G, Casazza G, Bossi I, et al. Long-term prophylaxis in hereditary angio-oedema: A systematic review. BMJ Open 2:pii: e000524, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gower R, Aygören-Pürsün E, Davis-Lorton M, et al. Hereditary angioedema caused by C1-esterase inhibitor deficiency: A literature-based analysis and clinical commentary on prophylaxis treatment strategies. World Allergy Organ J 4(suppl.):S9–S21, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cicardi M, Aberer W, Banerji A, et al. Classification, diagnosis, and approach to treatment for angioedema: Consensus report from the Hereditary Angioedema International Working Group. Allergy 69:602–616, 2014. [DOI] [PubMed] [Google Scholar]
- 5. Bork K, Barnstedt SE, Koch P, Traupe H. Hereditary angioedema with normal C1-inhibitor activity in women. Lancet 356:213–217, 2000. [DOI] [PubMed] [Google Scholar]
- 6. Zuraw BL, Bork K, Binkley KE, et al. Hereditary angioedema with normal C1 inhibitor function: Consensus of an international panel. Allergy Asthma Proc 33(suppl. 1):S145–S156, 2012. [DOI] [PubMed] [Google Scholar]
- 7. Xu YY, Zhi YX, Liu RL, et al. Upper airway edema in 43 patients with hereditary angioedema. Ann Allergy Asthma Immunol 112:539–544.e1, 2014. [DOI] [PubMed] [Google Scholar]
- 8. Bork K, Siedlecki K, Bosch S, et al. Asphyxiation by laryngeal edema in patients with hereditary angioedema. Mayo Clin Proc 75:349–354, 2000. [DOI] [PubMed] [Google Scholar]
- 9. Lumry WR, Castaldo AJ, Vernon MK, et al. The humanistic burden of hereditary angioedema: Impact on health-related quality of life, productivity, and depression. Allergy Asthma Proc 31:407–414, 2010. [DOI] [PubMed] [Google Scholar]
- 10. Aygören-Pürsün E, Bygum A, Beusterien K, et al. Socioeconomic burden of hereditary angioedema: Results from the hereditary angioedema burden of illness study in Europe. Orphanet J Rare Dis 9:99, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fouche AS, Saunders EF, Craig T. Depression and anxiety in patients with hereditary angioedema. Ann Allergy Asthma Immunol 112:371–375, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Craig T, Aygören-Pürsün E, Bork K, et al. WAO Guideline for the Management of Hereditary Angioedema. World Allergy Organ J 5:182–199, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lang DM, Aberer W, Bernstein JA, et al. International consensus on hereditary and acquired angioedema. Ann Allergy Asthma Immunol 109:395–402, 2012. [DOI] [PubMed] [Google Scholar]
- 14. Bernstein JA, Manning ME, Li H, et al. Escalating doses of C1 esterase inhibitor (CINRYZE) for prophylaxis in patients with hereditary angioedema. J Allergy Clin Immunol Pract 2:77–84, 2014. [DOI] [PubMed] [Google Scholar]
- 15. Zuraw BL, Busse PJ, White M, et al. Nanofiltered C1 inhibitor concentrate for treatment of hereditary angioedema. N Engl J Med 363:513–522, 2010. [DOI] [PubMed] [Google Scholar]
- 16. Bork K, Hardt J. Hereditary angioedema: Long-term treatment with one or more injections of C1 inhibitor concentrate per week. Int Arch Allergy Immunol 154:81–88, 2011. [DOI] [PubMed] [Google Scholar]
- 17. Greve J, Hahn J, Nordmann M, et al. Nanofiltered C1-esterase-inhibitor in the prophylactic treatment of bradykinin-mediated angioedema. Transfusion 56:1022–1029, 2016. [DOI] [PubMed] [Google Scholar]
- 18. Zuraw BL, Banerji A, Bernstein JA, et al. US Hereditary Angioedema Association Medical Advisory Board 2013 recommendations for the management of hereditary angioedema due to C1 inhibitor deficiency. J Allergy Clin Immunol Pract 1:458–467, 2013. [DOI] [PubMed] [Google Scholar]
- 19. Betschel S, Badiou J, Binkley K, et al. Canadian hereditary angioedema guideline. Allergy Asthma Clin Immunol 10:50, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cinryze (human C1-esterase inhibitor [human]) [prescribing information]. Exton, PA: ViroPharma Biologics, Inc., 2012. [Google Scholar]
