Abstract
Introduction:
The goal of this project was to evaluate the impact of immunoglobulin E (IgE) levels on outcomes in patients with chronic rhinosinusitis (CRS) who received maximal medical therapy (MMT).
Study Design:
Prospective cohort study.
Methods:
Thirty-eight patients who underwent MMT for CRS were assigned to three different cohorts based on their IgE levels: low IgE (<25 IU), moderate (>25 to <149 IU), and high (≥150 IU). The primary outcome evaluated was MMT failure with a surgical recommendation within each IgE cohort. Secondary outcomes included changes in pre- and post-MMT scores for the Rhinosinusitis Disability Index, Chronic Sinusitis Survey, and computed tomography–based Lund-Mackay evaluation. The cohorts were substratified based on the presence of nasal polyps and nasal allergies.
Results:
No significant difference was found when MMT failure was compared between the cohorts in terms of quality of life. When substratified based on the presence of nasal polyps and nasal allergies, there was no significant difference between the cohorts. In the high-IgE cohort, all patients regardless of presence of nasal polyps and nasal allergic disease, frequently failed MMT and were recommended for surgery.
Conclusions:
Overall, IgE levels did not seem to have a significant effect on the quality of life or outcomes of MMT in the patients with CRS. However, the presence of nasal allergies regardless of IgE levels seemed to result in more frequent recommendations for surgery after MMT. In the patients with higher-IgE levels (≥150 IU), MMT seemed to fail at high rates with or without the presence of polyps or allergic disease.
Keywords: Chronic rhinosinusitis, IgE levels, maximal medical therapy
The significant debilitating physical symptoms and disruption of quality of life (QoL) associated with chronic rhinosinusitis (CRS) have been reported.1 The etiology of this entity is complex and multifactorial, including infection, host systemic disorders, anatomic variations, and other causes in which atopy is included.2 Some reports2–4 postulate an association between atopy and CRS, whereas other reports3,6 describe a correlation between atopy and severity of disease. Atopy has been considered a risk factor or a negative prognostic factor in patients with CRS,3 but, a causal relationship has not been elucidated.4 Naclerio et al.,6 in 1997, explained the possible link between atopy and CRS to result from tissue edema and vascular congestion, with resulting sinus drainage obstruction.
Other studies report the association of immunoglobulin E (IgE), eosinophilia, and radio-allergo-sorbent-test (RAST) positive test results in extensive sinus disease.5,7 In addition, the relationship between total IgE levels and the thickness of sinus mucosa on computed tomography (CT) imaging in patients with CRS has also been described.7 Although, there may be a relationship between atopy and CT scores, other studies did not correlate disease severity measured by QoL scores with atopy.4 None of these studies investigated the association of the levels of IgE before or after maximal medical therapy (MMT). The purpose of this study was to test the hypothesis that patients with higher total IgE levels at diagnosis would have worse QoL scores and a poorer response to MMT that would require functional endoscopic sinus surgery (FESS).
METHODS
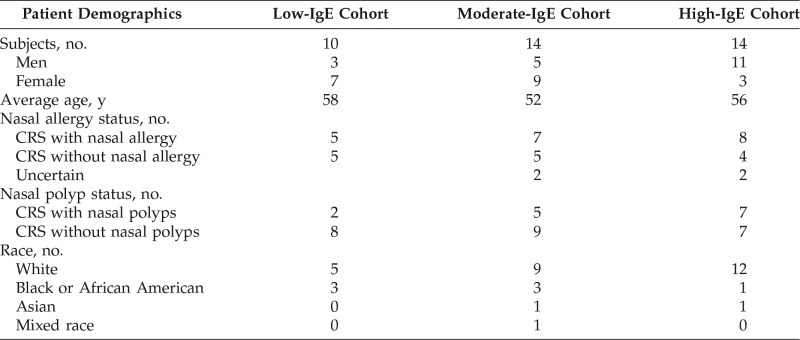
After the approval of the institutional review board of the University of North Carolina, subjects with objective evidence of CRS on either nasal endoscopy or radiographic imaging who presented to the otolaryngology clinics at the University of North Carolina Hospitals were recruited. The inclusion criteria were the following: age >18 years and a diagnosis of CRS8,9 that had not been treated with oral steroids or antibiotics in the previous 2 months (N = 44). Demographic information was recorded (Table 1). At the time of enrollment, all the subjects with objective evidence of CRS underwent CT sinus imaging and IgE titers. Patients who met the following criteria were excluded: allergic fungal sinusitis, primary ciliary dyskinesia, systemic vasculitis, granulomatous disease, history of cocaine abuse, or cancer, and patients who were pregnant or breast-feeding.
