Abstract
In the United States, there are in excess of 300,000 operations for diseases of the colon yearly. Minimally invasive colectomy became a reality early in the 21st century with the advent of laparoscopic colectomy. The goal of minimally invasive colectomy is to improve postoperative pain control, decrease length of hospital stay, decrease recovery time, decrease complications, and thereby decrease the cost of colon resections. There are many facets to laparoscopic colectomy, including completely laparoscopic approach versus hand-assisted approach and the medial versus lateral approach. These decisions are often based on the disease process, surgeon preference and comfort with technique, and patient considerations such as weight and prior operations. This article outlines the pros and cons of each of these factors.
Keywords: laparoscopy, laparoscopic colectomy, hand-assisted colectomy, colon resection
Each year in the United States, approximately 320,000 colectomies inclusive of laparoscopic colectomy and open colectomy (OC) are performed for both benign and malignant conditions. This number has remained relatively constant between 2001 and 2011.1 In 2004, the results of the COST trial that compared laparoscopic to open colectomy for colon cancer were published in the New England Journal of Medicine.2 This study showed equivalent recurrence rates (laparoscopic colectomy vs. open colectomy; 16 vs. 18%), overall survival (86 vs. 85%), complications, mortality, readmission frequency, and reoperation rate between laparoscopic and open colectomy. The laparoscopic group had a shorter length of stay (5 vs. 6 days) and less narcotic use. Since that time, the proportion of colectomies performed laparoscopically has continued to increase. In the years immediately preceding the COST trial (2001–2003), 6.2% of all colectomies for benign disease were performed laparoscopically, whereas in the years following (2005–2007) the COST trial, the percent of laparoscopic colectomy for benign disease increased to 11.8%. During the same periods, the biggest change came in the acceptance of laparoscopic colectomy for malignant disease. Prior to the publication of the COST trial, 2.3% of resections for malignant disease were performed laparoscopically, and after publication, this number climbed to 8.9%.3 Overall, however, by 2007, only 10% of all colectomies were performed laparoscopically. Soon thereafter, the numbers started to increase dramatically such that by 2009, 51.3% of all colectomies were performed laparoscopically and this number further climbed to 59.3% by 2012.4
During the decade or so from 2001 to 2012, we saw a stable overall number of colectomies but an increasing number performed laparoscopically. The laparoscopic learning curve has been well documented as requiring more than 50 procedures for a right-sided colectomy and upward of 60 procedures for a left-sided procedure.5 This steep learning curve is complicated by the fact that most general surgeons without specialty training do not perform a significant number of colectomies in any given year. This mathematically could take several years in practice for a general surgeon to attain proficiency in laparoscopic colectomy. These data started to become evident prior to the increased performance of laparoscopic colectomy. For instance, in a 1999 report describing practice patterns of general surgeons from Wallace Ritchie and the American Board of Surgery, it was reported that the average general surgeon (who actually practices general surgery) performs approximately 13 colectomies per year. Moreover, this report showed that the majority of advanced alimentary tract procedures are performed in urban settings by approximately 5 to 10% of the surgeon population.6 In an update to this study in 2011, it was found that complex procedures were more frequently performed by specialty surgeons and that general surgeons, although they occasionally performed complex procedures, performed less of these procedures due to increased specialization and that this trend potentially threatened access to general surgical care.7 Despite the trend that most of these procedures are performed by a small number of surgeons, there are benefits to laparoscopic colectomy and as a result all general surgeons should be able to offer this procedure to the appropriately selected patients. Laparoscopic colectomy has an increased learning curve, and as a result, there are several components to a successful procedure including patient selection, preoperative preparation, and intraoperative technique.