- 21. Riedl MA, Bygum A, Lumry W, et al. Safety and usage patterns of plasma-derived C1 esterase inhibitor concentrate in hereditary angioedema: Findings from the International Berinert® (C1-INH) Patient Registry. J Allergy Clin Immunol Pract 4:963–971, 2016.27286778 [Google Scholar]
- 22. Busse P, Bygum A, Edelman J, et al. Safety of C1-esterase inhibitor in acute and prophylactic therapy of hereditary angioedema: Findings from the ongoing international Berinert patient registry. J Allergy Clin Immunol Pract 3:213–219, 2015. [DOI] [PubMed] [Google Scholar]
- 23. Bork K, Witzke G. Long-term prophylaxis with C1-inhibitor (C1 INH) concentrate in patients with recurrent angioedema caused by hereditary and acquired C1-inhibitor deficiency. J Allergy Clin Immunol 83:677–682, 1989. [DOI] [PubMed] [Google Scholar]
- 24. Farkas H, Jakab L, Temesszentandrási G, et al. Hereditary angioedema: A decade of human C1-inhibitor concentrate therapy. J Allergy Clin Immunol 120:941–947, 2007. [DOI] [PubMed] [Google Scholar]
- 25. Helsing P, Nielsen EW. Hepatocellular focal nodular hyperplasia after danazol treatment for hereditary angio-oedema. Acta Derm Venereol 86:272–273, 2006. [DOI] [PubMed] [Google Scholar]
- 26. Hermans C. Successful management with C1-inhibitor concentrate of hereditary angioedema attacks during two successive pregnancies: A case report. Arch Gynecol Obstet 276:271–276, 2007. [DOI] [PubMed] [Google Scholar]
- 27. Gorman P. Hereditary angioedema and pregnancy: A successful outcome using C1 esterase inhibitor concentrate. Can Fam Physician 54:365–366, 2008. [PMC free article] [PubMed] [Google Scholar]
- 28. Tallroth GA. Long-term prophylaxis of hereditary angioedema with a pasteurized C1 inhibitor concentrate. Int Arch Allergy Immunol 154:356–359, 2011. [DOI] [PubMed] [Google Scholar]
- 29. Farkas H, Csuka D, Tóth F, et al. Successful pregnancy outcome after treatment with C1-inhibitor concentrate in a patient with hereditary angioedema and a history of four miscarriages. Eur J Obstet Gynecol Reprod Biol 165:366–367, 2012. [DOI] [PubMed] [Google Scholar]
- 30. Roldán Sevilla T, González Fernández MA, Roldán Rincón A, et al. The use of C1 esterase inhibitor in long term prophylaxis of recurrent acute hereditary angioedema exacerbated by tamoxifen. Atencion Farmaceutica 15:124–127, 2013. [Google Scholar]
- 31. Pedrosa M, Lobera T, Panizo C, et al. Long-term prophylaxis with C1-inhibitor concentrate in patients with hereditary angioedema. J Investig Allergol Clin Immunol 24:271–273, 2014. [PubMed] [Google Scholar]
- 32. Czaller I, Visy B, Csuka D, et al. The natural history of hereditary angioedema and the impact of treatment with human C1-inhibitor concentrate during pregnancy: A long-term survey. Eur J Obstet Gynecol Reprod Biol 152:44–49, 2010. [DOI] [PubMed] [Google Scholar]
- 33. Waytes AT, Rosen FS, Frank MM. Treatment of hereditary angioedema with a vapor-heated C1 inhibitor concentrate. N Engl J Med 334:1630–1634, 1996. [DOI] [PubMed] [Google Scholar]
- 34. Zuraw BL, Kalfus I. Safety and efficacy of prophylactic nanofiltered C1-inhibitor in hereditary angioedema. Am J Med 125:938.e1–7, 2012. [DOI] [PubMed] [Google Scholar]
- 35. Berinert (C1-esterase inhibitor [human]) [package insert]. Marburg, Germany: CSL Behring, GmbH, 2015. [Google Scholar]
- 36. Kalaria S, Craig T. Assessment of hereditary angioedema risk. Allergy Asthma Proc 34:519–522, 2013. [DOI] [PubMed] [Google Scholar]
- 37. Longhurst HJ, Tarzi MD, Ashworth F. C1 inhibitor deficiency: 2014 United Kingdom consensus document. Clin Exp Immunol 180:475–483, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]