Table 1.
Patient demographic information
IgE = Immunoglobulin E; CRS = chronic rhinosinusitis.
The MMT was set as 3 or 6 weeks of antibiotic treatment. Interestingly, there is little literature about the appropriate length of antibiotic therapy for patients with CRS.10 A recent publication by Sreenath et al.11 showed no significant difference in patients' QoL scores or Lund-MacKay (LM) CT scores in those patients who were given between 3 or 6 weeks of antibiotics therapy; for this reason, it was determined that either of the lengths of therapy would be equivalent. The patients with CRS without nasal polyps (CRSsNP) and the patients with CRS with nasal polyps (CRSwNP) received distinct management regimens based on previous established literature8–10,12–14 The patients with CRSwNP were prescribed doxycycline 100 mg orally taken twice a day, and the patients with CRSsNP were prescribed azithromycin 250 mg orally taken once daily. Sinus tissue from patients with CRSwNP and patients with CRSsNP showed different patterns of cells and cytokines, with more eosinophils and interleukin (IL) 5 in the patients with polyps and more hyperplasia and fibrosis in the patients without polyps.15
Doxycycline was chosen for CRSwNP due to its antimicrobial effects as well as it anti-inflammatory effects. Doxycycline is thought to be effective in the treatment of nasal polyposis due to the inhibition of the collagenase activity of metalloproteinases.14,16 Furthermore, doxycycline was reported to lower levels of IL-5 and metalloproteinase-915,17 in nasal secretions, which reduced the damage to nasal polyp tissue and eventually polyp size. Oral steroid tapers, beginning with 40 mg daily over 4 days, followed by 30 mg daily for 4 days, followed by 20 mg daily for 4 days, then 10 mg for 4 days were given as an adjunctive therapy in CRSwNP. Oral systemic therapy was provided based on evidence that oral steroids not only are anti-inflammatory agents but also decrease the number of eosinophils as well as markers of inflammation, such as eosinophilic cationic protein and IL-5, in sinus tissue, levels of which are elevated in patients with CRSwNP.12 Patients diagnosed with CRSsNP were treated with oral azithromycin. Azithromycin was chosen due the anti-inflammatory and antibacterial properties of macrolides and its reported benefit in the management of chronic sinusitis.15,17–19 No oral steroid was added due to the lack of evidence of benefit in CRSsNP.
Patients with purulence found on nasal endoscopy after flexible fiberoptic or rigid examinations underwent culture and subsequent assessment of antibiotic sensitivities. Microorganisms found in culture varied widely. However, the patients received culture-directed antibiotic therapy based on the sensitivity of the bacteria. In addition, all the patients received isotonic saline solution nasal rinses (NeilMed Sinus Rinse; NeilMed Pharmaceuticals, Inc., Santa Rosa, CA) and topical nasal steroid sprays for twice daily use. Patient compliance was evaluated by mid-treatment telephone calls and an exit survey at their follow-up post-MMT visit to verify if they had fully completed the treatment.