Patient Selection
Successful laparoscopic colectomy begins with selecting the appropriate patient. The most important factors are history of previous abdominal surgery, tumor size (for cases involving malignancy), associated phlegmon or abscess in inflammatory bowel/diverticular disease, and patient body mass index (BMI). Patients who have had previous surgery are still candidates for a laparoscopic procedure, but the nature of the previous operation is critical to success. If the patient has had previous open surgery in the area of concern, adhesions in this area often cause a significant impediment to the proposed laparoscopic colectomy. For example, an open cholecystectomy or open Roux-en-Y gastric bypass can impede the possibility of a laparoscopic right colectomy or a total/subtotal colectomy, respectively, whereas a previous hysterectomy can impede a sigmoid colectomy or a low anterior resection (LAR). On the other hand, the aforementioned upper abdominal surgeries often do not impede laparoscopic LAR/sigmoid colectomy, and laparoscopic pelvic surgery does not generally impede a right colectomy or subtotal colectomy. Patients with large tumors that extend into contiguous structures (bladder/uterus/pelvic sidewall) have a higher rate of conversion from laparoscopic to open colectomy. Additionally, those who have significant phlegmon/abscess/fistulous disease from inflammatory bowel disease (IBD) also have a higher rate of conversion especially in the early learning curve of the surgeon. Despite this, experienced laparoscopic surgeons have shown that even these factors are not insurmountable and that laparoscopic colectomy is possible even with significant IBD and diverticular disease. Careful interpretation of preoperative imaging can often guide to potential anatomic difficulties indicating whether laparoscopic colectomy is feasible. Finally, significant obesity makes laparoscopic colectomy more challenging. Patient BMI greater than 40 increases the difficulty substantially, especially early in the learning curve or for the surgeon who performs relatively few laparoscopic procedures.8
Preoperative Preparation
Preoperative preparation of the patient can make a laparoscopic colectomy easier. This preparation has three main components including bowel preparation, localization of the disease process, and placement of preoperative ureteral stents, if indicated. In the past, there has been controversy regarding the need for a bowel preparation. Recent evidence suggests that there is decreased surgical site as well as intra-abdominal infection with both a mechanical and an oral antibiotic bowel preperation.9 Moreover, eliminating the bulk of the feces from the colon makes it easier to manipulate the bowel during laparoscopic colectomy. More recent evidence supports the enhanced recovery after surgery (ERAS) pathway for easier and faster postoperative recovery.10 Current ERAS protocols for colorectal surgery have several components worth noting. Pain control is managed with a combination of epidural and local anesthesia as well as Tylenol, Gabapentin, and nonsteroidal anti-inflammatory drugs such as ketorolac and ibuprofen pre- and postoperatively. Intravenous fluid administration is limited both intra- and postoperatively and is goal directed during the operation with esophageal Doppler measurements of volume status. Additionally, volume administration includes liberal use of colloid solution after initial isotonic fluid resuscitation. Finally, patients are encouraged to drink a high carbohydrate-containing solution (ClearFast [ClearFast, BevMD, Encinitas, CA]: 200 g of carbohydrate) 2 hours before surgery to speed up gastrointestinal motility and gastrointestinal (GI) recovery postoperatively. On postoperative day 1, the diet is advanced from clear liquids to solids, as tolerated.11
Often a laparoscopic colectomy is performed for a flat tumor or an endoscopically unresectable flat polyp. Either or both of these may not be visualized on preoperative imaging and if the lesion is not seen then it cannot be assumed that the endoscopic localization is accurate. Even if a hand-assisted technique is used, it cannot be guaranteed that the lesion will be palpable so localization is paramount. Unless the lesion is in the cecum (ileocecal valve, appendiceal orifice, and crow's foot appearance of cecum) or the rectum, it needs to be localized and tattooed prior to the operation. Often, repeat colonoscopy and with tattooing in three locations evenly spaced apart on the bowel mucosa distal to the lesion will allow for easy laparoscopic identification. In case only one tattoo is placed at the base of the lesion, it can be difficult to identify the mass if it is located in the colonic flexures, if there is a robust omentum, or if the lesion is based on the mesenteric border of the bowel. All tattoos should be placed distal to the tumor, as this guides the extent of the resection and ensures that the lesion is removed.
Finally, ureteral identification can be difficult, and preoperative placement of ureteral stents can aid in ureteral localization. For hand-assisted cases, standard ureteral stents can be palpated, and for completely laparoscopic cases, lighted ureteral stents can be utilized. Patients undergoing sigmoid colectomy or LAR with a history of prior radiation, severe diverticulitis with chronic inflammation/abscess, or previous hysterectomy often benefit from ureteral stents. Patients undergoing an ileocolic resection for Crohn disease who have an active inflammatory phlegmon can also benefit from stenting. Finally, ureteral stenting can be useful in obese patients as well.