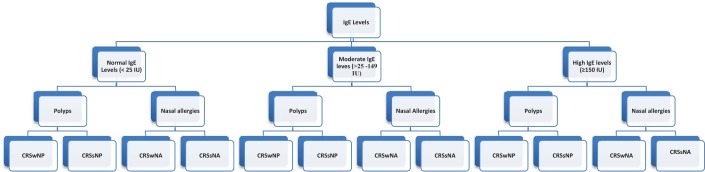
The subjects were assigned to three different cohorts based on the levels of IgE. Patients with levels of IgE of <25 IU were assigned to the normal-IgE cohort, IgE levels of >25 to 149 IU were assigned to the moderate-IgE cohort, and patients with IgE levels of ≥150 IU were assigned to the high-IgE cohort. In addition each cohort was substratified based on the presence of nasal polyps and nasal allergies (Fig. 1). A diagnosis of allergic rhinitis and the asthma status were based on a clinical history and the patient's reported medical history. The patients with total IgE levels of <25 IU have a low chance of having allergic inflammation. However, patients with higher levels have increasing likelihood of allergic rhinitis or asthma, which may serve as a proxy for sinonasal inflammation. According to Chung et al.,20 the positive predictive value of a total IgE level of >150 IU that identifies patients with positive in vitro testing is 88%.
Figure 1.
Division of groups and subgroups. CRSwNP = Chronic rhinosinusitis with nasal polyp; CRSsNP = chronic rhinosinusitis without nasal polyp; CRSwNA = chronic rhinosinusitis with nasal allergy; CRSsNA = chronic rhinosinusitis without nasal allergy.
To assess disease severity in the three cohorts, we used standardized and validated objective and subjective measures. For QoL measures, the patients were administered the Chronic Sinusitis Survey (CSS) and the Rhinosinusitis Disability Index (RSDI)21 at their initial visit and then after MMT was completed. Lower scores on the CSS and higher scores on the RSDI represented greater disease severity. For objective radiologic assessment, we used the LM grading system22,23 to evaluate all sinus CTs obtained at pre- and post-MMT visits. The mean change in LM and RSDI scores were calculated by subtracting the post-MMT scores from the pre-MMT scores. The mean changes in CSS scores were calculated by subtracting the pre-MMT scores from the post-MMT scores. At the post-MMT visit, decisions regarding whether or not MMT was successful were determined based on various factors, including patient symptoms, changes in QoL scores, and radiologic evidence of mucosal inflammation. The decision to proceed with surgery was made by a consensus between the patient and the practitioner in light of the patient's QoL, endoscopic examination and radiologic findings. Patients for surgery was defined as the primary outcome.
As other researchers reported,24,25 CRS not only encompasses physical symptoms but also great disruption in QoL. The major benefit of our study rationale was that it used real-life clinical decision-making practices and not those contrived for study purposes. Paired Student's t-tests were used to compare LM, RSDI, and CSS score differences in the two groups. Statistical significance was set at p = 0.05. The minimal clinically important difference for the RSDI and CSS based on this cohort of patients was determined by distribution-based methods26,27 by using the one-half standard deviation benchmark of the outcomes evaluated (for RSDI, a decrease of ≥11.67; for CSS, an increase of ≥10.45).
RESULTS
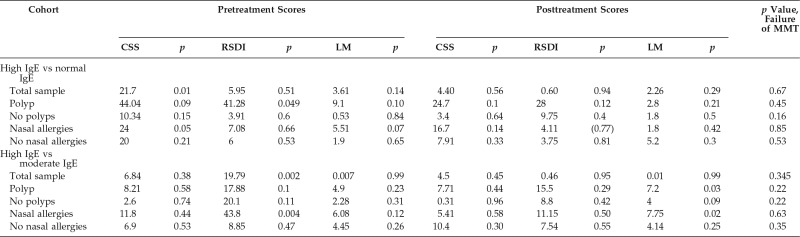
Forty-four patients were enrolled in the study at their initial clinic visit. Six patients were excluded: two were lost to follow-up, two had adverse effects from antibiotic treatment, and two did not have IgE levels obtained. The patients were stratified into three different cohorts based on their total serum IgE levels. Ten patients were included in the low-IgE cohort, 14 patients were in the moderate-IgE cohort, and 14 patients were in the high-IgE cohort. All the patients completed MMT, with 100% clinical follow-up achieved. The levels of IgE ranged from 2 to 764 IU in the total sample; 26% of the patients were in the low-IgE cohort, 36% were in the moderate-IgE cohort, and 36% were in the high-IgE cohort. The primary outcome was defined as a failure of MMT with a recommendation for surgical intervention. In the low-IgE cohort, medical management was considered to have failed in 60% of the subjects, and they were recommended to undergo surgical intervention. In the moderate cohort, MMT failed in 71% of the subjects, and, in the high-IgE cohort, 78% of patients were recommended to undergo surgical intervention after failure of MMT (Table 2). The between-group comparison (low-IgE versus high-IgE and moderate-IgE versus high-IgE cohorts) revealed no significant differences (p > 0.05) (Table 3).
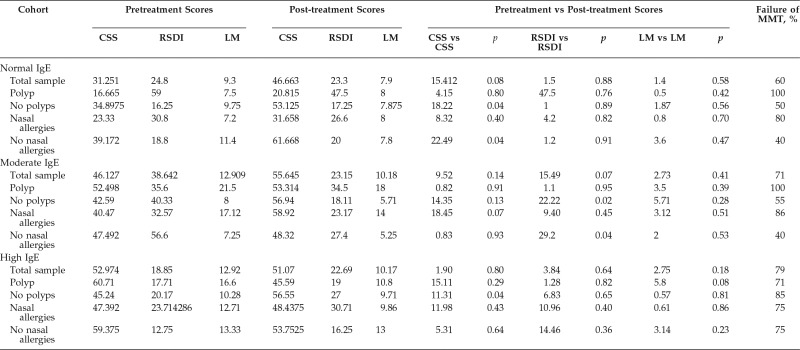
Table 2.
Pre- and posttreatment comparisons of CSS, RSDI, and LM scores in the three cohorts
CSS = Chronic Sinusitis Survey; RSDI = Rhinosinusitis Disability Index; LM = Lund-Mackay; MMT = maximal medical therapy; IgE = immunoglobulin E.
Table 3.
Comparison between the IgE cohorts
IgE = Immunoglobulin E; MMT = maximal medical therapy; CSS = Chronic Sinusitis Survey; RSDI = Rhinosinusitis Disability Index; LM = Lund-Mackay.
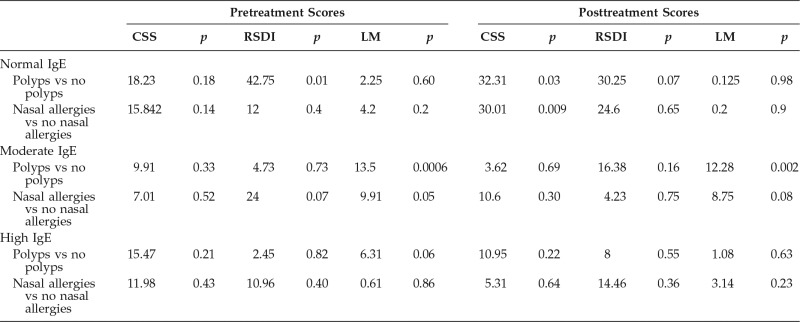
Subjective QoL measurements (i.e., CSS, RSDI scores) and objective disease measurements (i.e., LM scores) were evaluated before and after MMT in the total sample as well as between the polyp and nasal allergies subgroups. The results are shown in Table 2. The nonpolyp (p = 0.04) and no nasal allergies (p = 0.04) subgroups in the normal-IgE cohort had a significant difference before and after MMT in CCS scores. In the moderate-IgE cohort, CRSsNP and CRS without nasal allergy (CRSsNA) subgroups showed a significant difference in the QoL scores after MMT (for CRSsNP, p = 0.02, RSDI; for no CRSsNA, p = 0.049). The high-IgE cohort had a significant difference in CSS scores in patients with CRSsNP after MMT (p = 0.04). Furthermore, in the high-IgE cohort, MMT failed in 71% of the patients with CRSwNP and in 85% of the patients with CRSsNP, and MMT failed in 75% of the patients with CRSsNA and 75% of those with CRSwNA. Comparisons between cohorts (high-IgE versus normal-IgE and high-IgE versus moderate-IgE cohorts) (Table 3) did not show a significant difference in the main outcome. After MMT, the patients with high IgE levels with either nasal polyps or nasal allergies showed a significant difference in the LM scores (p = 0.03 and p = 0.02, respectively) but little difference in the QoL scores.