Patient Position
During laparoscopic colectomy, it is occasionally necessary to change the surgical approach by placing additional trocars to aid in a difficult dissection. As a result, placing the patient in the dorsal lithotomy position in yellow fin stirrups facilitates the ability to also stand between the legs. This gives an additional potential angle of dissection. Placing the patient on padded foam prevents the patient from sliding on the bed and thereby facilitates placing the patient in steep Trendelenburg or steep reverse Trendelenburg as well as left or right side up. Tucking the arms allows both the surgeon and the assistant to stand on the same side of the patient, often making retraction and dissection easier. Finally, a Foley catheter decompressing the bladder is essential for all pelvic laparoscopic colectomy (sigmoid and LAR) but not needed for right colectomy or subtotal colectomy.
Technical Specifics
The intraoperative technical aspects of a laparoscopic colectomy consist of:
Trocar position.
Extraction site.
-
Intracorporeal versus extracorporeal anastomosis.
When planning the laparoscopic surgical approach, the important factors to help delineate trocar position, extraction site, and anastomotic technique are:
Hand-assisted versus complete laparoscopic.
Medial-to-lateral or lateral-to-medial approach.
Benign or malignant pathology.
Taking all of these factors into account enables the surgeon to best plan a successful laparoscopic colectomy, also considering the surgeon's skill set and the goals of the operation (oncologic principles or removal of inflamed tissue). A careful description of each specific colectomy will outline these issues with advice for a successful laparoscopic colectomy.
Specifics of Main Types of Colectomy
Right Colectomy (Neoplasia)
The most important factor when performing a right colectomy is the disease process. All endoscopically unresectable polyps should be treated as if they represent malignancy, so oncologic principles should be followed with surgery. The vessels that are critical for a good oncologic right colectomy are the ileocolic, right colic, and branches of the middle colic vessels. Each of these vessels, as well as the final anastomosis following right colectomy, is in the upper abdomen. To get a good high ligation of the vessels and to easily perform the anastomosis, the focus of a right colectomy is in the supra-/peri-umbilical region. In general, to achieve this, the camera port is best positioned in the umbilicus or just above the umbilicus so that it can be included in the extraction site. The incision ends up being a vertical incision through which extraction and an extracorporeal anastomosis can be performed. If a lateral-to-medial approach is used, then the vessels can also be ligated through this incision during the extracorporeal phase of the operation.
In the lateral-to-medial approach, the main goal of the operation is to mobilize the colon adequately and then perform the vessel ligation and the anastomosis outside the abdominal cavity. As mentioned, the camera should be in the peri-umbilical area and then three additional 5-mm trocars should be placed in the right lower quadrant, suprapubic region, and then in the sub-xiphoid region probably a little to the left of the midline (Fig. 1A). The lower abdominal trocar positions allow for mobilization of the cecum, right colon, and terminal ileum from pelvic and iliac fossa attachments. The upper abdominal trocar allows for additional retraction and also for the lesser sac to be entered and aids in hepatic flexure mobilization. Once the colon is completely mobilized, then the camera port is extended, the key vessels are ligated, and the anastomosis is performed.
Fig. 1.
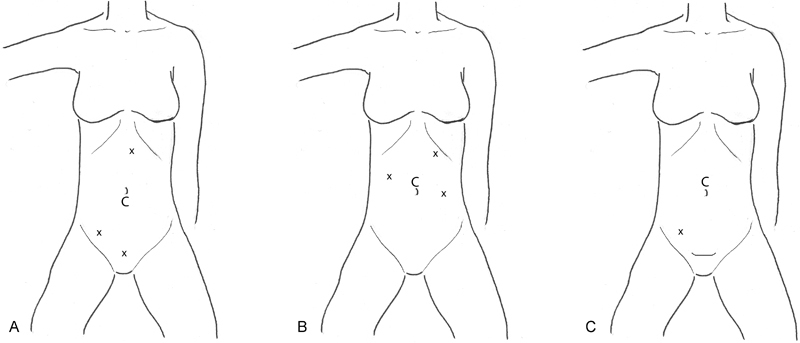
(A) Right colectomy lateral-to-medial approach for neoplasia. (B) Right colectomy medial-to-lateral approach for neoplasia. (C) Right colectomy for inflammatory bowel disease. C, camera; x, trocar site.