Comparisons within each IgE cohort were made based on polyp and nasal allergies status (Table 4). In the normal-IgE cohort, a comparison between the CRSwNP and CRSsNP subgroups showed a significant difference in RSDI scores (p = 0.01) before treatment and in the CSS after treatment (p = 0.03). As expected, the RSDI scores before MMT, overall, were higher (worst QoL) in patients with polyps. CSS after MMT in the patients without polyps had higher scores (better QoL) after treatment compared with patients with polyp. Patients with CRSsNA had overall better QoL (CSS) after MMT when compared with patients with CRSwNA (p = 0.009). In the moderate-IgE cohort, the LM scores were higher in the CRSwNP subgroups before and after MMT when compared with the CRSsNP subgroups (p = 0.0006 and p = 0.002). There was no difference in LM scores between patients with CRSsNA and patients with CRSwNA. In the high-IgE cohort, there was no significant difference in any QoL measurement when patients with polyp versus nonpolyp and nasal allergies versus no nasal allergies were compared.
Table 4.
Comparison between polyps or no polyps and nasal allergies or nonnasal allergies in the three cohorts
CSS = Chronic Sinusitis Survey; RSDI = Rhinosinusitis Disability Index; LM = Lund-Mackay; IgE = immunoglobulin E.
DISCUSSION
Analysis of data in a number of studies indicates that atopy can lead to a more extensive and severe CRS disease2,3,7, but none of these studies indicated a causal relationship. Our study evaluated the impact of IgE levels on outcomes after MMT not only as it related to recommendation for FESS but also as it related to changes in QoL. When evaluating the primary outcome, recommendation for FESS, we found that, in the low-IgE cohort, the positive predictive value for FESS recommendation was 60% compared with 71% in the moderate-IgE cohort and 79% in the high-IgE cohort. MMT had failed in the patients with CRSwNP in both the low- and moderate-IgE cohorts (low-IgE cohort, 100%; moderate-IgE cohort, 80%) compared with the patients with CRSsNP (low-IgE cohort, 50%; moderate-IgE cohort, 40%). Conversely, in the high-IgE cohort, the presence of polyps did not influence the main outcome (71% with CRSwNP and 85% CRSsNP were recommended for FESS), which indicated a more-severe disease process regardless of polyp status.
There is recent literature that CRSwNP and CRSsNP are distinct pathologic entities.15 Sinus tissue from patients with CRSwNP and those with CRSsNP had different patterns of cells and cytokines, with more eosinophils and IL-5 in patients with polyp and more hyperplasia and fibrosis in patients without polyps.15 In the present study, in the low- and moderate-IgE cohorts, polyp status resulted in differential outcomes (although not statistically significant) in terms of failure of MMT compared with the high-IgE cohort. It is postulated that this difference in the behavior of the groups may be due to the supraphysiologic IgE levels, which affect cytokine production in the local milieu. However, immunohistochemistry is needed to test this theory. When the cohorts were substratified by nasal allergies, the patients without nasal allergies tended to have improved QoL after treatment compared with those with allergic disease, which indicated that allergic disease likely played a role in symptomatology but did not influence the recommendation for surgery. In the high-IgE cohorts, both CRSwNA and CRSsNA resulted in MMT frequently failing and recommendations for FESS (nasal allergies, 75%; CRSsNA, 75%).