In the medial-to-lateral approach, the camera is placed in the same peri-umbilical position, and the additional three trocars are placed in the right mid-abdomen and left mid-abdomen both at the level of the umbilicus. An additional trocar can be placed in the left upper quadrant near the costal margin (Fig. 1B). The first step is to identify the origin of the ileocolic vessel. A good identifying landmark for this vessel is the duodenum in the retroperitoneum at the root of the transverse mesocolon mesentery. The vessel is just medial to the duodenum. Lifting the vessel up allows it to be followed out the ileocecum. Once this is confirmed, it should be ligated intracorporeally using a laparoscopic stapler or an energized tissue-sealing device (e.g., Ligasure [Medtronic, Minneapolis, MN], Harmonic Scalpel [Ethicon, Cincinnati, OH], or EnSeal [EnSeal, Ethicon, Cincinnati, OH]). Next, the retroperitoneal dissection over the head of pancreas and duodenum behind the hepatic flexure is accomplished and carried down the length of the ascending colon into the retrocecal region. After this is accomplished, the ileocolic vessel should be retracted superiorly up toward the liver, and one of the aforementioned energy devices should be utilized to dissect medially along the vessel out to the ileum. Once these steps are completed, the lesser sac can be entered by dividing the gastrocolic omentum to the left of the midline (where it is thinner than on the right). This gains access to the lesser sac, allowing transverse colon mobilization; the gastrocolic omentum can easily be divided from left to right with this approach. The small flimsy adhesions from the back wall of the stomach to the transverse mesocolon mesentery should also be divided, as this allows for better mobility of the transverse colon. Once this is done, dissection should continue along the gastrocolic omentum heading toward the inferior and lateral edge of the liver, thereby completing mobilization of the hepatic flexure. The surgeon's preferred energy device/tissue sealer can then be used to divide the lateral attachments to the ascending colon, which completes the mobilization of the right colon. The camera port in the upper midline can be expanded, the bowel exteriorized, and the anastomosis performed.
Right Colectomy (Inflammatory Bowel Disease)
For nonneoplastic processes which do not require oncologic mesenteric lymphadenectomy, the vessels do not need to be divided in the upper abdomen. A Pfannenstiel incision with a hand-assisted approach can be useful in this case. The camera should be placed in the peri-umbilical region, and a 5-mm trocar can be placed in the right lower quadrant. The surgeon's hand, via the hand port, is used to retract the colon and the lateral attachments are mobilized using the right lower quadrant port. The hepatic flexure does not usually need to be mobilized for ileocolic Crohn disease. The anastomosis can then be performed through the Pfannenstiel incision (Fig. 1C).
Sigmoid/Left Colectomy (Neoplasia and Inflammatory Bowel Disease)
Similar to a right colectomy, the disease process often influences the approach to a sigmoid or left colectomy. The main vessel to divide is the superior hemorrhoidal vessel, located in the lower abdomen just to the left of the midline. In this operation, the trocar position and extraction port are the same whether a medial-to-lateral or lateral-to-medial approach is utilized. The camera port should be placed in the umbilical region, and dissecting trocars should be placed in the suprapubic region, right lower quadrant (triangulated with the suprapubic port and camera port), and left lower quadrant (Fig. 2).
Fig. 2.