In terms of QoL, when the different cohorts were compared (low-IgE versus high-IgE and moderate-IgE versus high-IgE cohorts), there was only a significant statistical difference in CSS pretreatment scores between the normal-IgE and high-IgE cohorts. It seemed that patients in the low-IgE cohort were more symptomatic before MMT compared with patients in the high-IgE cohorts before treatment. When evaluating each cohort individually, there was no difference between pre- and posttreatment QoL and LM scores in the three cohorts. The subtle changes demonstrated in QoL after treatment in the low-IgE and moderate-IgE cohorts when compared with the high-IgE cohort was mainly the result of improvement in patients with CRSsNP polyps. Patients with CRSsNA also showed improvement in QoL scores in the normal-IgE (p = 0.04, CSS) and the moderate-IgE cohorts (p = 0.04, RSDI) after treatment; conversely, patients with CRSwNA did not show improvement after MMT. Based on these data, it seemed that patients' IgE status had little influence in disease severity as determined by the response to MMT. Within the high-IgE cohort, polyp and nasal allergy status did not influence the outcome after MMT.
Some literature to date indicates an association between atopy and sinonasal symptoms as well as higher CT scores.4,7 However, other studies failed to show a relationship between atopy and severity of the sinus disease.4 Some researchers reported more extensive disease in patients with low-IgE levels compared with patients with allergy. In our study, we found that patients with higher levels of IgE tended to have more polypoid inflammation, but, at high levels of IgE, the presence of polyps became less important in terms of response to MMT.
When nasal allergies were evaluated, the percentage of patients with nasal allergies in each cohort was similar (normal-IgE cohort, 50%; moderate-IgE cohort, 50%), with a slightly higher percentage in the high-IgE cohort (57%). The presence of allergic disease seemed to have played a role in recommending surgery in the low- and moderate-IgE cohorts. The failure rates of MMT were higher in the low- and moderate-IgE cohorts compared with those without allergic disease in those cohorts (Table 2). Again, analysis of these data indicated that the presence of allergic disease may contribute to worse symptomatology and poorer responses to MMT. Those patients in the high-IgE cohort with CRSwNA or with CRSsNA tended to respond similarly poor to MMT.
Researchers of previous studies indicate a relationship between nasal polyps and asthma and/or allergic rhinitis28,29 In the present study, we did not evaluate asthma status, which can be a confounding factor, given the high association of nasal polyps with this entity28,30 Nasal allergies diagnosed by clinical symptoms and clinical history had worst QoL scores when compared with CRSsNA in the low- and moderate-IgE cohort. It was possible that some patients misclassified their allergy status because nasal allergy symptoms may mimic that of CRSwNP. In addition, of the total sample, 79% of the patients with high IgE for whom MMT failed were recommended to undergo FESS. Based on these results, we could postulate that patients with higher counts of IgE had less likelihood of responding to maximal medical treatment and could, therefore, be considered surgical candidates earlier in the treatment algorithm, regardless of polyp or allergy status.
Before generalizing these results, there were some limitations of this study. The most important was the small sample size in the three different cohorts. Several pretrial power calculations were performed; however, the clinical impact and effect size of differing levels of serum IgE among the cohorts was unknown and no comparative studies exist. For a pretrial power calculation, an effect size of 0.5 was used to determine a cohort of 192 patients needed to achieve 80% power.31 However, the standard error of nonbinary outcomes is unknown because such comparative studies do not exist and, therefore, pretrial power calculations are at best an educated guess in such a situation. In addition, the power calculations mentioned were made by using standardize tables31 that greatly underestimate the true sample size needed to achieve appropriate power based on the actual effect size. Thus, we were unable to make a robust a priori calculation of the sample size. Future studies with larger sample sizes would likely provide a more-effective estimate of the effect size because there is no previous literature that evaluated the clinical difference between subjects that could be implemented. Also, immunohistochemical analysis of sinonasal tissue could provide useful data to assist stratifying patients based on the presence of local tissue eosinophils and/or IgE.