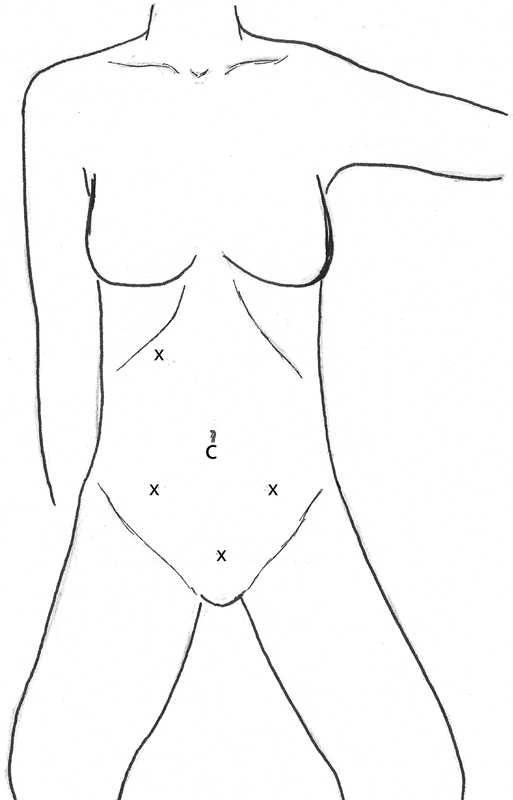
Sigmoid/Left colectomy lateral to medial or medial to lateral. C, camera; x, trocar site.
When performing the dissection utilizing the lateral-to-medial approach, the sigmoid colon is released from its lateral attachments all the way up to and around the splenic flexure, if needed. Care should be taken to not dissect too posterior or too lateral, as there is a tendency to stray lateral and posterior in the dissection, thereby often bringing the kidney up with the colon. At the splenic flexure, the omentum can be taken off the colon, which makes mobilization of the splenic flexure more complete. If needed, an additional trocar can be placed in the right upper quadrant to facilitate access to the lesser sac and division of the gastrocolic ligament, thereby completing splenic flexure mobilization. Once the descending colon and the splenic flexure have been released from their attachments, a Pfannenstiel or lower midline extraction port should be made, allowing extracorporeal ligation of the superior hemorrhoidal vessels and creation of a colorectal anastomosis.
In the medial-to-lateral approach, the same trocar positions are utilized, but the sequence of colonic mobilization is altered. In this approach, the sigmoid colon is lifted upward toward the abdominal wall and toward the pelvis, thereby displaying the course of the superior hemorrhoidal vessels. A window is then made underneath the vessels and the dissection is carried out into the retroperitoneum to identify the left ureter. Once the ureter is identified, a window is opened around the vessels, and they are divided with a laparoscopic stapler or an energy device. The next step is to dissect in the retroperitoneum, freeing the left colon from its retroperitoneal attachments. The mesentery of the left colon is divided medially along the aorta up to the lateral aspect of the distal duodenum to free up the mesentery. The lesser sac is then entered, and the gastrocolic omentum is divided. The lateral attachments are then released, completing the mobilization of the colon. A small Pfannenstiel or midline incision is made, and the anastomosis can be performed through this incision. If hand-assisted technique is used, the suprapubic port can be eliminated and the surgeon's hand utilized as the additional retractor. The remaining sequence of each approach is as described as previously.
Total/Subtotal Colectomy
Total abdominal colectomy and/or subtotal colectomy are generally performed for polyposis syndromes, multiple cancers/endoscopically unresectable polyps, slow transit constipation, or colitis (Crohn disease with spared rectum or as a first stage of a three-stage restorative proctocolectomy for chronic ulcerative colitis). The basic principles of this operation are a combination of the above sequence of events for the right and left colectomies as described. In general, the total or subtotal colectomy is more easily performed with a hand-assisted approach. A Pfannenstiel hand port is placed with a supraumbilical camera port and then right and left mid-abdomen trocars at the level of the umbilicus and a left subcostal trocar as well (Fig. 3). The sequence of the operation proceeds in the same fashion as the medial-to-lateral right colectomy with the hand used to assist in retraction. After the right colon is mobilized, the gastrocolic omentum division is completed from the right to the left, the transverse colon mesentery is completely divided, and the left colic gutter is then mobilized superiorly near the splenic flexure inferiorly toward the pelvis, taking the lateral attachments first and then the mesentery. After the left colon is mobilized, if the sigmoid is to be removed, then it is likewise mobilized as described previously. The anastomosis is then performed from the ileum to the sigmoid or upper rectum through the lower abdominal incision.
Fig. 3.

Total/subtotal colectomy. C, camera; x, trocar site.
Low Anterior Resection/Abdominoperineal Resection
Laparoscopic rectal resection often proves quite challenging as evidenced by the longer learning curve described in the introduction. The challenges of laparoscopic rectal resection are manifold. First, dissection in a narrow space in close vicinity to multiple important vessels, nerves, ureters, vagina, and prostatic urethra is challenging. Second, a good oncologic total mesorectal excision is not abrogated in rectal cancer, and in IBD cases, the rectum is often stiff and infiltrated with surrounding fat, making dissection and retraction even more difficult. After camera placement in the infra-umbilical position, the most important factor to consider is whether the sigmoid colon is redundant enough to reach the rectal stump in the pelvis to restore GI continuity when that is the goal. The mobilization is best done without a hand port, as this device would be in the lower midline placing the hand utilized for retraction directly in the way of the majority of the dissection. Moreover, in an abdominal-perineal resection for a distal rectal cancer or a proctectomy for IBD, the specimen can be removed through the perineum, thereby eliminating a larger abdominal extraction port. The trocars should be placed in similar positions to the sigmoid/left colon resection (Fig. 4), as described in the next paragraphs.
Fig. 4.
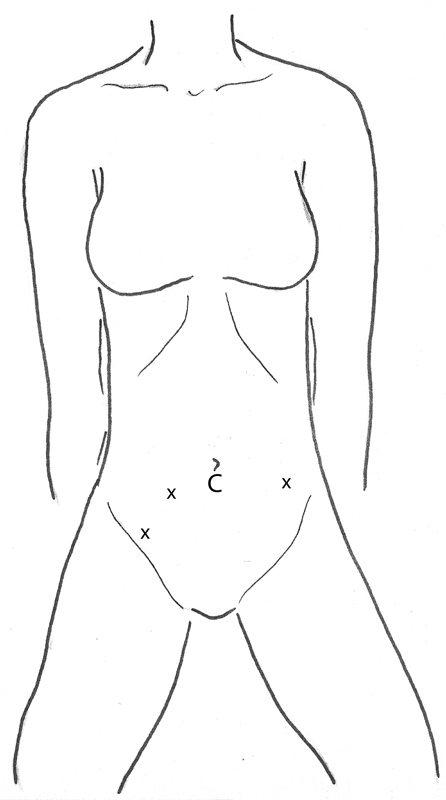
Low anterior resection/abdominoperineal resection. C, camera; x, trocar site.
The camera port should be placed in the umbilical region. If the distance between the umbilicus and pubic symphysis is short, the camera may be placed above the umbilicus. However, if this length is long, the camera can be at or just below the umbilicus. A retraction port should be placed in the left lower quadrant a single hands-breadth (8–10 cm) lateral and inferior to the camera port in the left lower quadrant, just medial to the anterior superior iliac spine. A dissecting and retracting port should each be placed in the right lower quadrant approximately one hands-breadth apart. The left lateral attachments should initially be left intact to serve as anatomic retraction. The colon should be retracted upward to the abdominal wall and toward the pelvis, so that the superior hemorrhoidal vessels can be identified. The peritoneum on the right at the root of these vessels is incised, and the retroperitoneal dissection posterior to this is accomplished allowing identification of the left ureter. After the vessels are divided, the retrorectal plane near the sacral promontory is entered. This can be done sharply with electrocautery or shears and should be extended appropriately distal to the lesion. Then the right lateral stalks should be divided in a similar fashion. Following this, dissection is performed in the anterior plane (recto-vaginal septum or between the seminal vesicles and rectum). Once this is accomplished, the right-sided ports can be used to retract the rectum to the right, and the left lower quadrant port can be used to take the left-sided stalks.
After this, a small suprapubic extraction port can be made, allowing stapling across the distal margin. The anvil for an end-to-end anastomosis (EEA) circular stapler can be sutured into the proximal bowel, and the anastomosis can be performed as can the air-leak test through this small open incision. Alternatively, a laparoscopic stapler can be used to divide distally in an anterior to posterior fashion, allowing a smaller incision to be made to extract the specimen and sew in the anvil. In a complete proctectomy for neoplasia or IBD, the perineal incision can be used as the extraction site. The perineal dissection can be done with a second team in the lithotomy position or after the ostomy is created with the patient in the prone position. The trocar sites can be incorporated into the ostomy site or vice versa as long as they are easy to use for the dissection and appropriately placed at the beginning of the operation. Also, one of the 5-mm trocar sites should be used for a pelvic drain if a drain is desired.