CONCLUSION
Overall, based on these data, IgE levels did not seem to have a significant effect on the QoL or outcomes of MMT in those patients with CRS. However, the presence of polyps and nasal allergies, regardless of IgE levels, seemed to result in more frequent recommendations for surgery after MMT. MMT seemed to fail in patients with higher IgE levels (≥150 IU) at high rates despite polyp or allergic status.
Footnotes
Oral presentation at the Triological Society Sections Meeting, January 22–24, 2016, Miami, Florida
No external funding sources reported
The authors have no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Lu-Myers Y, Deal AM, Miller JD, et al. Comparison of socioeconomic demographic factors in patients with chronic rhinosinusitis and allergic fungal rhinosinusitis. Otolaryngol Head Neck Surg 153:137–143, 2015. [DOI] [PubMed] [Google Scholar]
- 2. Tan BK, Schleimer RP, Kern RC. Perspectives on the etiology of chronic rhinosinusitis. Curr Opin Otolaryngol Head Neck Surg 18:21–26, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tan BK, Zirkle W, Chandra RK, et al. Atopic profile of patients failing medical therapy for chronic rhinosinusitis. Int Forum Allergy Rhinol 1:88–94, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Robinson S, Douglas R, Wormald PJ. The relationship between atopy and chronic rhinosinusitis. Am J Rhinol 20:625–628, 2006. [DOI] [PubMed] [Google Scholar]
- 5. Ramadan HH, Fornelli R, Ortiz AO, Rodman S. Correlation of allergy and severity of sinus disease. Am J Rhinol 13:345–347, 1999. [DOI] [PubMed] [Google Scholar]
- 6. Naclerio RM, deTineo ML, Baroody FM. Ragweed allergic rhinitis and the paranasal sinuses. A computed tomographic study. Arch Otolaryngol Head Neck Surg 123:193–196, 1997. [DOI] [PubMed] [Google Scholar]
- 7. Newman LJ, Platts-Mills TA, Phillips CD, et al. Chronic sinusitis. Relationship of computed tomographic findings to allergy, asthma, and eosinophilia. JAMA 271:363–367, 1994. [DOI] [PubMed] [Google Scholar]
- 8. Rosenfeld RM, Andes D, Bhattacharyya N, et al. Clinical practice guideline: Adult sinusitis. Otolaryngol Head Neck Surg 137(suppl.):S1–S31, 2007. [DOI] [PubMed] [Google Scholar]
- 9. Fokkens WJ, Lund VJ, Mullol J, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinol Suppl 23:3 p preceding table of contents, 1–298, 2012. [PubMed] [Google Scholar]
- 10. Dubin MG, Kuhn FA, Melroy CT. Radiographic resolution of chronic rhinosinusitis without polyposis after 6 weeks vs 3 weeks of oral antibiotics. Ann Allergy Asthma Immunol 98:32–35, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Sreenath SB, Taylor RJ, Miller JD, et al. A prospective randomized cohort study evaluating 3 weeks vs 6 weeks of oral antibiotic treatment in the setting of “maximal medical therapy” for chronic rhinosinusitis. Int Forum Allergy Rhinol 5:820–828, 2015. [DOI] [PubMed] [Google Scholar]
- 12. Van Zele T, Gevaert P, Holtappels G, et al. Oral steroids and doxycycline: Two different approaches to treat nasal polyps. J Allergy Clin Immunol 125:1069–1076.e4, 2010. [DOI] [PubMed] [Google Scholar]
- 13. Soler ZM, Oyer SL, Kern RC, et al. Antimicrobials and chronic rhinosinusitis with or without polyposis in adults: An evidenced-based review with recommendations. Int Forum Allergy Rhinol 3:31–47, 2013. [DOI] [PubMed] [Google Scholar]
- 14. Harvey RJ, Wallwork BD, Lund VJ. Anti-inflammatory effects of macrolides: Applications in chronic rhinosinusitis. Immunol Allergy Clin North Am 29:689–703, 2009. [DOI] [PubMed] [Google Scholar]
- 15. Banerji A, Piccirillo JF, Thawley SE, et al. Chronic rhinosinusitis patients with polyps or polypoid mucosa have a greater burden of illness. Am J Rhinol 21:19–26, 2007. [DOI] [PubMed] [Google Scholar]
- 16. Rempe S, Hayden JM, Robbins RA, Hoyt JC. Tetracyclines and pulmonary inflammation. Endocr Metab Immune Disord Drug Targets 7:232–236, 2007. [DOI] [PubMed] [Google Scholar]
- 17. Cervin A, Wallwork B. Macrolide therapy of chronic rhinosinusitis. Rhinology 45:259–267, 2007. [PubMed] [Google Scholar]
- 18. Fan Y, Xu R, Hong H, et al. High and low doses of clarithromycin treatment are associated with different clinical efficacies and immunomodulatory properties in chronic rhinosinusitis. J Laryngol Otol 128:236–241, 2014. [DOI] [PubMed] [Google Scholar]
- 19. Wallwork B, Coman W, Mackay-Sim A, et al. A double-blind, randomized, placebo-controlled trial of macrolide in the treatment of chronic rhinosinusitis. Laryngoscope 116:189–193, 2006. [DOI] [PubMed] [Google Scholar]
- 20. Chung D, Park KT, Yarlagadda B, et al. The significance of serum total immunoglobulin E for in vitro diagnosis of allergic rhinitis. Int Forum Allery Rhinol 2014:4:56–60, 2014. [DOI] [PubMed] [Google Scholar]
- 21. Senior BA, Glaze C, Benninger MS. Use of the Rhinosinusitis Disability Index (RSDI) in rhinologic disease. Am J Rhinol 15:15–20, 2001. [DOI] [PubMed] [Google Scholar]
- 22. Lund VJ, Mackay IS. Staging in rhinosinusitus. Rhinology 31:183–184, 1993. [PubMed] [Google Scholar]
- 23. Hopkins C, Browne JP, Slack R, et al. The Lund-Mackay staging system for chronic rhinosinusitis: How is it used and what does it predict? Otolaryngol Head Neck Surg 137:555–561, 2007. [DOI] [PubMed] [Google Scholar]
- 24. Schlosser RJ, Gage SE, Kohli P, Soler ZM. Burden of illness: A systematic review of depression in chronic rhinosinusitis. Am J Rhinol Allergy 30:250–256, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. DeConde AS, Soler ZM. Chronic rhinosinusitis: Epidemiology and burden of disease. Am J Rhinol Allergy 30:134–139, 2016. [DOI] [PubMed] [Google Scholar]
- 26. Wright A, Hannon J, Hegedus EJ, Kavchak AE. Clinimetrics corner: A closer look at the minimal clinically important difference (MCID). J Man Manip Ther 20:160–166, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bernstein JA, Mauger DT. The minimally clinically important difference (MCID): What difference does it make? J Allergy Clin Immunol Pract 4:689–690, 2016. [DOI] [PubMed] [Google Scholar]
- 28. Settipane GA. Epidemiology of nasal polyps. Allergy Asthma Proc 17:231–236, 1996. [DOI] [PubMed] [Google Scholar]
- 29. Settipane GA, Chafee FH. Nasal polyps in asthma and rhinitis: A review of 6,037 patients. J Allergy Clin Immunol 59:17–21, 1977. [DOI] [PubMed] [Google Scholar]
- 30. Pearlman AN, Chandra RK, Chang D, et al. Relationship between severity of chronic rhinosinusitis and nasal polyposis, asthma, and atopy. Am J Rhinol Allergy 23:145–148, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cohen J. Statistical Power Analysis for the Behavioral Sciences. Lawrence Erlbaw associates publishers, 2nd ed NJ, 1998. [Google Scholar]