Conclusion
Since the results of the COST trial were published in 2004, a greater percentage of colectomies have been performed utilizing a minimally invasive technique. These numbers depend on the disease process, surgeon's skill and preference, as well as hospital location and teaching affiliation, with larger teaching hospitals in urban settings performing a greater percentage of minimally invasive colectomies. Several approaches facilitate a minimally invasive approach for colon resection, including complete laparoscopy and the hand-assisted approach. The comfort level of the surgeon and specialty training also influence the chance that a colectomy is performed laparoscopically. As the drive to decrease hospital length of stay and complications continues, laparoscopic colectomy in combination with enhanced recovery pathways that have the potential to decrease complications and hasten patient recovery will continue to gain popularity.
References
- 1.Weiss A J, Elixhauser A. Trends in Operating Room Procedures in U.S. Hospitals, 2001–2011. Healthcare Cost and Utilization Project Statistical Brief #171. Agency for Healthcare Research and Quality March. 2014:1–14. [Google Scholar]
- 2.Clinical Outcomes of Surgical Therapy Study Group . A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004;350(20):2050–2059. doi: 10.1056/NEJMoa032651. [DOI] [PubMed] [Google Scholar]
- 3.Rea J D, Cone M M, Diggs B S, Deveney K E, Lu K C, Herzig D O. Utilization of laparoscopic colectomy in the United States before and after the clinical outcomes of surgical therapy study group trial. Ann Surg. 2011;254(2):281–288. doi: 10.1097/SLA.0b013e3182251aa3. [DOI] [PubMed] [Google Scholar]
- 4.Moghadamyeghaneh Z, Carmichael J C, Mills S, Pigazzi A, Nguyen N T, Stamos M J. Variations in laparoscopic colectomy utilization in the United States. Dis Colon Rectum. 2015;58(10):950–956. doi: 10.1097/DCR.0000000000000448. [DOI] [PubMed] [Google Scholar]
- 5.Tekkis P P, Senagore A J, Delaney C P, Fazio V W. Evaluation of the learning curve in laparoscopic colorectal surgery: comparison of right-sided and left-sided resections. Ann Surg. 2005;242(1):83–91. doi: 10.1097/01.sla.0000167857.14690.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ritchie W P Jr Rhodes R S Biester T W Work loads and practice patterns of general surgeons in the United States, 1995-1997: a report from the American Board of Surgery Ann Surg 19992304533–542., discussion 542–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valentine R J Jones A Biester T W Cogbill T H Borman K R Rhodes R S General surgery workloads and practice patterns in the United States, 2007 to 2009: a 10-year update from the American Board of Surgery Ann Surg 20112543520–525., discussion 525–526 [DOI] [PubMed] [Google Scholar]
- 8.Masoomi H, Moghadamyeghaneh Z, Mills S, Carmichael J C, Pigazzi A, Stamos M J. Risk factors for conversion of laparoscopic colorectal surgery to open surgery: does conversion worsen outcome? World J Surg. 2015;39(5):1240–1247. doi: 10.1007/s00268-015-2958-z. [DOI] [PubMed] [Google Scholar]
- 9.Cannon J A, Altom L K, Deierhoi R J. et al. Preoperative oral antibiotics reduce surgical site infection following elective colorectal resections. Dis Colon Rectum. 2012;55(11):1160–1166. doi: 10.1097/DCR.0b013e3182684fac. [DOI] [PubMed] [Google Scholar]
- 10.Cakir H, van Stijn M F, Lopes Cardozo A M. et al. Adherence to Enhanced Recovery After Surgery and length of stay after colonic resection. Colorectal Dis. 2013;15(8):1019–1025. doi: 10.1111/codi.12200. [DOI] [PubMed] [Google Scholar]
- 11.Feldheiser A, Aziz O, Baldini G. et al. Enhanced recovery after surgery (ERAS) for gastrointestinal surgery, part 2: consensus statement for anaesthesia practice. Acta Anaesthesiol Scand. 2016;60(3):289–334. doi: 10.1111/aas.12651. [DOI] [PMC free article] [PubMed] [Google Scholar]


